Abstract
Endoluminal treatment of infrapopliteal artery lesions is a matter of controversy. Bioabsorbable stents are discussed as a means to combine mechanical prevention of vessel recoil with the advantages of long-term perspectives. The possibility of not having a permanent metallic implant could permit the occurrence of positive remodeling with lumen enlargement to compensate for the development of new lesions. The present study was designed to investigate the safety of absorbable metal stents (AMSs) in the infrapopliteal arteries based on 1- and 6-month clinical follow-up and efficacy based on 6-month angiographic patency. One hundred seventeen patients with 149 lesions with chronic limb ischemia (CLI) were randomized to implantation of an AMS (60 patients, 74 lesions) or stand-alone percutaneous transluminal angioplasty (PTA; 57 patients, 75 lesions). Seven PTA-group patients “crossed over” to AMS stenting. The study population consisted of patients with symptomatic CLI (Rutherford categories 4 and 5) and de novo stenotic (>50%) or occlusive atherosclerotic disease of the infrapopliteal arteries who presented with a reference diameter of between 3.0 and 3.5 mm and a lesion length of <15 mm. The primary safety endpoint was defined as absence of major amputation and/or death within 30 days after index intervention and the primary efficacy endpoint was the 6-month angiographic patency rate as confirmed by core-lab quantitative vessel analysis. The 30-day complication rate was 5.3% (3/57) and 5.0% (3/60) in patients randomized for PTA alone and PTA followed by AMS implantation, respectively. On an intention-to-treat basis, the 6-month angiographic patency rate for lesions treated with AMS (31.8%) was significantly lower (p = 0.013) than the rate for those treated with PTA (58.0%). Although the present study indicates that the AMS technology can be safely applied, it did not demonstrate efficacy in long-term patency over standard PTA in the infrapopliteal vessels.
Keywords: Critical limb ischemia, Absorbable stent, Stent, Angioplasty, Revascularization, Peripheral artery disease, Below the knee, Infrapopliteal, Crural, Limb salvage
Introduction
Over the last years, a gradual change in treatment strategy for critical limb ischemia (CLI) has been observed: the endovascular approach has become more and more accepted as a valid alternative for surgical intervention [1]. The BASIL trial participants played a pivotal role in this shift toward the minimal invasive approach for patients with CLI due to infrainguinal disease. They were the first to publish results of a controlled, randomized study comparing bypass surgery results to those of percutaneous transluminal angioplasty (PTA) in CLI patients that yielded similar outcomes for both treatments, with angioplasty being the less expensive alternative in the short term [2]. The latter resulted in the statement in the renewed TASC-II guidelines that there is increasing evidence to support a recommendation for PTA in patients with CLI and infrapopliteal artery occlusion [3].
Although the use of stents is common in other peripheral vessels, the application of stents in the infrapopliteal bed remains highly controversial. The fear that early thrombosis and late luminal loss due to intimal hyperplasia formation potentially lead to insufficient long-term patency rates can explain the reluctance on implanting stents in these small-diameter vessels. Although the limited evidence available suggests very acceptable outcomes using either passive coated stents (PCSs) [4], balloon-expandable drug-eluting stents [5–8], or self-expanding nitinol stents [9–12], target lesion revascularization (TLR) is still required in a significant percentage of patients to enable limb salvage. The permanent presence of an artificial implant is believed to be the potential trigger for late restenosis. Recently the stenting technology has moved toward the development of temporary implants composed of biocompatible materials which mechanically support the vessel during the period of high risk for recoil and then completely degrade on the long-term perspective [13–19]. Therefore, bioabsorbable stents are discussed as a means to combine mechanical prevention of vessel recoil with various advantages of the long-term perspective compared to permanent implants, including the possibility of late outward vessel remodeling, and improved reintervention options [18]. The bioabsorbable magnesium-alloy stent developed by BIOTRONIK AG was the first of its kind for which it was proven that it could be implanted safely in infrapopliteal [17, 18, 20] and human coronary arteries [13, 21, 22] and that it was absorbed in the intended time frame. In the absorbable metal stent (AMS) below-the-knee study [20], Bosiers et al. presented the first clinical results using AMSs for treatment of infrapopliteal lesions in 20 patients with CLI. After 6 months, the resulting values for primary clinical patency and limb salvage indicated a promising performance for the treatment of below-the-knee lesions in CLI patients. Moreover, in the PROGRESS-AMS study the authors proved that bioabsorbable magnesium stents can achieve immediate angiographic results similar to the results with other permanent metal stents and is safely degraded after 4 months [13] in native coronary artery single de novo lesions.
Based on these findings, the study was set up to give further proof of AMS safety and efficacy for the treatment of CLI, and the AMS INSIGHT (Bioabsorbable Metal Stent Investigation in Chronic Limb Ischemia Treatment) randomized controlled trial was initiated. Therefore, in this article, safety results based on 1- and 6-month clinical follow-up and efficacy data based on 6-month angiographic results for the AMS INSIGHT in patients with CLI are presented and discussed (see Table 6 for a complete list of authors and their contributions). The primary aim of the study was to prove the superiority of the AMS stent compared to PTA alone for infrapopliteal indications.
Table 6.
Complete list of authors and authors’ contributions to this study
| Author name & affiliation | Conception & design | Analysis & interpretation | Data collection | Writing of article | Critical revision of article | Final approval of article | Statistical analysis | Obtaining funding | Overall responsibility |
|---|---|---|---|---|---|---|---|---|---|
| M. Bosiers, AZ St. Blasius Dendermonde, Belgium | X | X | X | X | X | X | X | X | |
| P. Peeters, Imelda Ziekenhuis Bonheiden, Belgium | X | X | |||||||
| O. D′Archambeau, Universitair Ziekenhuis Antwerpen, Belgium | X | X | |||||||
| J. Hendriks, Universitair Ziekenhuis Antwerpen, Belgium | X | X | |||||||
| E. Pilger, Medizinische Universität Graz, Austria | X | X | |||||||
| Ch. Düber, Universitätsklinikum Mainz, Germany | X | X | |||||||
| Th. Zeller,Herzzentrum Bad Krozingen, Germany | X | X | |||||||
| A. Gussmann, Humaine Kliniken Bad Saarow, Germany | X | X | |||||||
| P. N. M. Lohle, St. Elisabeth Ziekenhuis Tilburg, The Netherlands |
X | X | |||||||
| E. Minar, AKH Vienna, Austria | X | X | |||||||
| D. Scheinert, Universitätsklinikum Leipzig, Germany | X | X | |||||||
| K. Hausegger, A.ö. Landeskrankenhaus Klagenfurt, Austria | X | X | |||||||
| K. L. Schulte, Krankenhaus Herzberge, Berlin, Germany | X | X | |||||||
| J. Verbist, Imelda Hospital Bonheiden, Belgium | X | X | |||||||
| K. Deloose, AZ St. Blasius Dendermonde, Belgium | X | X | |||||||
| J. Lammer, AKH Vienna, Austria | X | X |
Materials and Methods
The AMS INSIGHT is a prospective, multicenter, randomized study, designed to evaluate the safety and performance of the first-generation AMS for the treatment of infrapopliteal lesions in patients with CLI. The results after AMS implantation are compared with the results after PTA alone.
The study was conducted according to the Declaration of Helsinki on investigation in humans and was approved by the institutional ethics committees at the different participating institutions. All patients provided written informed consent.
Patients
The study population consisted of patients with symptomatic CLI (Rutherford categories 4 and 5). They were eligible if they had de novo stenotic (>50%) or occlusive atherosclerotic disease of the infrapopliteal arteries and presented with a reference vessel diameter of between 3.0 and 3.5 mm and a lesion length of <15 mm (i.e., less than one stent length). An overview of all inclusion and exclusion criteria is presented in Table 1. Based on the diffusive nature of lesions and the difficulties during the enrollment process, the initial inclusion criterion for lesion length was expanded to be <20 mm. Moreover, the initial criterion considering a maximum of two lesions in one or more infrapopliteal vessel was also modified to allow the PTA treatment of other infrapopliteal lesions in nontarget vessels outside of the current study.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| Stenotic (>50%) or occlusive atherosclerotic disease of the infrapopliteal arteries |
| Length of lesion <15 mm (less than one stent length)a |
| Reference vessel diameter 3.0–3.5 mm |
| A maximum of two lesions in one infrapopliteal vessel treated in the study, or in two vessels of two different legsb |
| Symptomatic critical limb ischemia (Rutherford 4 and 5) |
| Patient ≥50 years of age |
| Life expectancy of >6 months |
| No child-bearing potential or negative serum pregnancy test within 7 days of the index procedure |
| Patient willing and able to return at the appropriate follow-up times for the duration of the study |
| Patient provision of written patient informed consent that is approved by the ethics committee |
| Exclusion criteria |
| Patient refusal of treatment |
| Reference segment diameter not suitable for available stent design |
| Length of lesion requiring more than one stent implantation |
| Previously implanted stent(s) or PTA at the same lesion site |
| Lesion lying within or adjacent to an aneurysm |
| Inflow-limiting arterial lesions left untreated |
| Patient has a known allergy to heparin, aspirin®, or other anticoagulant/antiplatelet therapies or a bleeding diatheses, or is unable, or unwilling, to tolerate such therapies |
| Patient taking phenprocoumon (Marcumar)® |
| Patient history of prior life-threatening contrast medium reaction |
| Patient currently enrolled in another investigational device or drug trial |
| Patient currently breastfeeding, pregnant, or intending to become pregnant |
| Patient mentally ill or retarded |
| Patient liable for military or civilian service |
Note: PTA, percutaneous transluminal angioplasty
aExpanded during course of investigation to <20 mm
bModified to allow PTA treatment of other infrapopliteal lesions in nontarget vessels outside of the current study
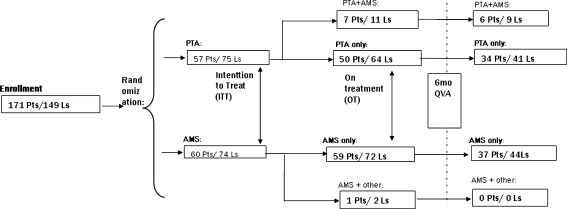
From June 30, 2005 (day of first patient enrollment), to February 2, 2007 (day of last patient enrollment), 117 CLI patients from 13 clinical sites in Belgium, the Netherlands, Austria, and Germany were enrolled in the trial. Fifty-seven patients were randomized to the PTA-only group, and 60 to the AMS group. In total, 149 lesions were treated in 117 patients, which resulted in a total of 74 lesions in the AMS arm and 75 lesions in the PTA control arm (Fig. 1).
Fig. 1.
Flowchart for patient distribution after randomization
The mean age of patients enrolled in the study was 73.1 ± 8.5 (range, 53–91) and 74.7 ± 7.8 (55–87) years in the PTA and AMS groups, respectively. Patient demographics are described in more detail in Table 2. Nicotine abuse was noted in 26 (45.6%) and 24 (40.0%) patients in the PTA and AMS groups, respectively. Comorbidities were arterial hypertension in 51 (89.5%) and 51 (85.0%), hyperlipidemia in 35 (61.4%) and 32 (53.3%), and diabetes mellitus in 39 (68.4%) and 43 (71.7%) patients in the PTA and AMS groups, respectively. In addition, Table 2 reports the patient distribution according to the Rutherford classification. The baseline characteristics of the randomized patients are statistically not different in the two treatment groups except for gender (p = 0.04).
Table 2.
Patient demographic distribution and patient baseline data at randomization in the AMS INSIGHT
| PTA only | AMS | p-value | |
|---|---|---|---|
| Age | |||
| Mean ± SD year | 73.1 ± 8.5 | 74.7 ± 7.8 | 0.31a |
| N/Ntotal; min–max | 57/117; 53–91 | 60/117; 55–87 | |
| Male, N/Ntotal (%) | 41/57 (71.9) | 31/60 (51.7) | 0.04b |
| Female, N/Ntotal (%) | 16/57 (28.1) | 29/60 (48.3) | |
| Diabetes mellitus, N/Ntotal (%) | 39/57 (68.4) | 43/60 (71.7) | 0.84b |
| Smoking, N/Ntotal (%) | 26/57 (45.6) | 24/60 (40.0) | 0.58b |
| Hyperlipidemia, N/Ntotal (%) | 35/57 (61.4) | 32/60 (53.3) | 0.45b |
| Hypertension, N/Ntotal (%) | 51/57 (89.5) | 51/60 (85.0) | 0.58b |
| Rutherford category, N/Ntotal (%) | |||
| 4 | 16/57 (28.1) | 16/60 (26.7) | 0.87c |
| 5 | 41/57 (71.9) | 44/60 (73.3) | |
| Ankle-brachial index | |||
| Mean ± SD (N) | 0.7 ± 0.3 (44) | 0.8 ± 0.5 (46) | 0.21a |
| Min–max | 0.2–1.8 | 0.3–2.5 | |
Note: PTA, percutaneous transluminal angioplasty; AMS, absorbable metal stent
at-test
bFisher exact test
cWilcoxon test
Materials
The devices used in the study were the first-generation AMS and the Pleon Explorer angioplasty balloon catheter, both developed by BIOTRONIK AG (Switzerland). The first-generation AMS is a balloon-expandable, tubular laser slotted, electropolished stent made of a bioabsorbable magnesium (Mg) alloy [13]. The first-generation AMS design is a special one that takes into consideration the specific mechanical properties of the bioabsorbable Mg alloy. The mechanical properties of the Mg stent are similar to those of permanent stainless-steel stents, including a high collapse pressure (0.8 bar), low elastic recoil (<8%), and minimum amount of shortening after inflation (<5%). The stents (10, 15, and 20 mm in length; 3.0 and 3.5 mm in diameter), premounted on a low-compliance fast-exchange delivery balloon catheter, were deployed after predilatation of the lesion. The two radio-opaque markers on the balloon ends were used for precise positioning since the AMS itself is not visible under fluoroscopy. The delivery system is compatible with guiding catheters with an inner diameter of at least 0.07 in. (1.78 mm). Magnesium was chosen as the main alloy component because of its hypothrombogenic properties [23] and good predictable local tissue tolerance. Additionally, the mechanical properties and corrosion of Mg alloy are controllable under physiologic conditions [14, 23] and match the high requirements for bioabsorbable stent implants.
The Pleon Explorer fast-exchange balloon catheter, used for the angioplasty arm (PTA group) and for lesion predilation in the stenting group, is designed for dilatation of stenotic segments in infrapopliteal arteries. The balloon has the same dimensions as the AMS (10, 15, and 20 mm in length and ϕ3.0 and ϕ3.5 mm in diameter). The working length of the Pleon Explorer balloon catheter is 140 cm. The inner distal lumen permits the use of guidewires of diameter 0.014 in. to facilitate advancement of the catheter to and through the lesions to be dilated. The Pleon Explorer balloon catheter is compatible with either coronary guiding catheters with an inner diameter of ≥0.056 in. (1.42 mm) or introducers with a minimal size of 5 Fr and length of 45 cm or 90 cm for antegrade or crossover procedure, respectively.
Procedural Description
Patients were randomly assigned to and treated with either PTA or AMS implantation. The randomization list was generated using PROC PLAN of SAS (Statistical Analysis Software). Sequentially numbered sealed envelopes contained information on the treatment to be applied. The sealed envelopes were opened only after the lesion was successfully crossed with the guidewire, and then patients were allocated either to stent or to PTA alone.
Vascular access was achieved, and all inflow-limiting lesions were treated according to the investigator’s standard clinical practice. Before lesion treatment, diagnostic angiography of the lesion area and distal runoff were performed. A long sheath or guiding catheter was used to advance the interventional catheters for the Pleon Explorer fast-exchange balloon catheter or the AMS delivery system.
In patients randomized to stenting, after measurement and then selection of a suitable balloon length, the lesion was predilated with the Pleon Explorer balloon under angiographic control. Predilatation was mandatory in this study. After dilatation, the stenosed area was treated by one AMS implant. Postdilatation was allowed at the discretion of the physician, for cases where angiographic control revealed suboptimal apposition of the AMS to the vessel wall or flow-limiting residual stenosis.
In patients randomized to PTA, the lesion was dilated with the Pleon Explorer balloon under angiographic control. In cases in which the residual stenosis after procedure was estimated to be >50%, balloon inflation was repeated and prolonged. If the stenosis persisted to be >50% or a flow-limiting dissection occurred, the patient underwent implantation of the AMS study stent and ended up in the crossover group.
Intra-arterial digital subtraction angiography (DSA) was performed to assess the different procedural steps. Immediate preprocedural and postprocedural images were obtained to assess lesion characteristics (lesion location, lesion length, stenosis percentage, outflow condition, etc.) and immediate postprocedural success, respectively, to document the final procedural outcome (Fig. 2). Angiographic images were shipped to an independent core lab for quantitative vessel analysis (QVA). Postprocedural vessel runoff was confirmed by angiography.
Fig. 2.
Angiographic control of (A) a pretreated 80% stenosis of the tibiofibular trunk, with (B) 5% residual stenosis after AMS implantation and (C) 36% in-stent restenosis at 6 months after index procedure
Endpoints
The primary safety endpoint of the AMS INSIGHT was defined as the absence of clinical complications at 1 month postprocedure. Complications were defined as major amputations or any cause of death. Major amputations were defined as amputations at or above the ankle. The primary efficacy endpoint of this study was to analyze and compare the 6-month angiographic patency rate after PTA alone or PTA followed by AMS implantation in patients with stenotic or occlusive atherosclerotic disease of the infrapopliteal arteries. Patency was defined as the absence of a hemodynamically significant restenosis (>50%) documented by digital subtraction angiography and confirmed by the core-lab QVA.
The secondary endpoints defined in the study design included (1) immediate angiographic procedural success, which was defined in both therapy groups as <30% final residual diameter stenosis of the target segment based on visual assessment of the planned treatment area; (2) late lumen loss (LLL) as diagnosed at 6-month angiographic control and defined by the difference between the in-stent minimal lumen diameter (MLD) postprocedure and the MLD at follow-up measured with angiography; (3) the limb salvage rates, defined as lack of major amputations at the different prescheduled follow-up visits until 12 months after index intervention; and (4) the primary patency rates at each visit as determined by color flow Doppler ultrasound (CFDU) and defined as either the absence of a hemodynamically significant restenosis (>50%) derived from the ratio of the peak systolic velocity (PSV) at the lesion segment to that at the proximal part [24], a major amputation, or a TLR.
The current article is limited to the analysis of the endpoints until 6 months.
Medication
Clopidrogel saturation was obtained prior to the procedure. Heparin was administered during the procedure according to standard practice. The postprocedure antithrombotic regimen was that used according to the protocol (Clopidogrel, 75 mg daily for 1 month; and aspirin, 75–300 mg daily lifelong).
Follow-Up Visits and Study Specific Investigations
After the intervention, all patients were closely monitored and controlled before discharge and at 1, 6, and 12 months after primary intervention, or when clinically indicated. At every visit (regular follow-up or any clinically indicated interim visit), clinical investigations were performed; these examinations included medication history assessment, clinical categorization of CLI, and the occurrence of in-stent restenosis investigated by CFDU. Additionally, at 6 months after the index intervention, patients were requested to undergo an intra-arterial DSA control. DSA images were analyzed at an external angiographic core laboratory (Jeffrey Popma, M.D., Brigham and Women’s Hospital, Boston, MA). Angiograms were performed according to the instruction of the core lab. A radio-opaque ruler was positioned in the imaging plane for precise size calibration. The ruler was present in all film analyzed sequences, including the follow-up. Moreover, images were obtained using the “best view” of stenosis, and additionally, a second view was considered in all film sequences to be analyzed. Finally, the identical imaging parameters were set and considered for the 6-month follow-up DSA control.
CFDU was performed at screening, before hospital discharge, and at scheduled 1-, 6-, and 12-month follow-up visits and any interim visit. Each examination comprised measurements of the maximum peak systolic velocity (PSV) 1 cm proximal to the lesion (“prestenotic”), within the lesion (“intrastenotic”), and 1 cm distal to the lesion (“poststenotic”). The ratio of the maximum intrastenotic PSV to the maximum prestenotic PSV (proximal peak velocity ratio PVRproximal = PSVintrastenotic/PSVprestenotic) determined the percentage stenosis by means of a look-up table [24]. For example, a PVR ratio ≥2.4 corresponds to a stenosis rateof >50–59%.
The 6-month results are composed of measurements performed at predefined protocol visits (6 months ± 30 days, including out-of-window visits) and any clinically indicated follow-up visits performed prior to 6 months ± 30 days due to nonpatency.
Statistics
The sample size calculation for this study was based on the hypothesis of a superior efficacy of the first-generation AMS system in maintaining a patent vessel lumen at 6 months compared to PTA alone. We assumed at 6 months a patency rate of 50% in the PTA arm and a clinical relevance effect of 25% in the AMS arm. With acceptance of a 10% dropout rate, a crossover rate of 30% in the PTA arm, a two-sided significance level α of 0.05, and 80% statistical power, a total of 117 patients were required.
Descriptive data summaries were used to present and summarize the collected study data. For categorical variables (e.g., gender), frequency distribution and cross tabulation were considered. For numeric variables (e.g., patient age), minimum, maximum, mean, median, and standard deviation were calculated. For all variables a 95% confidence interval for the relevant parameters of the underlying distribution were calculated. To show superiority regarding safety and efficacy endpoints of AMS over PTA alone, life tables according to the method of Kaplan–Meier are presented for all time-dependent events. To test differences in the cumulative survival probabilities between the study groups, the log-rank test was performed. To show statistical difference for the non-time-dependent variables, a t-test was performed for continuous variables. In the case of nonnormally distributed variables, a Wilcoxon test and, in the case of dichotomuous variables, a Fisher exact test were performed. Data were analyzed according to both “intention to treat” (ITT), i.e., the initial randomization schedule and “on-treatment” (OT), i.e., the actual treatment received. All statistical analyses were performed using SAS version 9.1 software.
Results
In the AMS INSIGHT a total of 117 patients (149 lesions) are enrolled and randomized to undergo endovascular treatment of infrapopliteal lesions in patients with CLI. As shown in Fig. 1, randomization assigned 57 patients (75 lesions) to PTA and 60 patients (74 lesions) to AMS. With respect to lesion morphology, the resulting patient cohorts were well matched. Description of the lesion morphology was based on the preprocedural angiographic images and is reported in Table 3. The reference vessel diameter (RVD) was 2.7 ± 0.5 (range, 1.4–4.4) and 2.6 ± 0.5 (range, 1.5–4.4) mm for the PTA and AMS groups, respectively. The in-lesion MLD before the procedure was 0.8 ± 0.3 (range, 0.0–1.6) and 0.8 ± 0.3 (range, 0.0–1.5) mm for the PTA and AMS groups, respectively. The mean preprocedure percentage diameter stenosis measured by QVA was 69 ± 12% (range, 51–100%) and 69 ± 11% (range, 51–100%) for the PTA and AMS groups, respectively. The mean lesion length was 12.0 ± 5.0 (range, 3.5–30.4) mm in the PTA group and 10.6 ± 4.9 (range, 3.1–27.6) mm in the AMS group. Lesion location distribution over the anterior tibial artery, tibiofibular trunk, fibular artery, and posterior tibial artery was 42.7%, 22.7%, 26.7%, and 8.0%, respectively, in the PTA group and, 44.6%, 17.6%, 28.4%, and 8.1%, respectively, for the AMS group (see Table 4). In one lesion (1.4%) in the AMS group, another lesion location was documented as popliteal. In total, 45.3% and 48.6% of lesions in the PTA and AMS groups were calcified.
Table 3.
Intention-to-treat primary efficacy endpoint: 6-month follow-up lesion patency based on quantitative vessel analysis (QVA) (pre- and postprocedure lesion QVA results)a
| PTA preproc | AMS preproc | PTA postproc | AMS postproc | 6-mo QVA: PTA | 6-mo QVA: AMS | |
|---|---|---|---|---|---|---|
| No. patients | 57 | 59 | 57 | 59 | 40 | 37 |
| No. lesions | 74 | 72 | 74 | 72 | 50 | 44 |
| Patency | na | na | na | na | 29/50 (58%) | 14/44 (31.8%) |
| p = 0.0134 | ||||||
| Non patency | na | na | na | na | 21/50 (42%) | 30/44 (68.2%) |
| Binary restenosis, mm | p = 0.0134 | |||||
| Lesion length | 12.0 ± 5.0 | 10.6 ± 4.9 | ||||
| Min–max | 3.5–30.4 | 3.1–27.6 | ||||
| Stenosis diameter, mm Mean ± SD | 68.7 ± 11.5 | 69.0 ± 10.7 | 21.9 ± 12.4 | 15.1 ± 10.2 | 47.8 ± 22.7 | 66.4 ± 27.1 |
| (Min–max) | (51.4–100) | (51.3–100) | (–7.2–53.6) | (−18.4–38.9) | (3.8–100) | (2.5–100) |
| MLD, mm Mean | 0.8 ± 0.3 | 0.8 ± 0.3 | 2.1 ± 0.5 | 2.2 ± 0.4 | 1.4 ± 0.7 | 0.9 ± 0.7 |
| (Min–max) | (0.0–1.6) | (0.0–1.5) | (1.0–3.2) | (1.4–3.4) | (0.0–2.9) | (0.0–2.9) |
| p = 0.0009 | ||||||
| RVD, mm Mean | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.5 | 2.6 ± 0.5 |
| (Min–max) | (1.4–4.4) | (1.5–4.4) | (1.5–4.6) | (1.5–4.6) | (1.8–4.5) | (1.9–3.9) |
| Late lumen loss, mm Mean ± SD | na | na | na | na | 0.7 ± 0.7 | 1.4 ± 0.8 |
| (Min–max) | (−0.3–2.9) | (−0.4–2.9) | ||||
| p = 0.0001 | ||||||
Note: PTA, percutaneous transluminal angioplasty; preproc, preprocedure; AMS, absorbable metal stent; postproc, postprocedure; MLD, minimal lumen diameter; RVD, reference vessel diameter
aFor three lesions (one PTA patient and two AMS patients), angiographic procedure data were not available
Table 4.
Lesion morphology characterization preprocedurea
| PTA only | AMS only | p-value | |
|---|---|---|---|
| Lesion site | |||
| Anterior tibial artery | 32/75 (42.7%) | 33/74 (44.6%) | 0.90b |
| Tibiofibular trunk | 17/75 (22.7%) | 13/74 (17.6%) | |
| Fibular artery | 20/75 (26.7%) | 21/74 (28.4%) | |
| Posterior tibial artery | 6/75 (8.0%) | 6/74 (8.1%) | |
| Other | 0/75 | 1/74 (1.4%) | |
| No. of lesions treated | |||
| 1 | 39/57 (68.4%) | 46/60 (76.7%) | 0.41b |
| 2 in 1 limb | 18/57 (31.6%) | 14/60 (23.3%) | |
| 2 in 2 limbs | 0 | 0 | |
| Calcification | 34/75 (45.3%) | 36/74 (48.6%) | 0.744b |
Note: PTA, percutaneous transluminal angioplasty; AMS, absorbable metal stent
aFor three lesions (one PTA patient and two AMS patients), angiographic procedure data were not available
bFisher test
As shown in Fig. 1, seven PTA group patients (7/57) with 11 lesions (11/75) crossed over to the other treatment (OT) arm due to dissections in at least one of the lesions and, in the case of one patient, due to significant residual stenosis. These patients were included in the PTA + AMS group, which is not considered in the OT data analysis in this paper. One patient randomized for stenting (1/60) with a double lesion (2/74) underwent implantation of a nonstudy stent (self-expanding) due to severe tortuosity of the iliac artery. Therefore, this patient is not considered in the OT analysis. The final OT cohort consisted of 50 patients with 64 lesions treated with PTA only and 59 patients with 72 lesions who underwent implantation of the study stent. Therefore, ITT procedural success, which was based on visual assessment, was achieved in 60 of 60 patients in the AMS group (100%) and in 55 of 57 patients for the PTA group (96.4%). For one PTA lesion, data on procedural success were not provided by the investigator. In a conservative manner, this patient was considered a nonsuccess. The OT procedural success was 96.0% (48/50) and 100% (59/59) of patients in the PTA and AMS arms, respectively. Procedural angiographic images of three lesions (one PTA, two AMS) were not made available by the investigator.
The primary safety endpoint, i.e., absence of major amputation and/or death within 30 days after index intervention was not significantly different between the AMS study group and the PTA control group (Table 5). At 1 month, a major amputation, defined as amputation at or above the ankle, was performed in four patients: two in the group of patients randomized to PTA alone (2/57) and two in the AMS randomized arm (2/60). One of 57 PTA patients and 1 of 60 AMS patients died before the time of 1-month follow-up. The ITT analysis of the complication rate within 30 days yielded values of 5.3% (3/57) and 5.0% (3/60) in patients randomized for PTA alone and PTA followed by AMS implantation, respectively (p = 1.0). The corresponding complication rates by OT analysis were 6.0% (3/50) and 5.1% (3/59), respectively (p = 1.0).
Table 5.
Primary safety endpoint: patient complication rate at 1-month follow-up
| Complication | Intention to treat (ITT) | On-treatment (OT) | ||||
|---|---|---|---|---|---|---|
| PTA | AMS | p-value | PTA | AMS | p-value | |
| Major amputation | 2/57 (3.5%) | 2/60 (3.3%) | 1.0a | 2/50 (4.0%) | 2/59 (3.4%) | 1.0a |
| Death | 1/57 (1.8%) | 1/60 (1.7%) | 1/50 (2.0%) | 1/59 (1.7%) | ||
| Total | 3/57 (5.3%) | 3/60 (5.0%) | 3/50 (6.0%) | 3/59 (5.1%) | ||
Note: PTA, percutaneous transluminal angioplasty; AMS, absorbable metal stent
aFisher’s exact test, two-sided
The study’s primary efficacy endpoint was the 6-month angiographic patency rate as confirmed by core-lab QVA (Table 3). Six-month QVA results (regular or delayed follow-up or clinically indicated visits) were available for 50 PTA lesions (40 patients) and 44 AMS lesions (37 patients) as presented in Table 3. The 6-month angiographic patency rate on an ITT basis was 58.0% (29/50 lesions) for the PTA and 31.8% (14/44 lesions) for the AMS (p = 0.013) group. Corresponding angiographic patency rates by OT analysis were 63.4% (26/41 lesions) and 31.8% (14/44 lesions) for PTA and AMS, respectively (p = 0.005).
The mean LLL at 6-month follow-up was, according to ITT, 0.7 ± 0.7 and 1.4 ± 0.8 mm, in PTA and AMS patients, respectively (p < 0.0001). By OT analysis, the corresponding LLL mean values were 0.6 ± 0.7 and 1.4 ± 0.8 mm (p < 0.0001).
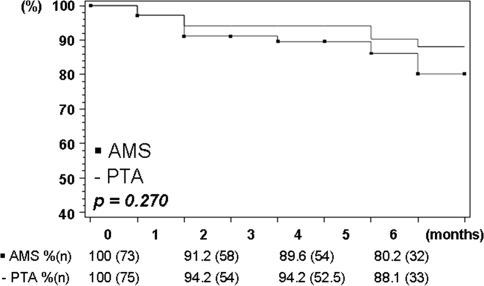
Kaplan–Meier estimation of the primary patency rate, as measured based on CFDU values as stipulated in the secondary endpoints, revealed a 6-month primary patency on an ITT basis (Fig. 3) of 88.1% for PTA only and of 80.2% for AMS implantation (p = 0.270). The corresponding rates for the OT analysis were 96.5% and 79.8% (p = 0.017).
Fig. 3.
Kaplan–Meier estimation (life-table method) of primary patency rate. Lesion-based color flow Doppler ultrasound, ITT analysis
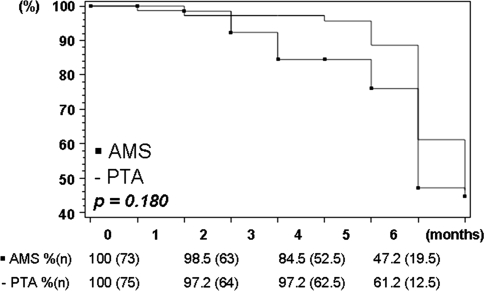
In comparison, the Kaplan–Meier analysis of the QVA measurements resulted in an ITT-based primary patency (Fig. 4) of 61.2% after PTA and 47.2% after AMS (p = 0.180) treatment. The OT primary patency rate was 70.3% for patients treated with PTA alone and 45.2% for patients stented with the AMS (p = 0.037).
Fig. 4.
Kaplan–Meier estimation (life-table method) of patency measured by quantitative vascular angiography. Lesion-based ITT analysis
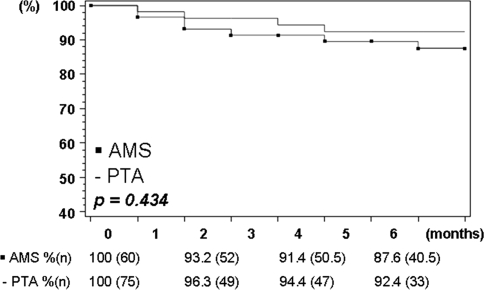
According to the Kaplan–Meier estimation, 6-month cumulative patient limb salvage rates (Fig. 5) were calculated on an ITT basis as 92.4% and 87.6% for PTA and AMS, respectively (p = 0.434). Corresponding OT 6-month limb salvage rates were 91.2% and 87.3%, respectively (p = 0.559).
Fig. 5.
Kaplan–Meier estimation (life-table method) of limb salvage. ITT analysis
The Kaplan–Meier estimate shows a 6-month survival rate of 92.5% and 91.3% for patients with PTA as ITT and AMS as ITT, respectively (p = 0.891), as presented in Fig. 6. By OT analysis the corresponding rates were 93.6% and 91.1%, respectively (p = 0.984).
Fig. 6.
Kaplan–Meier estimation (life-table method) for survival-death. ITT analysis
Considering the ITT analysis, the incidence of TLR at 6 months was 16.0% (12/75) in the PTA group and 31.1% (23/74) in the AMS group (p = 0.052), where, for PTA, 66.7% (8/12) and, for AMS, 78.3% (18/23) of lesion revascularizations were clinically indicated. By OT analysis, the corresponding TLR incidences were 10.9% (7/64) and 31.9% (23/72), respectively (p = 0.004), with clinical indication for reintervention in 71.4% (5/7) of PTA and 78.3% (18/23) of AMS lesions.
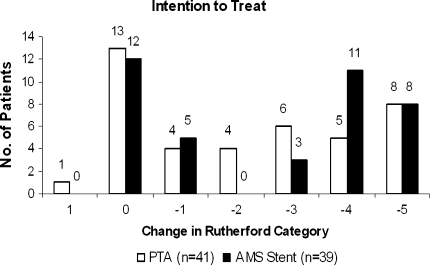
Clinical follow-up at 6 months was assessed in 41 of 57 (71.9%) and 39 of 60 (65.0%) initially enrolled PTA and AMS patients, respectively. The rate of patients who refused the 6-month angiogram was relatively high in both groups. Reasons for declination were diverse: patient renunciation to repeat angiography (16 patients), patient death (9 patients), major amputation (7 patients), health issues making the angiographic control problematic (5 patients), and difficulties analyzing angiograms at the core lab (3 patients). The patient’s 6-month clinical status was assessed by the evolution of the Rutherford category, and ITT results are summarized in Fig. 7. An improvement by at least one Rutherford category was observed in a total of 27 of 41 (65.9%) PTA and 27 of 39 (69.2%) AMS patients, without a statistically significant difference between treatment modalities, by either ITT or OT analysis.
Fig. 7.
Change in Rutherford category baseline vs. 6-month follow-up. ITT analysis
The mean resting ankle-brachial index (ABI) at baseline in PTA and AMS arms was 0.7 ± 0.3 and 0.8 ± 0.5, respectively (ITT data). It increased significantly, to 1.0 ± 0.2 and 1.0 ± 0.4 at 24 h after endovascular treatments and to 0.9 ± 0.3 and 0.9 ± 0.4 at 6-month follow-up, respectively.
Discussion
The AMS INSIGHT is the first to compare, in a randomized controlled setting, the safety and efficacy of the implantation of a balloon-expandable absorbable stent in the infrapopliteal bed. Earlier investigations have shown that the BIOTRONIK AG-developed bioabsorbable Mg-alloy stent is safe for use in infrapopliteal vessels and that it performed as intended. This study has succeeded in proving the safety of AMS technology (primary safety endpoint). The 30-day complication rate, as defined by the occurrence of major amputation and/or death, of patients treated with AMS (ITT, 5.0%) was not significantly different (p = 1.0) from the rate fr those treated with PTA alone (ITT, 5.3%).
Although the safety of the AMS technology has been shown, this study failed to prove the efficacy of the tested first-generation AMS. The results of the core-lab QVA even show that the 6-month outcome of PTA followed by AMS implantation does not equal the outcome after PTA alone. On n ITT basis the 6-month angiographic patency rate was significantly inferior (p = 0.013) for lesions treated with AMS (31.8%) compared to those treated with PTA alone (58.0%).
To date, only limited comparative QVA data for below-the-knee arteries are available in the literature. The 6-month binary restenosis rate after AMS was 68.2% (ITT), which is higher than restenosis rates published by Siablis et al. [6] and Scheinert et al. [8]comparing the effect of bare metal stents (BMSs) versus drug-eluting stents in the infrapopliteal bed. Siablis et al. reported a 55.3% binary restenosis rate 6 months after BMS and 4.0% after Sirolimus Eluting Stent implantation [6], while Scheinert et al. found a 6-month binary restenosis rate of 39.1% after coronary-type BMS and 0% after drug-eluting stent implantation [8]. In a single-arm controlled study published by Bosiers et al. [9], a 12-month binary restenosis rate of 20.45% was seen after the implantation of nitinol self-expanding stents in the same bed. The 6-month binary restenosis rate of the PTA control arm of the AMS INSIGHT (ITT, 42.0%) scores within the range of the published data for coronary-type BMS in the infrapopliteal region: 55.3% by Siablis et al. [6] and 39.1% by Scheinert et al. [8]. As reported in Table 3, postprocedural MLD was seen to be 2.2 ± 0.4 mm in the patient cohort receiving first-generation AMSs either ϕ3.0 or ϕ3.5 mm in diameter. The low postprocedural MLD might be attributed to the inadeaquate radial force of the first-generation AMS to withstand the initial vessel recoil.
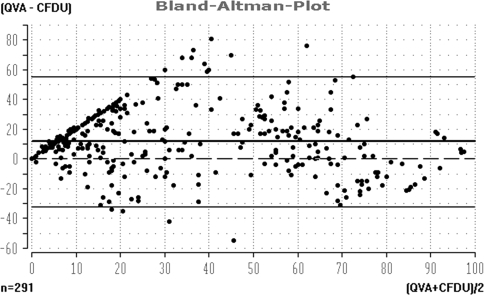
As shown in Fig. 8, there is a significant difference in the outcomes based on CFDU versus angiography (QVA). It was seen that there is no correspondence between the outcomes of both investigation methods. The untransformed values correlate significantly (r = 0.69, p < 0.001), but the Bland-Altman plot reveals a systematic overestimation by CFDU. Both methods differ significantly in their mean values (mean difference = 11.6). A significant number of patients diagnosed by CFDU to have <50% restenosis (PVR < 2.4) were observed to have significant restenosis measured by angiography. Other lesions with CFDU values indicating significant restenosis were shown to be well patent on angiographic core-lab control. Therefore it was decided to add a Kaplan–Meier estimation of primary patency based on the angiographic findings as provided by the core lab. A similar angiographic primary patency curve was first published by Siablis et al. [6] after the initiation of the AMS INSIGHT and has been used in different publications since then [4, 18, 25]. Two recent publications [26, 27] confirmed our finding that, although there is good agreement between CFDU and angiography in lower limb artery disease in general, there is only poor agreement in infrapopliteal regions.
Fig. 8.
Color flow Dopper ultrasound (CFDU) and quantitative vessel analysis patency correlation. The untransformed values correlate significantly (r = 0.69, p < 0.001), but the plot reveals a systematic underestimation by the CFDU method
Considering the Kaplan–Meier angiographic primary patency curves as an efficacy reference, the 6-month outcome of the AMS INSIGHT can be compared to other infrapopliteal PTA and/or stent study findings. The 6-month angiographic patency rate for the PTA (ITT, 61.2%) group is higher than the one published by Rand et al., which was only 45.6% for the PTA-only group [4]. The 6-month angiographic primary patency of the AMS (ITT, 47.2%) is well below the rates published for permanent balloon-expandable stent types in the crural arteries. A 6-month angiographic patency rate of 68.1% after BMS was found by Siablis et al. [6], and 79.7% was found by Rand et al. [4] after PCS.
As could be expected the limb salvage rates in the AMS trial are within the range of previously published studies on infrapopliteal endovascular interventions. In general, the 6-month limb salvage rates are far higher than according patency values and mostly lie at about 90% [9]. It could be understood that a nonhealing wound or viable limb does not require a full 6-month patency or reperfusion time to generate optimal healing or symptom relief. It is believed that, in combination with restarting the patient’s daily life activities, an approximate reperfusion lasting about 3 months would be sufficient for 6-month limb salvage. Nevertheless, long-term limb salvage undoubtedly benefits from extended patency of the treated lesion.
The findings from the AMS INSIGHT did not support the positive clinical outcome of the initial AMS findings in a small cohort of patients [17–19] and indicate that the current-generation stent does not meet the efficacy criteria. Therefore, the stent needs to be improved and re-evaluated before commercial use can be an option. The following criteria are believed to cause the problem. In the AMS INSIGHT, core-lab QVA showed a discrepancy between inclusion criteria and available stent sizes. The reference vessel diameter in the AMS randomized group was 2.6 ± 0.5 (range, 1.5–4.4) mm. Therefore, many lesions were too small in diameter and longer than the stent length. Hence, the lesion did not match the available nominal AMS sizes. It is possible that oversizing causing vessel trauma was the case. A small magnification error due to the ruler on the table can only partially explain the observed mismatch. New sizes and longer lengths for AMSs should be developed for below-the-knee use. This first-generation AMS is probably being absorbed too fast, inducing loss of stent scaffolding properties. The AMS PROGRESS [13] coronary study suggested fast degradation that initiated immediately after deployment, with nearly fully absorption after 4 months. Thus the restenosis in the AMS group can be attributed to early recoil in addition to neointima formation. To improve clinical results with the AMS technology, a second-generation AMS is under development, with optimization of the Mg alloy and the stent design resulting in a longer degradation time and increased radial force and stent sizes should be better adapted to peripheral lesions.
Study Limitations
The major limitation of the AMS INSIGHT was the selection of a CFDU investigation to determine the primary patency. During the course of the trial, it was discovered and evidence was obtained on hand that CFDU has only limited relevance in the diagnosis of infrapopliteal disease [23]. Therefore, it was decided during data analysis to use angiographic primary patency as the main comparator with other trials.
A second limitation of the trial might be the initially very strict angiographic inclusion criteria. It was decided, in order to have a good match with the limited length variations of the available AMS devices (the initial maximal stent length was 15 mm; later, 20-mm-long stents also became available) and to have each lesion covered by maximally one stent, to limit the maximal lesion length to 15 mm initially and to 20 mm later. Furthermore, it was decided to include only patients with a maximum of two lesions in one or more infrapopliteal vessel. As the majority of CLI patients represent with complex multilevel disease, it was the experience of the different investigators that these criteria allowed the inclusion of only a very restricted number of patients, not accurately reflecting the real-world CLI population. Therefore it was decided to amend the protocol and also allow the PTA treatment of other infrapopliteal lesions in nontarget vessels outside of the current study.
Third, repeated angiographic imaging could not be obtained in 34% of the study population. Similar high dropout rates have been published in other CLI studies [4, 25] and can be considered acceptable for this fragile population.
Conclusion
The AMS INSIGHT has shown that the AMS technology is a safe technique to use for treating peripheral arterial disease, but that the tested current-generation AMS did not show efficacy in long-term patency over standard PTA in infrapopliteal vessels. Therefore, important stent design modifications are required and further clinical trials should be performed before potential widespread application of the technology.
Acknowledgments
The authors take great pleasure in thanking the staff of the Flanders Medical Research Program (www.fmrp.be), with special regards to Koen De Meester for performing the systematic review of the literature and providing substantial support in the data analysis and the writing of the article. Moreover, the authors are indebeted to Evelyn Diessel (BIOTRONIK AG, Berlin) for study management and to Iker Mallabiabarrena (BIOTRONIK VI, Bülach-Zurich) for support in the writing of this publication. The sponsor, BIOTRONIK AG, funded the total study costs and was responsible for the study administration and monitoring of the study. The study was conducted according with the Declaration of Helsinki on investigation in humans and was approved by the institutional ethics committees at the different participating institutions.
Appendix
Footnotes
For a complete list of authors and their contributions, see the Table 6.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00270-009-9530-x
References
- 1.Bosiers M, Hart JP, Deloose K, Verbist J, Peeters P (2006) Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular 14(2):63–69 [DOI] [PubMed]
- 2.Adam DJ, Beard JD, Cleveland T et al (2005) Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 366(9501):1925–1934 [DOI] [PubMed]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG (2007) Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45(Suppl S):S5–S67 [DOI] [PubMed]
- 4.Rand T, Basile A, Cejna M et al (2006) PTA versus carbofilm-coated stents in infrapopliteal arteries: pilot study. Cardiovasc Interv Radiol 29(1):29–38 [DOI] [PubMed]
- 5.Commeau P, Barragan P, Roquebert PO (2006) Sirolimus for below the knee lesions: mid-term results of SiroBTK study. Cath Cardiovasc Interv 68(5):793–798 [DOI] [PubMed]
- 6.Siablis D, Kraniotis P, Karnabatidis D, Kagadis GC, Katsanos K, Tsolakis J (2005) Sirolimus-eluting versus bare stents for bailout after suboptimal infrapopliteal angioplasty for critical limb ischemia: 6-month angiographic results from a nonrandomized prospective single-center study. J Endovasc Ther 12(6):685–695 [DOI] [PubMed]
- 7.Bosiers M, Deloose K, Verbist J, Peeters P (2006) Percutaneous transluminal angioplasty for treatment of ‘‘below-the-knee’’ critical limb ischemia: early outcomes following the use of sirolimus-eluting stents. J Cardiovasc Surg (Torino) 47(2):171–176 [PubMed]
- 8.Scheinert D, Ulrich M, Scheinert S, Sax J (2006) Comparison of sirolimus-eluting vs. bare-metal stents for the treatment of infrapopliteal obstructions. EuroIntervention 2:169–174 [PubMed]
- 9.Bosiers M, Deloose K, Verbist J, Peeters P (2007) Nitinol stenting for treatment of ‘‘below-the-knee’’ critical limb ischemia: 1-year angiographic outcome after Xpert stent implantation. J Cardiovasc Surg (Torino) 48(4):455–461 [PubMed]
- 10.Tepe G, Zeller T, Heller S et al (2006) Self-expanding nitinol stents for treatment of infragenicular arteries following unsuccessful balloon angioplasty. Eur Radiol 18(4):295–298 [DOI] [PubMed]
- 11.Kickuth R, Keo HH, Triller J, Ludwig K, Do DD (2007) Initial clinical experience with the 4-F self-expanding XPERT stent system for infrapopliteal treatment of patients with severe claudication and critical limb ischemia. J Vasc Interv Radiol 18(6):703–708 [DOI] [PubMed]
- 12.Peregrin JH, Smirova S, Koznar B et al (2008) Self-expandable stent placement in infrapopliteal arteries after unsuccessful angioplasty failure: one-year follow-up. CardioVasc Interv Radiol 31(5):860–864 [DOI] [PubMed]
- 13.Erbel R, Di Mario C, Bartunek J et al (2007) Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet 369(9576):1869–1875 [DOI] [PubMed]
- 14.Waksman R, Pakala R, Kuchulakanti et al (2006) Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Cath CardioVasc Interv 68(4):607–617 [DOI] [PubMed]
- 15.Tsetis D, Belli AM (2004) The role of infrapopliteal angioplasty. Br J Radiol 77(924):1007–1015 [DOI] [PubMed]
- 16.Erne P, Schier M, Resink TJ (2006) The road to bioabsorbable stents: reaching clinical reality? CardioVasc Interv Radiol 29(1):11–16 [DOI] [PubMed]
- 17.Di MC, Griffiths H, Goktekin O et al (2004) Drug-eluting bioabsorbable magnesium stent. J Interv Cardiol 17(6):391–395 [DOI] [PubMed]
- 18.Peeters P, Bosiers M, Verbist J, Deloose K, Heublein B (2005) Preliminary results after application of absorbable metal stents in patients with critical limb ischemia. J Endovasc Ther 12(1):1–5 [DOI] [PubMed]
- 19.Bosiers M, Deloose K, Verbist J, Peeters P (2006) Will absorbable metal stent technology change our practice? J Cardiovasc Surg (Torino) 47(4):393–397 [PubMed]
- 20.Bosiers M, Deloose K, Verbist J, Peeters P (2005) First clinical application of absorbable metal stents in the treatment of critical limb ischemia: 12-month results. Vasc Dis Manage 2(4):86–91
- 21.Barlis P, Tanigawa J, Di MC (2007) Coronary bioabsorbable magnesium stent: 15-month intravascular ultrasound and optical coherence tomography findings. Eur Heart J 28(19):2319 [DOI] [PubMed]
- 22.Bose D, Eggebrecht H, Erbel R (2006) Absorbable metal stent in human coronary arteries: imaging with intravascular ultrasound. Heart 92(7):892 [DOI] [PMC free article] [PubMed]
- 23.Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A (2003) Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart 89(6):651–656 [DOI] [PMC free article] [PubMed]
- 24.Ranke C, Creutzig A, Alexander K (1992) Duplex scanning of the peripheral arteries: correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med Biol 18(5):433–440 [DOI] [PubMed]
- 25.Siablis D, Karnabatidis D, Katsanos K et al (2007) Sirolimus-eluting versus bare stents after suboptimal infrapopliteal angioplasty for critical limb ischemia: enduring 1-year angiographic and clinical benefit. J Endovasc Ther 14(2):241–250 [DOI] [PubMed]
- 26.Collins R, Burch J, Cranny G et al (2007) Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ 334(7606):1257 [DOI] [PMC free article] [PubMed]
- 27.Favaretto E, Pili C, Amato A et al (2007) Analysis of agreement between Duplex ultrasound scanning and arteriography in patients with lower limb artery disease. J Cardiovasc Med (Hagerstown) 8(5):337–341 [DOI] [PubMed]