Abstract
Objective
This study examined the effect of hormone therapy (HT) on the plasma concentration of remnant lipoprotein cholesterol (RLP-C) and high density lipoprotein (HDL) subpopulations and the contribution of HT-related changes in these lipoproteins to the progression of coronary heart disease (CHD) in postmenopausal women.
Methods
Study participants were 256 women who completed the Estrogen Replacement and Atherosclerosis (ERA) trial, a placebo-controlled, randomized trial that examined the effects of 3.2 years of conjugated equine estrogen (CEE, 0.625 mg/day) or CEE (0.625 mg/day) plus medroxyprogesterone acetate (MPA, 2.5 mg/day) on post-menopausal women with established coronary atherosclerosis. Quantitative coronary angiography and plasma RLP-C and HDL subpopulations were assessed at baseline and at follow-up.
Results
Relative to placebo, both CEE and CEE+MPA caused a significant reduction in plasma RLP-C concentrations and a significant increase in α1 and α2 HDL subpopulations. However, in the HT-treated subjects, faster progression of coronary atherosclerosis was observed in women who experienced the greatest reductions in RLP-C and in preβ1 HDL subpopulations.
Conclusions
Our data suggest that individual variability in RLP-C and HDL subpopulation response to HT is a predictor of CHD progression. Lipoprotein response to HT may be an indirect marker of susceptibility to other harmful effect of HT in postmenopausal women with established CHD or an indication of formation of dysfunctional lipoproteins.
Keywords: Lipoproteins, hormone therapy, coronary heart disease, angiography
INTRODUCTION
Randomized controlled trials conducted to assess the effect of hormone therapy (HT) on coronary heart disease (CHD) risk in postmenopausal women have concluded that HT does not reduce the overall risk of CHD (1–6). The lack of protection from CHD occurs in the context of HT-induced beneficial changes in intermediate markers of CHD risk, such as reductions in plasma low-density lipoprotein cholesterol (LDL-C) concentrations and increases in plasma high-density lipoprotein cholesterol (HDL-C) concentrations. The results of these randomized trials are in contrast with those of several previous observational studies and with those of studies conducted in animal models of atherosclerosis, which had suggested a protective role of HT (7, 8). It has recently been suggested that the discrepancy in findings between the randomized and observational studies may in part be reconciled by the observation that HT-related CHD risk tends to be lower in women who initiate HT close to menopause and higher in women who initiate HT distant from menopause (9), in conjunction with the fact that HT is usually initiated several years after menopause in the randomized trials but at the time of menopause in the observational studies. The age-dependent effect of HT on CHD risk may be related to the dual effects of estrogen: beneficial, such as reductions in plasma LDL-C and increases in HDL-C concentrations, and harmful, such as increased thrombogenicity, inflammation, and plaque instability (10–12). It has been hypothesized that, in women with CHD, the diseased endothelium may tilt the balance toward the harmful effects of HT (13).
Lipoprotein subfractions may be better indicators of CHD risk than triglyceride, LDL-C and HDL-C concentrations (14–16). We tested the hypothesis that variability in lipoprotein remnant and HDL subpopulation response to HT may play a role in the individual cardiovascular response to HT treatment.
METHODS
Subjects and study design
Subjects were women participating in the ERA trial, a placebo-controlled, randomized study (2, 17). Women were enrolled into the study if they were postmenopausal and had coronary artery disease, defined as ≥30% stenosis of at least one epicardial coronary artery by quantitative coronary angiography. Women with a history of uncontrolled diabetes or hypertension, deep-vein thrombosis or pulmonary embolism, kidney disease, symptomatic gallstones, or TG levels >400 mg/dL were excluded. A total of 309 women were enrolled in the ERA trial and were randomized to three parallel treatment arms: placebo, conjugated equine estrogen (CEE 0.625 mg/day, as Premarin, Wyeth-Ayerst), and CEE and medroxyprogesterone acetate (CEE 0.626 mg/day and MPA 2.5 mg/day, as Prempro, Wyeth-Ayerst). Paired coronary angiography assessments and plasma samples at baseline and at follow-up were available in 256 women (placebo: N=88; CEE: N=84; CEE+MPA: N=84).
Assessment of plasma lipids, remnant lipoproteins and HDL subpopulations
Fasting blood samples were obtained from study participants at the baseline visit and at the year-1 visit. Blood was drawn in tubes containing 0.1% EDTA and plasma was separated by centrifugation at 1000 × g for 30 min at 4 °C. Plasma total cholesterol (TC) and triglyceride (TG) concentrations were measured by automated assays (18). HDL-C levels were measured after precipitation of apo B-containing lipoproteins with heparin-manganese (19). Apo A-I in plasma and apo C-III in plasma and in HDL were measured with immunoturbidimetric assays (Wako Diagnostics, Richmond, VA) (20).
Plasma concentrations of remnant-like lipoprotein cholesterol (RLP-C) were measured using an immunoseparation technique (Polymedco, Cortlandt Manor, NY) (21).
Apo A-I-containing HDL subpopulations were measured by 2-dimensional gel electrophoresis, as previously described (22). This methodology allows for the separation of HDL into 8 subpopulations (preβ1–2, α1–3, preα1–3), according to charge, size, and composition. The concentration of each HDL subpopulation was calculated by multiplying its percentage with total plasma apo A-I concentration and expressed as mg/dL of apo A-I.
Coronary angiography
Quantitative coronary angiography was performed at baseline and after approximately 3.2 y of follow-up, as previously described (2, 17). Analysis of angiograms was performed in pairs with a previously validated system of cine-projection (SME 3500, Sony, Park Ridge, NJ). The mean intra-operator difference between blinded duplicate measurements of minimal diameter was 0.02 mm. The reference, minimal (the point of greatest narrowing), and average luminal diameters were obtained for 10 proximal epicardial coronary artery segments, as previously described (2, 17).
Statistical analyses
The SAS statistical package (version 9.1) was used for statistical analyses. All analyses were based on intent-to-treat. Skewed variables were log-transformed before analysis. Changes in plasma lipid levels, apolipoprotein levels, and mean minimum coronary artery diameter (MMD) were calculated as: Value follow-up − Value baseline. The effects of treatment on plasma concentration of lipids, apolipoproteins, and lipoprotein subspecies were tested by ANOVA. Mixed-model analysis of covariance was used to test the hypothesis of an association between changes in lipoprotein subspecies and changes in MMD. The model was adjusted for baseline minimal coronary lumen diameter, location of the segment in the coronary artery tree, age, body mass index (BMI), race, smoking, hypertension, diabetes, use of lipid-lowering medications, and prior percutaneous transluminal coronary angioplasty. Analyses of MMD change by tertiles of changes in RLP-C and HDL subpopulations were also carried out. These analyses were adjusted for the same CHD risk factors as listed in the mixed-model analysis. A P value <0.05 was set as statistically significant.
RESULTS
Women randomized to placebo, estrogen alone, or the combination estrogen plus progestin were comparable with respect to baseline characteristics (Table 1). Moreover, there were no differences in plasma concentrations of RLP-C, apo C-III, apo A-I, HDL-C, or HDL subpopulations in the three groups of women before randomization, with the only exception of a small difference in HDL preα1 particle levels (Table 2).
Table 1.
Characteristics of ERA participants at baseline (N=256).
| Placebo | Estrogen | Estrogen+Progestin | P value | |
|---|---|---|---|---|
| Age, years * | 66 (±7) | 66 (±8) | 65 (±7) | 0.82 |
| BMI, kg/m2 | 30.2 (±8.6) | 29.1 (±6.0) | 29.7 (±8.2) | 0.66 |
| Race, % | 0.33 | |||
| White | 82 | 83 | 85 | |
| Black | 12 | 12 | 15 | |
| Other | 6 | 5 | 0 | |
| Smoking, % | 22 | 16 | 18 | 0.31 |
| Diabetes, % | 33 | 21 | 26 | 0.24 |
| Lipid-lowering medications, % | 37 | 34 | 38 | 0.70 |
| Hypertension, % | 70 | 64 | 69 | 0.61 |
| Plasma lipids, mg/dL | ||||
| TC | 215 (±40) | 214 (±47) | 220 (±38) | 0.45 |
| TG | 207 (±122) | 196 (±115) | 170 (±82) | 0.10 |
| LDL-C | 134 (±36) | 133 (±40) | 138 (±34) | 0.40 |
| Minimal coronary artery Diameter, mm | 1.95 (±0.75) | 1.94 (±0.72) | 1.89 (±0.74) | 0.31 |
Data expressed as mean (±SD)
Table 2.
Plasma concentrations of RLP-C, apo C-III, apo A-I, and HDL subpopulations at baseline by randomization group.
| Placebo | Estrogen | Estrogen+Progestin | P value | |
|---|---|---|---|---|
| mg/dL | ||||
| RLP-C | 14.2 (±14.5) | 12.5 (±9.1) | 11.7 (±9.3) | 0.42 |
| Apo C-III | 14.1 (±4.5) | 14.3 (±5.8) | 13.7 (±5.0) | 0.66 |
| HDL apo C-III | 9.9 (±4.8) | 9.78 (±3.4) | 9.5 (±4.3) | 0.68 |
| Apo A-I | 125 (±21) | 125 (±17) | 128 (±20) | 0.55 |
| HDL-C | 43 (±12) | 43 (±11) | 46 (±14) | 0.14 |
| HDL supopulations | ||||
| preβ1 | 23.5 (±9.8) | 23.8 (±8.3) | 22.3 (±8.3) | 0.55 |
| preβ2 | 2.1 (±1.4) | 2.1 (±1.3) | 2.3 (±1.1) | 0.69 |
| α1 | 12.5 (±8.8) | 11.7 (±7.1) | 13.8 (±8.8) | 0.27 |
| α2 | 37.1 (±10.9) | 37.8 (±10.0) | 38.8 (±10.1) | 0.58 |
| α3 | 38.3 (±9.5) | 38.7 (±8.0) | 38.0 (±8.7) | 0.86 |
| preα1 | 2.4 (±1.9) | 2.3 (±2.2) | 3.0 (±2.3) | 0.05 |
| preα2 | 4.1 (±1.9) | 4.2 (±2.0) | 4.7 (±2.2) | 0.11 |
| preα3 | 4.7 (±2.1) | 4.8 (±1.8) | 5.0 (±1.9) | 0.29 |
values are mean (±SD)
HDL subpopulations expressed as mg/dL of total plasma apo A-I.
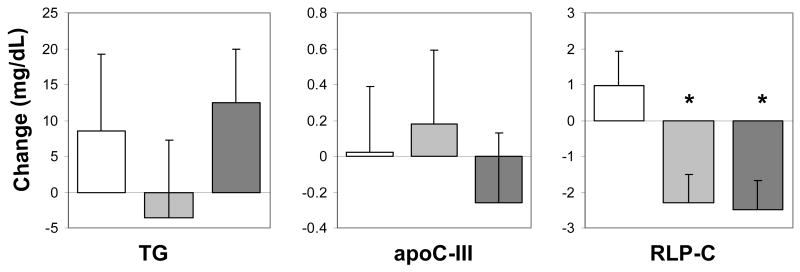
Over the course of the trial, no significant changes in plasma TG, apo C-III, or RLP-C levels were observed in the placebo arm of the study (Figure 1). Relative to placebo, active treatment with estrogen alone or with estrogen plus progestin was associated with significant overall reductions in plasma RLP-C concentrations (mean change: −2.3 mg/dL, range: −23 to +25 mg/dL; and mean change: −2.5 mg/dL, range: −44 to +16 mg/dL, respectively, P<0.001), but no significant changes in TG and apo C-III concentrations (Figure 1).
Figure 1.
Changes in plasma TG (panel A), apo C-III (panel B), and RLP-C (panel C) concentrations in the placebo (white), estrogen (light gray), and estrogen plus progestin (dark gray) arms of the ERA study; * P<0.001, relative to changes in the placebo arm. Data expressed as mean change±SE.
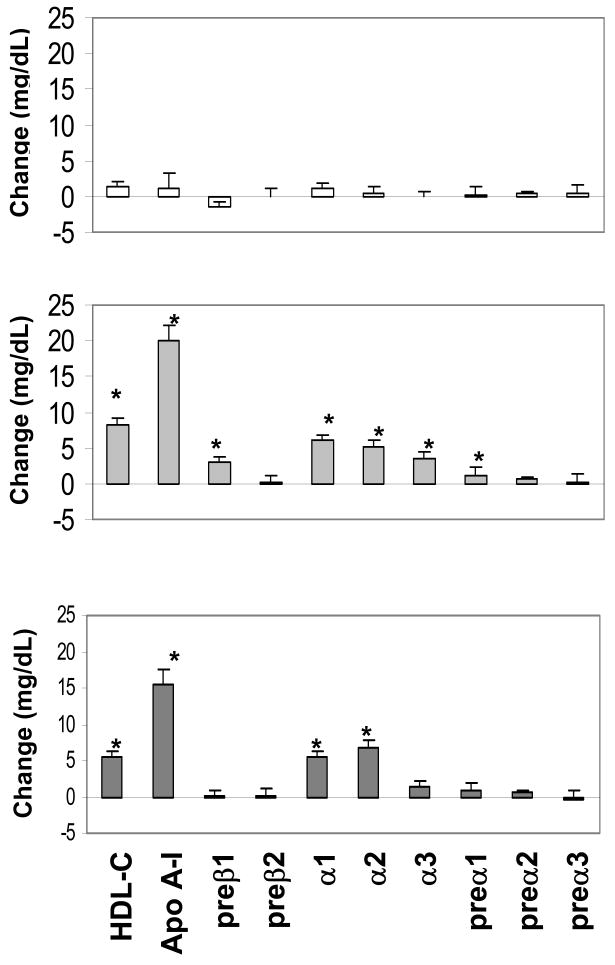
Relative to baseline, no significant changes in plasma HDL-C, apo A-I, and HDL subpopulation levels occurred in the placebo arm. Relative to placebo, estrogen alone caused significant increases in mean plasma concentrations of HDL-C, apo A-I, and the HDL subpopulations preβ1, α1, α2, α3, and preα1 (Figure 2). Relative to placebo, the combination treatment was associated with significant increases in plasma concentrations of HDL-C, apo A-I, and the HDL subpopulations α1 and α2 (Figure 2).
Figure 2.
Changes in plasma HDL-C, apo A-I, and HDL subpopulations in the placebo (upper panel), estrogen (middle panel), and estrogen + progestin (lower panel) arms of the ERA trial. HDL subpopulations are expressed as mg/dL of plasma apo A-I. * P<0.001, relative to changes in the placebo arm. Data expressed as mean change±SE.
The changes in plasma RLP-C concentrations were significantly and positively correlated with changes in plasma TG and preβ1 (Spearman’s rho=0.339 and 0.181, respectively, P<0.004), and inversely associated with changes in plasma α1 and α2 concentrations (rho=−0.302 and −0.200, respectively, P<0.002).
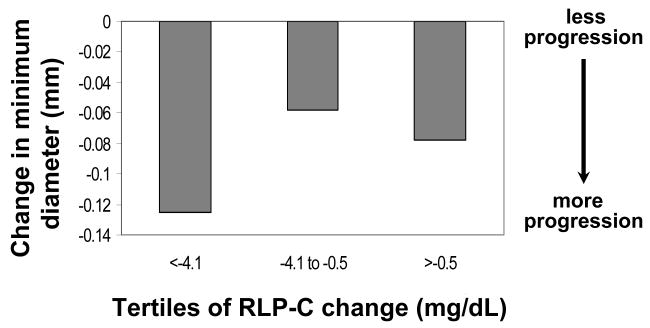
The changes in plasma RLP-C concentrations were significantly associated with changes in minimal coronary artery diameter in the whole population (P<0.008), reflecting faster progression of coronary atherosclerosis in women who experienced greater reductions in RLP-C concentrations on trial (0.0038 mm average reduction in MMD per 1 mg/dL reduction in RLP-C levels) (Table 3). Since estrogen and the combination treatment caused similar changes in RLP-C and HDL subpopulations, these two groups were combined in further analyses. When only subjects in active treatment arms were included in the analysis, the association between RLP-C and coronary disease progression remained highly significant (Table 4). Moreover, women in the tertile of larger HT-related reductions in RLP-C concentrations had significantly faster coronary atherosclerosis progression (P<0.016, after adjustment) (Figure 3). No association between change in minimal lumen diameter and change in RLP-C concentrations was observed in the placebo group (P=0.52). Changes in plasma TG and apo C-III levels were not associated with progression in coronary artery disease either in the whole population (Table 3) or in subjects in the active treatment arms (Table 4).
Table 3.
Associations between changes in plasma lipoprotein levels and progression of coronary atherosclerosis (all arms).
| Variable 1 (change) | Change in minimum diameter
|
|
|---|---|---|
| Estimate2 | P | |
| RLP-C | 0.0038 | 0.008 |
| Apo C-III | 0.0018 | 0.57 |
| HDL apo C-III | 0.0020 | 0.57 |
| TG | 0.0000 | 0.96 |
| Apo A-I | −0.0002 | 0.74 |
| HDL-C | −0.0025 | 0.10 |
| HDL supopulations | ||
| preβ1 | 0.0038 | 0.04 |
| preβ2 | −0.0011 | 0.46 |
| α1 | −0.0009 | 0.58 |
| α2 | −0.0014 | 0.31 |
| α3 | −0.0026 | 0.10 |
| preα1 | 0.0035 | 0.64 |
| preα2 | 0.0034 | 0.69 |
| preα3 | 0.0092 | 0.21 |
Change in variables expressed in mg/dL
Beta-hat from mixed-model analysis adjusted for mean minimal diameter at baseline, location in the artery segment, age, BMI, race, smoking, hypertension, diabetes, use of lipid-lowering medications, and prior percutaneous angioplasty
Table 4.
Associations between changes (and percent changes) in plasma lipoproteins and coronary atherosclerosis progression in subjects in the active treatment arms only.
| Variable 1 (change) | Change in minimum diameter
|
Variable(% change) | Change in minimum diameter
|
||
|---|---|---|---|---|---|
| Estimate2 | P | Estimate2 | P | ||
| RLP-C | 0.0074 | 0.0001 | RLP-C | 0.0011 | 0.007 |
| Apo C-III | 0.0030 | 0.47 | Apo C-III | 0.0026 | 0.54 |
| HDL apo C-III | 0.0046 | 0.29 | HDL apo C-III | 0.0002 | 0.57 |
| TG | 0.0000 | 0.91 | TG | 0.0000 | 0.99 |
| Apo A-I | −0.0006 | 0.47 | Apo A-I | −0.0004 | 0.65 |
| HDL-C | −0.0045 | 0.02 | HDL-C | −0.0016 | 0.05 |
| HDL supopulations | HDL subpolulations | ||||
| preβ1 | 0.0060 | 0.01 | preβ1 | 0.0010 | 0.07 |
| preβ2 | −0.0138 | 0.47 | preβ2 | 0.0000 | 0.75 |
| α1 | −0.0026 | 0.21 | α1 | −0.0001 | 0.37 |
| α2 | −0.0031 | 0.08 | α2 | −0.0001 | 0.08 |
| α3 | −0.0013 | 0.55 | α3 | −0.0003 | 0.66 |
| preα1 | 0.0008 | 0.93 | preα1 | 0.0000 | 0.27 |
| preα2 | −0.0046 | 0.68 | preα2 | −0.0002 | 0.56 |
| preα3 | 0.0129 | 0.19 | preα3 | 0.0002 | 0.65 |
Change in variables expressed in mg/dL
Beta-hat from mixed-model analysis adjusted for mean minimal diameter at baseline, location in the artery segment, age, BMI, race, smoking, hypertension, diabetes, use of lipid-lowering medications, and prior percutaneous angioplasty.
Figure 3.
Mean changes in mean minimum coronary artery diameter by tertiles of changes in plasma RLP-C levels (P<0.016) in women randomized to HT (N=152).
Changes in plasma apo A-I and HDL-C concentrations did not predict coronary atherosclerosis progression. However, changes in preβ1 HDL particle concentrations were associated with changes in minimum coronary artery diameter, suggesting faster coronary artery disease progression in women who had greater reductions in this HDL particle during the trial (Table 3). This association was still significant after exclusion of subjects on placebo (Table 4). While faster progression of coronary atherosclerosis was observed in women in the tertile of greater reductions in preβ1, this association was no longer significant after adjustment for other CHD risk factors (P=0.11). None of the other HDL particles were predictors of CHD progression either in the whole population (Table 3) or in the active treatment groups (Table 4). In the placebo group, changes in preβ1 particle concentrations were not related to changes in mean minimal diameter over the course of the trial (P=0.92). In the placebo group, a trend toward an association of the reduction in mean minimal lumen diameter with increases in α3 (estimate: −0.0034, P=0.08), and reductions in preα2 (estimate: 0.0244, P=0.06), and preα3 (estimate: 0.0179, P=0.09) concentrations was observed. These latter associations are in agreement with the suggested proatherogenic role of α3 and antiatherogenic role of preα particles (15, 16).
DISCUSSION
Clinical intervention trials of lipid-lowering medications have clearly established the causative association between LDL-C lowering and CHD risk reduction (23). The importance of HDL-C raising in the prevention of CHD has also been documented (24–26). Based on the documented LDL-C lowering and HDL-C raising effects of most HT preparations and the CHD risk reduction with HT in observational studies, it had been postulated that HT could lower the risk of CHD in postmenopausal women in part through its effects on plasma lipoproteins. However, randomized clinical trials of HT have consistently shown lack of protection against CHD in postmenopausal women, both in secondary (1, 3, 4) and primary prevention (5, 6) settings, in spite of documented beneficial changes in plasma lipid levels with HT. In agreement with the results of other randomized HT trials, the ERA study did not show cardiovascular benefit of HT in postmenopausal women with established CHD (2).
Effect of HT on plasma remnant lipoproteins
A significant reduction in plasma RLP-C concentrations was observed in both active treatment arms of the study, in agreement with most (27–30) but not all HT studies (31). In these studies, the reduction in plasma remnant lipoprotein concentrations occurred in the context of no change or elevation in plasma TG levels. Estrogen administration may cause elevations in plasma TG concentrations by increasing hepatic synthesis and secretion of TG-rich very low density lipoproteins (VLDL) (32). Estrogen also increases the hepatic expression of the LDL receptor (33). The reduction in remnant lipoproteins observed with HT may be attributed to their increased uptake via the interaction of apo E on their surface with the hepatic LDL receptor.
Effect of HT on plasma HDL subpopulations
Significant increases in HDL-C and apoA-I levels were observed in both active treatment arms of the ERA trial. HT increases HDL-C and apo A-I in part by increasing apo A-I production (34). The mechanism for the HT-related change in HDL subpopulation profile is not known, but it may be explained by both an increase in apo A-I synthesis and changes in the activity of enzymes and receptors involved in the continuous remodeling of HDL in plasma: estrogen lowers the expression of both the hepatic HDL receptor scavenger receptor class B type I (SR-BI) (35), which is likely to result in an increase in large HDL particles (36), and the enzyme hepatic lipase, which can further promote the formation of large HDL particles and also the increase in preβ1 particle.
Relation of changes in lipoprotein subpopulations to progression in coronary disease
The change in minimal coronary artery diameter over the course of the study was similar in the three treatment arms (2).
Contrary to expectations, women who experienced greater reductions in atherogenic remnant lipoproteins on HT showed faster progression in coronary disease. Similarly, women who experienced greater reductions in HDL preβ1 concentrations during HT had faster progression of coronary atherosclerosis.
Remnant lipoproteins are atherogenic, especially in women (14, 37). In the same ERA population, we have previously documented a significant positive association between elevated levels of remnant lipoproteins and extent of coronary artery disease at baseline (38). To our knowledge, only the WAVE study had examined the association between changes in RLP-C concentrations and progression of CHD in postmenopausal women on HT (31). However, in the WAVE study there were no significant changes in RLP-C with HT and no association with CHD progression was detected.
It is well established that higher HDL-C levels are associated with a reduction in CHD risk (39). Moreover, several studies have indicated that higher plasma α1 and α2 and lower preβ1 particle concentrations are better predictors of lower CHD risk than HDL-C (15, 16, 40). In contrast, we observed faster progression in disease in subjects who experienced greater HT-related reductions in preβ1 concentrations. Preβ1 is a small, lipid-poor apo A-I containing particle that acquire cholesterol through interaction with ABCA1, a transporter on plasma cell membranes, and it has been proposed that elevations in preβ1 reflect an impairment in HDL maturation (15).
The mechanism by which HT causes lipoprotein changes is different from that of other lipid-lowering medications that have been proven to beneficially influence CHD risk. Both statins and niacin remodel HDL by increasing the large α1 and α2 particles and reducing the small α3 and preβ1 particles (41, 42). In contrast, HT increased the concentration of both large and small HDL particles. It is possible that HT promotes the formation of dysfunctional HDL via different mechanisms: by promoting the formation of TG-enriched HDL (43), which have reduced anti-atherogenic potential; by reducing the hepatic SR-BI expression and SR-BI-mediated hepatic selective cholesterol removal; and by increasing HDL pro-inflammatory properties in the setting of systemic inflammation (44). Another possibility is that the degree of change in lipoprotein levels during HT is an indirect marker of “estrogen sensitivity” in women with CHD, identifying subjects with an increased inflammatory response to HT. In the ERA study, women in the tertile with greater RLP-C reductions had significantly greater on-trial changes in inflammatory markers than women in the other two tertiles (mean change in C-reactive protein: 0.44 μg/ml vs 0.28 μg/ml, respectively, P<0.03; mean change in intercellular adhesion molecule-1: −20 ng/ml vs −30 ng/ml, respectively, P<0.01). The molecular mechanisms that lead to an increased expression of LDL-R, apo A-I gene, and inflammatory genes by estrogen mostly involve non-genomic effects (33, 45). It is possible that estrogen causes the activation of gene transcription in lipid metabolism, coagulation, inflammation and plaque instability through mechanisms that are not mediated by the classical estrogen response element. Accordingly, women with “estrogen sensitivity” will have greater HT response in terms of plasma lipid levels, but also inflammation and thrombogenicity. In women with CHD, the harmful effects on coagulation, inflammation, and plaque instability can override the beneficial effects on lipid metabolism, while in women without CHD the beneficial changes in lipoproteins would prevail. In support of this, recent evidence indicates that HT promotes the progression of CHD in native coronary arteries but slows the disease in saphenous vein grafts in postmenopausal women with CHD, supporting the dual and opposite role of estrogen (46).
Even though our analyses were adjusted for covariates known to influence CHD risk, including baseline coronary lumen diameter and baseline plasma remnant lipoprotein cholesterol and preβ1 levels, we cannot rule out the presence of an unidentified confounder. In addition, multiple testing may have increased the likelihood of false positives.
It is unclear to what extent our results might be extrapolated to other estrogen replacement regimens differing in administration route and composition.
Clinical implications and conclusions
The mechanism by which estrogen lowers LDL-C and increases HDL-C concentrations is different from that of other lipid-lowering medications and may be the key to the documented lack of protection observed with estrogen replacement in postmenopausal women with CHD. Our results do not support the use of HT in postmenopausal women for the prevention of CHD progression.
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health/National Heart Lung and Blood Institute grant R01 HL70081 to S.L.-F., and by the U.S. Department of Agriculture, under agreement No. 58-1950-4-401. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
Disclosures: The authors do not have conflicts of interest related to the work reported in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hulley SB, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 2.Herrington DM, Reboussin DM, Brosnihan B, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. New England Journal of Medicine. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 3.Clarke S, Kelleher J, Lloyd-Jones H, et al. A study of hormone replacement therapy in postmenopausal women with ischemic heart disease: the Papworth HRT atherosclerosis study. British Journal of Obstetrics and Gynecology. 2002;109:1056–1062. doi: 10.1111/j.1471-0528.2002.01544.x. [DOI] [PubMed] [Google Scholar]
- 4.Angerer P, Stork S, Kothny W, et al. Effects of oral postmenopausal hormone replacement on progression of atherosclerosis. A randomized, controlled trial. Arteriosclerosis, Thrombosis & Vascular Biology. 2001;21:262–268. doi: 10.1161/01.atv.21.2.262. [DOI] [PubMed] [Google Scholar]
- 5.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative randomized controlled trial The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of epidemiologic evidence. Preventive Medicine. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson T, Anthony M, Wagner J. A comparison of tibolone and conjugated equine estrogens effects on coronary atherosclerosis and bone density of postmenopausal monkeys. Journal of Clinical Endocrinology & Metabolism. 2001;86:5396–5404. doi: 10.1210/jcem.86.11.8021. [DOI] [PubMed] [Google Scholar]
- 9.Rossouw J, Prentice R, Manson JA, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 10.Smith N, Keckbert S, Lemaitre R, et al. Esterified estrogens and conjugated equine estrogens and the risk of venous thormbosis. JAMA. 2004;292:1581–1587. doi: 10.1001/jama.292.13.1581. [DOI] [PubMed] [Google Scholar]
- 11.Seli E, Guzeloglu-Kayisli O, Cakman H, et al. Estradiol increases apoptosis in human coronary artery endothelial cells by up-regulating Fas and Fas ligand expression. Journal of Clinical Endocrinology & Metabolism. 2006;91:4995–5001. doi: 10.1210/jc.2006-1225. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Hennekens CH, Rifai N, et al. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100:713–716. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn M, Karas R. Molecular and cellular basis of cardiovascular gender differences. Science. 2007;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 14.McNamara JR, Shah PK, Nakajiam K, et al. Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154:229–236. doi: 10.1016/s0021-9150(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 15.Asztalos BF, Collins D, Cupples LA, et al. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arteriosclerosis, Thrombosis & Vascular Biology. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 16.Asztalos BF, Cupples LA, Demissie S, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arteriosclerosis, Thrombosis & Vascular Biology. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 17.Herrington DM, Reboussin DM, Klein K, et al. Estrogen Replacement and Atherosclerosis (ERA) study: study design and baseline characteristics of the cohort. Control Clin Trials. 2000;21:257–285. doi: 10.1016/s0197-2456(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 18.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma lipoprotein fractions. Clinica Chimica Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 19.Burstein M, Samaille J. Sur un dosage rapide du cholesterol lie aux alpha- et aux beta-lipoproteines du serum. Clinica Chimica Acta. 1960;5:609. doi: 10.1016/0009-8981(58)90020-2. [DOI] [PubMed] [Google Scholar]
- 20.Contois J, McNamara J, Lammi-Keefe C, et al. Reference intervals for plasma apolipoprotein A-I determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem. 1996;42:507–514. [PubMed] [Google Scholar]
- 21.McNamara JR, Shah PK, Nakajima K, et al. Remnant lipoprotein cholesterol and triglyceride reference ranges from the Framingham Heart Study. Clin Chem. 1998;44:1224–1232. [PubMed] [Google Scholar]
- 22.Asztalos BF, Sloop CH, Wong L, et al. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochimica et Biophysica Acta. 1993;1169:291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 23.Wilt TJ, Bloomfield HE, MacDonald R, et al. Effectiveness of statin therapy in adults with coronary heart disease. Archives of Internal Medicine. 2004;164:1427–1436. doi: 10.1001/archinte.164.13.1427. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S) Circulation. 1998;97:1453–1460. doi: 10.1161/01.cir.97.15.1453. [DOI] [PubMed] [Google Scholar]
- 25.Athyros V, Mikhailidis D, Papageorgiou A, et al. Effect of atorvastatin on high density lipoprotein-cholesterol and its relationship with coronary events: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) Study. Curr Med Res Opin. 2004;20:627–637. doi: 10.1185/030079904125003421. [DOI] [PubMed] [Google Scholar]
- 26.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events. VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 27.Lamon-Fava S, Postfai B, Asztalos BF, et al. Effects of estrogen and medroxyprogesterone acetate on subpopulations of triglyceride-rich lipoproteins and high-density lipoproteins. Metabolism. 2004;52:1330–1336. doi: 10.1016/s0026-0495(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 28.Sanada M, Nakagawa H, Kodama I, et al. The effect of hormone replacement therapy on metabolism of lipoprotein remants in postmenopausal women. Maturitas. 2000;34:75–82. doi: 10.1016/s0378-5122(99)00087-0. [DOI] [PubMed] [Google Scholar]
- 29.Westerveld H, Kock LAW, Rijn HJM, et al. 17 beta-estradiol improves postprandial lipid metabolism in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 1995;80:249–253. doi: 10.1210/jcem.80.1.7829621. [DOI] [PubMed] [Google Scholar]
- 30.Ossewaarde M, Dallinga-Thie G, Bots M, et al. Treatment with hormonal replacement therapy lowers remnant lipoprotein particles in healthy postmenopausal women: results from a randomized trial. Eur J Clin Invest. 2003;33:376–382. doi: 10.1046/j.1365-2362.2003.01163.x. [DOI] [PubMed] [Google Scholar]
- 31.Bittner V, Tripputi M, Hsia J, et al. Remnant-like lipoproteins, hormone therapy, and angiographic and clinical outcomes: The Women’s Angiographic Vitamin & Estrogen trial. American Heart Journal. 2004;148:293–299. doi: 10.1016/j.ahj.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Walsh BW, Schiff I, Rosner B, et al. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. New England Journal of Medicine. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 33.Bruning JC, Lingohr P, Gillette J, et al. Estrogen receptor alpha and Sp1 interact in the induction of the low density lipoprotein-receptor. Journal of Steroid Biochemistry and Molecular Biology. 2003;86:113–121. doi: 10.1016/s0960-0760(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 34.Lamon-Fava S, Postfai B, Diffenderfer M, et al. Role of the estrogen and progestin in hormonal replacement therapy on apolipoprotein A-I kinetics in postmenopausal women. Arteriosclerosis, Thrombosis & Vascular Biology. 2006;26:385–391. doi: 10.1161/01.ATV.0000199248.53590.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landschulz KT, Pathak RK, Rigotti AKM, et al. Regulation of scavenger receptor, class B, type 1, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. Journal of Clinical Investigation. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigotti A, Trigatti BL, Penman M, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukushima H, Kugiyama K, Sugiyama S, et al. Comparison of remnant-like lipoprotein particles in postmenopausal women with and without coronary artery disease and in men with coronary artery disease. The American Journal of Cardiology. 2001;88:1370–1373. doi: 10.1016/s0002-9149(01)02115-4. [DOI] [PubMed] [Google Scholar]
- 38.Lamon-Fava S, Herrington DM, Reboussin DM, et al. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arteriosclerosis, Thrombosis & Vascular Biology. 2008;28:575–579. doi: 10.1161/ATVBAHA.107.157123. [DOI] [PubMed] [Google Scholar]
- 39.The Expert Panel, Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 40.Miida T, Nakamura Y, Inano K, et al. Pre beta 1-high-density lipoprotein increases in coronary artery disease. Clin Chem. 1996;42:1992–1995. [PubMed] [Google Scholar]
- 41.Asztalos BF, Batista M, Horvath KV, et al. Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23:847–852. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]
- 42.Asztalos BF, Schaefer EJ. The effects of statins on high-density lipoproteins. Current Atherosclerosis Reports. 2006;8:41–49. doi: 10.1007/s11883-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 43.Tilly-Kiesi M, Kahri J, Pyorala T, et al. Responses of HDL subclasses, Lp(A-I) and Lp(A-I:A-II) levels and lipolytic enzyme activities to continuous oral estrogen-progestin and transdermal estrogen with cyclic progestin regimens in postmenopausal women. Atherosclerosis. 1997;129:249–259. doi: 10.1016/s0021-9150(96)06036-4. [DOI] [PubMed] [Google Scholar]
- 44.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Current Opinion In Lipidology. 2007;18:427–434. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 45.Lamon-Fava S, Micherone D. Regulation of apo A-I gene expression: mechanism of action of estrogen and genistein. Journal of Lipid Research. 2004;45:106–112. doi: 10.1194/jlr.M300179-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang P, Tardif JC, Herrington DM, et al. Randomized trial of hormone therapy in women after coronary bypass surgery. Evidence of differential effect of hormone therapy on angiographic progression of disease in saphenous vein grafts and native coronary arteries. Atherosclerosis. 2006;189:375–386. doi: 10.1016/j.atherosclerosis.2005.12.015. [DOI] [PubMed] [Google Scholar]