Abstract
Members of the Src family of nonreceptor protein tyrosine kinases (PTKs) have been implicated in the regulation of cellular excitability and synaptic plasticity. We have investigated the role of these PTKs in in vitro models of epileptiform activity. Spontaneous epileptiform discharges were induced in vitro in the CA3 region of rat hippocampal slices by superfusion with the potassium channel blocker 4-aminopyridine in Mg2+-free medium. In hippocampal slices treated in this fashion, Src kinase activity was increased and the frequency of epileptiform discharges could be greatly reduced by inhibitor of the Src family of PTKs, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), but not by the inactive structural analog 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3). 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine also reduced epileptiform activity induced by either 4-aminopyridine or Mg2+-free medium alone. These observations demonstrate a role for Src family PTKs in the pathophysiology of epilepsy and suggest potential therapeutic targets for antiepileptic therapy.
The epilepsies are heterogeneous disorders characterized by recurrent synchronous discharges of large neuronal populations. Prominent features of the natural history of these pathologies are functional changes leading to the development of areas of low seizure threshold (epileptic foci) and the evolution toward more pervasive and malignant forms. In the present study, we investigated the role of nonreceptor protein tyrosine kinases (PTKs) of the Src family in the generation of spontaneous epileptiform activity in the CA3 region of rat hippocampal slices. Members of this family of PTKs are expressed in the central nervous system and have been implicated in the regulation of glutamate N-methyl-d-aspartate (NMDA) receptors, affecting in turn neural excitability and plasticity (1–3). In fact, phosphorylation of certain NMDA NR2 subunits by Src and the related nonreceptor PTK Fyn has been shown to increase NMDA receptor channel activity (1, 4). Enhanced NMDA responses have been observed in the kindling model of epilepsy (5–8) and in human epilepsy (9). Activation of Src also induces potentiation of non-NMDA glutamate receptor responses in an NMDA- and Ca2+-dependent manner (1). Observations with mice in which Fyn was either genetically deleted or overexpressed also support a role for Src-family PTKs in the induction of kindling (10, 11), a process in which NMDA receptors have been shown to play a critical role (12, 13).
Depletion of extracellular Mg2+ from hippocampal slices results in enhancement of synaptically evoked responses in CA1 and CA3 and the appearance of spontaneous paroxysmal depolarization shifts in both areas (5, 14–16), whereas 4-aminopyridine (4-AP) induces epileptiform activity by enhancing the release of excitatory and inhibitory amino acids (17–19). We induced epileptiform activity in rat hippocampal slices with Mg2+-free medium in the presence of the potassium channel blocker 4-AP, a model of epileptogenesis previously used in neocortical slice preparations (17).
Application of Mg2+-free medium and 4-AP induced robust bursting in the CA3. Src kinase activity was induced in such a model, and spontaneous epileptiform activity could be strongly reduced by the Src-family PTK inhibitor (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) also known as AG 1879 (20). In some experiments, hippocampal slices were treated with either Mg2+-free medium or 4-AP alone. Epileptiform activity induced by either treatment also was reduced by PP2. These observations are likely to be relevant to the pathogenesis of hyperexcitability and possibly contribute to the identification of potential therapeutic targets for antiepileptic therapy.
Materials and Methods
Preparation of Rat Hippocampal Slices.
Hippocampal slices were prepared as described (21). Male Wistar rats (100–150 g) were anaesthetized with ether and decapitated. Their brains were rapidly removed, placed in ice-cold artificial cerebrospinal fluid (aCSF) and gassed with 95% O2, 5% CO2. The composition of aCSF was: 130 mM NaCl, 3.5 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgSO4⋅7 H2O, 2 mM CaCl2⋅2 H2O, 24 mM NaHCO3, and 10 mM glucose, pH 7.4. Transverse hippocampal slices (400 μm) were prepared with the use of a Vibratome (Campden Instruments, Loughborough, U.K.).
Experimental Protocol.
Hippocampal slices were incubated at room temperature for 1 h in aCSF (25°C) before use and then separated into control and experimental groups. We further incubated control slices in aCSF for 3 h while the experimental slices were incubated either in Mg2+-free aCSF containing 4-AP (50 μM) (Mg2+-free aCSF/4-AP) or in either Mg2+-free aCSF or 4-AP alone for 3 h. To test the role of Src-family PTK in epileptiform activity, the specific inhibitor PP2 (10 μM; Calbiochem) was added to the aCSF for 1 h and then to the Mg2+-free aCSF/4-AP for an additional 2–3 h during the preincubation period. After preincubation, hippocampal slices were transferred to an interface recording chamber for 15 min and then completely submerged and continuously superfused with warm (33–34°C) gassed aCSF at a constant flow rate of 2–4 ml/min. Slices preincubated in Mg2+-free aCSF/4-AP containing PP2, were perfused with the same solution and then washout of 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3). In some experiments, PP2 was added only during the perfusion (see Fig. 3). PP3, an inactive structural analogue of PP2, was used as a negative control (Calbiochem).
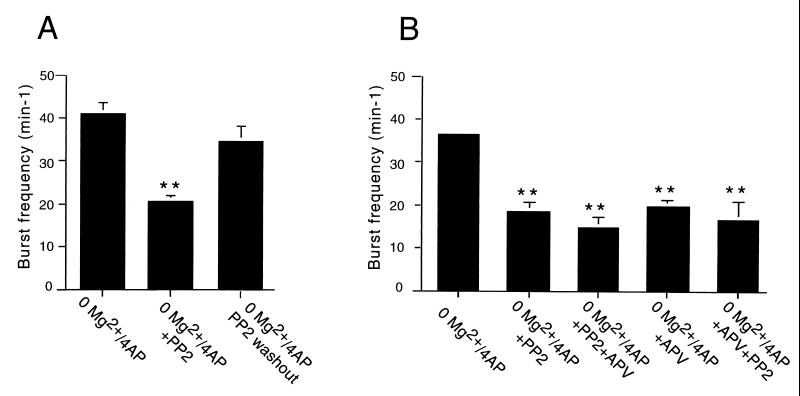
Figure 3.
Effect of the Src kinase inhibitor PP2 on epileptiform burst frequency activity in the CA3 region of hippocampal perfused with Mg2+-free medium containing 4-AP (50 μM). (A) The mean frequency of extracellularly recorded field bursts was significantly reduced (P < 0.01) in slices incubated with Mg2+-free aCSF/4-AP containing PP2 (10 μM). The burst frequency recovered significantly at 1 h of washout (P < 0.01 from slices treated with Mg2+-free aCSF/4-AP/PP2; P > 0.05 from slices treated with Mg2+-free aCSF/4-AP). (B) Addition of APV (50 μM) for 30 min to slices perfused in Mg2+-free aCSF/4-AP induced a reduction of spontaneous burst frequency comparable to PP2. Addition of PP2 (10 μM) for 30 min to slices perfused with Mg2+-free aCSF/4-AP containing APV did not result in a significant further reduction of burst frequency (P > 0.05). Consistently, addition of APV (50 μM) for 30 min to slices incubated in Mg2+-free aCSF/4-AP containing PP2 did not induce a significant additional reduction of epileptiform activity (P > 0.05). **, Different from Mg2+-free aCSF/4-AP (P < 0.01).
Electrophysiological Recordings.
We obtained extracellular recordings from CA3 stratum pyramidale by using 2- to 10-MΩ microelectrodes filled with aCSF and single orthodromic stimuli delivered via a bipolar stimulation electrode to the mossy fiber pathway at 0.2 Hz. Orthodromic-evoked field responses and spontaneous epileptiform burst waveforms were amplified with a Mangoni Biomedic Amplifier (Pisa, Italy) and filtered (dc 10 kHz). Signals were displayed on an oscilloscope and/or a chart recorder. Intracellular recordings were made with 50- to 60-MΩ thin-walled glass microelectrodes filled with 3 M potassium acetate. We performed intracellular current-clamp recordings with an Axoclamp-2B amplifier (Axon Instruments, Foster City, CA). Selected traces were stored on a PC for data analysis using the labview software package (National Instruments, Austin, TX). Statistical analyses were performed by ANOVA with statview software (Abacus Concepts, Berkeley, CA).
Src Kinase Assays.
Tissue extracts were obtained by sonication in 50 μl of lysis buffer containing 10 mM Tris⋅HCl (pH 7.6), 50 mM NaCl, 30 mM Na4P2O7, 1% NP-40, and protease (10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM PMSF) and phosphatase inhibitors (20 mM NaF, 1 mM activated Na3VO4). Homogenized samples were clarified by centrifugation for 10 min at 15,000 rpm at 4°C. Src kinase activity was determined in specific immunoprecipitates obtained with mouse mAb 327 (22) using either denatured enolase as a substrate (23) or a Src substrate peptide (Upstate Biotechnology, Lake Placid, NY) (24). Similar results were obtained with the two assays and, therefore, cumulative data are presented. Activated Src also was detected by Western blotting with an antibody (BioSource International, Camarillo, CA) raised against a phosphorylated peptide representing the catalytically active form of Src (pY418). Such antibody was used at a dilution of 1:1,000 in the presence of phosphatase inhibitors. Western blots were carried out as described (25, 26). After detection of phosphorylated Src, Western blots were stripped by incubation in 0.0625 M Tris⋅HCl (pH 6.8), 2% SDS, 0.7% β-mercaptoethanol at 56°C for 30 min and reprobed with an antibody to total Src (Santa Cruz Biotechnology) at a dilution of 1:1,000 for the purpose of control. Blots were exposed on Kodak Biomax MR film. Autoradiograms were scanned and the signal was quantified with the National Institutes of Health image 1.61 software package. Statistical analyses were performed as described above.
Results and Discussion
In control hippocampal slices perfused with normal aCSF, field potentials recorded in the stratum pyramidale of the CA3 region evoked by stimulation of mossy fibers consisted of positive field excitatory postsynaptic potentials (fEPSP) and single superimposed population spikes (PSs), indicating synchronous discharge of pyramidal cells (not shown). In these slices, we observed no spontaneous paroxysmal depolarization shifts (PDSs) or extracellular interictal spontaneous discharges for up to 3 h of recording from the CA3 region (n = 13). In slices incubated in Mg2+-free aCSF/4-AP, mossy fiber stimulation evoked fEPSPs and PSs of larger amplitude than in controls, followed by 2–4 additional PSs (not shown). In Mg2+-free aCSF/4-AP the CA3 region also exhibited spontaneous epileptiform events consisting of a series of PSs of variable amplitude riding on the positive wave. The intracellular correlates of the epileptiform discharge were 15- to 40-mV large, 50- to 100-ms long PDSs with a superimposed burst of fast action potentials followed by a long-lasting hyperpolarization of 1.5–2 sec (Fig. 1). The mean frequency of the extracellularly recorded field bursts was 41.0 ± 2.4 min-1 (n = 13) (Fig. 2A).
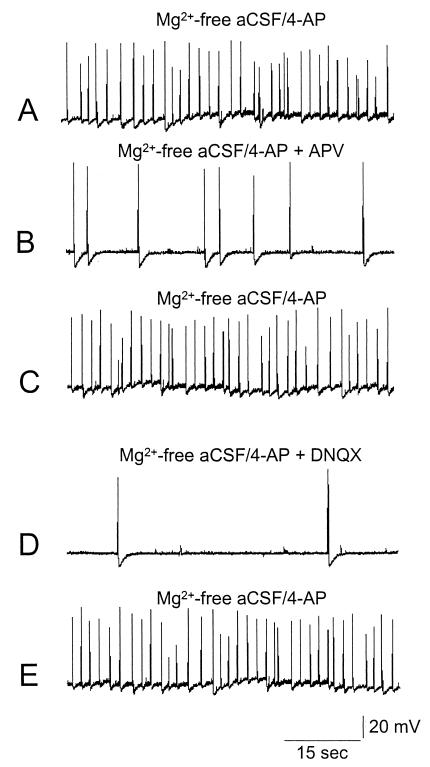
Figure 1.
Intracellular recordings of a representative CA3 pyramidal neuron during perfusion with Mg2+-free medium containing 4-AP (50 μM) and sequentially exposed to APV, an antagonist of NMDA glutamatergic receptors, and DNQX, an antagonist of non-NMDA receptors. (A) Spontaneous epileptiform activity was induced in the CA3 region of the hippocampus by perfusion with Mg2+-free aCSF/4-AP. (B) Such activity was reduced by application of APV (50 μM), and recovered upon washout (C). (D) DNQX (10 μM) also reduced burst frequency in a reversible way. (E) DNQX washout.
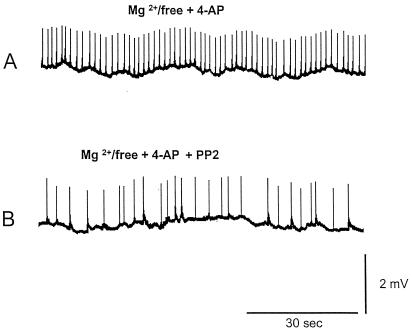
Figure 2.
Extracellular recordings of epileptiform activity in the CA3 region of hippocampal slices perfused with Mg2+-free medium containing 4-AP (50 μM) (low-speed chart recording). (A) Spontaneous epileptiform activity induced with Mg2+-free aCSF/4-AP in the CA3 region. A burst frequency of 41.0 ± 2.4 min-1 was observed. (B) Preincubation with the Src kinase inhibitor PP2 (10 μM) reduced burst frequency to 20.6 ± 2.4 min-1. Such a reduction was statistically significant (P < 0.01).
To test the involvement of glutamate receptor subtypes in the generation of epileptiform activity induced by Mg2+-free aCSF/4-AP in the CA3 region, we analyzed the effects of d-2-amino-5-phosphonovalerate (APV), an antagonist of NMDA glutamatergic receptors, and 6,7-dinitroquinoxaline-2,3-dione (DNQX), an antagonist of non-NMDA receptors. When APV (50 μM) was added to the perfusing solution, a marked decrease in the frequency of spontaneous epileptiform discharges was observed (Fig. 1). The frequency of spontaneous discharge fully recovered upon washout. Bath application of DNQX (10 μM) produced an even stronger blockage of epileptiform bursts, which also recovered upon washout (Fig. 1). APV, but not DNQX, also reduced paroxysmal depolarization shift duration (not shown).
We then investigated whether inhibition of Src-family PTKs played a role in the epileptiform activity seen in the CA3 region in this paradigm. The mean frequency of extracellular epileptiform discharges in slices preincubated in Mg2+-free aCSF/4-AP containing PP2 was 20.6 ± 1.2 min-1 (n = 16), which was significantly lower than in slices preincubated with Mg2+-free aCSF/4-AP, P < 0.01 (Figs. 2 and 3). About 1 h after washout of PP2, the frequency of spontaneous bursts recorded in the CA3 region increased, reaching 34.8 ± 3.2 min-1 (n = 5). Such a frequency was not significantly different from that of slices kept in Mg2+-free aCSF/4-AP (Fig. 3A). Preincubation of hippocampal slices with 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (n = 5), an inactive analogue of PP2, did not affect burst frequency in slices treated with Mg2+-free aCSF/4-AP (not shown). Addition of APV (50 μM) for 30 min to slices perfused in Mg2+-free aCSF/4-AP (n = 5) reduced spontaneous burst frequency to a level comparable to that seen in slices incubated with PP2 (Figs. 2B and 3B). Addition of PP2 (10 μM) for 30 min to slices perfused with Mg2+-free aCSF/4-AP containing APV (n = 5) did not result in a significant additional reduction of burst frequency (Fig. 3B). Similarly, if APV (50 μM) was added for 30 min to the slices incubated with PP2 (n = 5), no significant further reduction of activity was seen (Fig. 3B).
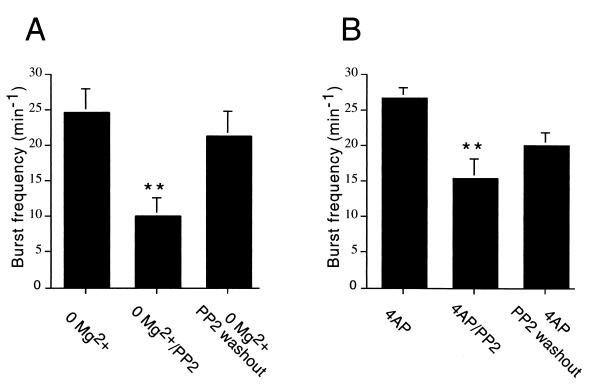
PP2 treatment was also capable of reducing epileptiform activity induced by either Mg2+-free or 4-AP medium alone (Fig. 4A). Spontaneous burst frequency in slices incubated in Mg2+-free medium was 24.8 ± 3.4 min-1 (n = 6). This frequency was about 59.7% of the one seen in slices treated with both Mg2+-free and aCSF/4-AP. Slices preincubated in Mg2+-free medium containing PP2 displayed a frequency of extracellular epileptiform discharges of 10.0 ± 2.5 min-1 (n = 7). Such a reduction was statistically significant (P < 0.01). After 1 h of washout from PP2, the frequency significantly (P < 0.01 from PP2 treatment; P > 0.05 from Mg2+-free) recovered to 21.2 ± 3.6 bursts min−1 (n = 7). When slices were incubated with 4-AP alone (Fig. 4B), the frequency of extracellular epileptiform discharges was 26.6 ± 1.5 min-1 (n = 5), or about 64.8% of the frequency seen with treatment with both Mg2+-free and 4-AP. Preincubation of slices with PP2 reduced significantly (P < 0.01) burst frequency to 15.3 ± 2.7 min-1 (n = 7). A recovery to 20.0 ± 1.8 min-1 (n = 7) was seen after PP2 washout, although frequency of extracellular epileptiform activity in these slices did not significantly differ from 4-AP-treated slices without PP2 or from slices incubated with both 4-AP and PP2 (P > 0.05 in both cases).
Figure 4.
Effect of the Src kinase inhibitor PP2 on epileptiform burst frequency activity in the CA3 region of hippocampal induced either by perfusion with Mg2+-free medium or by application of 4-AP (50 μM). (A) The frequency of extracellularly recorded field bursts induced by Mg2+-free medium was significantly reduced (P < 0.01) in slices incubated with PP2 (10 μM). The burst frequency recovered significantly at 1 h of washout (P < 0.01 from slices treated with Mg2+-free/PP2; P > 0.05 from slices treated with Mg2+-free). (B) The mean frequency of extracellularly recorded field bursts was significantly reduced (P < 0.01) in slices incubated with 4-AP containing PP2 (10 μM). The burst frequency recovered partially but not significantly at 1 h of washout (P > 0.05 from slices treated with 4-AP/PP2; P < 0.05 from slices treated with 4-AP). Thus although epileptiform activities induced by Mg2+-free medium or by application of 4-AP were lower than the activity induced with Mg2+-free aCSF/4-AP/PP2, both were significantly reduced by PP2 treatment. **, Different (P < 0.01) from slices treated with Mg2+-free medium (A) or with 4-AP (B).
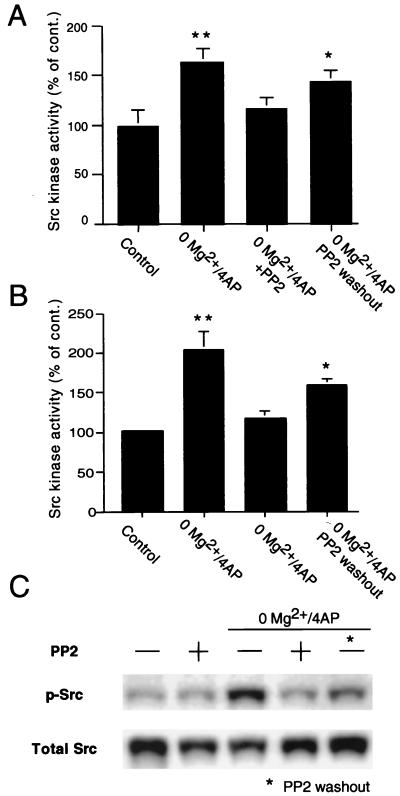
Src kinase activity was significantly increased (P < 0.01) in slices incubated in Mg2+-free aCSF/4-AP as measured either by in vitro kinase assay in tissue extracts or by Western blot detection of activated Src (Fig. 5). Such an increase was not observed in slices incubated with Mg2+-free aCSF/4-AP in the presence of PP2 (Fig. 5). In slices incubated in Mg2+-free aCSF/4-AP and PP2 but allowed to wash out PP2 in Mg2+-free aCSF/4-AP, we saw a recovery of Src kinase activity when compared with control slices. Similar results were obtained by in vitro kinase assays (n = 6–8) and detection of activated Src by Western blot (n = 4–6) (Fig. 5). Incubation of hippocampal slices in normal aCSF did not alter Src activity when compared with control slices in normal aCSF (Fig. 5C).
Figure 5.
Src kinase activity in extracts of hippocampal slices perfused with Mg2+/free medium containing 4-AP (50 μM) with or without PP2 (10 μM) and control slices. (A) Src kinase activity as measured by in vitro kinase assay in hippocampal extracts was significantly higher in slices perfused in Mg2+-free aCSF/4-AP (P < 0.01 from control slices incubated in normal aCSF). Incubation with PP2 prevented such an increase of Src activity (P < 0.01 from slices perfused in Mg2+-free aCSF/4-AP; P > 0.05 from control slices). One hour after PP2 washout, a partial recovery of Src kinase activity was seen. In fact, in these slices, Src activity was significantly higher than in control slices (P < 0.05), although not significantly different from slices treated with Mg2+-free aCSF/4-AP/PP2. (B) Similar results were obtained by quantification of catalytically active Src kinase in extracts of hippocampal slices by Western blot with an antibody specific for Src phosphorylated at the conserved residue Y418, which predicts kinase activation. Src phosphorylation was significantly induced in slices perfused in Mg2+-free aCSF/4-AP (P < 0.01 from control slices incubated in normal aCSF). Incubation with PP2 prevented such an increase of Src activity (P < 0.01 from slices perfused in Mg2+-free aCSF/4-AP; P > 0.05 from control slices). A recovery of Src phosphorylation was seen 1 h after PP2 washout (P > 0.05 from slices perfused in Mg2+-free aCSF/4-AP; P < 0.05 from control slices). (C) Representative Western blot. P-Src, phosphorylated Src (Y418); detection of total Src was used for control (total Src). With either method in the activity of Src kinase in slices treated with PP2 in normal aCSF did not differ from control slices in aCSF (not shown and C). *, Different from control P < 0.05; **, different from control P < 0.01.
In this study, epileptiform activity was induced in rat hippocampal slices by a exposure to Mg2+-free medium and application of the potassium channel blocker 4-AP, a model of epileptogenesis previously used in neocortical slices (17). Epileptiform activity then was monitored in the CA3 region of the hippocampus, which is considered to be the generation site for activity in CA1 and has been shown to be capable of spontaneous epileptic events in response to different convulsant stimuli including low Mg2+ and 4-AP (27–31). Both the NMDA antagonist APV and the non-NMDA antagonist DNQX affected epileptiform activity induced with Mg2+-free medium/4-AP in CA3 (Fig. 1), in agreement with observations by Siniscalchi et al. (17) on neocortical slices treated in the same fashion.
Spontaneous epileptiform activity induced in the CA3 region by Mg2+-free aCSF/4-AP could be greatly reduced by PP2, a specific inhibitor of the Src family of nonreceptor PTKs. This same inhibitor also reduced epileptiform activity induced by either Mg2+-free medium of 4-AP alone. Several members of the Src PTK family are expressed in the central nervous system, including Src, Fyn, Lyn, Yes, Abl, and Lck (2, 3). Some of them have been implicated in the regulation of cellular excitability and plastic changes (2, 3, 10, 11). In particular, Src and Fyn appear to play a role in the genesis of long-term potentiation (2, 3). Such activities appear to be related to the potentiation of NMDA currents brought about by tyrosine phosphorylation of this receptor channel (1, 4). Src kinase activity has been shown to increase NMDA receptor channel open probability and open time, leading Salter and coworkers (32) to propose that the increase in NMDA receptor function produced by the simultaneous activation of Src and raising intracellular sodium may be key to the regulation of excitatory neurotransmission. Such a mechanism could account for the reduced epileptiform activity that we observed in slices treated with the Src kinase antagonist PP2.
However, it is interesting to note that while long-term potentiation is impaired in Fyn null mutant mice, it is unaffected in Src knockouts (2), suggesting that a certain degree of redundancy or complementation within members of this PTK family may occur. Fyn knockout mice also showed a certain retardation in the rate of amygdala kindling while once the kindled state was established it remained stable, suggesting a role for this PTK in the induction of kindling (10). Consistently, enhanced seizure susceptibility was seen in transgenic mice overexpressing Fyn (11). In addition to its role in excitability, there is evidence that Src participates in neurotrophin signaling (33, 34). Neurotrophins such as nerve growth factor have been shown to play a role in the morphologic rearrangements that accompany seizure development in the kindling model of epilepsy (35). It is therefore possible that Src activation also may contribute to the functional synaptic reorganization believed to take place in epilepsy.
Low affinity NMDA receptor antagonists have shown potential in the management of epilepsy (36). Taken together with previous observations (32), our findings suggest that the Src kinase pathway could provide an adjunctive therapeutic target perhaps in combination with other agents affecting NMDA receptor function.
Acknowledgments
We thank Cindy Simpson, Alfredo Brancucci, Alvin King, and Marisa Roberto for experienced technical help. The present work was partially supported by National Institutes of Health Grants P50AA06420 (F.E.B., G.R.S., and P.P.S.) and MH44346 (G.R.S.), and by a grant from the Alcoholic Beverage Medical Research Foundation (P.P.S.).
Abbreviations
- 4-AP
4-aminopyridine
- PTK
protein tyrosine kinase
- NMDA
N-methyl-d-aspartate
- aCSF
artificial cerebrospinal fluid
- APV
d-2-amino-5-phosphonovalerate
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140219097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140219097
References
- 1.Salter M W. Biochem Pharmacol. 1998;56:789–798. doi: 10.1016/s0006-2952(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 2.Grant S G, O'Dell T J, Karl K A, Stein P L, Soriano P, Kandel E R. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y M, Roder J C, Davidow J, Salter M W. Science. 1998;279:1363–1367. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- 4.Kohr G, Seeburg P H. J Physiol (London) 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mody I, Heinemann U. Nature (London) 1987;326:701–704. doi: 10.1038/326701a0. [DOI] [PubMed] [Google Scholar]
- 6.Morrisett R A, Chow C, Nadler J V, McNamara J O. Brain Res. 1989;496:25–28. doi: 10.1016/0006-8993(89)91048-2. [DOI] [PubMed] [Google Scholar]
- 7.Martin D, McNamara J O, Nadler J V. J Neurosci. 1992;12:1928–1935. doi: 10.1523/JNEUROSCI.12-05-01928.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus J E, McNamara J O. Brain Res Mol Brain Res. 1998;61:114–120. doi: 10.1016/s0169-328x(98)00220-4. [DOI] [PubMed] [Google Scholar]
- 9.Avoli M, Bernasconi A, Mattia D, Olivier A, Hwa G G. Ann Neurol. 1999;46:816–826. doi: 10.1002/1531-8249(199912)46:6<816::aid-ana3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Cain D P, Grant S G, Saucier D, Hargreaves E L, Kandel E R. Epilepsy Res. 1995;22:107–114. doi: 10.1016/0920-1211(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 11.Kojima N, Ishibashi H, Obata K, Kandel E R. Learn Mem. 1998;5:429–445. [PMC free article] [PubMed] [Google Scholar]
- 12.Loscher W. Prog Neurobiol. 1998;54:721–741. doi: 10.1016/s0301-0082(97)00092-0. [DOI] [PubMed] [Google Scholar]
- 13.McNamara J O. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson W W, Lewis D V, Swartzwelder H S, Wilson W A. Brain Res. 1986;398:215–219. doi: 10.1016/0006-8993(86)91274-6. [DOI] [PubMed] [Google Scholar]
- 15.Tancredi V, Hwa G G, Zona C, Brancati A, Avoli M. Brain Res. 1990;511:280–290. doi: 10.1016/0006-8993(90)90173-9. [DOI] [PubMed] [Google Scholar]
- 16.Neuman R, Cherubini E, Ben-Ari Y. Brain Res. 1988;459:265–274. doi: 10.1016/0006-8993(88)90642-7. [DOI] [PubMed] [Google Scholar]
- 17.Siniscalchi A, Calabresi P, Mercuri N B, Bernardi G. Neuroscience. 1997;81:189–197. doi: 10.1016/s0306-4522(97)00178-4. [DOI] [PubMed] [Google Scholar]
- 18.Voskuyl R A, Albus H. Brain Res. 1985;342:54–66. doi: 10.1016/0006-8993(85)91352-6. [DOI] [PubMed] [Google Scholar]
- 19.Kapetanovic I M, Yonekawa W D, Kupferberg H J. Epilepsy Res. 1995;20:113–120. doi: 10.1016/0920-1211(94)00071-4. [DOI] [PubMed] [Google Scholar]
- 20.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 21.Berretta N, Berton F, Bianchi R, Capogna M, Francesconi W, Brunelli M. Exp Brain Res. 1990;83:124–130. doi: 10.1007/BF00232200. [DOI] [PubMed] [Google Scholar]
- 22.Lipsich L A, Lewis A J, Brugge J S. J Virol. 1983;48:352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper J A, Esch F S, Taylor S S, Hunter T. J Biol Chem. 1984;259:7835–7841. [PubMed] [Google Scholar]
- 24.Cheng H C, Nishio H, Hatase O, Ralph S, Wang J H. J Biol Chem. 1992;267:9248–9256. [PubMed] [Google Scholar]
- 25.De Logu A, Williamson R A, Rozenshteyn R, Ramiro-Ibanez F, Simpson C D, Burton D R, Sanna P P. J Clin Microbiol. 1998;36:3198–3204. doi: 10.1128/jcm.36.11.3198-3204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanna P P, Williamson R A, De Logu A, Bloom F E, Burton D R. Proc Natl Acad Sci USA. 1995;92:6439–6443. doi: 10.1073/pnas.92.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hablitz J J. J Neurophysiol. 1984;51:1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- 28.Miles R, Wong R K, Traub R D. Neuroscience. 1984;12:1179–1189. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- 29.Walther H, Lambert J D, Jones R S, Heinemann U, Hamon B. Neurosci Lett. 1986;69:156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- 30.Perreault P, Avoli M. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Psarropoulou C, Avoli M. Brain Res Dev Brain Res. 1996;94:52–59. doi: 10.1016/0165-3806(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 32.Yu X M, Askalan R, Keil G J, 2nd, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 33.Lazarovici P, Oshima M, Shavit D, Shibutani M, Jiang H, Monshipouri M, Fink D, Movsesyan V, Guroff G. J Biol Chem. 1997;272:11026–11034. doi: 10.1074/jbc.272.17.11026. [DOI] [PubMed] [Google Scholar]
- 34.Kremer N E, D'Arcangelo G, Thomas S M, DeMarco M, Brugge J S, Halegoua S. J Cell Biol. 1991;115:809–819. doi: 10.1083/jcb.115.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams B, Sazgar M, Osehobo P, Van der Zee C E, Diamond J, Fahnestock M, Racine R J. J Neurosci. 1997;17:5288–5296. doi: 10.1523/JNEUROSCI.17-14-05288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geter-Douglass B, Witkin J M. Psychopharmacology (Berlin) 1999;146:280–289. doi: 10.1007/s002130051118. [DOI] [PubMed] [Google Scholar]