Abstract
Hyaluronic acid (HA) is a glycosaminoglycan of tissue engineering importance that plays a vital role in mammalian development. In vitro kidney culture methods were utilized to investigate the importance of HA during renal organogenesis. We found that HA has the ability to simultaneously modulate ureteric bud (UB) branching, promote mesenchymal-to-epithelial transformation, and promote differentiation of both metanephric mesenchyme (MM) and the UB depending on the concentration and molecular weight (MW) of HA. Hyaluronidase inhibited branching morphogenesis in both isolated UB and whole kidney cultures, suggesting endogenous HA is required for branching morphogenesis. HA exhibited morphogen-like properties, stimulating branching morphogenesis at low concentrations (0.1%) and low MW (6.55 kDa) but inhibiting at high concentrations (3.75%) and high MW (234.4 kDa). Furthermore, HA of every MW tested promoted collecting duct differentiation as measured by AQP-2 expression. E-cadherin immunostaining and qPCR of nephron differentiation markers (OAT-1, NaPi-2, AQP-1, and THP), demonstrated that HA of a variety of MWs strongly promotes mesenchymal epithelialization and nephron differentiation in a concentration-dependent manner. Since the HA synthesis and degradation genes, has-2 and hyal-2, are highly expressed during kidney development, this data suggests that specific sizes and concentrations of HA may act to independently regulate UB branching and promote tubular maturation, representing a potential switch for ending branching morphogenesis, as well as initiating nephron differentiation. In addition, the ability of HA to promote in vitro embryonic kidney growth and maturation, together with the biocompatibility and crosslinking capability of HA, suggests a potential use of HA for both creating an instructive, 3D scaffold for in vitro kidney engineering from developmental tissues, as well as promoting tubule regeneration in injured or cryopreserved kidneys.
INTRODUCTION
Metanephric kidney development begins with the outgrowth of the ureteric bud (UB) from the Wolffian duct at week 5 in human development and approximately day 12 of rat development. The UB invades the surrounding metanephric mesenchyme (MM) and begins the process of branching morphogenesis. MM cells coalesce around the tips of the branching UB, thereby providing factors that promote UB branching morphogenesis and maturation, while the UB releases factors that promote epithelialization and differentiation of MM cells [1]. While many branching morphogenesis and mesenechymal-to-epithelial transformation (MET) regulatory factors have been identified [2–7], the mechanism(s) for stopping branching morphogenesis and beginning collecting duct and nephron differentiation is poorly understood. Here we demonstrate that hyaluronic acid (HA) is a molecule that depending on size and concentration can modulate branching morphogenesis and promote differentiation of both the collecting duct and nephrons. Since the majority of nephron formation occurs in the phases of renal development following branching morphogenesis, HA represents a possible switch molecule that stops branching morphogenesis and promotes nephron differentiation.
HA is a simple polysaccharide consisting of repeating disaccharide units of D-glucuronic acid (1-β-3) N-acetyl-D-glucosamine (1-β-4) [8] synthesized by three hyaluronic acid synthase genes, has-1, has-2 and has-3 [9]. Has-2 has been demonstrated to be the most important of these genes responsible for at least 97% of HA synthesized during murine embryonic development and murine embryos lacking the has-2 gene exhibit growth retardation evident by embryonic day 9 and lethality by day 9.5 (before ureteric bud outgrowth) [9]. Moreover, exogenous HA has been shown to improve the development of in vitro produced bovine embryos and porcine parthenogenic embryos [10, 11]. Together, this data indicates a vital role for HA during mammalian organogenesis and development. In renal organogenesis, HA has been implicated in the branching morphogenesis of ureteric bud (UB) cell lines and has been found to be expressed along with its receptor, CD44, along the basal surface of the branching UB [12]. Furthermore, HA has been demonstrated to be ubiquitously present throughout the interstitial space of the developing kidney [13]. However, much remains to be elucidated about the role of HA in kidney development.
Has-2 synthesizes HA chains between 2×105 – 2×106 Da, yet HA is known to exist in vivo at much smaller molecular weights and have different in vivo roles based on its molecular weight [14]. The molecular weight of HA is controlled in vivo by the degradative hyaluronidases (hyal). Although six different hyaluronidases are potentially involved in HA degradation, hyal-1 and hyal-2 are believed to be the primary functional hyaluronidases of mammalian somatic cells [15]. The different hyaluronidases play different roles in regulating HA; for example, hyal-1 is a secreted protein and breaks down HA to a tetrasaccharide [16], while hyal-2 exists as a cell anchored protein and degrades HA to 20 kDa fragments [16, 17]. If smaller molecular weights of HA (less than ~20kD) are regulatory molecules for kidney development, then hyal-2 would be expected to be expressed during kidney development.
HA has been researched extensively for a wide variety of tissue engineering purposes (reviewed in [18]). HA is a non-antigenic, immunocompatible extracellular macromolecule [19, 20] which has already demonstrated prevalent medical use as an injectible gel for viscosupplementation of osteoarthritic joints [21, 22] and as a dermatological treatment for wrinkles [23–25]. Because of its hydroxyl and carboxylic acid groups, HA is easily modified to achieve particular characteristics and easily crosslinked to form hydrogels [26–31]. The ability of HA to promote in vitro embryonic kidney growth and maturation demonstrated here, together with the proven biocompatibility and crosslinking of HA, provide evidence for the potential use of HA towards creating an instructive, 3D scaffold for in vitro kidney engineering from developmental tissues.
In this study, we used in vitro models of kidney development to examine the role of HA in kidney development and its potential role as a scaffold material for in vitro kidney tissue engineering. The temporal expression of has-2 and hyal-2 during in vivo kidney development was characterized by qPCR. Next, hyaluronidase and hyaluronic acid of various molecular weights and concentrations were added to both in vitro metanephric kidney and isolated ureteric bud culture systems. Morphometric analysis was used to quantify the effects on branching morphogenesis. UB/MM differentiation was measured by quantitative PCR of functional renal differentiation markers. We found that HA simultaneously modulates UB branching morphogenesis, induces MET, and promotes differentiation in both MM and UB depending on the MW and concentration of HA.
METHODS
Materials and Reagents
Primers were synthesized by Allele Biotechnology (San Diego, CA). Hyaluronidase from Streptomyces hyalurolyticus was obtained from Sigma (St. Louis, MO). HA of the following molecular weights were obtained from Lifecore Biomedical (Chaska, MN): 234.4, 132.3, 64.0, 17.0 and 6.55 kDa. BSN cell conditioned media (BSN-CM) was harvested as described previously [3]. Recombinant growth factors, fibroblast growth factor-1 (FGF1) and glial cell-derived neurotrophic factor (GDNF) were obtained from R&D Systems (Minneapolis, MN). Mouse anti-E-cadherin antibodies were from BD Biosciences Pharmingen (San Diego, CA) and goat anti-mouse AlexaFluor 594 was from Molecular Probes (Eugene, OR). FITC-conjugated dolichos biflorus (DB) lectin was from Vector Laboratories (Burlingame, CA). Unless otherwise noted, all other reagents are from Sigma (St. Louis, MO).
Organ Culture
Embryos from timed pregnant Holtzman rats (Harlan, Indianapolis, IN) at day 13 (E13) of gestation (day 0 being the day of appearance of the vaginal plug) were dissected free of surrounding tissues. The metanephric kidneys were isolated and placed on Transwell filters (0.4 μm pore size) (Costar, Cambridge, MA) in 12-well plates. The isolated kidneys were cultured at the media-air interface with 600 μL DMEM/F12 (GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, Utah) and 1% antibiotics (GIBCO-BRL, Grand Island, NY) below the filter at 37°C and 5% CO2/100% humidity. When samples were treated with hyaluronidase, the enzyme was added to the media for a final concentration of 50 U/mL. HA was dissolved in sterile 0.2 μm filtered and autoclaved water (Hyclone, Logan, Utah) and then diluted to a 1X DMEM solution, buffered with HEPES (pH 7.2). 270 μL of the HA solution were placed on top of the tissue sample for HA treatment. All treatments were present throughout the indicated culture period.
Isolated Ureteric Bud Culture
Ureteric buds were dissected from E13 kidneys, suspended in a growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) solution (1:1 Matrigel:DMEM/F12), and cultured with BSN-CM supplemented with 10% FBS, 1% antbiotics, 250 ng/mL FGF1, and 125 ng/mL GDNF as described previously [2, 3, 5, 32–34]. The low growth factor condition utilized a 1:2 BSN-CM:DMEM/F12 media with 30 ng/mL FGF1 (all else remained constant). When samples were treated with hyaluronidase, the enzyme was added to the media for a final concentration of 50 U/mL. For the HA conditions, a HA solution (prepared as described above) was used to dilute the Matrigel to 50% instead of DMEM/F12 to create a gel with the final HA concentration indicated.
Immunofluorescence and Lectin Staining
After the indicated number of days, kidney cultures were fixed for 30 min with 4% paraformaldehyde in PBS. Fixed kidney cultures were rinsed with TBS and extracted in absolute methanol at −20°C for 20 min Samples were then blocked for 1hr in 3% BSA in TBS-T at 4°C followed by incubation in anti-E-cadherin antibody (1:500 in blocking solution) for 24 hr at 4°C. Samples were then washed 3×8hr in TBS-T, followed by incubation in AlexaFluor594 antibody (1:2000) and FITC-DB (1:500) for 24 hr at 4°C, and a final 3×8hr washes in TBS-T at 4°C. Samples were then mounted on slides with ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA).
qRT-PCR Analysis
Embryonic kidneys were isolated from time-pregnanat Holtzman rats (Harlan, Indianapolis, IN) at embryonic days 13 – 20 and 8 weeks. The RNA from tissues (either freshly isolated or from tissue culture) was isolated using the RNAqueous Micro kit from Ambion (Austin, TX). Invitrogen’s (Carlsbad, CA) SuperScript III Platinum Two-Step qRT-PCR Kit with SYBR Green and the ABI 7500 Fast Real-time PCR System (Applied Biosystems, Foster City, CA) were used to perform qPCR analysis. All primers were designed using the Primer3 website. The following primer sets were used for qPCR: rat AQP-2 (sense and anti-sense: 5′-agagctcttcctgaccatgc-3′ and 5′-ccggtgaaatagatcccaag-3′, respectively), rat OAT-1 (sense and anti-sense: 5′-gaagagcaggagcagaggaa-3′ and 5′-accccactccctttagtgct-3′, respectively), rat NaPi-2 (sense and anti-sense: 5′-gccaatgtcatccagaaggt-3′ and 5′-tgctctggacaacaaacgtc-3′, respectively), rat AQP-1 (sense and anti-sense: 5′-cctggtctcagagcttcctg-3′ and 5′-actttggagtgagggggact-3′, respectively), rat THP (sense and anti-sense: 5′-atcacacgacaaggtgtcca-3′ and 5′-gccacaccaggttttctgtt-3′, respectively), and rat GAPDH (sense and anti-sense: 5′-aaggtcatcccagagctgaa-3′ and 5′-cctgcttcaccaccttcttg-3′, respectively). Samples were incubated at 50°C for 2 min followed by 95°C for 2 min followed by 45 cycles of 15 s at 95°C, 30 s at 56°C, 30 s at 72°C. Dissociation steps were run on all samples.
Imaging and Morphometric Analysis
Fluorescently stained samples were imaged using either the Nikon EZ-C1 confocal system or the FV300 Olympus 2-photon microscopy system. Image and tip number analysis were performed with ImageJ (NIH, Bethesda, Maryland). Isolated UB cultures were imaged by light brightfield microscopy and tips were counted using the Spot RT Slider digital camera attached to a Nikon microscope and Spot Advanced software v4.5 (Diagnostic Instruments, Sterling Heights, MI). The translucent isolated UB sample was divided into three distinct and non-overlapping planes of focus along the z axis. While focused on one plane, the tips of the other two planes were visible but blurred, supporting the view that tips were accounted for in only one plane. Tips were counted by focusing on each plane in sequence, finally summing the total number of tips from each plane to represent the whole 3-D structure.
Statistical analysis
Statistical significance among conditions was determined by analysis of variance (ANOVA). Additionally, the differences between means of counted tips and gene expression values were compared by Student’s t test, with P-values <0.05 considered significant. All P-values compare the experimental value to the untreated control unless otherwise noted with a connecting line. All tip counting experiments, isolated UB and organ culture, represent biological replicates of n ≥ 4 and gene expression data represents biological replicates of n ≥ 3. Error bars represent the standard error of the mean.
RESULTS
In vivo regulation of HA during kidney development
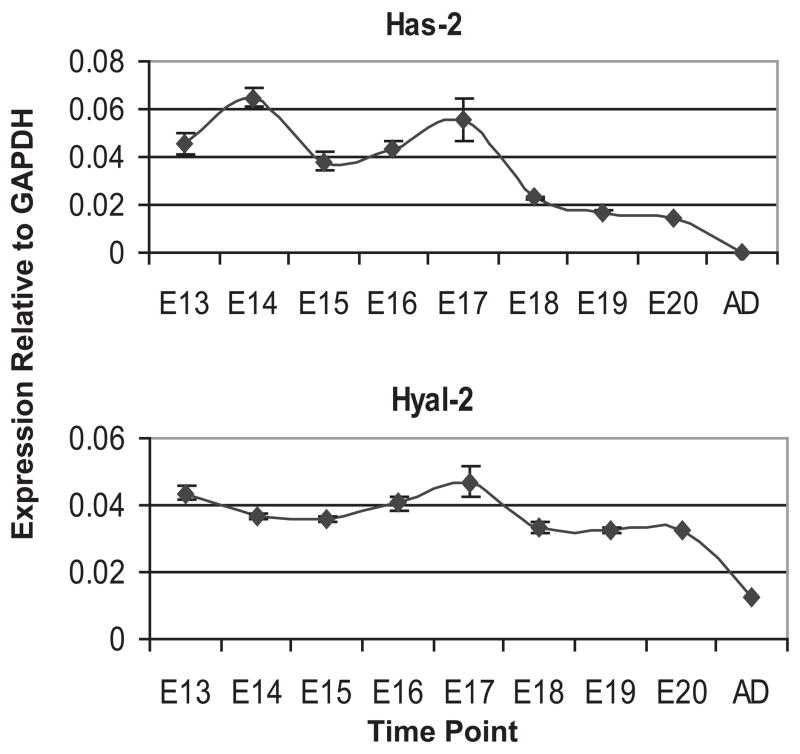
The expression pattern of has-2 was determined in the whole metanephric kidney throughout development. We performed a qPCR of has-2 for each day of kidney development from E13 to E20 and at adulthood (Fig. 1a). Has-2 is highly expressed in the kidneys during development, but nearly absent in adult kidneys. Next, we performed the same qPCR for hyal-2. Hyal-2 is a particularly interesting hyaluronidase because it reduces the molecular weight of HA rather than completely degrading HA [16]. In addition, hyal-2 exists as a glycosylphosphatidyl-inositol linked [17], cell anchored protein and therefore may allow the cell to tightly control the MW of the HA with which it interacts. Hyal-2 expression remained relatively constant throughout development, but dropped to one-third of the developmental level post-development (Fig. 1b). Thus, both the predominant HA synthesis gene (has-2) and its degradative enzyme (hyal-2) are developmentally regulated in the kidney.
Figure 1. Expression of has-2 and hyal-2 in the developing rat metanephric kidney.
Q-PCR analysis indicates that both has-2 and hyal-2 are highly expressed during kidney development.
HA-induced stimulation or inhibition of UB branching depending on molecular weight and concentration
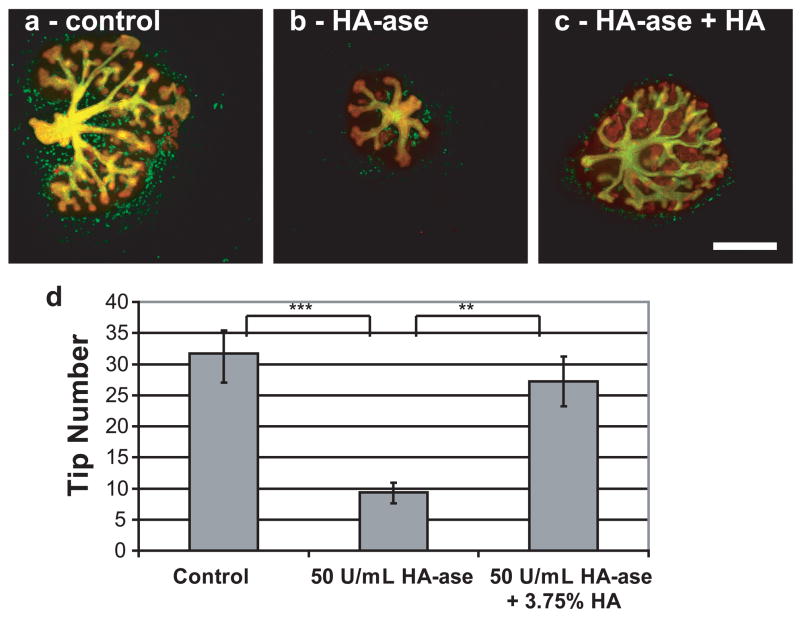
The has-2 gene knockout mouse is lethal prior to kidney development [9]; therefore, it is difficult to study the effect of HA deficiency on kidney development. One way to address this issue is to apply Streptomyces hyalurolyticus derived hyaluronidase (HA-ase), which is specific for HA and does not cleave chondroitin sulfates, to in vitro metanephric kidney cultures. Applying HA-ase to the whole embryonic kidney culture resulted in a 72% decrease in branching morphogenesis and kidney size as measured by tip number (Fig. 2). This decrease can be reversed by the addition of HA to the HA-ase.
Figure 2. Whole embryonic kidney with or without hyaluronidase.
(green = DB lectin, UB derived tissues; red = E-Cadherin, UB and MM derived epithelial tissues) (A) Control, (B) 50 U/mL hyaluronidase, (C) 50 U/mL hyaluronidase + 3.75% HA (MW = 234.4 kDa). (D) Plot of tip number vs. condition (ANOVA, P ≤ 0.05). Tip number measurements of each condition after 7 days of in vitro culture. ** = P ≤ 0.005, *** = P ≤ 0.0005. Scale bar corresponds to 500 μm.
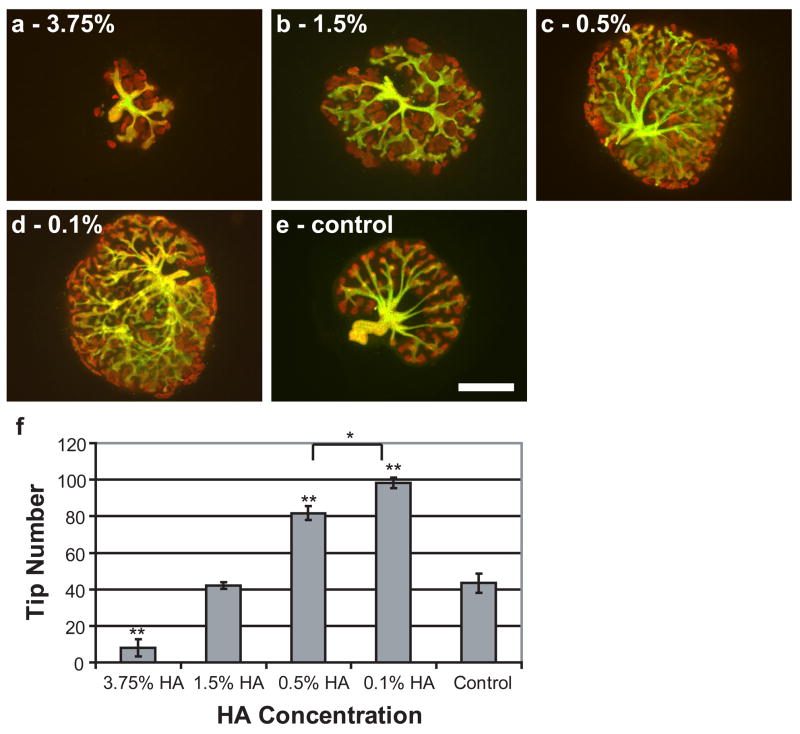
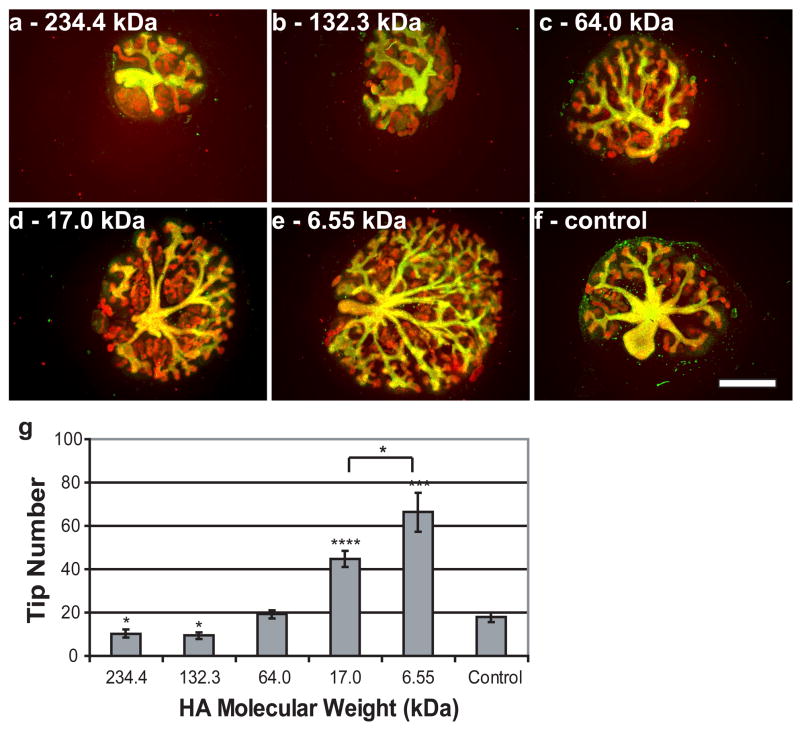
Since hyaluronidase decreases branching morphogenesis, it would appear to follow that the addition of exogenous HA would promote branching morphogenesis. However, when exogenous HA was added to in vitro cultured kidneys, it was found that HA could either promote or inhibit branching morphogenesis depending on the concentration and MW of the applied HA. Decreasing concentrations of exogenous HA, down to 0.1%, led to increases in branching morphogenesis (Fig. 3). Concentrations below 1.5% significantly increased the number of tips compared to untreated controls (nearly 2.5x more tips in kidneys treated with 0.1% HA), while concentrations above 1.5% dramatically reduced the number of tips in the organ culture compared to controls (>75% reduction with 3.75% HA) (Fig. 3). Similarly, decreasing molecular weights of exogenous HA led to increases in branching morphogenesis (Fig. 4). Molecular weights of 17.0 kDa and 6.55 kDa resulted in more than 200% and 300% increases in tip numbers, respectively, compared to controls (Fig. 4). Both the inhibitory and stimulatory effects of exogenous HA are reversible by the addition of hyaluronidase (Fig. 2c).
Figure 3. Effect of HA concentration on in vitro kidney growth and development.
Whole embryonic kidneys (green = DB lectin, UB derived tissues; red = E-Cadherin, UB and MM derived epithelial tissues) after 7 days in vitro culture with HA, 234.4 kDa. (A) 3.75% (B) 1.5% (C) 0.5% (D) 0.1% (E) control. (F) Plot of tip number vs. HA concentration (ANOVA, P ≤ 0.00001). * = P ≤ 0.05, ** = P ≤ 0.001. Scale bar corresponds to 500 μm.
Figure 4. Effect of HA molecular weight on in vitro kidney growth and development.
Whole embryonic kidneys (green = DB lectin, UB derived tissues; red = E-Cadherin, UB and MM derived epithelial tissues) after 7 days in vitro culture with 3.75% HA of the following molecular weights: (A) 234.4 kDa, (B) 132.3 kDa, (C) 64.0 kDa (D) MW 17.0 kDa, (E) 6.55kDa, (F) control. (G) Plot of tip number vs. HA molecular weight (ANOVA, P ≤ 0.00001). * = P ≤ 0.05, *** = P ≤ 0.0005, **** = P ≤ 0.0001. Scale bar corresponds to 500 μm.
To examine the effects of HA directly upon the UB and not in the presence of secondary effects via HA-based alterations to the MM, the effects of HA and HA-ase treatment were analyzed in the isolated UB culture system. Figure 5 shows that treatment of the isolated UB with HA-ase diminishes branching, although to a lesser extent than that seen in whole kidney culture (Fig. 2). When high MW exogenous HA (MW = 234.4 KD) was added to the isolated UB, branching morphogenesis was strongly inhibited in a concentration dependent manner (Fig. 5a,c,e); hyaluronidase could reverse this inhibition. When a more dilute BSN-CM and lower FGF concentration was used for isolated UB branching stimulation (1:3 diluted BSN-CM and 30 ng/mL FGF1), the effect of hyaluronidase was much more evident (Fig. 5g,h). This suggests that higher growth factor concentrations compensate for the removal of HA by HA-ase, raising the possibility that HA may act as a growth factor sink to promote UB branching. If HA is acting as a growth factor sink for branching tissues, then the effect of HA absence (i.e. HA-ase treatment) would be more evident in a growth factor depleted culture condition. Since, low MW and low concentration HA did not affect branching in the isolated UB system as was the case in whole kidney culture (data not shown), it is likely that the mechanism of high MW HA-mediated inhibition of branching is different from the low MW/low concentration HA-mediated stimulation of branching. Moreover, these results suggest that HA-mediated regulation of UB branching is independent of other effects on the kidney.
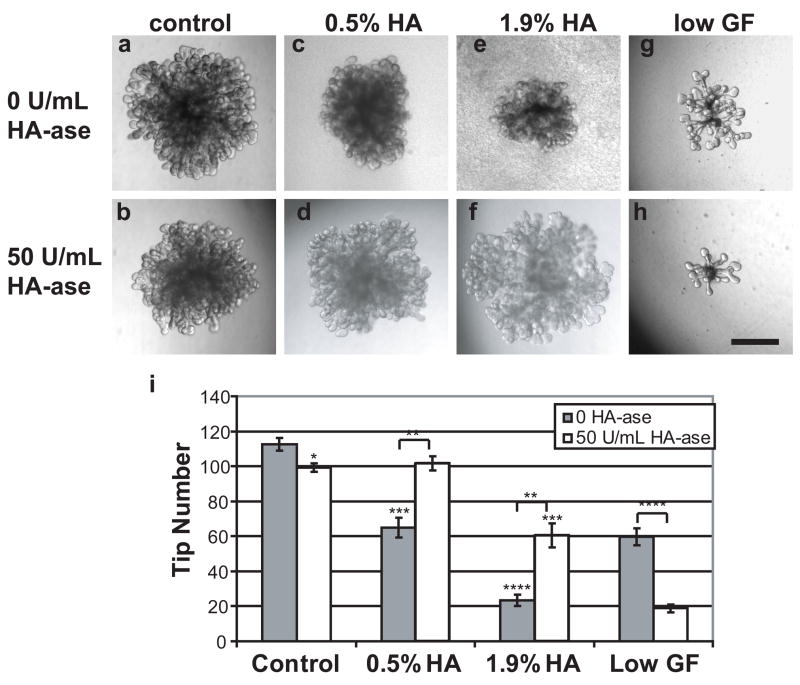
Figure 5. Isolated ureteric buds with HA and HA-ase.
UB suspended in 50% Matrigel without (A) or with HA-ase (B), 0.5% HA/50% Matrigel without (C) or with HA-ase (D), and 1.9% HA/50% Matrigel without (E) or with HA-ase (F). A low growth factor condition (no HA) without (G) and with HA-ase (H) accentuates the HA-ase inhibition. Tip measurements (I) and pictures were taken after 7 days in vitro culture. * = P ≤ 0.05, ** = P ≤ 0.005, *** = P ≤ 0.0005, **** = P ≤ 0.00005. Scale bar corresponds to 500 μm.
UB differentiation into collecting duct
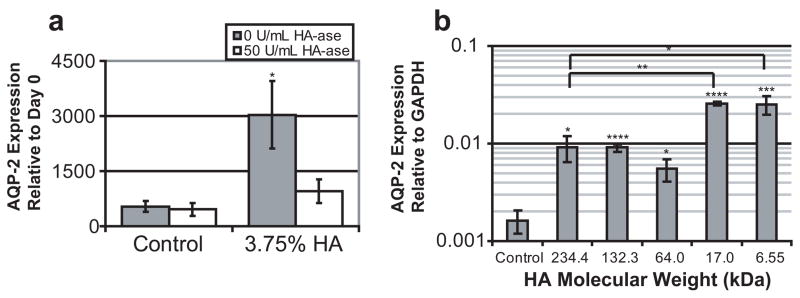
While HA was demonstrated to promote UB branching in certain conditions, we also investigated whether HA could promote collecting duct differentiation in addition to its effects on UB growth and branching. Aquaporin-2 (AQP-2), a transporter expressed only in mature collecting ducts [35], was used as a marker of UB maturation; initial expression of AQP-2 is seen at approximately embryonic day 17 of rat metanephric kidney development [36]. Quantitative PCR revealed that AQP-2 expression was 5.75 fold higher in HA treated kidneys than in untreated controls (Fig. 6). Hyaluronidase eliminated this increase, although it had no affect on untreated control kidneys. Every MW HA tested caused increases in AQP-2 expression; although, the lowest molecular weights tested (17.0 kDa and 6.55 kDa) resulted in additional increases in expression (more than 2 fold) over the 234.4, 132.3, and 64.0 kDa molecular weights. However, the data does suggest that HA is dispensable for collecting duct differentiation as exogenous HA-ase does not appear to reduce the level AQP-2 expression in control kidneys (Fig. 6). Interestingly, ureteric bud differentiation was stimulated by all tested MWs of HA, including those which inhibited branching morphogenesis, suggesting that UB branching and collecting duct differentiation are independent processes.
Figure 6. AQP-2 expression of embryonic kidney cultures.
(A) AQP-2 expression in control kidneys and HA (3.75%, 235 kDa) cultured kidneys with and without HA-ase. (B) AQP-2 expression in kidneys cultured with various MW HA solutions (ANOVA, P ≤0.00001). * = P ≤ 0.05, ** = P ≤ 0.005, *** = P ≤ 0.0005, **** = P ≤ 0.00005
Promotion of mesenchymal-to-epithelial transition and nephron differentiation by HA
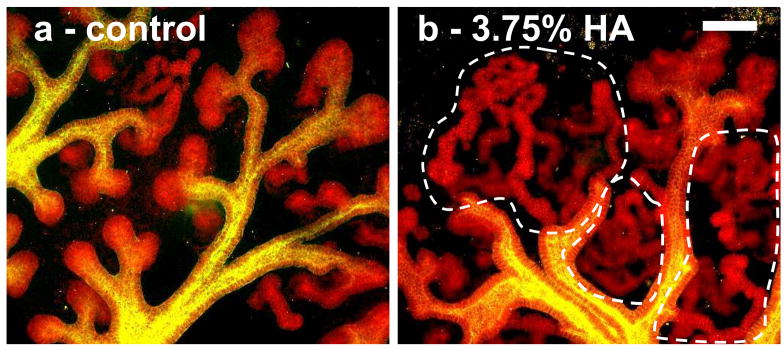
Metanephric mesenchyme differentiates into the tubular epithelium which ultimately forms the various segments of the nephron [1]. E-cadherin is a junction protein that is expressed by the collecting duct and by all segments of the epithelialized mesenchyme [37]. In kidney organ culture, HA appears to affect the MM by promoting MET evident by E-cadherin immunofluorescence (Fig. 7). After 7 days in culture, the mesenchyme of control kidneys have formed comma and s-shaped bodies with a few extended tubules, but the mesenchyme-derived tubules of HA treated kidneys are much longer and more convoluted (Fig. 7). This effect can be seen with HA of all molecular weights tested.
Figure 7. 2-photon microscopy images of HA-treated kidney cultures.
(green = DB lectin, UB derived tissues; red = E-Cadherin, UB and MM derived epithelial tissues) A) Control B) in 3.75% HA. HA treated kidneys contain longer, more convoluted tubules (noted by the dotted lines). Scale bar corresponds to 100 μm.
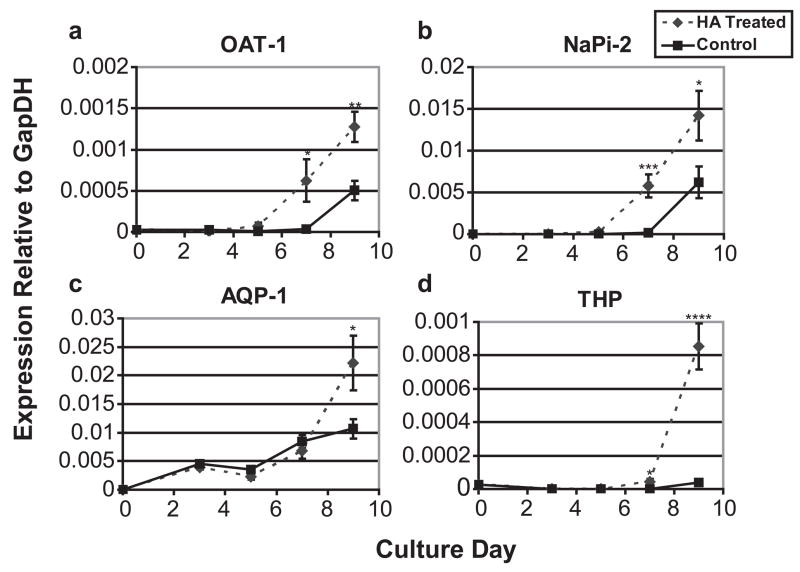
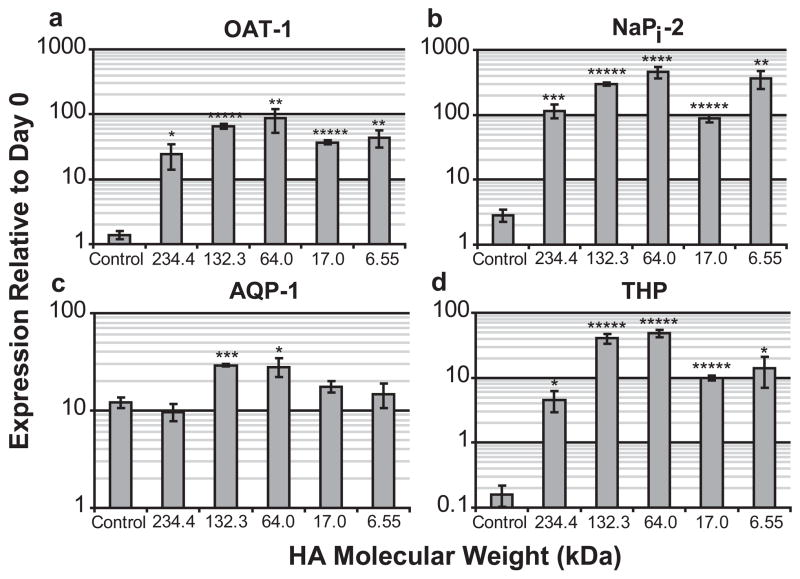
To test whether HA was only promoting MET or also promoting differentiation of the tubules into more mature nephrons, kidneys grown in various concentrations/MWs of HA were analyzed by qPCR for four proteins expressed by mature differentiated nephric tubules; organic anion transporter-1 (OAT-1), an early development proximal tubule marker; NaPi-2, a mid-development proximal tubule marker [38]; Aquaporin 1 (AQP-1), a late development proximal tubule marker [39]; and Tamm-Horsfall protein (THP), an ascending loop of Henle specific maker [40]. E13 rat kidneys cultured with or without a 3.75% HA (MW = 234.4 kDa) solution were analyzed by qPCR after 3, 5, 7, and 9 days of in vitro culture for expression of each of the four differentiation markers. Figure 8 shows that HA cultured kidneys develop at least 2 days faster than the control kidney cultures evident by the OAT-1 and NaPi-2 expression patterns. The ascending loop of Henle specific marker, THP, does not increase expression at all after 9 days of in vitro culture for the control kidneys, while HA treated kidneys display a 4.5 fold increase after 7 days and 86 fold after 9 days. Next, kidneys cultured for 7 days with different molecular weights of HA were tested for differentiation marker expression levels and compared to the untreated controls. With the exception of AQP1, HA appears to strongly promote MM differentiation at all tested molecular weights after 7 days (Fig. 9); however, HA treated kidneys display a 2 fold greater AQP-1 expression than controls after 9 days in culture (Fig. 8c).
Figure 8. Temporal gene expression of nephron differentiation markers in kidneys grown on a filter and kidneys grown in HA.
(A) OAT-1, (B) NaPi-2, (C) AQP-1, (D) THP. * = P ≤ 0.05, ** = P ≤ 0.005, *** = P ≤ 0.001, **** = P ≤ 0.0001
Figure 9. Gene expression of nephron differentiation markers after 7 days in culture for various molecular weights of HA.
(A) OAT-1 (ANOVA, P ≤ 0.001), (B) NaPi-2 (ANOVA, P ≤ 0.00001), (C) AQP-1 (ANOVA, P ≤ 0.0005), (D) THP (ANOVA, P ≤ 0.00001). * = P ≤ 0.05, ** = P ≤ 0.005, *** = P ≤ 0.001, **** = P ≤ 0.0001, ***** = P ≤ 0.00001
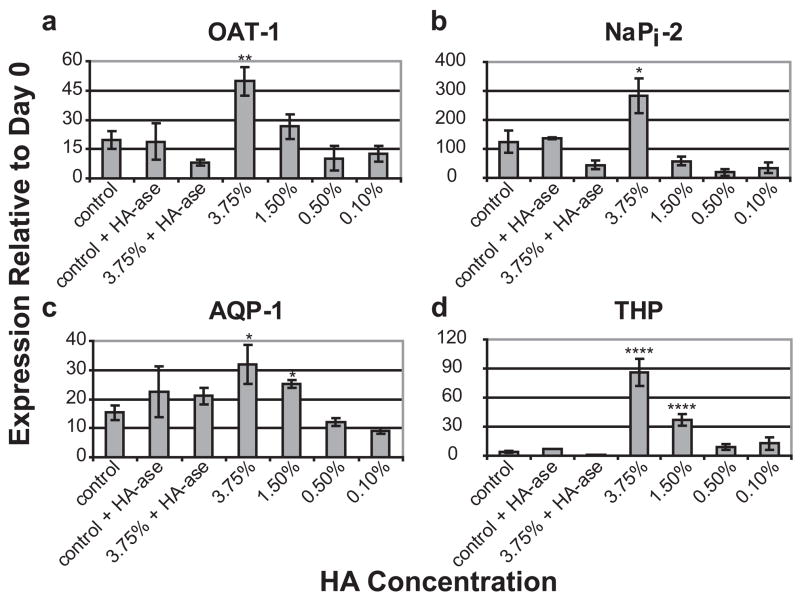
When the HA concentration was varied (MW was kept constant at 234.4 kDa), it was seen that the ability of HA to induce MM differentiation was concentration dependent and absent at concentrations at or below 0.5% HA (Fig. 10). Hyaluronidase treatment eliminated the expression increases caused by HA. However, it did not inhibit differentiation marker expression in untreated control kidneys suggesting that HA is not necessary for MM differentiation despite its strong enhancement of this process.
Figure 10. Effect of HA concentration on the expression of nephron differentiation markers after 9 days in culture.
* = P ≤ 0.05, ** = P ≤ 0.005, *** = P ≤ 0.001, **** = P ≤ 0.0001
DISCUSSION
Here we have demonstrated that has-2 and hyal-2 are genes highly expressed during kidney development. Along with the observations that HA is ubiquitously present throughout the interstitial space of the developing kidney [13] and that has-2 is known to be the predominant active enzyme in HA synthesis during embryogenesis [9], a key role for the involvement of has-2 and HA in kidney morphogenesis seemed plausible. To investigate the role of HA in kidney development, multiple in vitro models of kidney development, which mimic various aspects of renal morphogenesis (together and in isolation), were studied in detail.
The removal of HA from the in vitro models of kidney development by HA-ase treatment significantly inhibited branching morphogenesis in both whole metanephric kidney and isolated UB in vitro cultures (Fig. 2). However, HA-ase treated metanephric kidney cultures do not display decreases in the expression of MM maturation markers (i.e. OAT-1, NaPi-2, AQP-1, and THP) (Fig. 10) suggesting that the absence of HA inhibits UB branching morphogenesis, and thus kidney growth, independent of its effects on the MM in the in vitro developing kidney. This also suggests that HA may be vital for UB branching morphogenesis, but dispensable for MM maturation. Surprisingly, while HA may not be necessary for MM maturation, HA addition did stimulate MET and the expression of multiple differentiation markers.
Even though HA-ase inhibited branching morphogenesis, the effect of exogenous HA was bimodal, either promoting or inhibiting branching morphogenesis depending on the concentration of exogenous HA added. Low concentrations of HA (0.1%) tripled the number of tips seen in the metanephric kidney culture compared to controls, while high concentrations of HA (3.75 %) reduced the number of tips by 75%. The inhibition of branching morphogenesis by the high concentrations of HA was found to occur independent of effects upon the MM utilizing the isolated UB system. While the inhibitory effects of high concentration HA could be demonstrated with the isolated UB system, the stimulatory effects of low concentration and low MW HA could not. This can perhaps be explained by the composition of the extracellular matrix in which the isolated UB is grown. This matrix contains Matrigel, which may have interfered with the actions of HA. HA is known to bind various extracellular matrix proteins (including various collagens, fibronectin and chondroitin sulfates, all of which are present in Matrigel [41–46]) and may not have been able to freely diffuse through the matrix to come in to contact with the suspended UB and stimulate branching via signaling through CD44 or RHAMM (receptor for hyaluronic acid mediated motility) HA receptors. It is also possible that HA acts as a growth factor sink for the ureteric bud. If the exogenously added HA is not in the vicinity of the branching UB (because it is bound throughout the matrix), then it would not be attracting growth factors towards the UB. The latter may be supported by the amplified effect of HA-ase on the isolated UB system when lower growth factors concentrations are used. Higher growth factor concentrations in the media may be counteracting the effect of HA absence in the isolated UB system by negating the need for a growth factor sink.
Additionally, the discrepancy between the isolated UB system and metanephric kidney culture suggests that the mechanism for inhibition of UB branching by high concentration and high MW HA differs from the mechanism of stimulation by low concentration and low MW HA. While the stimulation of branching by HA may be the result of the growth factor binding capabilities of HA or by direct signaling via the CD44 or RHAMM receptors, the inhibition of branching morphogenesis by HA may be related to the physical characteristics of the HA and the highly viscous nature of the solution. High concentration and high molecular weight HA is very viscous and may be acting as a physical barrier to the growth required for UB branching morphogenesis; this observation could have tissue engineering and developmental relevance.
HA concentration profiles were also found to affect the UB and MM differently. The UB responds to very small or large doses of HA to promote or inhibit branching morphogenesis, respectively, while the enhanced MM differentiation occurs only in response to larger concentrations of HA (1.5% and above). This difference in concentration dependence between the UB and MM in response to HA may represent a mechanism through which HA could act as a developmental switch molecule. During kidney development, branching morphogenesis occurs first, followed by large scale MM differentiation [47]. It is possible that HA is present in relatively low concentrations early in development, but accumulates in the embryonic kidney as development continues.
This is supported by the high developmental expression of has-2. In this manner, HA would be promoting branching morphogenesis early in development, but as HA accumulates (either by has-2 synthesis of HA or perhaps by inhibition of hyaluronidases) branching is inhibited and MM differentiation is stimulated. It may also be possible that the UB and MM are independently controlling the local concentration of HA that is seen to achieve a desired effect. For instance, the UB may be keeping a higher local concentration of HA around stalks that have already branched to promote tubule differentiation, while keeping the concentration lower around the tips to promote branching morphogenesis.
If different MWs of HA play different roles in regulating kidney development, one would expect the hyal-2 gene to be expressed by the developing kidney. Here we have shown that hyal-2 is highly expressed during development (3-fold more than the adult kidney) suggesting the potential for HA molecular weight to be a factor in kidney development. Furthermore, we demonstrated that the different MWs of HA (at high concentrations) can have different effects on branching morphogenesis and maturation of the ureteric bud. While high MWs could inhibit branching morphogenesis, low MWs promoted branching morphogenesis. In addition, all MWs of HA tested induced increases in AQP-2 expression, although the lower MWs of HA (6.55 and 17.0 kDa) increased AQP-2 expression by an additional 2-fold compared to higher MWs.
While the multiple molecular weights of HA affected the UB in drastically different manners, the differentiation of MM was independent of the exogenous HA molecular weight. Since UB branching is dependent on HA molecular weight, but the stimulation of MM differentiation is not, it can be inferred that the HA stimulation of MM is independent of the HA effects on UB branching morphogenesis. This independence is important because it provides a second potential mechanism through which HA can direct the morphogenetic processes of the two renal progenitor tissues and suggests how the kidney could regulate HA molecular weight to act as a developmental switch. It may be possible that the one of the kidney progenitor tissues increases or decreases hyaluronidase action to achieve a response without inhibiting the effect of HA on the other tissue. Hyal-1 and hyal-2 are believed to be the two primary hyalurondiases that act in concert to regulate HA size and accumulation [15]. Glycosylphosphatidyl-inositol-anchored hyal-2 breaks down high molecular weight HA to 20 KD fragments at the cell surface, while hyal-1 further degrades HA to extinction [16, 17]. It may be possible that the UB and MM are controlling the size and concentration of HA that it sees at the cell surface. Early in development the UB may be using hyal-2 to keep HA molecular weight below 20 kDa, the size demonstrated to promote UB branching and differentiation, but as development continues, either hyaluronidase action decreases or HA accumulates faster than hyal-2 can cleave it, leading to a local HA molecular weight increase and therefore cessation of branching morphogenesis. Since MM is not affected by HA molecular weight, MM-related processes would not be affected by the increase in MW, and would continue to mature.
Regulation of HA molecular weight and modulation of HA concentration represent two possibilities of how HA may be acting as a switch or controlling the development of the UB and MM simultaneously, but independently. It is more likely that a combination of these two modes of action is occurring rather than just one or the other. Only a few developmentally relevant molecules are known to affect both branching morphogenesis and mesenchymal differentiation. For example, leukemia inhibitory factor (LIF) and transforming growth factor-β (TGF- β) have both been demonstrated to inhibit UB branching and promote MM differentiation [2, 6, 7]. However, unlike these growth factors, HA is also capable of stimulating branching morphogenesis and can be regulated by both concentration and molecular weight. A conditional knockout of has-2 and/or hyal-2 would assist in testing these ideas in vivo; however, the expression data and in vitro metanephric kidney culture presented here strongly support a role for HA in renal development, possibly as a switch molecule.
The potential of HA to be a guiding molecule in kidney development has many implications for organ development in general. In addition to gaining a further understanding of kidney development, these results may be useful for in vitro kidney engineering. The tissue engineering field has long been interested in HA as a biomaterial, and the results of this paper suggest additional reasons to pursue the application of HA towards in vitro organ engineering. In particular, some developmental approaches to kidney engineering involve the use of embryonic tissues as a therapy or as a source of tissues for in vitro kidney engineering [48–51]. Here we have demonstrated that HA is an extracellular molecule that guides and promotes in vitro kidney maturation and development. A HA-based scaffold could be designed specifically to promote the in vitro development of these kidney tissues. For example, since lower MWs, at high concentrations, promote branching morphogenesis and MM differentiation, a crosslinked HA scaffold could be designed with a high concentration of low MW HA molecules that are released as the matrix is degraded. Alternatively, one could create a bilayered scaffold containing an inner layer of UB promoting low concentrations or low MWs of HA and an outer layer consisting of higher MW molecules that will limit kidney growth to a specified size, yet continue to promote maturation.
Since HA promotes renal tubule development and differentiation, it may also prove useful during renal repair. HA may be able to promote formation of new nephrons in adult kidneys suffering from renal disease. HA receptors or HA analogs may represent a new set of drug targets or therapies to promote in renal tubule regeneration. Furthermore, the potential tubular regenerative capacity of HA may make it a useful additive to cryopreservation solutions. Kidneys used for transplantation are typically perfused with a solution containing an anionic, water absorbing substance to prevent cell swelling during transport [52]. HA could act both as the water absorbing, anionic substance to protect from cell swelling in addition to promoting tubule regeneration in damaged tubules caused by ischemia/reperfusion injury.
CONCLUSION
The mechanisms regulating the timing of events in kidney development are not well understood. However, the data we present here illustrates two possibilities of how the synthesis and size regulation of HA could act as a developmental mechanism that ends branching morphogenesis and promotes of nephron differentiation. Furthermore, the ability of HA to promote maturation in the in vitro models of kidney development, together with the prevalent use of HA as a biomaterial, suggests the potential of HA as an ideal scaffold for kidney tissue engineering.
Acknowledgments
This work was supported by NIH grants DK057286, DK065831and HL035018 (to S.K.N.). E.R. was supported by the NSF graduate research fellowship. We also acknowledge Dr. Kevin Bush and Dr. Hiro Sakurai for their critical reading of this manuscript. We would like to thank the UCSD Neuroscience microscopy shared facility and NINDS grant NS047101 for providing us access to their 2-photon imaging facilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1(3):385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 2.Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, et al. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266(2):285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc Natl Acad Sci U S A. 1999;96(13):7330–7335. doi: 10.1073/pnas.96.13.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai H, Bush KT, Nigam SK. Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development. 2001;128(17):3283–3293. doi: 10.1242/dev.128.17.3283. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai H, Bush KT, Nigam SK. Heregulin induces glial cell line-derived neurotrophic growth factor-independent, non-branching growth and differentiation of ureteric bud epithelia. J Biol Chem. 2005;280(51):42181–42187. doi: 10.1074/jbc.M507962200. [DOI] [PubMed] [Google Scholar]
- 6.Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, et al. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999;99(4):377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- 7.Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO. TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development. 2001;128(7):1045–1057. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]
- 8.Laurent TC, Fraser JR. Hyaluronan. Faseb J. 1992;6(7):2397–2404. [PubMed] [Google Scholar]
- 9.McDonald JA, Camenisch TD. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconj J. 2002;19(4–5):331–339. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- 10.Furnus CC, de Matos DG, Martinez AG. Effect of hyaluronic acid on development of in vitro produced bovine embryos. Theriogenology. 1998;49(8):1489–1499. doi: 10.1016/s0093-691x(98)00095-8. [DOI] [PubMed] [Google Scholar]
- 11.Toyokawa K, Harayama H, Miyake M. Exogenous hyaluronic acid enhances porcine parthenogenetic embryo development in vitro possibly mediated by CD44. Theriogenology. 2005;64(2):378–392. doi: 10.1016/j.theriogenology.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Pohl M, Sakurai H, Stuart RO, Nigam SK. Role of hyaluronan and CD44 in in vitro branching morphogenesis of ureteric bud cells. Dev Biol. 2000;224(2):312–325. doi: 10.1006/dbio.2000.9783. [DOI] [PubMed] [Google Scholar]
- 13.Song HK, Kim WY, Lee HW, Park EY, Kim J. Developmental Expression of Hyaluronan in Rat Kidney; American Society of Nephrology National Meeting 2006; San Diego: CA. 2006. [Google Scholar]
- 14.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274(35):25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 15.Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20(8):499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- 16.Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273(35):22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- 17.Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci U S A. 2001;98(8):4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12(8):2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 19.Richter W. Non-immunogenicity of purified hyaluronic acid preparations tested by passive cutaneous anaphylaxis. Int Arch Allergy Appl Immunol. 1974;47(2):211–217. doi: 10.1159/000231214. [DOI] [PubMed] [Google Scholar]
- 20.Richter AW, Ryde EM, Zetterstrom EO. Non-immunogenicity of a purified sodium hyaluronate preparation in man. Int Arch Allergy Appl Immunol. 1979;59(1):45–48. doi: 10.1159/000232238. [DOI] [PubMed] [Google Scholar]
- 21.Balazs EA. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Int. 2004;12:278–289. [PubMed] [Google Scholar]
- 22.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005321. doi: 10.1002/14651858.CD005321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe NJ, Grover R. Injectable hyaluronic acid implant for malar and mental enhancement. Dermatol Surg. 2006;32(7):881–885. doi: 10.1111/j.1524-4725.2006.32190.x. [DOI] [PubMed] [Google Scholar]
- 24.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19(3):141–50. doi: 10.1111/j.1529-8019.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 25.Lupo MP. Hyaluronic acid fillers in facial rejuvenation. Semin Cutan Med Surg. 2006;25(3):122–126. doi: 10.1016/j.sder.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zheng Shu X, Prestwich GD. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26(23):4737–4746. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Vercruysse KP, Marecak DM, Marecek JF, Prestwich GD. Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjug Chem. 1997;8(5):686–694. doi: 10.1021/bc9701095. [DOI] [PubMed] [Google Scholar]
- 29.Tomihata K, Ikada Y. Crosslinking of hyaluronic acid with water-soluble carbodiimide. J Biomed Mater Res. 1997;37(2):243–251. doi: 10.1002/(sici)1097-4636(199711)37:2<243::aid-jbm14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Larsen NE, Pollak CT, Reiner K, Leshchiner E, Balazs EA. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27(9):1129–1134. doi: 10.1002/jbm.820270903. [DOI] [PubMed] [Google Scholar]
- 31.Leshchiner EABaA. Crosslinked gels of hyaluronic acid and products containing such gels. United States Patent #4. 1986;582:865. [Google Scholar]
- 32.Pohl M, Sakurai H, Bush KT, Nigam SK. Matrix metalloproteinases and their inhibitors regulate in vitro ureteric bud branching morphogenesis. Am J Physiol Renal Physiol. 2000;279(5):F891–900. doi: 10.1152/ajprenal.2000.279.5.F891. [DOI] [PubMed] [Google Scholar]
- 33.Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, et al. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol. 2004;272(2):310–327. doi: 10.1016/j.ydbio.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, et al. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238(2):289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- 35.Kishore BK, Terris JM, Knepper MA. Quantitation of aquaporin-2 abundance in microdissected collecting ducts: axial distribution and control by AVP. Am J Physiol. 1996;271(1 Pt 2):F62–70. doi: 10.1152/ajprenal.1996.271.1.F62. [DOI] [PubMed] [Google Scholar]
- 36.Stuart RO, Bush KT, Nigam SK. Changes in global gene expression patterns during development and maturation of the rat kidney. Proc Natl Acad Sci U S A. 2001;98(10):5649–5654. doi: 10.1073/pnas.091110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125(5):803–812. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- 38.Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int. 2006;69(5):837–845. doi: 10.1038/sj.ki.5000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wintour EM, Earnest L, Alcorn D, Butkus A, Shandley L, Jeyaseelan K. Ovine AQP1: cDNA cloning, ontogeny, and control of renal gene expression. Pediatr Nephrol. 1998;12(7):545–553. doi: 10.1007/s004670050502. [DOI] [PubMed] [Google Scholar]
- 40.Sikri KL, Foster CL, MacHugh N, Marshall RD. Localization of Tamm-Horsfall glycoprotein in the human kidney using immuno-fluorescence and immuno-electron microscopical techniques. J Anat. 1981;132(Pt 4):597–605. [PMC free article] [PubMed] [Google Scholar]
- 41.McDevitt CA, Marcelino J, Tucker L. Interaction of intact type VI collagen with hyaluronan. FEBS Lett. 1991;294(3):167–170. doi: 10.1016/0014-5793(91)80660-u. [DOI] [PubMed] [Google Scholar]
- 42.Kielty CM, Whittaker SP, Grant ME, Shuttleworth CA. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992;118(4):979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura M, Mishima H, Nishida T, Otori T. Binding of hyaluronan to plasma fibronectin increases the attachment of corneal epithelial cells to a fibronectin matrix. J Cell Physiol. 1994;159(3):415–422. doi: 10.1002/jcp.1041590305. [DOI] [PubMed] [Google Scholar]
- 44.Turley EA, Erickson CA, Tucker RP. The retention and ultrastructural appearances of various extracellular matrix molecules incorporated into three-dimensional hydrated collagen lattices. Dev Biol. 1985;109(2):347–369. doi: 10.1016/0012-1606(85)90461-0. [DOI] [PubMed] [Google Scholar]
- 45.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 46.Schittny JC, Kresse H, Burri PH. Immunostaining of a heterodimeric dermatan sulphate proteoglycan is correlated with smooth muscles and some basement membranes. Histochem Cell Biol. 1995;103(4):271–279. doi: 10.1007/BF01457411. [DOI] [PubMed] [Google Scholar]
- 47.Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Dev Dyn. 2004;231(3):601–608. doi: 10.1002/dvdy.20143. [DOI] [PubMed] [Google Scholar]
- 48.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, et al. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9(1):53–60. doi: 10.1038/nm812. [DOI] [PubMed] [Google Scholar]
- 49.Rogers SA, Lowell JA, Hammerman NA, Hammerman MR. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54(1):27–37. doi: 10.1046/j.1523-1755.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 50.Steer DL, Bush KT, Meyer TN, Schwesinger C, Nigam SK. A strategy for in vitro propagation of rat nephrons. Kidney Int. 2002;62(6):1958–1965. doi: 10.1046/j.1523-1755.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- 51.Steer DL, Nigam SK. Developmental approaches to kidney tissue engineering. Am J Physiol Renal Physiol. 2004;286(1):F1–7. doi: 10.1152/ajprenal.00167.2003. [DOI] [PubMed] [Google Scholar]
- 52.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83(10):1289–1298. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]