Abstract
This multi-center correlational prospective study examined early neonatal predictors of neurodevelopment in 59 premature infants (mean birth weight=1713.8±1242.5 g; mean gestational age=31.2±3.6 weeks) suspected to have sustained brain injury at birth. The mental and motor development of the infants selected from five university-affiliated hospitals was assessed at baseline (59 infants), 12 (55 infants), and 18 months (46 infants) using Bayley II scales. Factors correlating with Bayley II scores at 12 and 18 months included head circumference, results of neurological and magnetic resonance imaging (MRI) examination at baseline, environmental factors such as mother–infant interactions and levels of parental stress, and infant medical factors such as Apgar scores at 5 min and length of hospital stay. Multiple regression analyses distinguished the most significant predictors of mental and motor development.
The best predictors of mental and motor development at 18 months were head circumference, neurological examinations, and MRI results. These findings suggest that in infants suspected of brain injury at birth, neurological assessments and head circumference measurements are just as predictive of developmental outcome at 18 months as MRI, and this is especially relevant in developing countries or other locations where MRI is not possible. The presence of this information may offer the potential of early tailored interventions to improve the mental and motor development of children in developing countries or other facilities where MRI is unavailable.
Keywords: Brain injury, MRI, Neurodevelopment
1. Introduction
Brain injury in infants, estimated at.2 to.5% of live births, occurs most frequently as a consequence of a perinatal hypoxic–ischemic insult [1,2]. The mechanisms contributing to brain injury, especially in preterm infants, are not well understood [1]. Irrespective of pathology, approximately 20 to 30% of infants who sustain brain injury at or near birth develop significant neurological impairments [2–4], the degree of which depends on the extent, nature, and location of the injury, as well as gestational age at the time of insult [5–7].
When the hypoxic–ischemic insult is localized to specific brain regions, long-term neurodevelopmental outcome is often accurately determined. In general, however, nonspecific, widespread damage precludes long-term prognosis. Clinical markers (e.g., intrapartum distress, intubation or resuscitation in the delivery room, Apgar scores <5 at 5 min) do not reliably predict cognitive or motor outcome [8–10]. Similarly, although environmental factors (e.g., stress, socioeconomic status, education of parents, and interaction of mother with infant) correlate with developmental outcome in preterm and high-risk infants, they do not consistently predict long-term progress in premature infants suspected of brain injury [11–14].
Results of brain magnetic resonance imaging (MRI) examination are objective and MR techniques such as diffusion tensor imaging (DTI) can be used to characterize brain white matter. In contrast to other potential predictors of long-term development, results of MRI examinations are useful in determining long-term outcome in infants with suspected brain injury [15–21]. Infants with severe white matter injury, especially to basal ganglia regions, show persistent neurological abnormalities at school age [22–23]. In addition, converging evidence suggests that reduced white matter volume measured by MRI is associated with adverse outcomes [22,24]. Abnormal neurological findings are also effective predictors of long-term outcome; evidence of encephalopathy or seizures in the early neonatal period correlate with the subsequent development of mental retardation, cerebral palsy, and various other developmental disabilities [6,7,25–28].
Few if any studies have investigated the separate and combined significance of clinical and environmental factors and the results of MRI and neurological examinations in predicting developmental outcome in children suspected of brain injury at birth. Consequently, the objective of this study was to assess early neonatal determinants of neurodevelop-ment in infants suspected of brain injury at or near birth.
2. Methods
2.1. Study design and setting
Developmental outcome at 12 and 18 months corrected gestational age in infants suspected of brain injury at birth was assessed using descriptive prospective correlational analysis. Premature infants suspected to have sustained brain injury at birth were recruited over a three-year period from 5 Southern California University hospitals with level 3 Neonatal Intensive Care Units (NICU). Although five hospitals with large NICUs (20–40 beds) assisted with the recruitment of infants, the process of enrollment was slower than expected due to low incidence of brain injury and reluctance of parents to enroll their high risk infants in a longitudinal research study. The difficulties inherent to recruiting high-risk neonates to research studies arising from equipoise and informed consent were recently discussed by Degos et al. [29]. Institutional Review Board approval was obtained at each hospital; informed consent was acquired from all parents with infants participating in the study. With the exception of MRI and neurological examinations (conducted in the pediatric clinics of the University of California at Los Angeles; UCLA), mother and infant variables were collected in the home environment.
2.2. Subjects
The initial sample consisted of 59 infants and their mothers. Mothers (17–40 years old) were from a variety of socioeconomic and ethnic backgrounds. Infants were recruited if they were between 28 and 37 weeks gestational age and either 1) met three of the criteria outlined in Table 1 [30] or 2) had any abnormalities observed in MR images or electroencephalogram (EEG) recordings (as performed in the NICU). Infants were excluded if they had Grade IV intraventricular hemorrhage with periventricular leukomalacia, evidence of profound cortical destruction or atrophy, or any congenital anomalies (see Table 1); these conditions are associated with severe long-term disabilities and high mortality rates. Furthermore, the fragility of infants sustaining such damage may hinder MR image acquisition [7,27].
Table 1.
Criteria for infant inclusion or exclusion.
| Inclusion criteria: the presence of at least three of the following characteristics |
|---|
| Intrapartum distress: |
| Placental abruption, thick stained meconium amniotic fluid, and/or abnormal fetal heart rate (e.g., bradycardia, <100 beats/min). |
| Within 5–10 min of birth: |
| APGAR score <5 (10 minute APGAR score acceptable if 5 minute appraisal unavailable). |
| Within 1 h of birth: |
| Intubation and/or resuscitation. |
| Within 72 h of birth: |
| pH<7.0; cord blood, base deficit of −10 meq/L or more; cord blood, or pH of <7.1; arterial blood. |
| Any time during NICU stay: |
| Abnormal neurological exam. |
| Any time during NICU stay: |
| Abnormal head ultrasound, abnormal EEG, abnormal CT scan, or abnormal MRI scan. |
| Anytime during NICU stay: |
| Seizure activity. |
| Intraventricular hemorrhage: |
| Grade III or IV with NO evidence of periventricular leukomalacia or severe cortical destruction/atrophy. |
| Exclusion criteria: the presence of any one of the following conditions |
| Cochlear or pacemaker implants. |
| Congenital orthopedic anomalies requiring surgery. |
| Congenital CNS anomalies. |
| Congenital cardiac anomalies (severe or at multiple sites). |
| Genetic conditions (e.g., trisomy 18, trisomy 21, fragile X syndrome). |
| Genetic syndromes indicating errors in metabolism (e.g., phenylketonuria). |
| Intraventricular hemorrhage: |
| Grade IV with evidence periventricular leukomalacia or severe cortical destruction/atrophy. |
Fifty infants were assessed at 12 months corrected for gestational age (1 infant died, 3 were excluded due to non-compliance, 5 were lost to follow-up), and 46 infants were assessed at 18 months corrected for gestational age (4 were lost to follow-up; Fig. 1). Although efforts were made to minimize attrition (e.g., developing bonds with the family, collecting addresses of the extended family, neighbors, and friends, flexibility in time and place of visit, frequent phone calls to families, and empowering and supporting parents during visits); [31], 22% of the infants were lost to follow-up. The characteristics of the infants and mothers who remained in the study were the same as those lost to follow-up (Table 2).
Fig. 1.
Number of subjects followed over 18-months.
Table 2.
Characteristics of the sample (mean±SD) for baseline variables at the different time points.
| Characteristic/variable | Baseline (n=59) | 12 months (n=50) | 18 months (n=46) | p value |
|---|---|---|---|---|
| Birth weight (grams) | 1713.8±1242.5 | 1709.5±1087.7 | 1708.2±907.7 | NS |
| Gestational age (weeks) | 31.2±3.6 | 30.5±3.8 | 31.7±4.2 | NS |
| APGAR score (5 min) | 6.2±3.5 | 6.1±3.9 | 6.6±3.2 | NS |
| Head circumferencea | 30.5±6.6 | 30.2±8.5 | 31.6±7.8 | NS |
| Male/female | 34/25 | 27/23 | 24/22 | NS |
| Prenatal care (2nd trimester) | 43 of 59 | 39 of 50 | 37 of 46 | NS |
| Maternal age (years) | 27.1±3.7 | 29.2±3.7 | 28.8±3.4 | NS |
| Maternal education (NGrade 12) | 25 of 59 | 22 of 50 | 20 of 46 | NS |
| Days in hospital | 66.3±10.5 | 68.9±9.6 | 69.1±10.2 | NS |
| Hispanic | 42 of 59 | 34 of 50 | 26 of 46 | NS |
| Parental Stress Index | 75.8±11.9 | 77.6±16.9 | 76.6.±19.8 | NS |
| Perceived Social Support | 69.2±12.8 | 65.6±9.4 | 64.8±10.6 | NS |
| Mother/infant interaction | 61.3±8.2 | 59.9±9.1 | 60.2±10.2 | NS |
| The HOME | 34.2±5.5 | 35.7±5.3 | 37.2±7.4 | NS |
| Bayley motor score | 88.3±18.7 | 84.1±20.4 | 86.3±16.9 | .05* |
| Bayley mental score | 82.6±19.5 | 89.6±20.2 | 90.2±22.3 | .04* |
NS = not significant.
Wilcoxon p test significnt at p<.05.
2.3. Data collection
A bilingual (Spanish–English) developmental specialist blinded to the objectives of the study and trained to 90% reliability on all scales collected all data excluding neurological and MRI examination. Medical information was obtained from the medical records at baseline and assessments including questionnaires (e.g., parental stress, social support), and direct observation (e.g., Bayley II scales, mother–infant interactions, quality of the home environment) were obtained at baseline (i.e., discharge, typically 2 to 19 weeks after birth), and at 12 and 18 months corrected for gestational age. Questionnaires and other materials used in this study were available bilingually [32].
2.4. Dependent variables
Cognitive and motor development of infants was assessed using Bayley II scales; these scales are widely employed to measure development of infants and toddlers aged 16 days to 42 months, and require approximately 30 to 60 min to complete [33]. The validity and reliability of these scales are well established, and have been used to successfully assess infants and toddlers from various ethnic and socioeconomic groups. The Bayley II scales yield 2 measurements: the Mental Developmental Index (BSID-II-MDI) and the Motor Developmental Index (BSID-II-PDI), these were the dependent variables of the study. Average BSID-II-MDI and BSID-II-PDI scores in healthy infants and children are 100±15.
2.5. Independent variables
2.5.1. Neurological examination/head circumference
An experienced pediatric neurologist (DS), blinded to MRI findings and medical history performed a neurological examination at the three time points (i.e., baseline, 12, and 18 months). Information collected included head circumference (in centimeters, assessed with a tape measure), head and limb tone, motor abilities, reflexes, and visual and auditory acuity. Results were classiffied based on the Amiel-Tison neurological assessment [34] as normal (0), mildly abnormal (1), or severely abnormal (2; e.g., 2=abnormal limb tone + abnormal visual acuity + abnormal head circumference).
2.5.2. Magnetic resonance imaging acquisition
No sedation was given to infants; rather, mothers were instructed to feed infants after 4 h of fasting immediately before the scan session. Infants were wrapped with an inflatable blanket that molded into their shape, and wore earplugs and headphones with acoustical damping to protect them from MR scanning noise. After ensuring infants were asleep, their heads were placed in the MR head coil; vital signs were monitored using electrocardiography, and oxygen saturations using pulse-oximetry. An anesthesiologist and a pediatrician were present during all scan sessions.
Infants were scanned at baseline on a 1.5 T GE scanner (Milwaukee, MI, USA; Version 5.8); these scans were independent of any that may have been performed in the NICU. For each infant, structural scans acquired included a sagittal localizer, axial T1-weighted images (3D), and axial T2-weighted FAST Spin Echo images. Images were transferred via secure intranet to off-line computers for image processing. An experienced radiologist (SS) classified images as either normal (0) or abnormal (1) based on the presence of lesions or other signs of overt damage. Individual voxels (2×2 milliliters; mL) were automatically categorized as predominantly gray matter, white matter, or cerebrosp-inal fluid (CSF) based on threshold values established from the T2-weighted images, and then averaged across the entire brain to compute total volume in mL3 (i.e., cm3’s) of three brain tissue classes.
Every attempt was made to image infants as soon as possible after discharge from the NICU (between 2 and 19 weeks after birth). However, only 41 neonatal scans were complete and could be read and analyzed for this study (1 infant died soon after discharge, 3 scan sessions could not be read due to motion artifacts, and 14 did not have complete scans for various reasons including transportation and scheduling difficulties). Because 8 of the infants who remained in the study at 18 months did not have baseline MRI scans, we used results from the MR scans acquired in the NICU. Two infants who completed the study did not have MR data (further discussed in the Results section).
2.5.3. Mother–infant interaction
The Nursing Child Assessment Feeding Scale (NCAFS) was used to assess mother–infant interactions [35]. This scale consists of 149 items that are answered yes/no and yield a summary score. The predictive validity of the NCAFS has been established with the Bayley II Scales of Infant Development (r=.72), the Preschool Behavior Questionnaire (r =.79), and the Home Observation for Measurement of the Environment (HOME; r=.76; 34).
2.5.4. Parenting stress
The Parenting Stress Index (PSI; short version) was designed to evaluate the degree of stress related to parenting [36]. The PSI requires rating on a 5-point scale the validity of a particular statement describing parents, their child, or their environment. The scale has adequate concurrent and construct validity with a test–retest range of.68–.85 and internal reliability range of.80–.87 [36].
2.6. Intervening variables
2.6.1. The home environment
The quality of the home environment was assessed using the Caldwell “HOME” inventory [37]. The 45 items of the HOME assess both the response of the mother to the child and the quality of the home environment. The 45 items are summarized to six subscales and a single total score. The six subscales include; Responsivity, Acceptance, Organization, Learning Materials, Involvement and Variety. Only the total score was used in this study. Earlier studies have found the HOME to be significantly correlated with cognitive ability at 3 years of age [38]. Inter-rater reliability is at 90% agreement and internal consistency coefficients range between.44 and.89 for the subscales and.89 for the total scale.
2.6.2. Social support
Social support was assessed using the Perceived Social Support Scale (PSS; 39). The multidimensional PSS evaluates the degree of satisfaction with the support received from family members, friends, and significant others. It is composed of 12 items that describe perceived support from those the respondent turned to if he/she had experienced problems in the past six months. It has high internal consistency (α=.91) and validity and has been used in several studies with various ethnic groups [39].
2.6.3. Demographic and background variables
Parental, perinatal, and neonatal data were obtained from medical records and by maternal interview. Information obtained included the background of the parents (income, education, language spoken at home, and number of children), as well as the medical history of the mother and infant (e.g., Apgar scores, gestational age, birth-weight, and length of stay in the hospital).
2.6.4. Data analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS). First, univariate analysis was used to describe the sample characteristics. Second, bivariate analysis to test for multicollinearilty between the BSID-II-MDI and BSID-II-PDI scores at 12 and 18 months and baseline independent and intervening variables (Table 3) were performed. Third, to determine which variables best predicted outcome, the independent or intervening variables that were significantly correlated with either the 12 month BSID-II-MDI or the BSID-II-PDI or with the 18 month BSID-II-MDI or BSID-II-PDI were included in four sets of multiple regression analysis; two for the BSID-II-MDI at 12 and 18 months and two for the BSID-II-PDI at 12 and 18 months.
Table 3.
Correlation matrix between independent and dependent variables (r values presented).
| Independent variables | Dependent variables |
|||||
|---|---|---|---|---|---|---|
| BSID-II-PD |
BSID-II-MDI |
|||||
| Baseline | 12 months | 18 months | Baseline | 18 months | 12 months | |
| Birth weight | .23* | .17 | .17 | .21 | .12 | .14 |
| Gestational age | .15 | .07 | .18 | .27* | .16 | .18 |
| Length of hospital stay | −.27* | −.14 | −.19 | −.25* | −.26* | −.22 |
| APGAR scores | .26* | .29* | .19 | .32* | .21 | .25* |
| Head circumference | .21 | .30* | .34* | 21 | .29* | .34* |
| Socioeconomic status | .19 | .03 | .13 | 14 | .10 | .19 |
| Parental Stress Index | −.09 | −22* | −.15 | −.24* | .26* | −.09 |
| Maternal ethnicity | .04 | .10 | .02 | .06 | .12 | .04 |
| Maternal education | .13 | .16 | .19 | .12 | .09 | .18 |
| Perceived Social Support | .24* | .20 | .26* | .29* | .24 | .16 |
| Mother/infant interaction | .23* | .21 | .25* | .26* | .20 | .15 |
| The HOME | .19 | .18 | .15 | .11 | .09 | .13 |
| Neuro exam – baseline | −.38* | −.56** | −.52** | −.39* | −.52** | −.46** |
| MRI results | −.43 | −.51** | −.47** | −.40** | −48** | −.45** |
| Neuro exam – 12 months | −.31* | −.49** | −.46** | −.31* | −.44** | −.40** |
| Neuro exam – 18 months | −.39 | −.58* | −.47* | −.36* | −.51* | −.43* |
p<.05.
p<.01.
3. Results
The characteristics of the sample population are presented in Table 2. At baseline, infants had a mean birth weight of 1713.8±1242.5 (range=1234.5–2923.9 g), a mean gestational age of 31.2±3.5 weeks (range=28–37 weeks), and a mean Apgar score at 5 min of 6.2±3.5. Approximately 73% of the sample was of Hispanic origin; 13% were Caucasian and 12% were African-American. Seventy-four percent of mothers had prenatal care in the second trimester, 90% received antenatal steroids, and 68% had cesarean deliveries. There were no significant differences at the 12 and 18 month time points on baseline characteristics of the infants sampled, indicating that those who remained in the study were not different than those lost to follow-up.
MRI (n=41) scans at baseline yielded the following: 2 (5%) infants had apparently normal MRI results, but abnormal neurological findings that persisted until 18 months, 10 (24%) infants had apparently normal MRI results, but abnormal neurological signs at baseline that were no longer evident at the 12 or 18 month time-points, and 29 (71%) infants revealed abnormal MRI results which were associated with abnormal neurological findings that persisted until 18 months.
Correlation matrices revealed significant correlations between BSID-II-MDI and BSID-II-PDI scores at 12 (n=50) and 18 (n=46) months and the following variables at baseline (Table 3): length of hospital stay, Apgar scores at 5 min, NCAFS, PSI, head circumference, neurological examination, and MRI results; these variables were entered into four sets of multiple regression analyses. Gestational age, birth weight, socioeconomic status, ethnicity, maternal education, social support and HOME were not correlated with the 12 or 18 month BSID-II-MDI and BSID-II-PDI scores.
3.1. Predictors of motor (BSID-II-PDI) outcome
Table 4 indicates the R2 significance and standardized beta weights for each of the predictor variables of BSID-II-PDI scores at 12 and 18 months. For the BSID-II-PDI scores at 12 months, the model accounted for 37% of the variance (F (7/48)=5.58, p<.05). MRI results, neurological examinations, head circumference, and Apgar scores were significantly related to the BSID-II-PDI scores (β=.29, p<.01; β=.23, p<.05; β=.22, p<.05; β=.19, p<.05 respectively). The remaining variables did not contribute significantly to the BSID-II-PDI scores. At 18 months, the same trend was noted with the exception of Apgar scores, which were no longer significant. Forty-three percent of the variance was explained by the predicting variables (F (7/39)= 5.25, p<.01); MRI scans (β=.26, p<.05), neurological exams (β=.25, p<.05), and head circumference (β=.24, p<.05). This indicates that the best predictors of motor outcome in premature infants suspected of brain injury at birth were the MRI scans performed at baseline, neurological assessments, and head circumference.
Table 4.
Predictors of BSID-II-PDI and BSID-II-MDI scores.
| R2 | BSID-II-PDI |
BSID-II-MDI |
||
|---|---|---|---|---|
| 12 months |
18 months |
12 months |
18 months |
|
| .38 |
.42 |
.39 |
.40 |
|
| β | β | β | β | |
| MRI results | 28** | .25* | .31** | .26** |
| Neuro exam | .22* | .27* | .23* | .23* |
| Head circumference | .21* | 23* | .24* | .19* |
| APGAR scores | .19* | 06 | .17* | .09 |
| Length of hospital stay | −.08 | −.07 | −.20* | −.08 |
| Parental stress | −.09 | −.11 | −.04 | −.06 |
| Mother/infant interaction | .10 | .08 | .09 | .03 |
p<.05.
p<.01.
3.2. Predictors of mental (BSID-II-MDI) outcome
Apgar scores, length of hospital stay, head circumference, NCAFS, PSI, MRI results, and neurological examinations were entered into a multiple regression analysis and explained 38% of the variance in the BSID-II-MDI scores at 12 months (F (7/48)=6.6, p<.01). MRI results (β=.32, p<.01), neurological examinations (β=.25, p<.05), head circumference (β=.21; p<.05), Apgar scores (β=.17. p<.05) and length of hospital stay (β=.20, p<.05) were all significantly related to the mental scores. At 18 months, the same model accounted for 40% of the variance in predicting the BSID-II-MDI scores (F (7/39)=6.33, p<.01) with MRI results (β=.25, p<.01), neurological examinations (β=.22, p<.05), and head circumference (β=.20, p<.05) significantly related to the mental scores. This indicates that the best predictors of mental outcome in premature infants suspected of brain injury at birth were the MRI scans performed at baseline, neurological assessments, and head circumference.
3.3. Brain tissue volume and neurodevelopment
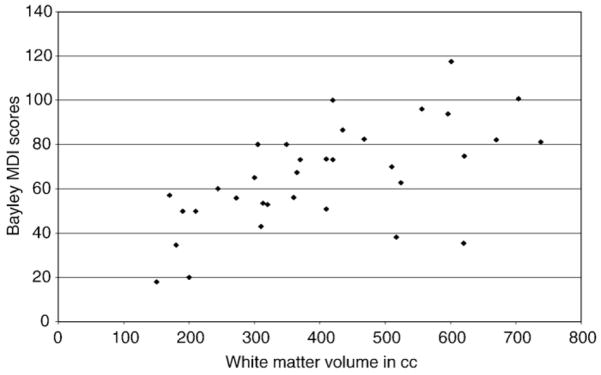
Developmental outcome as quantified using BSID-II-MDI and BSID-II-PDI scores at 12 and 18 months was assessed with respect to brain tissue volumes as quantified from the baseline MR scans. White matter volume significantly correlated with BSID-II-PDI (12 months: r=.31, p<.05; 18 months: r=.29, p<.05) and BSI-II-MDI (12 months: r=.36, p<.05; 18 months: r=.31, p<.05; Fig. 2). Greater white matter volume was associated with higher (i.e., better) BSID-II-PDI and BSID-II-MDI scores at 12 and 18 months.
Fig. 2.
White matter total volume (cm3) and MDI scores at 18 months (N=33).
4. Discussion
The purpose of this study was to determine which variables best predict neurodevelopmental outcome in infants suspected to have sustained brain injury at birth. The results revealed that at both 12 and 18 months, MRI scans, neurological examinations, and head circumference were significantly related to outcome which is in accordance with earlier studies [18,28,40–42]. What is novel is that this study demonstrates that neurological examinations, conducted by an experienced neurologist are as significant in predicting neurodeve-lopmental outcome as results of MRI scans. Infants with abnormal neurological evaluations at baseline are more likely to develop neurological abnormalities at 12 and 18 months than those with normal neurological examinations. This finding is especially relevant for situations in which MR imaging is unavailable [41,43]. Likewise, we noted that head circumference is strongly associated with neurode-velopmental outcome, a finding supported by research findings which document the association between suboptimal head growth, the presence of brain lesions, and poor neurodevelopmental outcome [28,40]. This study also suggests that while Apgar scores at 5 min and length of hospital stay are useful measures in predicting short-term motor and mental scores [29,44], they do not significantly predict long-term outcome (i.e., BSID-II-PDI and BSID-II-MDI scores at 18 months) [45–47]. Of note is that although the clinical criteria used for inclusion in this study have previously been used to diagnose brain injury [41], 31% of the infants recruited had apparently normal MR images at baseline. Indeed, two infants with “normal” MRI scans at baseline presented with neurologic abnormalities at 12 and 18 months (cerebral palsy and mild hemiplegia) which lends support to the idea that neurological assessments are a more reliable index of outcome.
Other plausible explanations for the current findings are that the clinical observations or histories obtained at baseline were inaccurate or that the MR image resolution was insufficient to allow for the detection of subtle brain insults. Furthermore, the predictive capacity of MRI is hindered when the brain insult is subtle, especially in newborn infants [19,20,27]. To date neither the positive predictive value of MRI scans nor their specificity in newborns with brain injury are 100% [48].
Consistent with previous findings, a correlation between brain white matter volume at discharge and neurodevelopmental outcome, as quantified using BSID-II-PDI and BSID-II-MDI scores was demonstrated [18,49,50]. MR studies indicate that mild white matter injury is associated with relatively normal development, while moderate to severe white matter injury is linked to cognitive delays, motor impairments, and cerebral palsy [51,52].
Environmental factors (i.e., socioeconomic background, stress levels, ethnicity, education of mothers, mother–infant interaction, and social support) did not contribute significantly to predicting the developmental outcome of infants with suspected brain injury, in contrast to previous reports (e.g., [32,53,54]). A potential explanation is that this study included infants with suspected brain injury, while previous studies included high-risk preterm infants. Thus, while high-risk premature infants may have the resilience and the potential to benefit from higher parental education and a rich home environment, infants with a brain injury do not seem to have a similar malleability. Furthermore, using the results of MRI scans and neurological examinations to predict outcome may have dwarfed the predictive contributions of environmental variables [17,24,25]. Bendersky and Lewis [12] noted that the severity of intraventricular hemorrhage remained the most significant predictor of motor and language development at three years compared to family risk factors and socioeconomic status. Finally, the period of follow-up (18 months) may have been too early to observe the influence of environment on neurodevelopmental outcome [53–55]. The potential for environmental factors to influence neurodevelopment at later time points warrants further investigation.
The main limitation of this study was the relatively small sample size, especially considering the number of variables evaluated. However, despite the small sample size, 42–48% of the variance in predicting BSID-II-PDI scores and 37–44% of the variance in predicting BSID-II-MDI scores were accounted for by results of MRI and neurological examinations. A similar limitation was that MRI scans were not collected for all infants; this is especially problematic as the least stable infants were the least likely to have completed the MR imaging session.
In conclusion, abnormal MRI findings, results of neurological examinations completed by an experienced neurologist, and head circumference are accurate predictors of neurodevelopmental outcome in infants with suspected brain injury. Although improvements in MR technology will assist in making MRI the best predictive method available, it is neither economical nor practical in many situations. Neurological examinations, on the other hand, are convenient and as accurate in predicting neurodevelopmental outcome as MRI findings [19,27]. The same is true for head circumference which can easily be assessed. It is suggested that when possible, infants suspected of brain injury at or near birth undergo MR, neurological, and head circumference assessments before discharge from the NICU. This information may help in tailoring interventions that might improve the development of infants suspected of brain injury.
Acknowledgments
Sources of support: This study was funded by a grant from the NICHD, R01NCT00006516 and a grant from NIH, MO1RR00425.
I wish to thank my daughter, Natalie Zahr, PhD for her patience in editing this manuscript. I would also like to acknowledge all the nurses and physicians who helped recruit and follow-up the infants for this study.
References
- 1.Ferriero D. Neonatal brain injury. N Engl J Med. 2005;351(19):1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 2.Gäddlin PO, Finnström O, Samuelsson S, Wadsby M, Wang C, Leijon I. Academic achievement, behavioural outcomes, and MRI findings at 15 years of age in very low birthweight children. Acta Paediatr. 2008;84:343–9. doi: 10.1111/j.1651-2227.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 3.Pierrat V, Haouari N, Liska A, Thomas D, Subtil D, Truffert P. Groupe d’Etudes en Epidemiologie Perinatale. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed. 2005:F257–61. doi: 10.1136/adc.2003.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–93. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 5.Carli G, Reiger I, Evans N. One-year neurodevelopmental outcome after moderate newborn hypoxic ischemic encephalopathy. J Pediatr Child Health. 2004;40(4):217–20. doi: 10.1111/j.1440-1754.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- 6.du Plessis AJ, Volpe J. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15(2):151–7. doi: 10.1097/00019052-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Volpe J. Perinatal brain injury: from pathogenesis to neuro-protection. Ment Retard Dev Disabil. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Fotopoulos S, Xanthou M, Pavlou K, Skouteli H, Papassotirou I, Lipsou N. Early markers of brain damage in premature low-birth-weight neonates who suffered from perinatal asphyxia and/or infection. Biol Neonate. 2001;79:213–8. doi: 10.1159/000047094. [DOI] [PubMed] [Google Scholar]
- 9.Janota J, Stranak Z, Simak J, Hackajl D NEOMOD. New Apgar score? A multi-centre study dealing with the evaluation of the neonate NEOMOD scoring system for the first day of life. Ceska Gyneko. 2004;69(Suppl 1):85–90. [PubMed] [Google Scholar]
- 10.Johnston M. Clinical disorders of brain plasticity. Brain Develop. 2004;26(2):73–80. doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 11.Aylward GP. Neodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26(6):427–40. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Bendersky M, Lewis M. Effects of intraventricular hemorrhage and other medical and environmental risks on multiple outcomes at age three years. J Dev Behav Pediatr. 1995;16(2):89–96. [PubMed] [Google Scholar]
- 13.Whitaker AH, Feldman JF, Lorenz JM, Shen S, McNicholas F, Nieto M, et al. Motor and cognitive outcomes in nondisabled low-birth-weight adolescents: early determinants. Arch Pediatr Adolesc Med. 2006;160(10):1040–6. doi: 10.1001/archpedi.160.10.1040. [DOI] [PubMed] [Google Scholar]
- 14.Zahr LK. Predictors of development in premature infants from low-income families: African Americans and Hispanics. J Perinatol. 1999;19(4):284–9. doi: 10.1038/sj.jp.7200159. [DOI] [PubMed] [Google Scholar]
- 15.Krageloh-Mann I. Imaging of early brain injury and cortical plasticity. Exp Neurol. 2004;190(Suppl 1):S84–90. doi: 10.1016/j.expneurol.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, et al. Neurological and perceptual-motor outcome at 5–6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33(5):242–8. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- 17.Arthur R. Magnetic resonance imaging in preterm infants. Pediatr Radiol. 2006;36 (7):593–607. doi: 10.1007/s00247-006-0154-x. [DOI] [PubMed] [Google Scholar]
- 18.Iwata O, Iwata S, Tamura M, Nakamura T, Hirabayashi S, Fueki N, et al. Periventricular low intensities on fluid attenuated inversion recovery imaging in the newborn infant: relationships to the clinical data and long-term outcome. Pediatr Int. 2004;46 (2):150–2417. doi: 10.1046/j.1442-200x.2004.01873.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147 (5):609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Liauw L, van der Grond J, van den Berg-Huysmans AA, Laan LA, van Buchem MA, van Wezel-Meijler G. Is there a way to predict outcome in (near) term neonates with hypoxic–ischemic encephalopathy based on MR imaging? Am J Neuroradiology. 2008;29:1789–94. doi: 10.3174/ajnr.A1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russ A, Hand IL. Preterm brain injury: imaging and neurodevelopmental outcome. Am J Perinato. 2004;21(3):167–72. doi: 10.1055/s-2004-823772. [DOI] [PubMed] [Google Scholar]
- 22.Bartha AI, Yap KR, Miller SPe, Jeremy RJ, Nishimoto M, Vigneron DB, et al. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. Am J Neuroradiol. 2007;28(6):1015–21. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65–74. doi: 10.1542/peds.2007-2822. [DOI] [PubMed] [Google Scholar]
- 24.Iwata S, Iwata O, Bainbridge A, Nakamura T, Kihara H, Hizume E, et al. Abnormal white matter appearance on term FLAIR predicts neuro-developmental outcome at 6 years old following preterm birth. Int J Dev Neurosci. 2007;25(8):523–30. doi: 10.1016/j.ijdevneu.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Bodensteiner JB, Johnsen SD. Magnetic resonance imaging (MRI) findings in children surviving extremely premature delivery and extremely low birthweight with cerebral palsy. Int J Child Neurol. 2006;21(9):743–7. doi: 10.1177/08830738060210091101. [DOI] [PubMed] [Google Scholar]
- 26.Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen L, Tammela O, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116(6):1391–1397.27. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 27.Mercuri E, Guzzetta A, Haataja L, Cowan F, Rutherford M, Counsell S, et al. Neonatal neurological examination in infants with hypoxic ischaemic encephalopathy: correlation with MRI findings. Neuropediatrics. 1999;30(2):83–9. doi: 10.1055/s-2007-973465. [DOI] [PubMed] [Google Scholar]
- 28.Talati AJ, Yang W, Yolton K, Korones SB, Bada HS. Combination of early perinatal factors to identify near-term and term neonates for neuroprotection. J Perinat. 2005;25(4):245–50. doi: 10.1038/sj.jp.7211259. [DOI] [PubMed] [Google Scholar]
- 29.Degos V, Loron G, Mantz J, Gressens P. Neuroprotective strategies for the neonatal brain. Anesth Analg. 2008;106(6):1670–80. doi: 10.1213/ane.0b013e3181733f6f. [DOI] [PubMed] [Google Scholar]
- 30.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 31.Zahr LK, Parker S, Cole J, Engler C. Follow-up of premature infants of low socioeconomic status. Nurs Res. 1989;38(4):246–7. doi: 10.1097/00006199-198907000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Zahr LK. Predictors of development in premature infants from low-income families: African Americans and Hispanics. J Perinatol. 1999;19(4):284–9. doi: 10.1038/sj.jp.7200159. [DOI] [PubMed] [Google Scholar]
- 33.Bayley N. Bayley scales of infant development manual. 2. San Antonio: The Psychological Corp; 1993. [Google Scholar]
- 34.Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol. 2002;27(3):196–212. doi: 10.1016/s0887-8994(02)00436-8. [DOI] [PubMed] [Google Scholar]
- 35.Barnard K, Hammond M, Sumner G, Kang R, Johnson-Crowley N, Snyder C, et al. Helping parents with preterm infants: field test of a protocol. Early Child Dev Care. 1987;27:255–90. [Google Scholar]
- 36.Abiddin RR. Parenting Stress Index. Charlottesville, Va: Pediatric Psychology Press; 1986. [Google Scholar]
- 37.Caldwell BM, Bradley RH. Screening the environment. Am J Orthopsychiatr. 1978;8 (1):114–30. doi: 10.1111/j.1939-0025.1978.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DL, Swank P, Howie VM, Baldwin CD, Owen M, Luttman D. Does HOME add to the prediction of child intelligence over and above SES? J Genet Psychol. 1993;154(1):33–40. doi: 10.1080/00221325.1993.9914719. [DOI] [PubMed] [Google Scholar]
- 39.Canty-Mitchell J, Zimet GD. Psychometric properties of the Multidimensional Scale of Perceived Social Support in urban adolescents. Am J Community Psychol. 2000;28(3):391–400. doi: 10.1023/A:1005109522457. [DOI] [PubMed] [Google Scholar]
- 40.Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121(6):1534–40. doi: 10.1542/peds.2007-2671. [DOI] [PubMed] [Google Scholar]
- 41.Miller SP, Latal B, Clark H, Barnwell A, Glidden D, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190(1):93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman SA, Miller SP, Ferriero DM, Glidden DH, Barkovich AJ, Partridge JC. Encephalopathy as a predictor of magnetic resonance imaging abnormalities in asphyxiated newborns. Pediatr Neurol. 2003;28(5):342–6. doi: 10.1016/s0887-8994(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 43.Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen L, Tammela O, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116(6):1391–7. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 44.Forsblad K, Kallen K, Marsal K, Hellstrom-Westas L. Apgar score predicts short-term outcome in infants born at 25 gestational weeks. Acta Paediatr. 2007;96(2):166–71. doi: 10.1111/j.1651-2227.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 45.Mercuri E, Rutherford M, Barnett A, Foglia C, Haataja L, Counsell S, et al. MRI lesions and infants with neonatal encephalopathy. Is the Apgar score predictive? Neuropediatrics. 2002;33(3):150–6. doi: 10.1055/s-2002-33412. [DOI] [PubMed] [Google Scholar]
- 46.The American College of Obstetrics and Gynecologists. Neonatal encephalopathy and cerebral. Washington DC: Library of Congress; 2003. [Google Scholar]
- 47.Janota J, Stranak Z, Simak J, Hackajlo D, Vyzkumna S NEOMOD. New Apgar score? A multi centre study dealing with the evaluation of the neonate NEOMOD scoring system for the first day of life. Ceska Gyneko. 2004;69(Suppl 1):85–90. [PubMed] [Google Scholar]
- 48.El-Ayouty M, Abdel-Hady H, El-Mogy S, Zaghlol H, El-Beltagy M, Aly H. Relationship between electroencephalography and magnetic resonance imaging findings after hypoxic–ischemic encephalopathy at term. Am J Perinatol. 2007;24(8):467–73. doi: 10.1055/s-2007-986686. [DOI] [PubMed] [Google Scholar]
- 49.Yung A, Poon G, Qiu DQ, Chu J, Lam B, Leung C, et al. White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr Res. 2007;61(6):732–6. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]
- 50.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 51.Drobyshevsky A, Bregman J, Storey P, Meyer J, Prasad PV, Derrick M, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29(4–5):289–601. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 52.Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63(5):642–51. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 53.Goyen TA, Lui K. Longitudinal motor development of “apparently normal” high-risk infants at 18 months, 3 and 5 years. Early Hum Dev. 2002;70(1–2):103–15. doi: 10.1016/s0378-3782(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan MC, Msall ME. Functional performance of preterm children at age 4. J Pediatr Nurs. 2007;22(4):297–09. doi: 10.1016/j.pedn.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyce G, Saylor C, Price C. School-age performance for early intervention participants who experienced intraventricular hemorrhage and low birth weight. Child Health Care. 2004;33(4):257–74. [Google Scholar]