Abstract
Aims
In this article we present our experience with radiofrequency ablation (RFA) in the treatment of 105 renal tumors.
Materials and Methods
RFA was performed on 105 renal tumors in 97 patients, with a mean tumor size of 32 mm (11–68 mm). The mean patient age was 71.7 years (range, 36–89 years). The ablations were carried out under ultrasound (n = 43) or CT (n = 62) guidance. Imaging follow-up was by contrast-enhanced CT within 10 days and then at 6-monthly intervals. Multivariate analysis was performed to determine variables associated with procedural outcome.
Results
Eighty-three tumors were completely treated at a single sitting (79%). Twelve of the remaining tumors were successfully re-treated and a clinical decision was made not to re-treat seven patients. A patient with a small residual crescent of tumor is under follow-up and may require further treatment. In another patient, re-treatment was abandoned due to complicating pneumothorax and difficult access. One patient is awaiting further re-treatment. The overall technical success rate was 90.5%. Multivariate analysis revealed tumor size to be the only significant variable affecting procedural outcome. (p = 0.007, Pearson χ2) Five patients had complications. There have been no local recurrences.
Conclusion
Our experience to date suggests that RFA is a safe and effective, minimally invasive treatment for small renal tumors.
Keywords: Kidney, Computed tomography, Kidney neoplasms, Therapeutic radiology, Radiofrequency ablation
In recent years radiofrequency ablation (RFA) has continued to evolve into an effective image-guided tool for the minimally invasive destruction of small-volume, discrete tumors. While the vast majority of experience has been gained in the treatment of hepatocellular carcinoma (HCC) and colorectal metastases in the liver, recent attention has turned to renal tumors [1–4]. Renal tumors represent 3% of all human tumors [5] and the 5-year survival rate for RCC has increased from 34% in 1954 to 62% in 1996 [6]. There has also been a 126% increase in the incidence of renal cell carcinoma in the United States since 1950 [6]. Both the increased incidence and the improved survival are largely attributable to the radiologic detection of early-stage disease [7]. In addition, despite other strategies, this detection is largely serendipitous at cross-sectional imaging studies for other symptomatology. Some series have suggested that up to 85% of all renal tumors are in fact detected incidentally [8]. The improved outcome from smaller-volume tumors has been reflected by the TNM classification system, whereby the upper size limit for T1 tumors was increased to 7 cm in 1997 [9] and a subgroup of discrete tumors < 4 cm in diameter was classified as stage T1a, which rarely metastasize [7].
A growing body of opinion has acknowledged the morbidity and mortality of radical surgery for often small and potentially low-grade tumors [10]. This has paved the way for nephron-sparing surgery, however, partial nephrectomy can be a more technically demanding procedure than standard nephrectomy and carries its own, not insignificant, morbidity [11–13]. Laparoscopic partial nephrectomy represents a further evolution in minimally invasive therapies for small-volume renal cancer, however, it remains a technically demanding and time-consuming operation. Image-guided thermal ablation techniques including RFA, cryotherapy, and microwave ablation are now emerging as practical, safe, and effective ablative tools.
RFA has been widely used in the treatment of small-volume hepatic tumors. It achieves tumor destruction through thermally induced coagulative necrosis at temperatures of 60 to 110°C. In 1999, we initiated a program for the percutaneous RF ablation of small (<4-cm) renal tumors at our institution. This paper outlines our experience with procedural technique, complications, and oncologic outcomes.
Materials and Methods
In 1999, ethics committee approval was granted for the commencement of a renal tumor RFA program. Inclusion criteria for possible RFA included patients unfit for major surgical intervention, or for whom the surgeon deemed that resection would be problematic due to tumor location (interpolar or deep-set positions), multifocal disease (n = 6), or in the setting of a solitary kidney (n = 20). In only one case in this series was RFA used to treat a tumor in the setting of metastatic disease to the dorsal spine.
The patients attended for outpatient assessment, including a clotting screen (INR ≤1.4 was sought) and creatinine measurement and a review of their recent radiologic staging. A degree of renal impairment did not preclude treatment but was carefully monitored following RFA. Fully informed consent was obtained. The decision to treat was based on established CT criteria, namely, average density >20 Hounsfield units (HU) and enhancement of >20 HU after contrast [14], with or without additional criteria such as absence of fat and contour deformation [7].
At the time of reporting, we have treated 105 renal tumors in 97 patients. The mean age of the patients was 71.7 years (range, 36–89 years). Sixty-five patients were male and 32 were female. Although the majority of tumors treated were small, on occasions tumors larger than 4 cm were ablated (n = 12), according to individual clinical indications. The mean tumor size was 32 mm (median, 30 mm; range, 11–68 mm).
The tumors were assessed for ultrasonographic visibility and ease of access. Some of the lesions (n = 43) were treated under ultrasound guidance but those that were more difficult to visualize with ultrasound or that were in close proximity to bowel (n = 62) were treated with a combination of CT and ultrasound guidance. Most small-volume cortical tumors lend themselves to ultrasound-guided treatment in the prone oblique position, however, more recently we have tended toward a combined ultrasound- and CT-guided approach to absolutely confirm probe position with respect to adjacent structures. An initial contrast-enhanced study was performed with 100 ml of iodinated contrast medium (300 mg I/ml; Omnipaque; GE Healthcare, UK) at a rate of 4.0 ml per second. The subsequent CT-guided intervention was performed using small (5-mm-collimation) diagnostic spiral acquisitions.
Eighty-six tumors were exophytic and 19 were intraparenchymal or more centrally located. Tumors were deemed exophytic when 25% or more of their diameter protruded outside the cortical margin [15]. Twenty-three tumors were located within the upper pole of the kidney, 33 were in the lower pole, and 49 were categorized as “interpolar” in location. Fifty-seven tumors were located in the left kidney and 48 in the right.
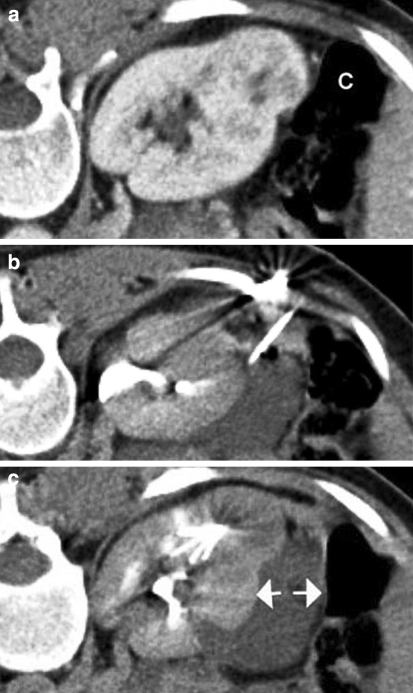
Occasionally anterior interpolar tumors are closely approximated to the gut and it has been our practice to increase the safety margin around these tumors using “hydrodissection” with 5% dextrose solution ± air (nine cases) (Figs. 1a–c). In these cases, the related retroperitoneal space is needle-punctured and 250 to 750 ml of 5% dextrose solution is instilled to displace the bowel, so creating a safety margin for thermal ablation.
Fig. 1.
Prone contrast-enhanced CT images illustrating the technique of “hydrodissection.” a Image showing an exophytic renal tumor lying in close proximity to the colon (C). b A needle has been introduced into the perirenal space and 5% dextrose is being instilled. c The 5% dextrose has created a safety margin (arrows) between the tumor and adjacent colon. The tines of an expandable RFA probe can be seen within the lesion to be treated
In the case of two larger tumors, embolization was performed prior to RFA with Embospheres (500–700 μm; Biosphere Medical, Paris) using a co-axial microcatheter technique to selectively target tumor vascularity. Both tumors that were pre-embolized in this cohort were >5 cm in size, although a lower threshold has been suggested [16]. The renal, adrenal, and lumbar arteries were assessed for accessory collateral supply and, if identified, were also embolized to stasis. In one case additional coiling (Embolization Microcoils Soft Platinum Type-C; Cook, Denmark) of a “parasitized” retroperitoneal feeding vessel was performed.
All patients receive 24 h of broad-spectrum intravenous antibiotic cover with metronidazole (500 mg three times daily) and cefuroxime (750 mg three times daily) followed by a 10-day course of oral ciprofloxacin (500 mg twice daily). At our hospital most RFA procedures have been performed under conscious sedation (dose titrated to individual patient requirement), with midazolam and fentanyl citrate (Sublimaze; Janssen-Cilag, Belgium; n = 67), although more recently we have shifted toward general anesthesia for larger-volume or multifocal treatments (n = 38).
Two RFA systems have been employed in this series: Tyco/Radionics (Boulder, CO, USA) single (n = 21) or cluster (n = 37) needle probes with 3- and 2.5-cm exposed tips, respectively, and RITA (Mountainview, CA, USA) expandable needle probes (Starburst and Starburst XL; n = 47) at the initial treatment. In the case of Tyco/Radionics needle probes, treatments were applied using an impedance-regulated 200W generator (CC1 generator; Tyco, Boulder, CO, USA) in 12-min aliquots for between 12 and 36 min. With the RITA probe, a time- and temperature-regulated 150- or 200-W generator (models 1500 and 1500X; RITA) was used, aiming for a mean target temperature of 105°C, for 3- to 5-cm treatment cycles, depending on the size of the lesion. A thermal “track ablation” was performed at the end of the treatment episode to reduce the potential risk of track seeding. Patients were monitored for 4 h in the recovery area and then observed overnight on a ward before being discharged home. RFA was performed by one of two experienced interventional radiologists (D.J.B., J.E.I.C.) and the technique and probe, as outlined above, were chosen at the discretion of the operator and were not related to tumor characteristics.
The overall follow-up period ranged from 1 to 76 months (mean, 16.7 months in surviving patients). Follow-up assessment was by contrast-enhanced CT (reported by one of the two operators: D.J.B. or J.E.I.C.) and treatment adequacy determined by the area of nonenhancement with respect to the tumor mass [15]. Careful comparison was made with the most recent preprocedural imaging. (Figs. 2a, b). Subsequent CT assessment was made at approximately 6-monthly intervals. This involved precontrast imaging of the tumor followed by images of the abdomen in arterial phase (35 s) and subsequent imaging of the chest, abdomen, and pelvis in the portal venous phase (65 to 70 s) after administration of 100 ml of iodinated contrast medium (300 mg I/ml; Omnipaque; GE Healthcare, UK) at a rate of 4.0 ml per second, with a collimation of 1.25 mm (Siemens Somatom Sensation 16-slice scanner; Siemens AG, Medical Solutions, Germany). Residual tumor was defined as enhancing tumor remnants within the volume of the lesion as appreciated at preprocedural imaging (Fig. 3a, d). If the patient had significant renal impairment, then iso-osmolar contrast was used (Visipaque; GE Healthcare, UK) and in one case gadolinium-enhanced MR (T1-weighted, breath-hold volume sequence) imaging was utilized.
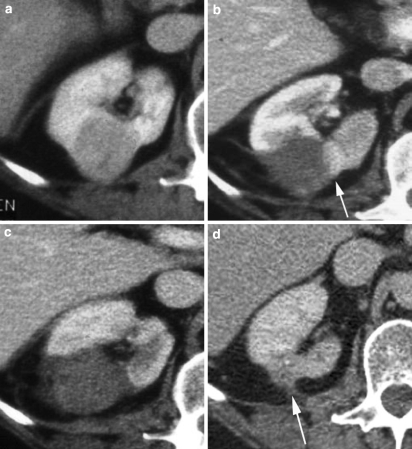
Fig. 3.
a Axial CT image of a 3-cm exophytic right interpolar renal tumor prior to RFA. b Ten days following RFA, contrast-enhanced CT reveals that the majority of the lesion is nonenhancing, consistent with necrosis, but there is a residual crescent of enhancing tumor within the medial aspect of the lesion (arrow). c One week after re-treatment of the residual crescent of tumor, the whole lesion is nonenhancing, in addition to a wedge-shaped area of adjacent cortex. This is consistent with complete necrosis of the tumor. d Five years post RFA: the lesion shows typical involution, with dispersal into the perirenal fat (arrow). There is no evidence of tumor recurrence
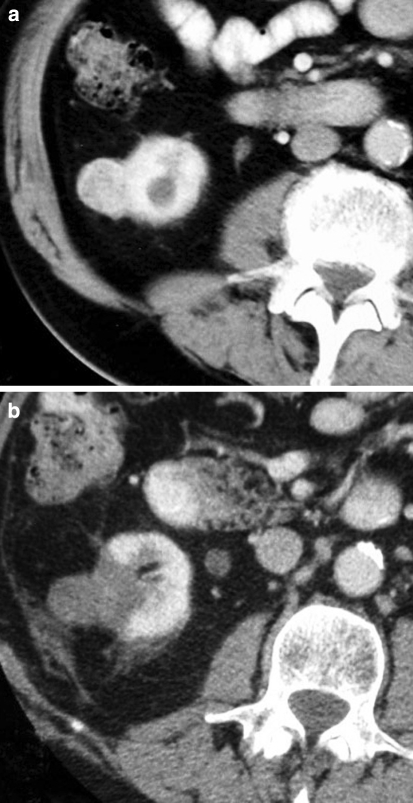
Fig. 2.
a Contrast-enhanced CT revealing a small exophytic tumor arising from the lower pole of the right kidney. b Contrast-enhanced CT performed 3 days after RFA shows a typical wedge-shaped area of nonenhancement, involving the tumor and immediately adjacent cortex. This appearance indicates coagulative necrosis and hence a completely treated lesion
Multivariate analysis was performed on the data to determine any association between treatment variables (namely, tumor size, imaging modality, probe type, central/exophytic position, and polar location within the kidney) and the likelihood of successful tumor ablation. Statistical Package for the Social Sciences (SPSS, version 10.0) software was used for the analysis.
Results
Eighty-three of 105 tumors were completely treated at a single sitting. In five elderly patients with thin residual crescents of viable tumor, a clinical decision was made not to re-treat. In one case of subtotal treatment, the patient proceeded to nephrectomy, and in another where contralateral nephrectomy had been performed for multiple oncocytomas, the decision was made not to pursue further intervention.
Fourteen tumors were re-treated, the majority under CT guidance (n = 12), and one patient is awaiting re-treatment. In 12 cases the residual tumor crescent was successfully ablated (one patient required two re-treatments for complete tumor ablation). The overall technical success rate of this cohort is therefore 95 of 105 tumors (90.5%). In one case of an elderly patient with kyphoscoliosis, the re-treatment procedure was complicated by pneumothorax and abandoned due to difficult subsequent access. One patient had a persistent small residual area of tumor enhancement that will be reassessed at interim CT, however, it is thought likely that further treatment will be required.
Tumor size was clearly predictive of complete treatment, with all tumors <3.5 cm (n = 73) in size being successfully ablated at a single treatment sitting barring two cases where lesions proved difficult to target. The mean size of tumors completely ablated at a single sitting was 28 mm (range, 14–46 mm) and the mean size of those in whom the initial treatment was subtotal was 41 mm (range, 18–68 mm). A statistically significant association was seen between a tumor size of ≤3 cm and complete treatment at a single session (p = 0.007, Pearson χ2; odds ratio, 3.99).
Of the 22 subtotal treatments, 9 were ultrasound-guided and 13 were performed with a combination of CT and ultrasound guidance. No statistically significant association between imaging modality utilized during RFA and treatment outcome was observed (p = 0.996).
No statistically significant association was seen between tumor location within the kidney, in terms of either whether it was central or exophytic (p = 0.222, Fisher’s exact test) or whether it was upper, lower, or interpolar (p = 0.823, Pearson χ2) and successful tumor ablation at first sitting. Similarly, there was no association observed between the type of probe used for RFA (single, cluster, or expandable) and a successful initial outcome (p = 0.538, Pearson χ2).
In this series, there was an overall complication rate of 5 of 120 treatment episodes (4.2%; 3 CT-guided, 2 ultrasound-guided). One patient developed profuse but self-limiting hematuria. Another patient, in whom a scoliotic deformity and a renal hematoma had complicated the procedure, sustained a thermal injury to the duodenum that required laparotomy and repair. He has subsequently made a full recovery. A further patient was found to have moderate hydronephrosis due to a proximal ureteric stricture on a follow-up CT approximately 4 months post-RFA. This was treated by temporary placement of a ureteric stent. Two of the five complications occurred at second treatments. In one case, the re-treatment was performed under CT guidance in the prone-oblique position but complicated by a marked kyphoscoliotic deformity. This led to poor visualization of the upper pole target tumor and resulted in a pneumothorax that was treated by simple chest tube drainage. A further patient with a scoliosis and a 2.5-cm, deeply set tumor developed a calyceal leak and subsequent urinoma, which were treated by percutaneous drainage and placement of a temporary ureteric stent. Of note, three of the five complications occurred in the setting of marked scoliotic deformity.
Within our follow-up study group there have been 13 deaths to date. Six of these were from completely unrelated pathologies, namely, chronic lymphatic leukemia (two cases), mesothelioma, obstructive airways disease, myocardial infarction, and cholangitis. The cause of death was not investigated or records were unobtainable in six cases, however, follow-up imaging within 6 months of death had demonstrated no recurrent or metastatic disease in these patients. One death occurred due to disseminated metastatic disease in a patient with von Hippel-Lindau disease who had previously undergone a contralateral nephrectomy for renal cell carcinoma. She had three small tumors in her remaining kidney, which were treated with RFA at a single sitting. The patient was offered a second nephrectomy but declined on the grounds that she did not wish to become dialysis-dependent. At follow-up, a further metachronous renal tumor became apparent and was successfully ablated. This patient subsequently developed disseminated metastases and died. In this case it was impossible to determine which of the renal tumors had metastasized. One further patient (a 78-year-old woman) developed lung metastases under follow-up. She had undergone previous contralateral nephrectomy for a large clear cell carcinoma 6 months prior to RFA of a 2.5-cm tumor in the remaining kidney. Small lung metastases were seen on a CT performed 3 months after RFA. There have been no other cases of metastatic disease and no local recurrences.
Discussion
In recent years a number of groups have studied the use of RFA in the management of small renal tumors [1, 2]. We have reviewed our experience since 1999 in the treatment of a large cohort of 105 renal tumors with regard to technical success, complications, and midterm oncologic efficacy.
The overall technical success rate for the procedure in this series was 95 of 105 tumors (90.5%). This is comparable with the results of Gervais et al. [1], who reported a complete tumor necrosis rate of 90%. Matsumoto et al. [2] pooled data from three major North American urological centers in their report on the treatment outcome for 109 tumors and reported an overall technical success rate of 100%, with only two patients requiring re-treatment for completion of the ablation. These procedures were carried out percutaneously in 63 cases and at laparoscopy in 46 cases. This outcome may be explained in part by the small tumor size in this report, which ranged from 0.8 to 4.7 cm (mean, 2.4 cm). This compares to a mean tumor size of 3.2 cm in both this study and the series of Gervais et al. [1].
This cohort of 105 tumors has permitted multivariate analysis of the factors likely to predict successful outcome. It has been widely reported that exophytic tumors are more amenable to thermal ablation, likely due to their smaller “vascular pedicle” with the underlying renal parenchyma [1]. We utilised Gervais’ definition [15] of central and exophytic tumors, yielding 19 central and 86 exophytic tumors in this series, but we could not confirm a statistically significant association between tumor location and outcome using Fisher’s exact test (p = 0.222). Likewise regarding polar tumor location (23 upper pole, 33 lower pole, and 49 interpolar), no significant association with outcome was confirmed (p = 0.823, Pearson χ2). In addition, we found no significant association between probe type (cluster, single, expandable) and likelihood of complete tumor ablation (p = 0.996, χ2). Logistic regression revealed that the only statistically significant variable associated with successful outcome was tumor size. A tumor size of ≤3 cm was associated with complete treatment at a single session (p = 0.007, Pearson χ2; odds ratio, 3.99).
All 105 tumors have been treated by one of two operators (D.J.B., J.E.I.C.), in 43 cases with ultrasound guidance alone and in 62 instances using CT guidance combined with adjunctive ultrasound to facilitate probe placement. It has been our experience that the latter technique expedites tumor treatment and increases operator certainty with regard to the position of closely related bowel loops, particularly during the use of expandable, multitined probes.
Of the initial treatments, 3 of 105 (2.9%) incurred complications. This parallels other large series such as that of Matsumoto et al. [2], who, in their series of 109 tumors, experienced a complication rate of 2.8%. Gervais et al. [1] incurred 4 major complications in the treatment of 100 tumors. Contrary to the findings in that series, we did not encounter significant perirenal hemorrhage. In one case, there was brisk but self-limiting hematuria and in another, a temporary ureteric stricture, related to a lower pole tumor treatment, was managed by temporary ureteric stenting. In the third case, the target tumor was in the “deeply set” kidney of a patient with a scoliotic deformity. An initial biopsy caused a moderate hematoma resulting in some obscuration of the tumor for the purposes of probe placement. The patient re-presented 3 days following treatment and CT revealed a contained retroperitoneal duodenal perforation, which was immediately repaired at laparotomy. We have now changed our practice such that when biopsy is required, the RF needle is usually placed prior to biopsy to ensure good probe position. This avoids obscuration of the tumor by postbiopsy hematoma.
Two further complications occurred in the re-treatment group, thereby yielding an overall complication rate of 5 of 120 treatment episodes (4.2%) but resulting in a higher complication rate, 2 of 14 (14%), among this subset. In one case, a patient with scoliosis, a calyceal leak caused a urinoma that was managed by drainage and ureteric stenting. In the second case, re-treatment of a poorly visible tumor at the upper pole of the kidney was attempted under ultrasound and CT guidance. This patient had a significant scoliotic deformity; attempted probe placement resulted in a pneumothorax and the procedure had to be abandoned. It is notable that three of these five overall complications occurred in scoliotic patients when attempting to treat tumors in the more poorly accessible, or “deep-set” kidney.
The case remains that RFA is an in situ technique, reliant on devascularization of the tumor, as confirmed by nonenhancement at postprocedural CT to determine treatment adequacy. No cases of late local recurrence have been encountered and the authors believe that this is attributable to meticulous postprocedural imaging assessment with the use of multidetector CT and careful multiplanar assessment.
The use of nonenhancement of the treated tumor and peritumoral changes along with ablative changes to the adjacent cortex as surrogates of treatment adequacy has been criticized. This has, however, been borne out by the steady involution of the treated tumors in this series under CT follow-up. In 22 cases, incomplete treatment was evidenced by residual enhancing tumor “crescents,” and secondary ablations were successfully performed in 12 of these cases. A recent study by Michaels et al. [17] suggested that residual viable tumor was present in all 20 resected small-volume renal tumors on the basis of hematoxylin and eosin staining and in 4 of 5 of the most recent tumors on nicotinamide adenine dinucleotide (NADH) diaphorase staining. There has been some criticism of the adequacy of the RF treatment cycles used by these authors, where the mean treatment time was 9.1 min. In addition, hematoxylin and eosin staining has been repeatedly criticized as an inadequate determinant of thermally induced cell death [18]. Our mid- to long-term case follow-up has also demonstrated steady involution of the vast majority of treated lesions, and to date, with a mean follow-up of 18 months, no revascularization of treated tumors has been detected.
This patient cohort has been actively followed up, with approximately 6-monthly CT surveillance over a period of 1 to 76 months. Thirteen patients have died, yielding a mean follow-up among the survivors of 16.7 months. Only two patients have developed metastatic renal cancer. One patient suffered from von Hippel Lindau syndrome and had undergone a previous nephrectomy. She refused a further nephrectomy and proceeded to RFA of three renal tumors in the remaining kidney. A fourth metachronous tumor was subsequently treated. She presented approximately 18 months after the initial RFA treatment with disseminated metastatic disease. Postmortem suggested new “interval” disease within the remnant kidney. In the other case, nephrectomy for a 7-cm tumor had been carried out 6 months prior to a referral for RFA of a new 3-cm tumor in the contralateral kidney. RFA for this second lesion was successful, but at CT 3 months after RFA, a lung base metastasis was noted. It remains indeterminate as to whether this related to the earlier larger tumor or the smaller contralateral tumor treated by RFA, but in view of the natural history of RCC, the former seems more likely.
The imaging diagnosis of renal tumors has been repeatedly shown to be more accurate than biopsy [19, 20] due to false-negative specimens. However, even with careful CT radiologic technique, it remains the case that a proportion (∼10%–15%) of resected or ablated lesions will prove to be benign in nature such as fat-poor angiomyolipomas and oncocytomas [7]. The lack of biopsy data in this series could be considered a limitation of this study, however, it could be argued that the inclusion of a small number of benign lesions does not invalidate the results of the technical efficacy of the procedure with regard to tumor necrosis. As commented upon by Matsumoto et al. [2], even benign tumors such as oncocytomas have the potential to recur locally. Some authors would argue that with the increasing detection of small renal tumors, which may not be easily characterized at CT, there is an increasing role for preprocedural percutaneous biopsy [21].
As yet there are few published long-term outcome data for ablative techniques. In one recent study [22] of 16 biopsy-proven RFA cases with a minimum of 4 years’ follow-up, 5 patients died under surveillance and the remainder were well, with no tumor recurrence. This experience concurs with our accruing follow-up data.
Conclusion
These interim results in 105 tumors appear to confirm the efficacy of RFA but formal 5-year follow-up data are awaited. There does, however, appear to be a real place for the image-guided ablation of sub-4-cm renal tumors in patients who are less suitable for surgical resection.
Acknowledgments
We would like to gratefully acknowledge Dr. R. Mehta and Dr. G. Yadegarfar for their assistance with the statistical analyses for this case series.
References
- 1.Gervais DA, McGovern FJ, Arellano RS, et al. (2005) Radiofrequency ablation of renal cell carcinoma. Part 1. Indications, results and role in patient management over a 6-year period and ablation of 100 tumors. AJR 185:64–71 [DOI] [PubMed]
- 2.Matsumoto ED, Johnson DB, Ogan K, et al. (2005) Short-term efficacy of temperature-based radiofrequency ablation of small renal tumors. Urology 65(5):877–881 [DOI] [PubMed]
- 3.Mayo-Smith WW, Dupuy DE, Parikh PM, et al. (2003) Imaging-guided percutaneous radiofrequency ablation of solid renal masses: technique and outcomes of 38 treatment sessions in 32 consecutive patients. AJR 180:1503–1508 [DOI] [PubMed]
- 4.Farrell MA, Charboneau WJ, DiMarco DS, et al. (2003) Imaging-guided radiofrequency ablation of solid renal tumors. AJR 180:1509–1513 [DOI] [PubMed]
- 5.Jemal A, Tiwari RC, Murray T, et al. (2004) Cancer statistics. CA Cancer J Clin 54:8–29 [DOI] [PubMed]
- 6.Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal cell carcinoma. J Urol 166:1611–1623 [DOI] [PubMed]
- 7.Zagoria RJ (2000) Imaging of small renal masses. AJR 175:945–955 [DOI] [PubMed]
- 8.Jayson M, Sanders H (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51:203–205 [DOI] [PubMed]
- 9.Hermanek P, Hutter RVP, eds (1997) TNM atlas. Ed. 3. Springer Verlag, New York
- 10.Belldegrun A, Tsui KH, de Kernien JB, et al. (1999) Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol 17:2868–2875 [DOI] [PubMed]
- 11.Van Poppel H, Bamelis B, Oyen R, et al. (1998) Partial nephrectomy for renal cell carcinoma can achieve long-term tumoral control. J Urol 160:674–678 [DOI] [PubMed]
- 12.Hafez KS, Norvick AC, Butler BP (1998) Management of small unilateral renal cell carcinomas: impact of central versus peripheral tumor location. J Urol 159:1156–1160 [DOI] [PubMed]
- 13.Uzzo RG, Novick AC (2001) Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol 166:6–18 [DOI] [PubMed]
- 14.Silverman SG, Lee BY, Seltzer SE, et al. (1994) Small (≤3cm) renal masses: correlation of spiral CT features and pathologic findings. AJR 163:597–605 [DOI] [PubMed]
- 15.Gervais DA, McGovern FJ, Wood BJ, et al. (2000) Radiofrequency ablation of renal cell carcinoma: early clinical experience. Radiology 217:665–672 [DOI] [PubMed]
- 16.Mahnken AH, Rohde D, Brkovic D, Günther RW, Tacke JA (2005) Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol 46:208–214 [DOI] [PubMed]
- 17.Michaels MJ, Rhee HK, Mourtzinos AP, et al. (2002) Incomplete renal tumor destruction using radio frequency interstitial ablation. J Urol 168:2406–2410 [DOI] [PubMed]
- 18.Goldberg SN, Gazelle GS, Compton CC, et al. (2000) Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 88(11):2452–2463 [DOI] [PubMed]
- 19.Goethuys H, Van Poppel H, Oyen R, et al. (1996) The case against fine-needle aspiration cytology for small solid kidney tumors. Eur Urol 29:284–287 [DOI] [PubMed]
- 20.Wood BJ, Khan MA, McGovern F, et al. (1999) Imaging guided biopsy of renal masses: indications, accuracy and impact on clinical management. J Urol 161:1470–1474 [DOI] [PubMed]
- 21.Silverman SG, Gan YU, Mortele KJ, et al. (2006) Renal masses in the adult patient: the role of percutaneous biopsy. Radiology 240(1):6–22 [DOI] [PubMed]
- 22.McDougal WS, Gervais DA, McGovern FJ, Mueller PR (2005) Long-term follow up of patients with renal cell carcinoma treated with radiofrequency ablation with curative intent. J Urol 174(1):61–63 [DOI] [PubMed]