Abstract
Recent evidence suggests that nonsteroidal antiinflammatory drugs (NSAIDs) may prevent colorectal cancer. The mechanism of action of NSAIDs in chemoprevention is unknown but may be linked to their effect on mucosal prostaglandin levels. Levels of five major prostaglandin metabolites were measured by gas chromatography–mass spectrometry in biopsy specimens of flat rectal mucosa from four patients with familial adenomatous polyposis (FAP) before and after sulindac therapy and from five healthy individuals. The prostaglandin present at highest concentration in rectal mucosa from FAP and control subjects was prostaglandin E2. The concentration of thromboxane B2 alone was significantly elevated in FAP patients compared to controls (P = 0.016). In FAP patients treated with sulindac, all prostaglandin metabolite levels were significantly reduced compared to pretreatment levels (P < 0.05) except prostaglandin D2 (P = 0.07). Prostaglandins D2, E2, F2α, and 6-ke to-F1α levels also were significantly reduced in FAP patients on sulindac compared to healthy controls (P < 0.05). However, interpatient heterogeneity of response to sulindac was evident with changes ranging from +19% to −89%, and the patient with the greatest reductions after sulindac developed colorectal cancer after 35 months of therapy. Sulindac treatment, at drug doses shown to regress colorectal adenomas in FAP patients, has heterogeneous effects on the level of major prostaglandins in their rectal mucosa and may not prevent colorectal cancer due to uncoupling of prostaglandin levels and carcinogenesis.
Keywords: familial adenomatous polyposis, prostaglandins, nonsteroidal antiinflammatory drugs, sulindac, polyps
Several lines of investigation support the concept that nonsteroid al antiinflammatory drugs (NSAIDs), which inhibit the synthesis of prostaglandins, can prevent colorectal cancer (1). Specifically, NSAIDs inhibit cell growth in cell culture (2-4), decrease the multiplicity and incidence of colon tumors in carcinogen-induced murine models (5-9), decrease the relative risk of the incidence and mortality of colorectal cancer in users of NSAIDs in human epidemiologic studies (10, 11), and regress adenomas in patients with familial adenomatous polyposis (12-16).
The mechanism of action of NSAIDs in the chemoprevention of colorectal cancer is unknown but may be linked to the effect of these agents on mucosal prostaglandin levels. Earlier evidence suggests that prostaglandins may modulate tumor cell growth and facilitate tumor cell progression (17-19). Prostaglandins, particularly prostaglandin E2 (PGE2), are produced in increased amounts in human colonic tumor cells (20, 21). In animal models of colorectal cancer carcinogenesis, normal-appearing mucosa in cancer-bearing animals has increased levels of PGE2 (22) compared to controls, and PGE2 is higher in the tumor tissue compared to the mucosa in carcinogen-treated animals (23). In humans, tissue concentrations of PGE2 are significantly increased in colorectal adenomas compared to flat mucosa in controls, and even greater levels were found in malignant polyps and cancers (24, 25). An increase of PGE2 is also found in flat colorectal mucosa of patients with adenocarcinoma (24). On the other hand, prostaglandin levels are found to be independent of the effects of NSAIDs in studies of experiment colorectal carcinogenesis and growth of colorectal cancer cell lines (26, 27).
In patients with familial adenomatous polyposis (FAP), sulindac has been shown to result in regression of colorectal adenomas, but limited data exist on the influence of NSAIDs on colorectal mucosal prostaglandin levels (13, 28, 29). Therefore, to understand the effect of NSAIDs on a profile of colorectal mucosal prostaglandin levels, we utilized gas chromatography and mass spectrometry, a methodology not previously employed, to measure the concurrent levels of five major prostaglandin metabolites in biopsy specimens. Flat mucosa of patients with FAP before and after sulindac treatment and of healthy controls was studied.
MATERIALS AND METHODS
Subjects
The study population consisted of four Caucasian FAP patients with previous colectomy and ileorectal anastomosis who had adenomas in the rectum (mean age 37.4 ± 11 SD; two men, two women). Mucosal prostaglandin levels were measured in these patients immediately before the initiation of sulindac treatment and after therapy with sulindac 150 mg orally twice a day for three months. Polyp regression was noted in all patients after three months of therapy. However, one of the patients (patient 4 in Figure 1 below) developed rectal cancer while on sulindac 35 months after initiation of the drug. The control population was five otherwise healthy Caucasian individuals with normal colonoscopic examinations performed for the evaluation of abdominal pain or hematochezia or for risk of FAP (persons at risk for FAP had negative APC gene tests) (mean age 26.7 ± 15 SD; two men; three women).
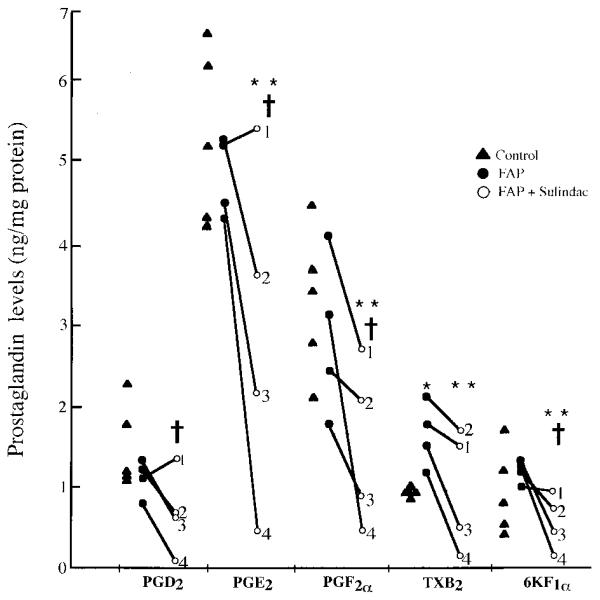
Fig 1.
Prostaglandin levels (ng/mg protein) in colorectal mucosa. PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; TXB2, thromboxane B2; 6-keto-F1, 6-keto-prostaglandin F1α. FAP patients 1–4 are noted. * P = 0.016 control vs FAP; ** P < 0.05 FAP vs FAP and sulindac; † P < 0.05 control vs FAP and sulindac. Controls are five otherwise healthy Caucasians with normal colonoscopic examinations. FAP patients are four Caucasian patients with previous colectomy and ileorectal anastomosis. Patient 4 developed colorectal cancer after 35 months of sulindac treatment.
All patients were prepared for the endoscopic procedure with a clear liquid diet and oral cathartic solution. Enemas that could influence mucosal biochemistry were not given. In each patient, six rectal mucosal biopsies were taken from flat mucosa 10–12 cm from the anal verge to minimize potential differences that might occur from specimens taken at different colorectal sites. Four rectal mucosal specimens were snap frozen in liquid nitrogen for prostaglandin analysis, and two were placed in formalin for histopathologic examination. Histopathology showed colorectal mucosa without adenomatous epithelium or aberrant crypt foci in all nine individuals.
Specimen Processing
Rectal mucosal biopsy specimens were analyzed for prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), thromboxane B2 (TXB2), and the principal prostaglandin I2 metabolite, 6-keto-prostaglandin F1α (6-keto-F1α). Briefly, specimens were rinsed with cationic-free Hanks' balanced salt solution (HBSS; containing 138 mM NaCl, 5 mM KCl, 4 mM NaHCO3, 5.6 mM D-glucose, 0.3 mM Na2HPO4, and 0.3 mM KH2PO4). Samples were then manually homogenized in a glass microhomogenizer in 50 μl HBSS containing 1 mM CaCl2. The homogenizer was then rinsed with 60 μl HBSS containing 1 mM CaCl2. Of the combined 110 μl homogenate, 10 μl was used to determine protein concentration by the Bradford protein assay (Bio-Rad). The remaining 100 μl was then incubated at 37°C for 15 min, followed by the addition of 50 μl of deuterated prostaglandin standards. The sample was then equally divided. Following the addition of 250 μl acetone to each half, the specimens were dried under a steady stream of nitrogen gas. At the point of complete drying, 25 μl oximating reagent (2% O-methoxylamine HCl in pyridine) was added immediately to the sample. Each vial was then sealed with a Teflon-lined closure, and the contents were vigorously mixed with the use of a vortex mixer and left at room temperature for 12–16 hr for oximation of the carbonyl moieties of PGD2, PGE2, TXB2, and 6-keto-F1α. The samples were stored at −20°C until delivery to the mass spectrometry laboratory for quantitation of prostaglandin levels.
Determination of Prostaglan dins by GC-MS
Samples stored at −20°C were first brought to room temperature, followed by evaporation of the pyridine solvent of the oximating reagent under a nitrogen stream. Subsequent to evaporation of pyridine, the residue of each vial was treated with reagents for synthesis of the pentafluorobenzyl ester–trimethylsilyl ether derivatives of the prostanoid analytes for gas chromatography–mass spectrometric (GC-MS) analysis as described (30, 31). Briefly, a Finnagan MAT SSQ710 combined gas chromatograph–mass spectrometer (Finnigan MAT, San Jose, California) was operated under electron capture negative conditions. Characteristic M−, −181 fragmentions of each of the prostaglandin derivatives and those of the deuterate d prostaglandin analogs used as internal standards were monitored simultaneously. The levels of each of the prostaglandins in the samples were based upon the use of known quantities of deuterated prostaglandins employed as internal standards. Other instrument parameters for combined GC-MS analysis and commercial sources of reagents for derivatization have been reported previously (30, 31).
Statistical Analysis
Differences in prostaglandin levels between controls and FAP patients before sulindac treatment were compared by Mann-Whitney U test. Reductions in FAP patients after sulindac treatment as compared to baseline were compared by paired one-tailed Student's t test. Significance between groups was defined as P < 0.05.
RESULTS
Of the five prostaglandin metabolites measured (Table 1 and Figure 1), PGE2 was present at the highest concentration in rectal mucosa from FAP patients before sulindac treatment and from controls. The concentration of TXB2 was statistically elevated in untreated FAP patients compared to controls (P = 0.016), but the concentrations of PGD2, PGE2, PGF2α, and 6KF1α were not statistically different.
Table 1.
Prostaglandin Levels in Rectal Mucosal Biopsy Specimens*
|
Prostaglandin ng/mg protein |
Baseline change (%)† |
|||
|---|---|---|---|---|
| Control | FAP | FAP & SUL | ||
| PGD2 | 1.541 ± 0.461 | 1.136 ± 0.182 | 0.729 ± 0.450c | −38.8 |
| PGE2 | 5.355 ± 1.015 | 4.832 ± 0.454 | 2.930 ± 1.820bc | −32.3 |
| PGF2a | 3.345 ± 0.791 | 2.917 ± 0.881 | 1.618 ± 0.888bc | −43.6 |
| TXB2 | 0.945 ± 0.042 | 1.673 ± 0.329a | 1.005 ± 0.643b | −45.2 |
| 6KPGF1a | 0.960 ± 0.482 | 1.184 ± 0.108 | 0.567 ± 0.307bc | −49.5 |
Results are mean ± SD. FAP is familial adenomatous polyposis; FAP & SUL is FAP patients treated with sulindac.
P = 0.016 FAP compared to control
P = 0.05 FAP compared with FAP treated with sulindac
P = 0.05 FAP treated with sulindac compared to control.
Percent change from baseline in patients before and after sulindac treatment.
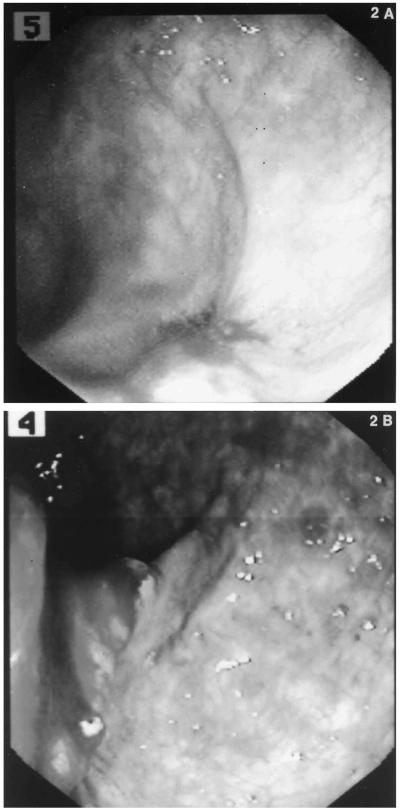
In FAP patients treated with sulindac for three months, all prostaglandin metabolite levels except PGD2 were statistically significantly lower than before therapy (P < 0.05); the concentration of PGD2 level was marginally lower (P = 0.07). Mean PGD2, PGE2, PGF2α, and 6-keto-F1α concentrations in FAP patients treated with sulindac also were significantly reduced compared to those of healthy controls (P < 0.05) . These effects correlated with complete adenoma regression in all patients. Mean change from base line after sulindac therapy was found to decline for all prostaglandins (Table 1). When interpatient variation was considered, patient 1 (Figure 1) showed changes from base line ranging from +19% for PGD2 to −34% for PGF2. The greatest response to sulindac therapy occurred in patient 4 (range : −89% for PGE2 to −82% for PGF2), who subsequently developed an ulcerating flat rectal adenocarcinom a but no adenomatous polyps while on sulindac therapy for 35 months (Figure 2A and B).
Fig 2.
(A) Endoscopic view of the rectum in patient 4 at 33 months of sulindac treatment revealing flat erythematous lesion. Mucosal biopsies revealed tubular adenoma and fibroinflammatory exudate. No polypoid adenomas were present. (B) Endoscopic view of the rectum at 35 months of sulindac treatment. Ulce rating rectal adenocarcinoma shown without concomitant re ctal polypoid adenomas.
DISCUSSION
We examined a profile of prostaglandin metabolites in human colorectal mucosa. In contrast to other investigators who have utilized radioimmunoassay to determine colorectal mucosal prostaglandin levels (13, 28, 29, 32), we employed gas chromatography coupled with mass spectrometry. The accuracy of radioimmunoassay is dependent upon antibody specificity, and the limitation of this approach is evidenced by the wide variation of absolute values for prostaglandin levels between published studies. In contrast, our methodology allowed the convenient, simultaneous, and accurate measurement of a series of prostaglandins. Difficulties with metabolite stability are minimized by the use of internal standards employed in this technique . Examination of a mucosal prostaglandin profile may also have future importance in understanding which of these compounds might best serve as biomarkers of human colorectal carcinogenesis and as indicators of patient compliance with medication in clinical trials. More over, this approach may also be useful in testing compounds thought to act via pathways independent of prostaglandin synthesis.
In our study, PGE2 was present in greatest concentration in rectal mucosa not only in those affected by FAP but also in healthy individuals, a finding not previously reported. Of note , a significantly higher level of TXB2, but not other prostaglandins, was found in the flat mucosa of FAP patients compared to healthy controls. These findings contrast with those in animal models of colorectal cancer, in which carcinogen-treated rats have persistently higher levels of PGE2 in normal flat mucosa compared to control animals (23). The significance of increased TXB2 levels in FAP patients awaits further investigation.
We found that sulindac taken orally by FAP patients significantly decreased mean rectal mucosal levels of all the major prostaglandin metabolites except PGD2 (although PGD2 had a marginally significant decrease, P = 0.07). This finding is consistent with the work of Rigau et al (13), who measured PGE2 and 6-keto-F1α in flat colorectal mucosa of FAP patients before and after sulindac treatment and found a significant decrease in both prostaglandins after sulindac therapy. Winde et al (28) and Nugent et al (29) analyzed flat colorectal mucosal PGE2 and PGF2α and also noted a significant reduction in the PGE2 after treatment. Importantly, in our study, sulindac administration significantly decreased levels of several prostaglandins (D2, E2, F2, 6-keto-F1α) below concentrations in healthy control subjects. These effects correlated with the regression of colorectal adenomas in the sulindac-treated FAP patients.
Some investigators have indicated that colorectal prostaglandin levels are not affected in patients taking similar NSAID doses that cause tumor regression. Although our data demonstrate a significant decrease in mean prostaglandin levels in rectal mucosa following sulindac treatment, substantial interpatient heter-ogeneity was noted. Patient 1 showed little or no reduction in several prostaglandin levels in the face of regression of adenomas and high medication compliance. Of even greater interest, patient 4, who had the greatest reduction in prostaglandin levels, developed a ulcerating flat rectal adenocarcinoma despite the absence of polypoid adenomas 35 months after starting sulindac. Other patients developing rectal cancer while on sulindac therapy have been reported (33, 34). In addition, investigators have questioned the importance of prostaglandin inhibition in colorectal chemoprevention. Ahnen et al (26) noted that the sulfone metabolite of sulindac (FGN-1) inhibited experimental colon carcinogenesis in the rat by a prostaglandin-independent process (26). Further investigation revealed reduced tumor incidence, multiplicity, and tumor burden in sulindac sulfone -treated rats compared to controls (36). Moreover, there was no decrease in PGE2 or significant inhibitory effects on cyclooxygenase, lipoxygenase or phospholipase A2 (36). These observations are supported by Hanif et al, who noted, in colorectal cancer cell lines, that growth inhibition was not associated with prostaglandin inhibition (27). Similar findings have been noted with sulindac sulfone in murine models of mammary carcinoma (37). The uncoupling of prostaglandin levels from carcinogenesis raises the possibility that other biochemical effects of NSAIDs are important. Sulindac has been shown to increase apoptosis without changing the proliferative rate as measured by immunohistoche mistry for proliferating cell nuclear antigen (35). Therefore, NSAIDs may have regulatory effects on the apoptotic pathway independent of prostaglandin synthesis.
Our results suggest that future work evaluating an array of chemoprotective compounds should include an analysis of prostaglandin levels to determine if the semetabolites are affected in unpredicted ways. Long-term studies are also needed to correlate the development of human colorectal neoplasia with mucosal prostaglandin levels on a longitudinal basis in patients randomized to prostaglandin inhibitory drugs and placebo.
ACKNOWLEDGMENTS
We thank Linda M. Welch for secretarial support and Jeffery Floyd for laboratory assistance.
Supported by the Clayton Fund and NIH grants CA62924, CA53801, CA63721.
REFERENCES
- 1.Giardiello FM, Offerhaus GJA, DuBois RN. Role of nonsteroidal drugs in colon cancer prevention. Eur J Cancer. 1995;31A:1071–1076. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- 2.Hial V, Demello MC, Horakova Z, Beaven MA. Antiproliferative activity of antiinflammatory drugs in two mammalian cell culture cell lines. J Pharmacol Exp Ther. 1977;202:446–454. [PubMed] [Google Scholar]
- 3.Bayer BM, Beaven MA. Evidence that indomethacin reversibly inhibits cell growth in the G1 phase of the cell cycle. Biochem Pharmacol. 1979;28:441–443. doi: 10.1016/0006-2952(79)90112-6. [DOI] [PubMed] [Google Scholar]
- 4.Bayer BM, Kruth HS, Vaughan M, Beaven MA. Arrest of cultured cells in the G1 phase of the cell cycle by indomethacin. Pharmacol Exp Ther. 1979;210:106–111. [PubMed] [Google Scholar]
- 5.Pollard M, Luckert PH. Indomethacin treatment of rats with dimethylhydrazine-induced intestinal tumors. Cancer Treat Rep. 1980;64:1323–1327. [PubMed] [Google Scholar]
- 6.Moorghen M, Ince P, Finney KJ, Sunter JP, Appleton DR, Watson AJ. A protective effect of sulindac against chemically-induced primary colonic tumors in mice. J Pathol. 1988;156:341–347. doi: 10.1002/path.1711560411. [DOI] [PubMed] [Google Scholar]
- 7.Skinner SA, Penny AG, O'Brien PE. Sulindac inhibits the rate of growth and development of colon tumors in the rat. Gastroenterology. 1990;100:A403. doi: 10.1001/archsurg.1991.01410330048007. [DOI] [PubMed] [Google Scholar]
- 8.Reddy BS, Rao CV, Rivenson A, Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993;14:1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby RF, Marshall, Newton MA, Novakovic K, Tutsch K, Cole CE, Lubet RA, Kellof GH, Verma A, Moser AR, Dove WF. Chemoprevention of spontaneous intestinal adenomas in APC Min mouse model by the nonsteroidal antiinflammatory drug piroxicam. Cancer Res. 1996;56:710–715. [PubMed] [Google Scholar]
- 10.Thun M, Namboodiri M, Heath C. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 13.Rigau J, Pique JM, Rubio E, Planas R, Tarrech JM, Bordas J. Effects of long-term sulindac therapy on colonic polyposis. Ann Intern Med. 1991;115:952–954. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- 14.Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 15.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJA. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;398:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 16.Nugent KP, Farmer KCR, Spigelman AD, Williams DB, Phillips RKS. Randomized controlled clinical trial of sulindac on intestinal polyposis in FAP. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 17.Honn KV, Bockmann RS, Marnett LJ. Prostaglandin and cancer a review of tumor initiation through tumor metastasis. Prostaglandin. 1981;21:833–864. doi: 10.1016/0090-6980(81)90240-9. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe BM. Prostaglandin and cancer: An update. Prostaglandin. 1974;6:453–461. doi: 10.1016/s0090-6980(74)80055-9. [DOI] [PubMed] [Google Scholar]
- 19.Nariswa T, Kusaka H, Yamazaki Y, Takahashi M, Koyama H, Koyama K, et al. Relationship between blood plasma prostaglandin E2 and liver lung metastases in colorectal cancer. Dis Colon Rectum. 1990;33:840–845. doi: 10.1007/BF02051919. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe BM, Parker CW, Philpott GW. Immunochemical measurement of prostaglandin or prostaglandin-like activity from normal and neoplastic cultured tissue. Surg Forum. 1971;22:90–92. [PubMed] [Google Scholar]
- 21.Bennett A, Del Tacca M, Stamford IF, Zebro T. Prostaglandin from tumours of human large bowel. Br J Cancer. 1977;35:881–884. doi: 10.1038/bjc.1977.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanka M, Nazkazawa S, Koike M. Investigation of endogenous prostaglandin in DMH-induced colonic cancer in rats. J Gastroenterol. 1985;82:592–598. [PubMed] [Google Scholar]
- 23.Yamaguchi A, Ishida T, Nishimura G, Katoh M, Miyazaki I. Investigation of colonic prostaglandin in carcinogenesis in the rat colon. Dis Colon Rectum. 1991;34:572–576. doi: 10.1007/BF02049897. [DOI] [PubMed] [Google Scholar]
- 24.Pugh S, Thomas GAO. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earnest DL, Hixson LJ, Finley PR, Backwell GG, Einspahr J, Emerson SS, Alberts DS. Arachidonic acid cascade inhibitors in chemoprevention of human colonic cancer: Preliminary studies. In: Wattenberg L, Lipkin M, Boone CW, Kelloff GJ, editors. Cancer Chemoprevention. CRC Press; Boca Raton, Florida: 1992. pp. 165–180. [Google Scholar]
- 26.Ahnen D, Alberts D, Hixson L, Paranka N, Burt R, Panukcu R. The sulfone metabolite of sulindac (FGN-1) inhibits rat colonic carcinogenesis but does not inhibit either prostaglandin synthesis or colonic proliferation. Gastroenterology. 1995;108:A444. [Google Scholar]
- 27.Hanif R, Pittas A, Feng Y, Koutsos M, Shiff S, Staiano-Coico L, Rigas B. NSAIDs inhibit the growth of colon cancer cell lines by a prostaglandin independent pathway. Gastroenterology. 1995;108:A478. [Google Scholar]
- 28.Winde G, Schmid KW, Schlegel W, Fischer R, Osswald H, Bunte H. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment. Dis Colon Rectum. 1995;38:813–830. doi: 10.1007/BF02049838. [DOI] [PubMed] [Google Scholar]
- 29.Nugent KP, Spigelman AD, Phillips RKS. Tissue prostaglandin levels in familial adenomatous polyposis patients treated with sulindac. Dis Colon Rectum. 1996:659–662. doi: 10.1007/BF02056946. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard WC, Litterst CL, Liu MC, Bleecker ER, Eggleston JC, McLemore TL, Boyd MR. Profiling of prostaglandin bio-synthesis in biopsy fragments of human lung carcinoma and human lung tissues by capillary gas chromatography–negative ion chemical ionization mass spectrometry. Prostaglandins. 1986;32:889–906. doi: 10.1016/0090-6980(86)90097-3. [DOI] [PubMed] [Google Scholar]
- 31.Liu MC, Bleecker ER, Proud D, Mclemore TL, Hubbard WC. Profiling of bisenoic prostaglandins and thromboxane B2 in bronchoalveolar fluid from the lower respiratory tract of human subjects by combined capillary gas chromatography–mass spectrometry. Prostaglandins. 1988;35:69–81. doi: 10.1016/0090-6980(88)90275-4. [DOI] [PubMed] [Google Scholar]
- 32.Finley PR, Bogert CL, Alberts DS, Einspahr J, Earnest DL, Blackwell G, Girodias K. Measurement of prostaglandin E2 in rectal mucosa in human subjects: A method study. Cancer Epidemiol Biomarkers Prev. 1995;4:239–244. [PubMed] [Google Scholar]
- 33.Thorson AG, Lynch HT, Smyrk TC. Rectal cancer in FAP patient after sulindac. Lancet. 1994;343:180. doi: 10.1016/s0140-6736(94)90974-1. [DOI] [PubMed] [Google Scholar]
- 34.Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994;107:854–857. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 35.Pasricha PJ, Bedi A, O'Connor K, Rashid A, Akhtar AJ, Zahurak ML, Piantadosi S, Hamilton SR, Giardiello FM. The effects of sulindac on colorectal proliferation and apoptosis in familial adenomatous polyposis. Gastroenterology. 1996;109:994–998. doi: 10.1016/0016-5085(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 36.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, Bogert C, Guillen JM, Brendel K, Gross PH, Sperl G, Ritchie J, Burt RW, Ellsworth L, Ahnen DJ, Pamukeu R. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 37.Thompson HJ, Jiang C, Lu JX, Menta RG, Piazza GA, Paranka NS, Panukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits Mammary carcinogenesis. Cancer Research. 1997;57:267–271. [PubMed] [Google Scholar]