Abstract
Recent studies have shown that sulforaphane, a naturally occurring compound that is found in cruciferous vegetables, offers cellular protection in several models of brain injury. When administered following traumatic brain injury (TBI), sulforaphane has been demonstrated to attenuate blood-brain barrier permeability and reduce cerebral edema. These beneficial effects of sulforaphane have been shown to involve induction of a group of cytoprotective, Nrf2-driven genes, whose protein products include free radical scavenging and detoxifying enzymes. However, the influence of sulforaphane on post-injury cognitive deficits has not been examined. In this study, we examined if sulforaphane, when administered following cortical impact injury, can improve the performance of rats tested in hippocampal- and prefrontal cortex-dependent tasks. Our results indicate that sulforaphane treatment improves performance in the Morris water maze task (as indicated by decreased latencies during learning and platform localization during a probe trial) and reduces working memory dysfunction (tested using the delayed match-to-place task). These behavioral improvements were only observed when the treatment was initiated 1 hr, but not 6 hr, post-injury. These studies support the use of sulforaphane in the treatment of TBI, and extend the previously observed protective effects to include enhanced cognition.
Keywords: TBI, Nrf2, prefrontal cortex, hippocampus, spatial memory
Introduction
Cells possess endogenous protective mechanism(s) as a defense against environmental insults and injury. These cellular defenses often involve induction of cytoprotective genes whose protein products scavenge and/or neutralize toxins. A few regulatory pathways have been identified involving specific transcription factors that induce the expression of cytoprotective proteins and enzymes [11]. The transcription factor Nrf2 (nuclear factor E2-related factor 2) binds to the antioxidant/electrophilic response element (ARE/EpRE) and regulates the expression of multiple cytoprotective proteins including antioxidant and glutathione generating enzymes [8;15]. Nrf2 is normally sequestered in the cytoplasm through its interaction with KEAP1 where it is rapidly turned-over by the ubiquitin-proteosome system [9;16]. The naturally occurring compound sulforaphane, which is present in cruciferous vegetables such as broccoli, has been shown to reduce the proteosomal degradation of Nrf2, resulting in its nuclear accumulation and increased expression of Nrf2-driven genes [16].
Our previous studies have shown that systemic administration of sulforaphane results in increased expression of Nrf2-driven genes in rodent brains and microvasculature [17;18]. Consistent with this, post-injury administration of this compound to rodents with traumatic brain injury (TBI) attenuates blood-brain barrier permeability and reduces brain edema [17;18]. In the present study, we investigated if post-injury sulforaphane administration improves cognitive function. Our results indicate that sulforaphane administration initiated at 1 hr, but not at 6 hr, post-cortical impact injury improves spatial learning and memory, and reduces working memory dysfunction.
Materials and Methods
Materials
Male Sprague Dawley rats (300 g) were purchased from Harlan (Indianapolis, IN). Sulforaphane (Catalog No. S8044) was purchased from LKT Laboratories, Inc. (St. Paul, MN). Anti-4-Hydroxy-2-noneal (4-HNE) antibody was purchased from EMD Chemicals, Inc. (Gibbstown, NJ).
Controlled cortical impact injury and drug administration
All experimental procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals. A controlled cortical impact (CCI) device was used to cause brain injury as previously described [3]. Bilateral craniectomies were positioned midway between the bregma and lambda and animals received a single impact (2.7 mm deformation, 6 meters/sec) on the right parietal lobe. Sulforaphane was dissolved in corn oil and administered by intraperitoneal (i.p.) injection.
Assessment of cognitive function
Spatial memory was tested using the standard hidden platform version of the Morris water maze task (MWM) (beginning on day 14 post-injury) followed by a working memory task (beginning on day 25 post-injury). Animals were randomly assigned to either drug or vehicle groups. For the MWM task, animals were trained for 7 consecutive days and were given 4 training trials (60 seconds maximum) per day, with an inter-trial interval (iti) of 4min [2;5]. If the animal failed to locate the platform within 60sec on any given trial, it was led there by the experimenter where it was allowed to rest for 30sec. If no significant differences were detected during the first 5 days, training was discontinued. Twenty-four hours following the last day of training, animals were tested for retention by administering a 60sec probe trial. Movement within the maze was monitored using a video camera linked to tracking software (Ethovision, Noldus Information Technology, Leesbury, VA, USA). The time to cross the site at which the platform was located during training, the number of platform crossings, and quadrant preference was calculated.
Following MWM training and testing, animals were tested in a delayed match-to-place spatial working memory task [6]. For each testing pair of trials, animals were given a location trial, in which the platform and start location were randomized. Animals were then allowed to search for the platform for a period of 60sec (location trial) and once found, the animal was allowed to rest on it for 10sec. The animal was removed from the water maze and after a 5sec delay, allowed to once again search for the hidden platform (match trial). This procedure of location and match trial was repeated 5 times (each pair separated by a 4min iti) with the platform in a novel location for each pair.
Immunohistochemistry
Sulforaphane (5mg/kg) or vehicle was injected i.p. 1 hr and 24hr after injury. One hour following the last injection, animals were deeply anesthetized with sodium pentobarbital (100mg/kg) and transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were removed, and coronal sections prepared on a cryostat. Sections were incubated with primary antibodies (anti-4-HNE or anti-NeuN) in 2.5% goat serum in Tris buffered saline containing 0.1% triton X-100 for 48hr at 4°C. Immunoreactivity was detected by species-specific secondary antibodies conjugated to Alexafluors. Photomicrographs (2 sections/animal) were taken of the cortex and the dorsal hippocampus for quantification of 4-HNE immunoreactivity. Camera settings remained constant for all animals. NeuN immunoreactivity was used to define hippocampal subfields. Immunoreactivity was assessed using Image J (available from NIH) and calculated as optical density/mm2.
Statistical Analysis
For evaluation of behavioral data, a two-way repeated measures analysis of variance was utilized to determine group differences. A Holm-Sidak method for multiple comparisons was used to determine data points with significant differences. Optical density measurements for immunohistochemistry were evaluated using a Student’s t-test for unpaired variables. Data were considered significant at p < 0.05 and presented as Mean ± Standard Error of the Mean (S.E.M.).
Results
Early, but not delayed, sulforaphane administration following injury significantly improves spatial memory
We have previous demonstrated that sulforaphane given 6hr post-injury significantly reduces post-TBI blood-brain barrier permeability and cerebral edema [17;18]. To test if this administration routine is associated with a cognitive improvement, rats were injured and injected with either sulforaphane (5mg/kg) or vehicle 6hr post-injury (n=10/group) (Figure 1A). Beginning on day 14 post-injury, animals were tested in the standard hidden platform version of the Morris water maze. Figure 1A shows that there was no significant difference in water maze performance between the sulforaphane- and vehicle-treated animals when the compound was administered 6hr post-injury (group main comparison: F(1,9)=0.495, p=0.499).
Figure 1. Post-injury administration of sulforaphane improves water maze performance.
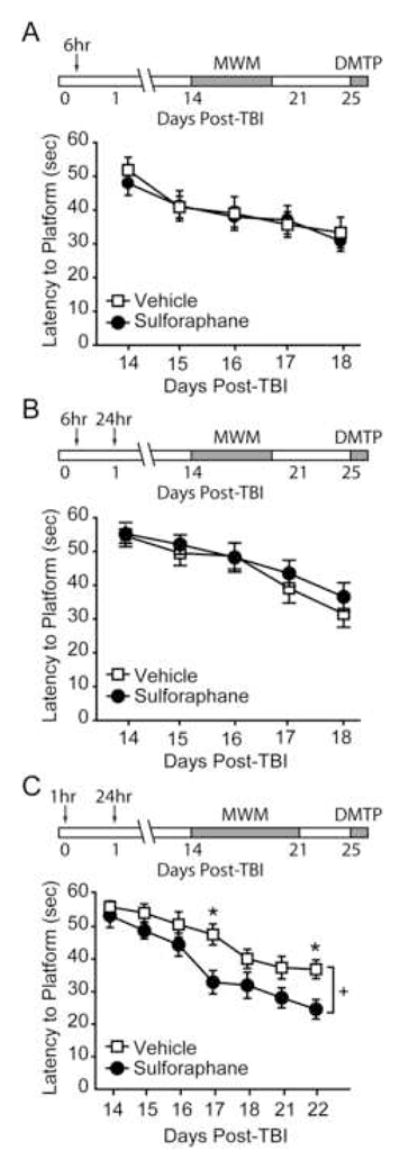
Performance in the Morris water maze task was tested 14 days after injury. Sulforaphane (5 mg/kg) or vehicle was administered by i.p. injection at either A) 6 hr, B) 6hr and 24hr, or C) 1hr and 24hr post-injury. ↓, time of injection. MWM: Morris water maze. DMTP: delayed match-to-place task. +, significant group main effect. *, p <0.05 between vehicle and sulforaphane groups.
To prolong the effectiveness of the drug, injured animals (n=10/group) were given a dose of sulforaphane (5 mg/kg) or vehicle at 6hr post-injury, followed by a second injection at 24hr post-injury. Figure 1B shows that the addition of a second sulforaphane injection also had no significant effect on water maze performance (group main comparison: F(1,9)=0.603, p=0.457). In contrast, when the drug was administered earlier, injured animals receiving sulforaphane (n=9) at 1hr and 24hr post-injury performed significantly better than their vehicle-treated counterparts (n=10) (group main effect: F(1,8)=6.750, p=0.032). As these rats had significantly improved performance after 5 of training, they were given an additional 2 days of training to ensure that the differences in learning would be maintained (Figure 1C; group main effect: F(1,8)=10.270, p=0.013). These differences in performance were not due to changes in swimming speed (swimming speed at end of training: vehicle, 28.10 ± 1.04 cm/sec; sulforaphane, 30.42 ± 1.90 cm/sec, n.s.)
To determine if the improved performance in the 1hr and 24hr sulforaphane-treated animals was associated with measures of platform localization, a probe test was given 24hr following the completion of training. The representative traces shown in figure 2A demonstrate the initial swimming paths taken by a vehicle- and sulforaphane–treated animal during the probe trial (Figure 2A). When analyzed for quadrant dwell times, a significant preference for the target quadrant (I) (quadrant that contained the hidden platform) was found for the sulforaphane-treated animals (Figure 2B). In contrast, the vehicle infused animals only had a significant difference in dwell time between the target quadrant (I) and the start quadrant (III). The probe test was further analyzed for localization differences between the groups by determining the latencies to, and number of times the animals entered, concentric rings of decreasing diameter centered on the platform location. The summary graph shown in figure 2C shows that while the sulforaphane-treated animals proceed from the outermost ring (4×) to the platform with minimal delay, the vehicle-injected animals had significantly longer latencies to reach the inner rings (+, interaction between group and latency: F(3,48)=2.949, p=0.042). Consistent with this, the sulforaphane-injected animals were found to cross the site of the platform location significantly more often than the vehicle-injected animals (vehicle, 1.00 ± 0.33 crossings; sulforaphane, 2.33 ± 0.44 crossings, p=0.028; Figure 2D). These measures of platform localization were not detected in animals injected with sulforaphane at either 6hr, or 6hr and 24hr, post-injury.
Figure 2. Improved water maze performance is associated with enhanced measures of spatial localization.
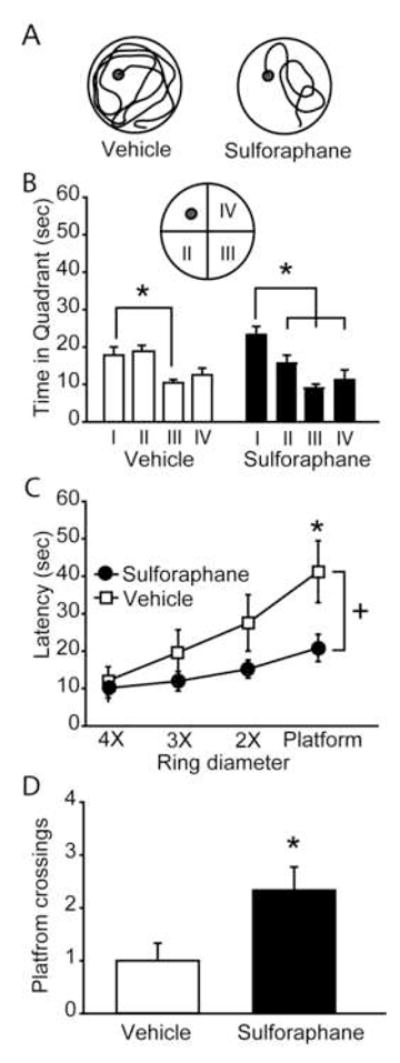
Twenty-four hours following the completion of training, rats were tested in a probe trial. A) Representative probe traces of swimming paths for a sulforaphane and a vehicle injected animal. The trace shows the path taken from the time of maze entry to the first crossing of the platform location. B) Quantification of time spent in each quadrant of the water maze during the probe trial. *, significant differences in dwell time. C) The latency to enter concentric circles (4X, 3X, 2X or 1X the diameter of the platform) centered on the platform (+, significant group main effect). D) Platform crossings during the probe trial. Data are represented as mean ± SEM. *, p <0.05 between vehicle and sulforaphane groups.
Sulforaphane administration following injury improves spatial working memory
Beginning on day 25 post-injury, the animals treated at 1hr and 24 hr post-injury were further tested using the delayed match-to-place working memory task (Figure 3A). Figure 3B shows representative traces from vehicle- and sulforaphane-treated rats during the location and match trials. Vehicle-injected animals did not show any significant decline in latency between the location and match trials (location trial: 36.08 ± 5.25 sec; match trial: 39.24 ± 9.63 sec; p=0.110), indicating impaired working memory. In contrast, animals receiving sulforaphane reached the platform during the match trial significantly faster than during the location trial (location trial: 41.18 ± 2.48 sec; match trial: 25.94 ± 2.48 sec; p=0.001). Comparison of the two groups revealed a significant interaction between group and trial (F(1,8)=15.669, p=0.004).
Figure 3. Post-injury administration of sulforaphane improves working memory. A).
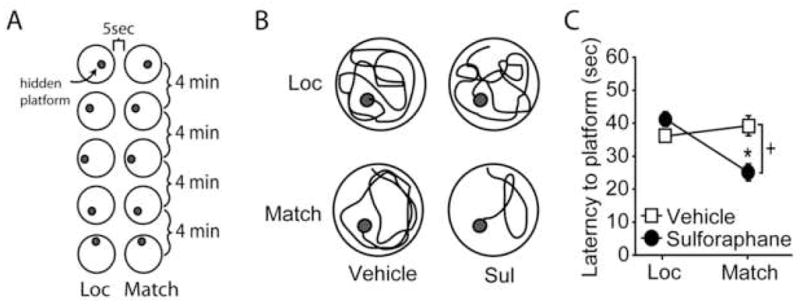
Schematic diagram showing the protocol of delayed match-to-place testing consisting of location (Loc) and match trials, separated by a 5 sec delay. Five pairs of location and match trials were performed, with the platform position in a novel site for each pair. Each pair of trials was separated by a 4 min inter-trial interval. B) Representative traces of a vehicle and a sulforaphane (Sul)-treated animal during the location and match trials. C) Summary data showing average latencies to the platform during the location and match trials. +, significant interaction between trial number and treatment groups; *, p<0.05 between location and match trials. Data are represented as mean ± SEM.
Systemic administration of sulforaphane attenuates TBI-associated oxidative damage in the brain
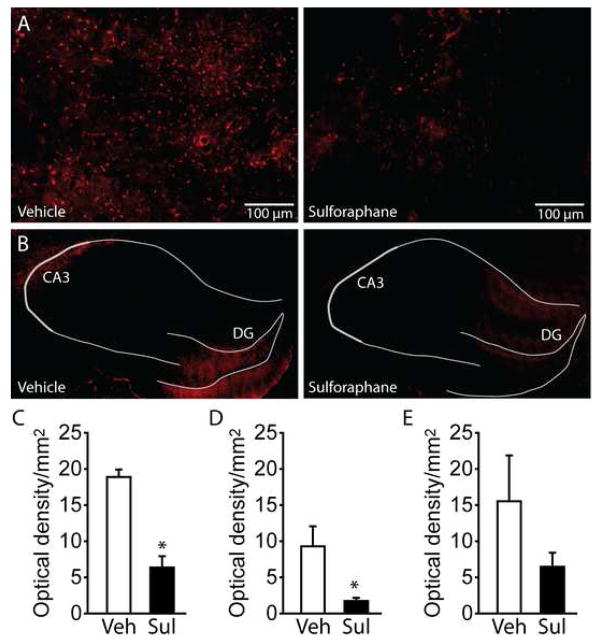
Since a number of Nrf2-driven genes have antioxidant functions, we examined if the cognitive improvement observed following sulforaphane administration was associated with reduced oxidative damage in the brains of TBI animals. Animals were injured (n=4/group) and at 1hr and 24hr post-injury, treated with either 5mg/kg sulforaphane or vehicle. One hour after the last injection, brains were removed for immunological evaluation of 4-Hydroxynonenal (4-HNE), a marker of lipid peroxidation [4]. Sections were double-labeled with anti-NeuN to identify neuronal layers. Figure 4 shows representative images of the cerebral cortex immediately adjacent to the injury core (Figure 4A) and dorsal hippocampus (Figure 4B) from a TBI animal treated with vehicle and a TBI animal treated with 5 mg/kg sulforaphane. As the immunoreactivity for 4-HNE appeared primarily in the CA3 (an area highly vulnerable to TBI) and dentate gyrus subfields of the hippocampus in vehicle-treated animals, these areas were chosen for quantification. Figure 4C shows that 4-HNE immunoreactivity (calculated as optical density/mm2) was significantly reduced in the cortex of sulforaphane-treated animals (Student’s t-test: p=0.0006). Likewise, 4-HNE immunoreactivity was found to be significantly reduced in the CA3 subfield of the hippocampus (Student’s t-test: p=0.035). No significant difference was found in the dentate gyrus (Student’s t-test: p=0.220), presumably due to the high degree of variability detected in the vehicle-treated animals (Figure 4D).
Figure 4. Post-injury administration of sulforaphane decreases oxidative damage in the brain.
Representative images showing the relative immunoreactivity of 4-HNE in an injured animal receiving vehicle, and an injured animal receiving 5mg/kg sulforaphane 1hr and 24hr post-injury. Images were taken from the A) cerebral cortex immediately adjacent to the injury location, and B) the ipsilateral hippocampus. Hippocampal subfields were identified by NeuN double-label immunohistochemistry and indicated by gray lines. Summary date for quantification of 4-HNE immunoreactivity from C) the ipsilateral cortex adjacent to the site of injury, D) the CA3 subfield, and D) the dentate gyrus. *, P <0.05. Veh: vehicle; Sul: sulforaphane; DG: dentate gyrus
Discussion
To defend against exogenous toxins and harmful products of cellular respiration, cells contain a large number of antioxidant and detoxifying enzymes whose expressions are rapidly increased in response to threats. Many of these genes contain binding sites for the transcription factor Nrf2 and can therefore be regulated by the Nrf2-activating compound sulforaphane [8;15]. Our laboratory has previously shown that post-injury administration of sulforaphane reduces TBI-associated blood-brain barrier permeability and attenuates cerebral edema [17;18]. In the present study, we extend these findings to show that sulforaphane, when given to rats following cortical impact injury, improves hippocampal- and prefrontal cortex-dependent cognitive functions. These preclinical findings further support the use of sulforaphane as a potential treatment option following brain injury.
Increased generation of reactive oxygen or nitrogen species, such as superoxide, hydroxyl radicals, and peroxynitrite are widely recognized as important contributors to the pathology of brain trauma. Because of this, several studies have evaluated the protective effects of free radical scavenging compounds, altered expression of detoxifying enzymes, and other neuroprotective agents as putative treatments for TBI [10;12;13]. For example, overexpression of Cu/Zn superoxide dismutase was found to decrease blood-brain barrier permeability, cerebral edema, and motor dysfunction in a dose-dependent manner following a weight-drop model of brain injury. Sulforaphane, a naturally occurring compound generated from cruciferous vegetables such as broccoli, is a potent inducer of antioxidant and detoxifying enzymes [15]. As such, this compound has been suggested to provide broad protection against a variety of cellular threats. For example, application of sulforaphane to neuron-astrocyte co-cultures protects neurons against nonexcitotoxic glutamate and hydrogen peroxide toxicity [7]. Consistent with this ability of sulforaphane to reduce oxidative damage, we observed that sulforaphane administered 1hr and 24hr post-injury significantly reduced 4-HNE immunoreactivity in the CA3 subfield of the hippocampus and the cortex surrounding the injury site. The CA3 subfield has been demonstrated to be vulnerable to lateral cortical impact injury, and damage to this area is thought to contribute to hippocampal-dependent learning and memory dysfunction [1].
We have previously demonstrated that a single, post-injury injection of sulforaphane is sufficient to stimulate the expression of Nrf2-dependent genes and reduce TBI-associated blood-brain barrier permeability and cerebral edema [17;18]. While encouraging, the consequences of sulforaphane on post-injury cognition were not tested. In this study, we present data to demonstrate that in addition to vascular protection, post-injury sulforaphane treatment preserves neurologic function in injured animals. This improvement was evidenced by enhanced learning and memory, and by improved performance in a working memory task. Although the systemic nature of the drug treatment paradigm employed in this study prevents ascribing these neurologic improvements to a specific brain structure, both the Morris water maze task and the delayed match-to-place task are dependent on the hippocampus, suggesting a preservation of this structure’s function. However, working memory is heavily dependent on the transient storage and manipulation of information by the prefrontal cortex. Thus, the improved performance observed in the delayed match-to-place task may also reflect a reduction of prefrontal cortex dysfunction. Future studies using targeted infusions of sulforaphane may be required to determine the contribution of individual structures to the effects we observed.
Based on our previous observations that sulforaphane could offer protection against blood-brain barrier compromise when delayed by as much as 6 hr post-injury [17;18], and the premise that the components of the neurovascular unit (a functional unit composed of neurons, astrocytes, oligodendrocytes and brain endothelial cells) work in concert to maintain brain function, we anticipated that cognitive improvement would be seen when sulforaphane was administered 6hr post-injury. However, hippocampal-dependent behavioral improvements were only observed when sulforaphane treatment was initiated within 1 hr of injury. The reason for the dissociation between vascular protection and cognitive improvement is not clear at present. As a number of factors are required for improvement of cognitive function, simply improving vascular permeability may not be sufficient. Furthermore, although oxidative damage may occur in the neurons and endothelial cells with similar time courses [4], there is evidence to suggest that neurons may be particularly sensitive to injury. For example, using the expression of hsp70 as a marker of injury, it has been shown that brief periods of ischemia results in hsp70 induction first in neurons, followed by glia, and finally in endothelial cells [14]. Therefore, sufficient oxidative damage may have occurred within neurons by 6hr post-injury to make this delay incapable of improving cognitive function. Regardless of the underlying reasons for the observed differences in time windows, our results on the behavioral and pathophysiological improvements offered by post-injury sulforaphane administration suggest that this compound is a promising therapeutic option for the treatment of brain injury patients.
Acknowledgments
This work was supported, in part, by grants (MH072933, NS053588, NS049160) from the National Institutes of Health to P.K.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colicos MA, Dixon CE, Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- 2.Dash PK, Moore AN, Dixon CE. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 4.Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- 5.Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 6.Hamm RJ, Temple MD, Pike BR, O’Dell DM, Buck DL, Lyeth BG. Working memory deficits following traumatic brain injury in the rat. J Neurotrauma. 1996;13:317–323. doi: 10.1089/neu.1996.13.317. [DOI] [PubMed] [Google Scholar]
- 7.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 9.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 10.Mikawa S, Kinouchi H, Kamii H, Gobbel GT, Chen SF, Carlson E, Epstein CJ, Chan PH. Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- 11.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royo NC, LeBold D, Magge SN, Chen I, Hauspurg A, Cohen AS, Watson DJ. Neurotrophin-mediated neuroprotection of hippocampal neurons following traumatic brain injury is not associated with acute recovery of hippocampal function. Neuroscience. 2007;148:359–370. doi: 10.1016/j.neuroscience.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp FR, Kinouchi H, Koistinaho J, Chan PH, Sagar SM. HSP70 heat shock gene regulation during ischemia. Stroke. 1993;24:I72–I75. [PubMed] [Google Scholar]
- 15.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 16.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]