Abstract
Mounting evidence suggests that parallels between normal stem cell biology and cancer biology may provide new targets for cancer therapy. Prospective identification and isolation of cancer initiating cells from solid tumors has promoted the descriptive and functional identification of these cells allowing for characterization of their response to contemporary cancer therapies including chemotherapy and radiation. In clinical radiation therapy, the failure to clinically eradicate all tumor cells (e.g. a lack of response, partial response or non-permanent complete response by imaging) is considered a treatment failure. As such, biologists have explored the characteristics of the small population of clonogenic cancer cells that can survive and are capable of re-populating the tumor after sub-curative therapy. Herein, we discuss the convergence of these clonogenic studies with contemporary radiosensitivity studies that employ cell surface markers to identify cancer initiating cells. Implications for and uncertainties regarding incorporation of these concepts into the practice of modern radiation oncology are discussed.
Keywords: cancer initiating cells, stem cells, radiobiology, γH2AX, DNA repair, Comet Assay, CD133, flow cytometry, radiocurability, radiation toxicity, clonogens
I. Introduction
A stem cell is a single cell from which all other committed cells derive. It continually renews itself thereby retaining unlimited replicative capacity in an undifferentiated state. It has been proposed (and heatedly-debated) that cancers may arise from stem cells either through the misregulation of a normal solid organ stem cell or through the de-differentiation and acquisition of stem cell properties of a terminally differentiated cancer cell 1. The great hope in the burgeoning field of cancer stem cells is that a greater understanding of the factors that promote stem cell survival will lead to new and highly-targeted therapies with increased efficacy.
For the purposes of this review, we will refer to pluripotent cancer cells capable of recapitulating the tumor from which they were derived as “cancer-initiating cells” (CICs), self-renewing single pluripotent tumor cells as cancer stem cells, and single pluripotent, self-renewing normal cells as normal stem cells. Herein, we address the hypothesis that CICs are important cells that mediate recurrence following sub-curative therapies and that existing cancer therapies have not been always been directed against this specific population.
II. Pre-Clinical and Clinical Assays for Clonogenic Survival and Relation Local Tumor Control
Ionizing radiation (IR) has been described as an ideal cytotoxic agent to study normal stem cell biology relating to tissue regenerative responses which offset radiation toxicity 2. Following irradiation, repopulation of normal stem cells is required to drive the maturation of functional cells in order to recreate a well orchestrated, cell-tissue hierarchy and resumption of normal tissue function(s). This has been most studied in the irradiated intestine whereby surviving normal stem cells in gut crypts repopulate the length of intestinal villi with a mature cell population to renew the ability for the gut to perform nutrient uptake. The stem cells which survive can be quantitated using an in vivo crypt cell clonogenic assay in which the crypt cell survival is directly related to dose 2.
Similarly, the use of experimental radiotherapy protocols using human tumor xenografts or spontaneous murine tumors allows for the interrogation of CIC biology in relation to local tumor radiocurability. In clinical radiotherapy studies, a partial response of the gross tumor (e.g. volume reduction based on imaging as might be achieved with sequential chemotherapy) is defined as a local failure, even if it represents the maximal effect of treatment. This is because the failure of radiotherapy (and surgery) to eradicate all cells capable of re-growing the tumor will lead to recurrence due to cancer cell repopulation. Curative external beam radiotherapy must therefore use sufficient total doses to sterilize all cells capable of repopulation to provide permanent complete local control of the tumor. This total dose is typically delivered as a series of fractionated daily treatments over a period of 6-8 weeks. Normal and tumor tissue responses to IR are governed by the 5 “R’s of Radiotherapy”: radiosensitivity, repair, re-oxygenation, redistribution and repopulation. In the next sections we will endeavor to merge these concepts with the radiocurability and radioresponse of cancer-initiating cells (CIC) and normal stem cells, respectively.
(i) Clonogens
Radiation biologists have studied the cells capable of ongoing replicative capacity in vitro after IR for decades as clonogens: cells capable of generating a colony of greater than 50 cells (as a measure of unlimited proliferation resulting in a minimum of 5 cell doublings) from a single cell. Since radiation therapy generally leads to cell killing through mitotic catastrophe rather than apoptosis in epithelial tumors, it is sometimes necessary to wait until at least 4 or more mitoses to know if a clonogenic cell has survived. Repopulation of the tumor during or after therapy is therefore due to these surviving cells which are presumed to be represented in vitro by clonogens (discussed below). Repopulation of normal tissues also occurs over the same period due to the expansion of normal stem cell populations (e.g. hematopoietic cells and gut cells) to protect against permanent loss of normal tissue function.
(ii) Clonogenic Assays and Clinical Correlates
Regardless of whether the cell of origin of a clonogenic colony is a stem/progenitor cell or a differentiated cell, the concept that a small sub-population of tumor cells (e.g. 0.01-1% of the cells in a tumor volume) limits the effectiveness of exponential cell killing during fractionated radiotherapy has had a dramatic impact on the practice of radiation oncology. Using mathematical calculations relating to tissue repair, clinical experience data, and based on the concept of equal levels of cell kill per radiotherapy fraction, the total dose and dose per fraction for given biologic effects have been determined to improve tumor control within the setting of Phase III randomized trials 3-5.
Furthermore, clonogenic assays ex vivo based on pre-treatment patient biopsies from cervix and head and neck cancers were found to be in vitro predictors of local control. For most tissues, intrinsic radiosensitivity can be summarized by the relative survival following 2 Gy (SF2) within the lower-dose shoulder region of the clonogenic survival curve 6. However, based on available preclinical and clinical data, the clonogenic radiation survival of tumor cells ex vivo may correlate with tumor radiocurability and clonogen survival in vivo following fractionated radiotherapy treatment (see below; 7-9 reviewed in 10).
(iii) Clonogenic Assays and Stemness
The quantitation of clonogenic radiation survival in tissue culture can be accomplished using ex vivo and in vitro clonogenic assays as single cells or spheroid cultures (see Figure 1, review 10). The in situ quantitation of CIC requires TCD50 assays in vivo (i.e. radiotherapy dose required for 50% local tumor control) in spontaneous murine or xenograft models; the latter models can be used to study both the genetic and microenvironmental determinants of CIC radiation survival 10. An important unanswered question for radiobiologists today is to what extent clonogens or clonogenic colonies represent cancer stem cells or CICs.
Figure 1.
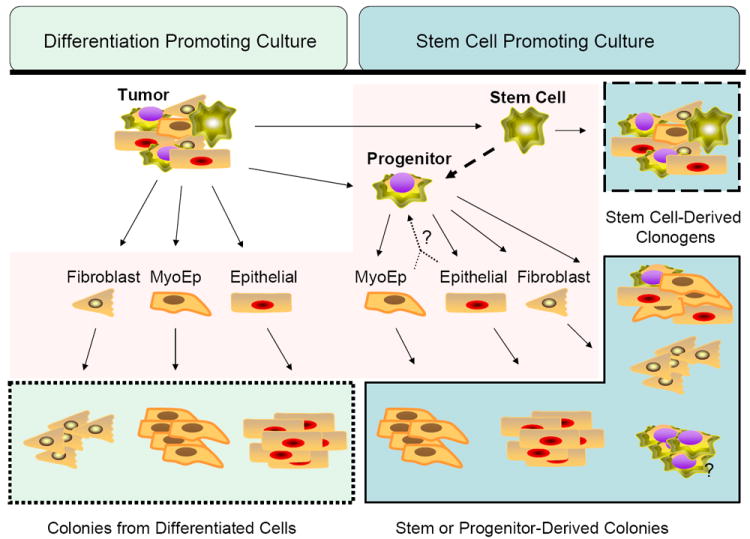
Potential value of incorporating stem cell promoting culture into clonogenic assays. Clonogenic assays are performed by digesting tumors or trypsinizing cell lines to isolate single cells. In this example, the possible single cells (multipotent and terminally differentiated) are highlighted in the center area (shaded light pink). Possible clonogenic colonies derived from each type of single cell are highlighted in blue boxes. On the left are in vitro colonies that would be generated from cells surviving radiation if the culture conditions (standard monolayer) did not promote the survival or expansion of the stem or progenitor cells. In this case, all colonies would represent differentiated cells capable of multiple mitoses after plating/radiation. This would provide no information about the radioresistance of the stem/progenitor population. On the right are colonies that would be generated in vitro from cells surviving radiation if the culture conditions (3D serum free growth factor enriched) promote the survival or expansion of the stem or progenitor cells and differentiated cells. In this case, colonies would represent differentiated cells capable of multiple mitoses after plating/radiation and colonies from the stem/progenitor population.
Based on this model, measuring the effect of radiation on the stem/progenitor population would be possible only in culture that promotes stem/progenitor cells and not the differentiated cells.
In a merging of the concepts of clonogenicity and stemness, there is pre-clinical data to support that the number of clonogenic cells and/or CD133+ cells within a tumor population correlates to the ability to form brain tumors11. Similarly, the number of clonogens determines the dose at which local control is achieved by fractionated radiotherapy based on TCD50 assays 7,10,12. An important concept is that the SF2 clonogenic survival values derived from patient biopsies varies between patients and even within a given tumor histology 6,9,13,14. It follows that if the number and radiosensitivity of clonogenic tumor cells tested ex vivo reflects the number and radiosensitivity of cancer-initiating cells, then there is also heterogeneity in radiosensitivity and colony forming ability within CIC populations. This argues against the simplistic concept that CIC populations are uniformly radioresistant and instead suggests that there is further complexity and heterogeneity in CIC radiosensitivity. The study of CIC markers and clonogenic survival ex vivo on a patient-by-patient basis and correlation of these findings to local control following radical radiotherapy is required to resolve whether CIC and tumor clonogens represent the same entity and can predict radiotherapy response.
In consolidating simultaneous approaches to stemness and clonogenicity, Dontu et al have proposed a 3D culture system to propagate cells capable of multi-lineage differentiation and self renewal 15. Spheres generated from normal mammary gland or tumor cells under these conditions have been termed mammospheres or tumorspheres respectively. Adapting this culture technique one could assess the number of tumorspheres remaining after irradiation ex vivo to predict response similar to the ex vivo soft agar-based assays (i.e. Courtenay-Mills assays) by West and colleagues which predicted cervix cancer radiocurability 9. For example, the number of spheres may reflect the number of self-renewing cells while the number of cells in the spheres reflects the self-renewing capacity of each of those cells 15,16. In this type of assay, one can track clonogenic survival on the basis of specific CIC and normal stem cell biomarkers, and incorporation of similar approaches may be a necessary modern approach to understand stem cell radiobiology (Figure 1). It could also drive the discovery of potential novel targets for the sensitization or protection of irradiated malignant and normal tissues, respectively.
So, are all cancer stem cells clonogens and/or vice versa? The difficulty is proving that the single cell that formed a colony was, or was not, a cancer or normal stem cell in the setting of imperfect stem cell biomarkers that largely fail to isolate pure CICs at the single cell level. It is therefore difficult to measure simultaneously all of the qualities of both “stemness” and “clonogenicity”. The development of a clonogenic colony from a stem cell may well include the production of differentiated cells leading to a heterogeneous group of cells from which the cell of origin and its label “stem cell” may not be easily confirmed. Similarly, the assays one might employ to show the initial cell was a stem cell do not allow for the functional study of clonogenicity. In the field of quantum physics it is accepted that one cannot know both the position and the momentum of an atom at the same time since measuring one makes the other uncertain (the “Heisenberg Uncertainty Principle”). It may be said given the tools currently available, that the effect of measuring stemness may make quantification of its radioresistance uncertain and that measuring radioresistance may similarly make measuring stemness uncertain.
How then should the question “are cancer stem cells resistant to radiation” be answered? How much uncertainty is tolerable and to what degree can we accept a quantum state in biology? These are questions that radiobiologists and radiation oncologists must address together in order to predict therapeutic efficacy with pre-clinical biomarkers and assays. We will now review the cell biology and cell biomarkers linked to normal and cancer stem cells that could be used for such translational studies.
III. Differential Radiosensitivity of CICs and Normal Stem Cell Subpopulations
(i) Normal stem cell radiosensitivity
The evidence for the existence of a normal murine mammary gland stem cell was bolstered by the demonstration that a single prospectively-identified cell (marker lineage-CD24+ and CD29+) from the mammary gland could be transplanted into the mammary fatpad of a mouse (after the native mammary epithelia had been removed) and a new functional mammary gland could be regenerated only by cells expressing this phenotype 17. Interestingly, colonies in Matrigel could be produced from CD24+ cells with low or high CD29 expression. In a companion study using lin-CD49f+CD24+ cell surface markers to identify mammary gland repopulating cells, the colony forming unit (assayed in differentiation-promoting Matrigel) was demonstrated to be distinct from the repopulating unit based on the level of CD24 expression18. These data suggest that different cell populations are responsible for total, functional glandular repopulation versus formation of colonies in Matrigel or on plastic. If one considers the formation of a functional mammary gland a stem cell assay, it could then be said that a stem cell and a clonogenic cell are indeed different. A stem cell may be a clonogenic cell, but a clonogenic cell may not be a stem cell and may require unique environmental factors that are not provided for within in vitro assays.
Additional studies of the mouse mammary gland have confirmed the repopulating ability in additional populations of cells selected for expression of Stem Cell Antigen-1 (Sca1, 19,20) within a “side population”: the latter is a flow cytometry based assay to select for stemness in cells that extrude Hoechst 33342 via a cell membrane pump 21-23. It can be concluded that these populations are enriched for mammary gland repopulating units. Woodward et al have shown that therapeutic doses of irradiation of normal mammary epithelial cells enriched for side population and Sca1+ cells, but not lin-CD24+CD29+ cells. This suggests that the Sca1+ and side population cells are resistant to radiation as selective enrichment following irradiation was a function of cell death of unmarked populations and these were observed to decrease with increasing doses of radiation (and to exhibit increased foci of DNA damage; see below) when compared to Sca1+ cells 24.
(ii) CIC radiosensitivity
In human solid tumors, evidence for radiation resistance of stem or progenitor cells has been reported for breast cancer 24,25, glioma 11,26, medulloblastoma 27, and atypical teratoid/rhabdoid tumors 28. MCF-7 cells and MDA-231 cells grown as progenitor promoting spheres 15 were more resistant to radiation than cells grown in monolayers 25 and demonstrated increased DNA damage foci (assayed as γH2AX intensity) in monolayer cells compared to sphere cultures 25. The work by Phillips et al 25 includes an important correlation between increased percentage of cells reported to be tumor-initiating based on xenograft studies from metastatic breast cancer cells (lin-CD44+CD24lo, 29) and clonogenicity given that surviving fraction was correlated to the percentage of tumor-initiating cells. A potential caveat to these data is the effect of sphere structure itself on clonogenicity. Independent of progenitor propagation, it has been observed in 3-dimensional cultures in soft agar that survival fraction is increased 30 and that radiation sensitivity of 3D organoid cultures is dependent on beta1-integrin, CD29 31. Using the breast cancer cell line MCF-7 side population cells and CD24+CD29+ cell have also been shown to be enriched by radiation in vitro 24. Subsequent work in the p53 heterozygous murine tumor model has demonstrated CD24hiCD29hi represents the CIC population in these tumors and confers the ability to form mammospheres. Clonogenic assays comparing mammospheres from these tumors to the same cells grown in monolayer culture with serum demonstrates complete radioresistance of the mammospheres up to 6 Gy 32.
While radiation data specific to stem/progenitor cells from human primary breast cancers have not been reported, breast tumor biopsies taken after neoadjuvant chemotherapy have been reported to contain higher percentages of lin-CD44+CD24lo cells than paired biopsies taken before chemotherapy (0.5 vs. 5.9% 16). Importantly, these authors also confirmed the work of Al-Hajj et al (from metastatic breast cancer cells) 29 demonstrating that serial xenograft transplant potential resides selectively in the lin-CD44+CD24lo cells 16.
In brain tumors, stem/progenitor cells have been isolated from normal mammalian brains by a neurosphere assay 33,34. In a study of 14 pediatric brain tumors Singh et al 35 utilized the neurosphere assay to demonstrated that the population of tumor precursor cells capable of self-renewal, proliferation, and differentiation were prospectively identified by the expression of CD133 (AC133, Prominin-1). CD133- cells neither formed neurospheres nor expressed an alternative neural primitive cell marker, nestin, and in contrast to CD133+ cells they were not capable of initiating brain tumors in vivo in NOD/SCID mice35.
From primary human gliomas, CD133+ cells were reported to be enriched for neurosphere formation and to initiate xenografts from as few as 500 cells 11. Bao et al reported that irradiation of intracranial D456MG glioma xenografts (in vivo) and human glioma tumor (ex vivo) increased the percentage of CD133+ cells determined by flow cytometry and that increasing the fraction of CD133+ cells injected intracranially decreased the time to neurological symptoms in mice and the gross appearance of tumor at necropsy 11. Although baseline colony formation appeared to be equivalent between CD133+ and CD133- cells, the authors presented cell staining data after 5 Gy and suggested that surviving colonies may be reduced in CD133- cells when compared to CD133+ cells. However, quantitative clonogenic survival data was not provided in this study and it is difficult to evaluate in some of the images if there may be a greater difference in size of colonies rather than the resulting number of colonies.
Examination of stem/progenitor characteristics of CD133+ cells has also been undertaken in clinical samples of atypical teratoid/rhabdoid tumor (AT/RT), a malignancy of the central nervous system that occurs in infancy and childhood28. CD133+ cells were isolated from nine patients with AT/RT. CD133+ cells had higher proliferative rates and in vitro invasiveness, and exhibited greater clonogenicity in soft agar compared to CD133- cells. Xenograft tumors were serially transplanted from as few as 300 CD133+ cells compared to formation from 100,000 implanted CD133- cells. CD133+ cells formed neurospheres while CD133- cells did not, and CD133+ cells were capable of tri-lineage differentiation. In vitro radiation studies revealed higher survival of CD133+ cells in standard clonogenic assays (survival curves 0 -10Gy), and xenografts after in vivo irradiation were volumetrically larger from mice injected with CD133+ cells than CD133- cells. In fact, there was no significant difference in the growth of irradiated CD133+ tumors than in unirradiated mice bearing tumors from injections of CD133+ cells. In five patients who relapsed after chemotherapy and radiation, the percentage of CD133+ cells was significantly higher in the relapse biopsy (approximately 10% vs. 50%, numeric data not provided 28). Taken together, these studies provide strong clinical data that in this disease, CD133 is a marker for radioresistant cells.
In the intestine, musashi-1 encodes a RNA binding protein involved in asymmetric cell division of stem cells. It is a positive regulator of Notch signaling through inhibition of numb, a Notch inhibitor. In the APCmin/+ colorectal cancer mouse models, musashi-1 was upregulated in tumors compared to normal tissues 36. In a study of human colon xenografts (HCT116 cells implanted subcutaneously), siRNA knockdown of musashi-1 led to increased mitotic catastrophe in musashi-negative tumor cells treated with radiation compared to controls 37. This was assayed 24 hours after radiation through co-immuno-staining with phospho-histoneH3 (a marker of mitosis) and Tunel staining (a marker of apoptosis). Approximately 20-25% of cells treated with siRNA for musashi-1 underwent mitotic catastrophe assayed by co-immuno-fluorescence. These data suggest that musashi-1 expression may protect colon tumor cells from radiation.
The tissue and tumor microenvironment are likely critical mediators of the effects of radiation and few studies exist which examine the radiation resistance of putative stem/progenitor markers cells in the context of their environment. Using three different sonic hedgehog mediated wild-type p53 mouse models of the relatively radiosensitive pediatric brain tumor, medulloblastoma, Hambardzumyan et al describe that cell death after radiation occurs in the proliferating, PCNA+, Nestin- cells of the tumor bulk27. A subset of tumor bulk cells with a low proliferative rate survived in areas of extensive nodularity, and the remaining surviving cells after 2 Gy resided in the perivascular niche. The cells in the perivascular niche express PCNA prior to radiation suggesting their resistance is not a function of quiescence (a characteristic of hematopoetic normal and malignant stem cells which has not been demonstrated in solid tumor stem cell biology). These cells were also GFAP, Nestin, and Olig2 positive and appear to arrest post-radiation. Examination of proliferation and apoptosis markers at numerous time points following 2 Gy revealed that cells are completely arrested by 12h post-radiation and re-enter the cell cycle after 72 hours. These in vivo data are compelling in that they demonstrate that the increase in progenitor marker-positive cells is not a function of proliferation but rather increased survival of marker-positive cells explicitly in the perivascular niche in this model.
Demonstration of the relative radiation resistance of putative stem/progenitor-marked cells in vitro argues that the cells themselves may have inherent radioresistance. There is less data on the role the microenvironment plays in mediating radioresistance. For example, to date it has not been proven whether a stem cell or CIC niche may protect cells from radiation cell death and/or whether migrating cells or growth factors may also play a role in stem cell or CIC radiosensitivity. Mesenchymal stem cells are multipotent bone marrow-derived stem-like cells that migrate to and proliferate in response to tumors and inflammation. In a xenograft model of bilateral 4T1 breast carcinomas subjected to unilateral irradiation (2 Gy), homing of post-irradiation injected mesenchymal stem cells was increased 34% in irradiated tumors38. Cytokines secreted into the media of irradiated 4T1 cells induced the migration of mesenchymal stem cells in a transwell migration assay in a dose dependent fashion. The effect of mesenchymal stem cells on resistance has not been fully explored, but is required to understand the full nature of the microenviroment for the response of stem cells to cancer therapy.
IV. DNA Damage Sensing and Repair in CICs and Normal Stem Cells
Fractionated radiotherapy maximizes cell killing in tumors by allowing for increased sublethal damage cellular repair (in the SF2 region) in slowly proliferating tissues (e.g. late-reacting tissues such as kidney or neural tissues) which have decreased capacity for regeneration. This repair is increased over that of rapidly proliferating tumor cells during therapy. Normal tissue cells with rapid proliferation (e.g. gut, skin) will also be killed during radiotherapy, but these have a high capacity for regeneration. Additionally, at the molecular level the capacity for DNA repair can be measured using DNA rejoining and DNA repair foci assays. Taken together, the data available suggest that the relative DNA repair capacity of tumor and normal tissue clonogens within cell lines or tissues derived from radiotherapy patients can be correlated with clonogenic survival in vitro 39-41.
It is useful to briefly review the molecular response of cells to ionizing radiation which results in the production of a variety of DNA lesions including lethal DNA double-strand breaks (DNA-dsbs). These DNA-dsbs need to be repaired to prevent cellular apoptosis, mitotic catastrophe and/or terminal growth arrest/senescence 42,43. In response to DNA breaks, human cells undergo cell cycle checkpoints driven by the ATM-CHK2-p53 and ATR-CHK1 pathways in the G1, S and G2 phases of the cell cycle to allow for the repair of DNA damage 44. IR-induced DSBs are initially sensed by the MRE11 protein which activates ATM, DNA-PKcs and ATR kinase activity and the phosphorylation of the histone, γH2AX within chromatin regions distal to the DNA-dsb 44. Gamma-H2AX foci within the nucleus can be used as a sensitive measure of DNA-dsbs within single cells and this measure is useful for interrogating DNA damage and repair in rare CIC or stem cell populations 43. Following initial DNA-dsb sensing, a DNA-dsb is repaired primarily through two pathways: the S- and G2-phase specific homologous recombination (HR) pathway or the non-homologous end joining (NHEJ) pathway which predominates in G1 phase 42.
The sensing and repair of DNA damage in normal stem cell and CIC populations relative to cells without pluripotent potential is of great interest given the potential role of DNA repair in cellular carcinogenesis and therapeutic response 45. For example, the HR-related, BRCA1 DNA repair protein is required for the differentiation of ER-negative stem progenitor cells to functional ER-positive luminal cells in breast tissues. The authors of this paper suggested that a lack of BRCA1 leads to increased carcinogenesis and ultimately breast cancer due to a lack of protection from genetic instability within stem cell populations 46. Similar observations were made within hematopoetic stem cell populations derived from DNA-repair deficient mice in that stem cell function and stability during the process of aging required intact DNA repair activity 47-49.
Additionally, studies using a variety of cancer cell lines have shown that hypoxia can alter the expression and function of DNA-dsb and mismatch repair proteins leading to altered radio- and chemosensitivity and tumor progression50 (reviewed in 51). The proportion of stem cells may be elevated within hypoxic niches (see below). Importantly, it was recently reported that hypoxia down-regulates mismatch repair and increases genetic instability in murine and human stem cells suggesting that the tumor microenviroment might alter DNA repair in normal stem cells 52.
Inherent alterations in DNA damage sensing and repair was reported by Bao et al who observed radioresistance in glioma CICs associated with a hyperactive DNA damage response (e.g. increased CHK1 and CHK2 phosphorylation in CD133+ cells when compared to CD133- cells) 11. Using the COMET assay and scoring γH2AX foci, the authors observed increased residual DNA double strand breaks (DNA-dsbs) in CD133- cells compared to CD133+ cells; however, the data for absolute numbers of DNA-dsbs was not shown (only relative DNA-dsbs were shown) and tumor cell clonogenic assays were not published for the same cells in order to directly correlate clonogenicty and the altered DNA damage response in glioma CICs. Whether CICs have altered initial recognition of DNA-dsbs is not known. Future studies are needed to rigorously determine initial and residual DNA-dsbs in relation to clonogenic survival in CIC populations derived directly from patients. This could then be correlated to radiotherapy outcome within specific tumor sites
There is controversy regarding the capacity of DNA repair in normal stem cells. Woodward et al 24 observed increased numbers of initial γH2AX foci following 2 Gy in Sca1- murine mammary cells when compared to Sca1+ cells, but subsequent repair of the DNA-dsbs and clonogenic survival was not described. Increased clonogenic survival has been observed for human mesenchymal stem cells in which the HR and NHEJ pathways and ATM activation was intact, but in contrast to the above glioma CIC studies, these repair pathways were not hyper-stimulated 53.
Murine ES cells may differ in their DNA-dsb, mismatch and nucleotide excision repair capacity when compared to differentiated murine cells (reviewed by 54). Two recent reports using human ES cells for DNA-dsb repair studies differ in their conclusions. Maynard et al 55 reported increased expression of NHEJ and HR genes and more efficient repair of damage following 3 Gy using the Comet assay when compared to malignant cell lines. However, an extensive study by Banuelos and colleagues 56 using COMET and γH2AX assays showed that murine ES cells have a DNA-dsb rejoining defect associated with altered DNA-PKcs kinase activity. In this study, the capacity for DNA-dsb repair in human ES cells remained intact. More studies are required in human ES and differentiated cells in order to understand the relationship of DNA and cellular repair in normal stem cells and acute and late normal tissue radiotoxicity. Such studies will also determine whether human stem cells respond in a similar manner as murine stem cells during repopulation to determine the late effects following experimental radiotherapy.
The capacity for stem cells to repair radiotherapy induced tissue damage is very exciting and there may be potential for manipulation of neural stem cell niches, either by cell transplantation or appropriate chemokines and growth factor supplementation, to offset late neurotoxicity 57. Furthermore, studies by Coppes and co-workers 56,57 have used murine models to show that salivary gland ablation following experimental radiotherapy could be decreased by mobilization of bone marrow derived stem cells by growth factors (G-CSF) and direct stem cell transplantation (see also article in this issue). If successful in humans, this could potentially offset debilitating xerostomia following head and neck radiotherapy. Such approaches may be able to cross a variety of tissue toxicities given the potential totipotency of embryonic and adult stem cells.
V. Targeting CICs During Clinical Radiotherapy
There is increasing interest in delivering non-homogenous radiotherapy doses to human tumors based on the functional imaging of radioresistant subpopulations. The multi-modality use of PET and functional MRI and CT scanning, along with the use of 4-D image tracking software, afford clinicians the ability to track and adapt therapy to intra-tumoral changes in tumor cell death, proliferation, hypoxia or other biological endpoints 58. However, the resolution of these current imaging technologies may be too low to image single CICs within tumor volumes unless they aggregate within larger biological niches that are more amenable to serial imaging.
Examples of biological niches that could contain an increased fraction of CICs amenable for imaging include hypoxic tumor sub-volumes (imaged by BOLD-MRI or PET-MISO/FAZA) or vascular and perivascular niches (imaging of tumor angiogenesis (Chan et al; 2007; 10,27,59,60). The relative radiosensitivity of CICs may further be affected by tumor oxygenation in that acutely hypoxic cells are 2-3 times more resistant to radiotherapy than oxic cells. Note that the proportion of CD133+ cells in mixed medulloblastoma and glioma populations can increase under hypoxia 59,60. Normally, hypoxic CICs would undergo re-oxygenation during fractionated radiotherapy reducing the fraction of surviving CICs and residual hypoxic volumes potentially containing CIC niches at the end of a radiotherapy protocol could be targeted with increased radiotherapy dose. With the development of CIC biomarkers and increasing resolution of tumor cell imaging (such as MRI techniques to detect single CIC or stem cells; 61), the targeting of CICs during precision radiotherapy, alone or in combination with CIC-specific molecular-targeting agents, may one day be possible.
VI. Discussion
New markers and isolation techniques for the prospective identification and culture of CICs as potential cancer stem cells have re-invigorated the interest in solid cancer stem cell biology. However, many assumptions from the hematopoetic stem cell literature -including the quiescence of cancer stem cells and the characterization of the cancer stem cell niche - have been transferred, but not demonstrated, for solid tumors. Nonetheless, the data from radiobiologic studies contribute enormously to our understanding of cancer stem cell biology. It is clear that single and fractionated radiotherapy targets clonogenic cells, and possibly also decreases the bulk of more differentiated cells within 3-D tumor or tissue hierarchies. Highly-quantitative radiobiologic studies have almost universally incorporated tumor cure and clonogenic eradication as endpoints, in contrast to more qualitative endpoints that may be solely representative of the treatment of differentiated tumor cells (e.g. time to progression, growth delay and clinical or radiographic response). Presumably, all stem cells may be clonogenic in the right environment with the right stimulus; although the reverse may not be true of all clonogenic cells and a further understanding of this relationship will no doubt drive the development and discovery of surrogate biomarkers predictive of therapeutic efficacy.
While many questions are still outstanding, the case for the radioresistance of CICs is growing. In vitro clonogenic data from primary human AT/RT tumors and primary murine p53+/- mammary tumors demonstrate classic radiation resistance of cells selected based on CICs surrogates (CD133+ and mammosphere formation respectively)28,32. In situ pathologic examination of irradiated murine medulloblastoma models reveals localization of cell death in proliferating tumor bulk away from the vessels with surviving cells localized to the perivasculature and associated with markers of undifferentiated cells27. Progenitor and CIC marked cells are enriched by radiation in normal murine and human cell lines from multiple tumor types11,24,25,62, and successful radiosensitizing of these populations by targeting stem cell survival signaling and DNA repair has been described in preclinical work11,25,36.
While the explosion of new data are exciting, many questions remain unanswered (see Table 1) and significant translational work is needed to best determine which patients to treat with novel agents that could maximize targeted radiosensitization of CICs or radioprotect normal stem cells. Additional efforts to integrate clonogenicity and stem-ness are required to optimize screening assays for targeted cancer stem cell radiosensitizers and normal stem cell radioprotectors. Many patients are clearly cured of cancer with contemporary therapy, and strategies to select patients for clinical trials will be critical to the success of these agents. Ultimately, the success or failure of the clinical use of stem cell biomarkers and targeted agents may determine the verdict on the significance of uncertainty in stem cell biology. Future trials that effectively test unique biomarkers for the stratification of patients into subgroups based on unique stem cell markers will be critical.
Table 1.
Unanswered Questions
|
Figure 2.
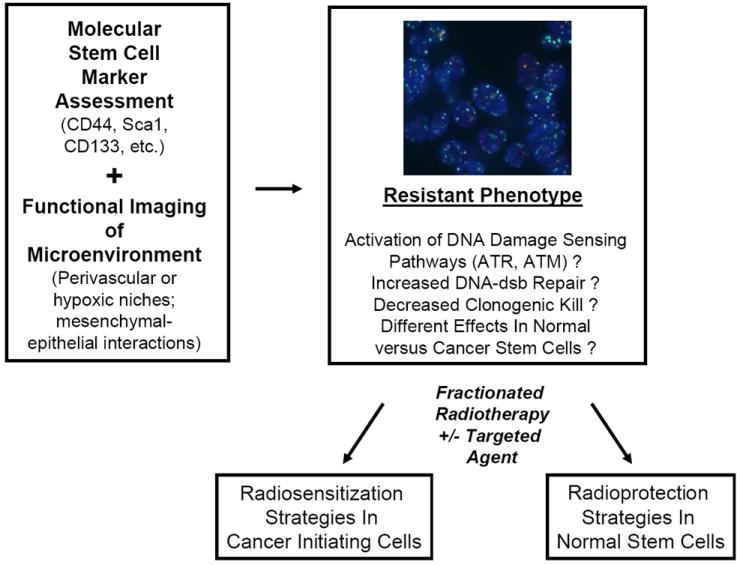
A model for incorporating functional stem cell information into clinical radiotherapy protocols. Marrying biomarkers of CIC and normal stem cells and functional imaging of stem cell niches may allow for the identification and tracking of stem cells and adaptive radiotherapy. Cancer and normal stem cells may have hyperactivated DNA damage sensing and improved DNA repair leading to radioresistance. Relative DNA repair could be tracked using DNA repair foci ex vivo (shown are DU145 prostate cancer cells stained for intranuclear DNA (blue DAPI signal), γH2AX (green foci) and 53BP1 (red foci) at 30 minutes following 2 Gy). In normal tissues, intervention with augmentation of this resistance could lead to radioprotection and decreased acute and late toxicity. In tumor cells, this resistance could be targeted with radiosensitization protocols using CIC-targeted agents. Assays of both CIC identification and radiobiological function should be built into any clinical trial that tests these clinical hypotheses.
Acknowledgments
WW is supported by The State of Texas Grant for Rare and Aggressive Cancer; The National Institute of Health (K12-5611B administered by the University of Texas Health Sciences Center) and the Susan G. Komen Breast Cancer Foundation, KG071287. RGB is supported by operating grants from the Canadian Cancer Society-National Cancer Institute of Canada, the Terry Fox Foundation, The Prostate Cancer Research Foundation of Canada. RGB is a Canadian Cancer Society Research Scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 2.Potten C. Radiation, the ideal cytotoxic agend for studying the cell biology of tissues such as the small intestine. Radiation Research. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 3.Bentzen SM. Steepness of the radiation dose-response curve for dose-per-fraction escalation keeping the number of fractions fixed. Acta Oncol. 2005;44(8):825–8. doi: 10.1080/02841860500374471. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 5.Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25(26):4096–103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 6.Deacon J, Peckham MJ, Steel GG. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother Oncol. 1984;2(4):317–23. doi: 10.1016/s0167-8140(84)80074-2. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RG, Hill RP. Comparison between in vitro radiosensitivity and in vivo radioresponse in murine tumor cell lines. II: In vivo radioresponse following fractionated treatment and in vitro/in vivo correlations. Int J Radiat Oncol Biol Phys. 1990;18(2):331–45. doi: 10.1016/0360-3016(90)90098-5. [DOI] [PubMed] [Google Scholar]
- 8.Geara F, Girinski TA, Chavaudra N, Cosset JM, Dubray B, Brock WA, Malaise EP. Estimation of clonogenic cell fraction in primary cultures derived from human squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 1991;21(3):661–5. doi: 10.1016/0360-3016(91)90684-v. [DOI] [PubMed] [Google Scholar]
- 9.West CM. Invited review: intrinsic radiosensitivity as a predictor of patient response to radiotherapy. Br J Radiol. 1995;68(812):827–37. doi: 10.1259/0007-1285-68-812-827. [DOI] [PubMed] [Google Scholar]
- 10.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8(7):545–54. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 11.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 12.Hill RP, Milas L. The proportion of stem cells in murine tumors. Int J Radiat Oncol Biol Phys. 1989;16(2):513–8. doi: 10.1016/0360-3016(89)90353-2. [DOI] [PubMed] [Google Scholar]
- 13.Taghian A, Suit H, Pardo F, Gioioso D, Tomkinson K, DuBois W, Gerweck L. In vitro intrinsic radiation sensitivity of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1992;23(1):55–62. doi: 10.1016/0360-3016(92)90543-q. [DOI] [PubMed] [Google Scholar]
- 14.Brock WA, Baker FL, Wike JL, Sivon SL, Peters LJ. Cellular radiosensitivity of primary head and neck squamous cell carcinomas and local tumor control. Int J Radiat Oncol Biol Phys. 1990;18(6):1283–6. doi: 10.1016/0360-3016(90)90298-x. [DOI] [PubMed] [Google Scholar]
- 15.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 17.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 18.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 19.Welm B, Behbod F, Goodell MA, Rosen JM. Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 2003;36(Suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245(1):42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 21.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101(3):781–6. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99(19):12339–44. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104(2):618–23. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 26.Kang M, BHur B, Ko M, Kim C, Cha S, Kang S. Potential identity of multi-potential cancer stem-like subpopulation after radiation of cultured brain glioma. BMC Neuroscience. 2008;9(15):1–12. doi: 10.1186/1471-2202-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiou S, Kao C, Chen Y, Chien C, Hung S, Lo J, Chen Y, Ku H, Hsu M, Wong T. Identification of CD133-positie radioresistant cells in atypical teratoid/rhabdoid tumor. PLos One. 2008;3(5):1–13. doi: 10.1371/journal.pone.0002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand RE, Olive PL. Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods Cell Biol. 2001;64:211–33. doi: 10.1016/s0091-679x(01)64015-9. [DOI] [PubMed] [Google Scholar]
- 31.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66(3):1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, Medina D, Tsimelzon A, Hilsenbeck S, Green JE, et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 2008;68(12):4674–82. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds BA, Rietze RL. Neural stem cells and neurospheres - Re-evaluating the relationship. Nature Methods. 2005;2(5):333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 36.Potten C, Booth C, Tudor G, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative instestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 37.Sureban S, May R, George R, Dieckgraefe B, McLeod H, Ramalingam S, Bishnupuri K, Natarajan G, Anant S, Houchen C. Knockdown of RNA binding protein musashi-1 leads to tumor regression in vivo. Gastroenterology. 2008;134:1448–1458. doi: 10.1053/j.gastro.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 38.Klopp A, Spaeth E, Dembinski J, Woodward W, Munshi A, Meyn R, Cox J, Andreeff M, Marini F. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67(24):11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiltie AE, Orton CJ, Ryan AJ, Roberts SA, Marples B, Davidson SE, Hunter RD, Margison GP, West CM, Hendry JH. A correlation between residual DNA double-strand breaks and clonogenic measurements of radiosensitivity in fibroblasts from preradiotherapy cervix cancer patients. Int J Radiat Oncol Biol Phys. 1997;39(5):1137–44. doi: 10.1016/s0360-3016(97)00545-2. [DOI] [PubMed] [Google Scholar]
- 40.Klokov D, MacPhail SM, Banath JP, Byrne JP, Olive PL. Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother Oncol. 2006;80(2):223–9. doi: 10.1016/j.radonc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Zaffaroni N, Orlandi L, Villa R, Bearzatto A, Rofstad EK, Silvestrini R. DNA double-strand break repair and radiation response in human tumour primary cultures. Int J Radiat Biol. 1994;66(3):279–85. doi: 10.1080/09553009414551211. [DOI] [PubMed] [Google Scholar]
- 42.Bristow RG, Ozcelik H, Jalali F, Chan N, Vesprini D. Homologous recombination and prostate cancer: a model for novel DNA repair targets and therapies. Radiother Oncol. 2007;83(3):220–30. doi: 10.1016/j.radonc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Liu SK, Olive PL, Bristow RG. Biomarkers for DNA DSB inhibitors and radiotherapy clinical trials. Cancer Metastasis Rev. 2008;27(3):445–58. doi: 10.1007/s10555-008-9137-8. [DOI] [PubMed] [Google Scholar]
- 44.Kastan MB. DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol Cancer Res. 2008;6(4):517–24. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- 45.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26(17):2839–45. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci U S A. 2008;105(5):1680–5. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niedernhofer LJ. DNA repair is crucial for maintaining hematopoietic stem cell function. DNA Repair (Amst) 2008;7(3):523–9. doi: 10.1016/j.dnarep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447(7145):686–90. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 49.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 50.Chan N, Milosevic M, Bristow RG. Tumor hypoxia, DNA repair and prostate cancer progression: new targets and new therapies. Future Oncol. 2007;3(3):329–41. doi: 10.2217/14796694.3.3.329. [DOI] [PubMed] [Google Scholar]
- 51.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Jimenez FJ, Moreno-Manzano V, Lucas-Dominguez R, Sanchez-Puelles JM. Hypoxia Causes Down-Regulation of Mismatch Repair System and Genomic Instability in Stem Cells. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1016. [DOI] [PubMed] [Google Scholar]
- 53.Chen MF, Lin CT, Chen WC, Yang CT, Chen CC, Liao SK, Liu JM, Lu CH, Lee KD. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys. 2006;66(1):244–53. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 54.Tichy ED, Stambrook PJ. DNA repair in murine embryonic stem cells and differentiated cells. Exp Cell Res. 2008;314(9):1929–36. doi: 10.1016/j.yexcr.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maynard S, Swistikowa AM, Lee JW, Liu Y, Liu ST, DAC A, Rao M, de Souza-Pinto N, Zeng X, Bohr VA. Human Embryonic Stem Cells have Enhanced Repair of Multiple Forms of DNA Damage. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banuelos CA, Banath JP, Macphail SH, Zhao J, Eaves CA, O’Connor MD, Lansdorp PM, Olive PL. Mouse but not human embryonic stem cells are deficient in rejoining of ionizing radiation-induced DNA double-strand breaks. DNA Repair (Amst) 2008;7(9):1471–83. doi: 10.1016/j.dnarep.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Nieder C, Andratschke N, Astner ST. Experimental concepts for toxicity prevention and tissue restoration after central nervous system irradiation. Radiat Oncol. 2007;2:23. doi: 10.1186/1748-717X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verheij M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev. 2008;27(3):471–80. doi: 10.1007/s10555-008-9131-1. [DOI] [PubMed] [Google Scholar]
- 59.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67(1):1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 60.Platet N, Mayol JF, Berger F, Herodin F, Wion D. Fluctuation of the SP/non-SP phenotype in the C6 glioma cell line. FEBS Lett. 2007;581(7):1435–40. doi: 10.1016/j.febslet.2007.02.071. [DOI] [PubMed] [Google Scholar]
- 61.Arbab AS, Janic B, Knight RA, Anderson SA, Pawelczyk E, Rad AM, Read EJ, Pandit SD, Frank JA. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. Faseb J. 2008 doi: 10.1096/fj.07-105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120(Pt 3):468–77. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]


