Synopsis
Prevention and control of sexually transmitted infections (STIs) has proven effective in reducing HIV infection when treatment is available promptly for symptomatic persons in conditions of an emerging infection. Biologically, it is assumed that reduced genital tract inflammation reduces infectiousness for HIV as well as reducing susceptibility in HIV-uninfected persons. Other strategies may not work however, such as periodic mass chemotherapy for STIs. Male circumcision has been demonstrated effective in reducing risk for HIV infection in three separate trials from South Africa, Kenya, and Uganda. Global expansion of STI treatment and male circumcision programs is a vital tool for control of HIV infection; current research priorities are presented.
Keywords: HIV, AIDS, prevention, sexually transmitted infections, circumcision, public health
Introduction
There were an estimated 39.5 million persons living with HIV/AIDS worldwide by the end of 2006 [1]. The first years of new millennium have seen a massive global effort to improve the coverage and reach of antiretroviral therapy [ART] for treatment of HIV/AIDS in resource-limited settings [2,3]. Despite unprecedented and concerted global care and treatment efforts, most persons in need of ART do not now have access to it. UNAIDS estimates that 4.3 million individuals were newly infected with HIV in 2006, while 2.9 million persons died in that year [1]. More than three million persons are infected yearly than are placed onto ART programs. About 95% of the approximately 15,000 new infections that occurred every day in 2006 were in developing world, amongst whom almost half were women. Almost half of all new infections occur in individuals twenty-five years or younger [1].
Despite the proven effectiveness of current approaches, fewer than one in five people worldwide have the knowledge of, and access to the tools to prevent HIV infection [4]. Furthermore, many of the current approaches, like condom use, are not popular with men; and typically, women in developing countries do not have the power in their gender relationships to negotiate condom use. There is an increasing realization that there exists a huge and unmet need for prevention of HIV infection, and that a rigorous approach for developing, evaluating and implementing future prevention efforts is required.
Modelers predict that a stronger global commitment to expanded HIV prevention programs started now could avert 28 million new HIV infections through 2015 [5]. This figure is more than half of the new infections that might otherwise occur during this period in 125 low- and middle-income countries. Preventing these new infections would require investing an estimated US$122 billion over this period, but is deemed cost-effective in that it would reduce the need for treatment and care in the future, would keep families intact, and blunt the economic devastation of HIV/AIDS [5].
Here we seek to provide an overview of the current state and future directions of two key public health interventions to contain the HIV/AIDS epidemic globally: control and prevention of sexually transmitted infections (STIs) and the surgical removal of the male foreskin (circumcision). First, we present the evidence that treatment of STIs promptly and offering male circumcision can each reduce HIV transmission. Second, we discuss the prospects for scaling up STI control and surgery for male circumcision. Finally, we highlight research in progress and research that is needed to further these approaches to HIV control.
Conceptual framework for HIV prevention
From a public health standpoint, one can consider prevention in three phases: (i) primary prevention, mainly directed towards persons hitherto uninfected by HIV, (ii) secondary prevention, which includes early detection of HIV infection in those who have been newly (‘acutely’) infected by HIV with a goal to initiate appropriate prevention strategies (reducing transmission to others, termed prevention in positives, or “positive prevention”) and to reduce complications in the infected person, and (iii) tertiary prevention targeting persons with chronic HIV infection [Table 1]. Most often, more than one approach for prevention is directed towards a target group since risk behaviors for HIV are multidimensional and overlapping [6,7]. A host of interventional strategies have been shown to be effective. These strategies can be applied at the micro (individual patient, sexual or needle-sharing partners, family) level, meso (community) level, and macro (policy or structural) level. We present a simple conceptual framework for prevention based on evidence of effectiveness of each strategy (Table 1).
Table 1.
| Prevention strategy and target groups | |||
|---|---|---|---|
| Level | Primary prevention for hitherto HIV-uninfected individuals | Secondary prevention for recent HIV seroconverters/‘acutely infected’ individuals | Tertiary prevention for individuals with established HIV-infections/AIDS |
| Micro level [Individual patient, and partners/family] | Approach: Improving access to healthcare and individual empowerment through appropriate education and socio-economic development. | ||
|
|
|
|
| Meso level [Community] | Approach: Broaden individual and family-centric interventions in a community- development framework | ||
|
|
|
|
| Macro level [Policy] | Approach: Developing and sustaining policy frameworks that support individual and community-based interventions | ||
|
|
|
|
Notes: ABC = Abstinence, Behavior Change and Correct and Consistent Condom use; VCT = Voluntary Counseling and Testing services, IEC = Information Education and Communication; ART = Antiretroviral treatment; STI = Sexually Transmitted Infections; OI = Opportunistic Infections
Public health interventions to end the epidemic
Most prevention programs worldwide are targeted towards hitherto HIV-uninfected (negative) individuals to reduce the incidence of HIV acquisition [8]. Counseling and interventions to reduce individuals’ risk behaviors are the overarching strategies that are applied to all target populations and individuals. Delaying onset of sexual debut through abstinence, coupled with skill-building and motivational counseling for esteem development is a key strategy for preventing new infections especially among young people [9,10]. Programs targeting youth include school and recreation-areas based interventions that have been shown to be successful if tied to local customs, traditions and social norms [10]. Reduction in sexual intermixing rates among the sexually active men and women from the general population may be achieved by promoting ‘be faithful’ messages that advocate monogamy; correct and consistent use of condoms rounds out the basic prevention message [11, 12]. The ‘ABC’ (Abstinence, Be faithful and correct and consistent Condom use) strategy promulgated for prevention of sexual transmission of HIV is believed to have had measurable effects in reducing HIV incidence and prevalence in settings like Uganda, Senegal, Thailand and Cambodia [11,13–17]. Prevention programs for especially high-risk persons, like commercial sex workers, injection drug users, migrant laborers, clients of sex workers, and men who have sex with men are designed with a strategic focus towards risk and harm reduction [18–20]. These include other prevention strategies discussed by other contributors to this issue of Infectious Disease Clinics of North America, including needle exchange and treatment for drug addiction for injection drug users.
There is limited evidence from randomized clinical trials that treatment of STIs as an effective means for preventing HIV, but the evidence is more convincing in settings with low to emerging HIV epidemics and where there is a high background prevalence of STIs [13, 21–27]. Since there are other compelling reasons why STI treatment services should be strengthened, and the coherence of the STI control and prevention strategies with HIV/AIDS ‘ABC’ approaches are highly synergistic; when a STI health service intervention is effectively implemented, it can substantially improve quality of care and education, contributing in turn to HIV prevention [13].
A wealth of observational evidence from many epidemiological studies and the recent randomized clinical trials in South Africa, Uganda and Kenya have demonstrated the efficacy of adult male circumcision to prevent HIV acquisition [28–37]. We highlight both STI treatment and adult male circumcision as HIV prevention modalities later in this review.
The role of confidential and easily accessible voluntary counseling and testing [VCT] services is a critical primary and secondary modality for prevention. In community-based settings, randomized controlled trials have shown the efficacy of VCT services for improving the access to prevention and care services in general as has been proven in a variety of clinic and community based settings [38–45]. Currently in progress, the HIV Prevention Trials Network (HPTN; http://www.hptn.org) trial 043 is conducting a structural intervention of intensive community-based educational advocacy for VCT and evaluate the extent to which this intervention results in increased VCT, reduced risk behaviors, and reduced seroincidence in study communities compared to comparison communities. Nearly 200,000 persons will be tested for HIV in this multi-site, multi-continent study.
An enabling environment for people who have recently seroconverted to HIV involves wider access to confidential voluntary counseling and testing [VCT] services and self-help and peer-support groups [46]. It has been shown that the proportion of HIV-infections that occur via acute seroconverters may constitute a high attributable fraction of the preventable HIV infections [47]. Therefore enabling approaches for early detection of HIV through confidential testing, rapid HIV tests and effective linkages to care and social support programs can promote ‘acute seroconverters’ to reduce risky sexual behaviors and contain the spread of HIV [48]. The Center for HIV/AIDS Vaccine Immunology (CHAVI; http://www.chavi.org) is supporting HIV behavioral risk reduction research, in collaboration with the HPTN, to assess the receptivity of acutely infected persons (detected with pooled PCR in high risk venues such as Sexually Transmitted Disease [STD] clinics) to risk reduction messages.
While a full summary of all prevention strategies for HIV is beyond the scope of this review, we would like to highlight a simple model to illustrate how prevention of HIV can work through multiple modalities and populations (Figure 1). Reducing viral load through ART or by reducing immune activation by treatment of co-infections can reduce sexual, needle-related, and perinatal transmission. A clinical trial to demonstrate whether ART can reduce transmission between discordant couples began in 2006 (HPTN 052). Barriers to transmission can protect HIV-uninfected persons. Male condoms and clean needles and uncontaminated blood and blood products are well defended by the literature, while other modalities in use have less evidence to document effectiveness, such as female condoms. A trial in Zimbabwe of the female diaphragm to block HIV infection is nearing completion in 2007. Other methods of physical and/or chemical barriers such as microbicides and pre-exposure antiretroviral drugs are being studied in pre-clinical and clinical research, as are immunological barriers like HIV vaccines (Figure 1). Treating STIs and reducing high risk behaviors can reduce both infectiousness and susceptibility. Circumcision can help protect men from getting infected, but this, in turn, helps protect women who may be exposed to fewer infected men, if community circumcision coverage is substantial and men do not increase their high risk activities, i.e., disinhibition. While Figure 1 suggests interventions at the individual level, it does not do justice to the challenges for community-level interventions, or those promulgated at the policy or societal level, termed structural interventions.
Figure 1.
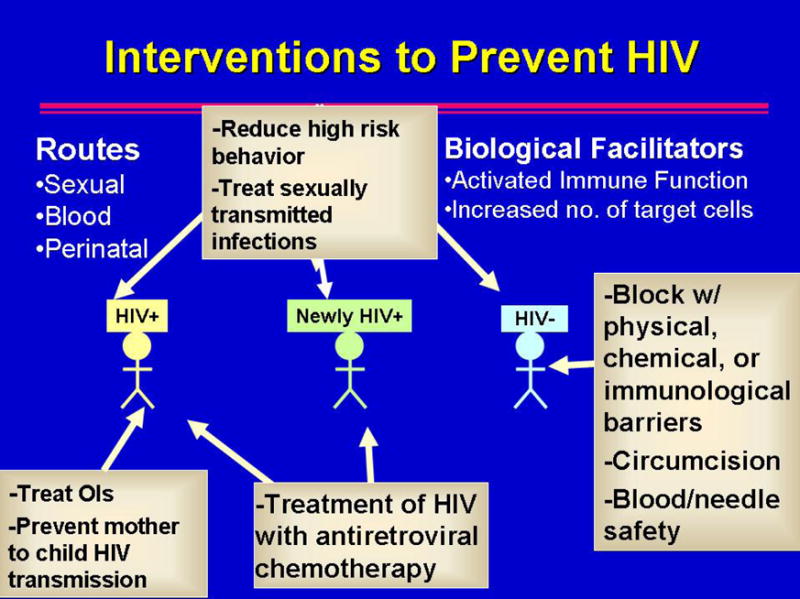
A simple model of HIV prevention strategies that are either proven to work to reduce HIV incidence [e.g., behavior change to reduce risk, condoms as a physical barrier, needle exchange and treatment of opiate dependency to reduce injection drug-related risk] or that are in research development [e.g., physical barriers like the diaphragm for women, chemical barriers like microbicides, or immunological barriers like vaccines]. “OIs” refers to opportunistic infections whose treatment is associated with a small decline [0.2–0.5 log10 viral copies/mL plasma] in HIV viral load, thereby probably making persons less infectious.
The most important community-level or structural intervention would be to promote broader gender-equality in sexual relations. Laws and policies that nurture socio-economic development and poverty alleviation are additional macro-level, long-term interventions that can be expected to reduce HIV transmission [49–52]. If HIV is tackled as a socioeconomic development, women’s empowerment, and human rights issue, it is easer to imagine long-term, sustained success in HIV prevention programs [53–55]. However, it is complex and unprecedented to influence deeply entrenched traditions, social norms, and beliefs; for policy makers to create an environment that reduces risk activities as well as promotes the well being of people living with HIV/AIDS is a challenge that such nations as Uganda and Thailand have met most successfully among those with serious epidemics [11, 14, 15, 46, 56]. Gender equity and poverty alleviation are long-term goals of a truly comprehensive HIV/AIDS prevention and care agenda.
Treatment of STIs
STIs are an important cause of morbidity worldwide with substantial economic, social, and health consequences, particularly for women and their reproductive outcomes [57]. STIs have ranked an important cause of disability-adjusted life years (DALYs) in men and women as reported by the World Health Organization (WHO). There are an estimated 340 million new cases of curable STIs (i.e., syphilis, gonorrhea, trichomoniasis, chancroid, and chlamydia) and over a billion cases of chronic viral STIs (i.e., genital herpes simplex virus type 2 (HSV-2), hepatitis B virus, genital human papillomavirus, and HIV). The three important strategies for the control of STIs include: (i) reducing the rate of exposure to the STI, particularly the frequency with which persons have sex beyond stable partnerships (sexual mixing); (ii) reducing the efficiency of transmission for each exposure, and (iii) reducing the duration of infectiousness for the specific STI [58, 59]. Reducing risky sexual behaviors involves the ‘ABC’ strategy at the individual level, coupled with community level efforts for promoting changes in social norms and beliefs to reduce number of sexual partners, delaying onset of sexual activity and avoiding high-risk partners [60–62]. Key structural changes to reduce STIs could include political leadership for encouraging reduced male sexual predation and promiscuity, education of girls and women, economic empowerment of women, condom use policies that reduce risk, especially in higher risk settings. We wish to highlight the merits of structural improvements to the health care system to screen, diagnose, and treat STDs, as well as to perform contact tracing for persons exposed sexually to STI-infected individuals. These improvements are well within the global capacity to establish, as has been done with oral rehydration therapy and improved vaccine coverage worldwide.
The earliest evidence of STIs’ role to impact the increase in sexual transmission of HIV came from observational epidemiologic studies [63,64]. The biologic basis of this observation is rooted in the documentation that a substantially increased HIV viral load is detected in the genital secretions with either urethral or cervical inflammation [66,67]. Furthermore, treatment of STIs results in reduction in genital tract HIV viral loads, thereby reducing the infectiousness of the index case and lowering the probability of transmission [66]. It has been shown that ulcerative STIs (e.g., syphilis, chancroid, HSV-2) disrupt the integrity of the epithelial mucosa and facilitate the contact of HIV with the lymphatic and circulatory systems. While inflammatory and exudative STIs (those without frank ulcers, such as chlamydia, gonorrhoea and trichomoniasis) are less disruptive of the epithelial tissues, the infection causes inflammation and associated recruitment of large volumes of cervical or urethral discharge filled with HIV-susceptible while blood cells (exudate) [67,68]. Inflammation results in microulcerations and engorgement of capillaries, facilitating contact of HIV with target cells. While ulcerogenic STIs increase the risk of sexual transmission of HIV by five-to-ten fold compared to a two-to-five fold increase due to inflammatory, nonulcerogenic STIs, the attributable risk is substantial for the latter due to their high prevalence in the population. HIV-1 infection, particularly in the setting of advancing immunosuppression, may lead to an increase in susceptibility to some STIs. Many HIV-1-infected individuals have higher-than-typical rates of STIs, probably because of shared behavioral risk factors that facilitate both HIV-1 and STI transmission [69–70]. The targeting of all treatable STIs is thus a logical target for prevention program for HIV. Suppression of HSV-2 with oral acyclovir to reduce risk for HIV seroconversion is being attempted in a multinational placebo-controlled clinical trial (HPTN039).
The magnitude of the effect of improved STI treatment on HIV transmission at a population level was tested during the nineties in three community-randomized trials in rural East Africa, where rates of HIV infection were high and STI control was suboptimal [21–23]. Improved syndromic management of symptomatic STIs was associated with a reduction in incidence of HIV infection of 38% (95% CI: 15% to 55%) over the course of two years in the Mwanza region, Tanzania [21, 27]. Over a similar length of time and with seemingly comparable proportional reductions in treated STIs, periodic (every 10 months) mass treatment in Rakai, Uganda, did not reduce the incidence of HIV infection (3% reduction; 95% CI: −16% to 19%) [22]. Several hypotheses have been put forward to explain these contrasting findings, including one that syndromic treatment was a more effective strategy than periodic mass treatment [25,26]. However, the results of a third trial, in Masaka district, Uganda, that neighbors Rakai, demonstated that syndromic treatment had no effect on incidence of HIV infection in Masaka (0% reduction, for syndromic treatment plus health education vs. the control arm; 95% CI, −58% to 37%)[23]. A simulation modeling study of HIV and STI transmission confirmed that the low trial impact in Rakai and Masaka could be explained by low prevalence levels of curable STIs resulting from lower-risk sexual behavior in Uganda [25]. The mature HIV epidemics in Uganda, with most HIV transmission occurring outside core groups with high STI rates, also may have contributed to the lower impact on HIV incidence [26]. Simulated impact on HIV was much greater in Mwanza, even higher than that predicted from STI reductions [25]. Another alternative explanation highlights the high herpetic ulceration rates in Uganda compared to Tanzania, exacerbated by HIV-related immunosuppression the more substantial Ugandan epidemic; antibiotic treatment would not have an impact on HSV-2 lesions [26]. Declining frequencies of high risk behaviors, the mature HIV epidemic, and the mass therapy approach that left symptomatic STDs unattended for many months may have explained the lack of impact of STI treatment on HIV incidence in Uganda in the 1990s. Although the three studies provided a complex interpretation of the impact of STI treatment on HIV incidence, the biological links between STI and HIV infectivity and susceptibility, the successes in Mwanza, and the broad benefits of STD programs reinforce the need to include STI treatment in an integrated response to HIV/AIDS [13].
There remain significant challenges for approaches to delivering STI services in resource-constrained settings where there are innumerable infrastructure, personnel and supply constraints. The implementation strategies for STI control are two-fold: [i] improving technologies for actual clinical management, and [ii] improving service delivery strategies for both the general population and specific high risk groups [71]. The WHO-recommended standardized STI treatment flowcharts are based on the syndromic management approach used in the Mwanza and Masaka studies [72]. This enables a majority of providers who often lack access to adequate examination or laboratory facilities to manage symptomatic patients. The syndromic management approach is simpler than the classic dermato-venereology approach [71]. Using the syndromic approach, treatment is provided during the same clinic visit as the screening evaluation, preventing promptly the risk of further spreading the infection as well as decreasing the risk of sequelae that may develop from untreated infections [72]. This also obviates the need to return for laboratory results which also results in cost savings for the system and the patient.
Women represent a major challenge in STI control in the syndromic management approach especially due to the asymptomatic nature of many STIs in women and the relatively poor specificity of the clinical manifestations, if any [73, 74]. Syndrome-based flowcharts for lower genital tract infections in women are less valid, and laboratory tests are desirable for detecting STIs in asymptomatic and high-risk women [75]. Although there is wide availability of low-cost, rapid and simple tests for diagnosis of syphilis, they remain widely underutilized, often due to programmatic and managerial obstacles [76–78]. There remains an unmet need for point-of-care diagnostics for diagnosis of chlamydial and gonorrhea in women [78, 79]. In settings where access to laboratory tests is feasible, assessing risk prior to testing could be cost effective approaches [72]. Rapid testing for HSV-2 would be an important tool to enable more aggressive antiviral suppression, especially if the HPTN039 study proves acyclovir to be helpful in preventing HIV transmission in HSV-2 seropositive persons, or if the parallel discordant couples study reduces transmission from HSV-2, HIV co-infected persons to their uninfected partners if the infected person receives acyclovir suppressive therapy.
There have been many demonstration projects and well designed studies that have shown the effectiveness of improvising the service delivery and uptake of STI treatment strategies, especially those in Peru [80, 86], Cameroon [81], Zaire [63], Kenya [77], India [82], Thailand [83], Nepal [84], Haiti [85], Brazil [87], Congo [39], Jamaica [88] and other resource limited settings. The effective integration of STI treatment and HIV prevention and care programs is long overdue.
Adult male circumcision
Adult male circumcision is the surgical removal of most or all of the foreskin [or prepuce] from the penis [89]. The inner mucosa of the foreskin has less keratinized skin compared to the exposed external skin surface. It also has a higher density of target cells for HIV infection in the form of dendritic and Langerhans cells; the foreskin may have comparatively greater susceptibility to traumatic epithelial disruptions [tears] during intercourse, providing a portal of entry for pathogens including HIV [31,32]. Additionally, the micro-environment of the preputial sac between the unretracted foreskin and the glans penis may be conducive to viral survival, keeping infected vaginal or anal fluid in a warm, moist state [91]. Finally, the higher rates of sexually transmitted genital ulcerative disease, such as syphilis and HSV-2, observed in uncircumcised men may also increase susceptibility to HIV infection [33, 92].
An association of higher rates of circumcision in regions with lower prevalence of HIV has been noted, as in North Africa and the Middle East, and in selected parts of central/west Africa [93]. To account for other behavioral and biological factors and to quantify the strength of association, multiple ecological (population-based), cross sectional, case control and cohort studies were designed in the past decade, but most studies were limited by potential selection biases (as with Moslems who may have more conservative lifestyles and also have near-universal circumcision rates), and variable study quality [33,34].
A systematic review and meta-analysis that focused on heterosexual transmission of HIV in Africa was published in 2000 [32]. It included 19 cross-sectional studies, five case-control studies, three cohort studies, and one partner study. A substantial protective effect of male circumcision on risk for HIV infection was noted, along with a reduced risk for genital ulcer disease. After adjusting for confounding factors in the population-based studies, the relative risk for HIV infection was 44% lower in circumcised men. The strongest association was seen in high-risk men, such as patients at STD clinics, for whom the adjusted relative risk was 71% lower for circumcised men [32].
A later systematic review published in 2003 included stringent assessment of 10 potential confounding factors and was stratified by study type or study population [33]. Of the 35 observational studies included in the review, most from Africa, sixteen studies in the general population had inconsistent results. The one large prospective cohort study in this group showed a significant protective effect, with the odds of infection being 42% lower in circumcised men [35]. The nineteen other studies were conducted among high-risk men and found a consistent, substantial protective effect, which increased with adjustment for confounding. Four of these were cohort studies: all demonstrated a protective effect with two studies showing statistically significance. Overall, after adjusting for potential confounders the adjusted risk ratio (RR) was 0.56 (95% CI: 0.44 to 0.70) in general populations and the adjusted RR for high-risk populations was 0.29 (95% CI: 0.20 to 0.41). [35]
Ecologic studies also indicate a strong association between lack of male circumcision and HIV infection at the population level. Although links between circumcision, culture, religion, and risk behavior may account for some of the differences in HIV infection prevalence, the countries in Africa and Asia with prevalence of male circumcision of <20% have HIV-infection prevalence several times higher than countries in those regions where ≥80% of men are circumcised. [94]
The results of these observational studies through systematic reviews provided suggestive evidence that circumcised men were at lower risk of HIV, but could not control for potential confounding factors [32,33]. Three proof-of-efficacy clinical trials in Africa were conducted in high HIV incidence settings [28–30, 93].
The French-funded Orange Farm trial in South Africa published in 2005 is hailed as the first landmark randomized controlled evidence of the impact of adult male circumcision on HIV incidence [30]. In this trial, 3128 HIV-negative men aged 18–24 were randomized to immediate versus delayed circumcision and followed up prospectively while receiving STI/HIV prevention counseling and voluntary counseling and testing services. The trial was stopped after recommendation by the Data Safety and Monitoring Board (DSMB) on interim analyses results that found that men in the intervention (immediate circumcision) arm were at strongly reduced risk of HIV-infection (RR = 0.4, 95% CI: 0.2–0.7, p<0.0001) [30]. Despite these results, there was still debate among clinicians, policy makers and the international community over whether these results could be generalized to different populations. In December 2006, NIAID-sponsored trials NIAID-funded trials in Uganda and Kenya provided additional scientific evidence for the role of adult male circumcision in HIV prevention [28,29]. Just like the South African trials, these trials were also stopped by their DSMB after interim analyses revealed among men in these trials, adult male circumcision reduced the risk of acquiring HIV infection by 48 percent in the Ugandan study and by 53 percent in the Kenyan study. Given these results, both trials were halted and men in the control group were offered circumcision. In order to understand the long term impact of adult male circumcision, the studies will continue to measure HIV infection rates and to study the risk-taking behavior and attitudes of participants. While the methods in these trials differed (foreskin clamp method in Kenya versus the sleeve method in Uganda), it was reported that circumcisions were safe when performed by trained medical personnel and with appropriate post-surgical follow-up to ensure management of any infections or other problems with wound healing [28].
It is not known whether there are other potential impacts of promulgating adult male circumcision in real world settings. There is a potential for behavioral disinhibition wherein circumcised males may engage in high risk behavior without the use of condoms under the false ‘reassurance’ that their circumcised status may prevent them from acquiring HIV. In fact, the South African study participants in the circumcised group actually reported higher risk sexual behaviors; nevertheless, the group had lower incidence of HIV, suggesting further the validity of the result that circumcision in highly protective. Although efficacious in trials, the real world implementation of adult male circumcision will be determined by the social context, cultural and religious acceptability and relative availability and acceptance of other modalities of prevention. It will be critical for the development of targeted and tailored communication messages to highlight both the benefits and limitations of this method that is, as of the end of 2006, the closest alternative to a preventive HIV vaccine. If promulgated properly, male circumcision is of enormous potential for the at-risk uninfected, especially when coupled with the ABC strategy at a population level [94].
The demand for male circumcision has reportedly increased in some countries with high HIV incidence, such as Swaziland and Zambia, after the results of the South African study [95]. The UN Work Plan on Male Circumcision is focusing on improving the safety of current male circumcision practices and assisting countries to obtain the data necessary for informed and evidence-based decision-making regarding the role of male circumcision in HIV-prevention programming [96]. The magnitude of benefit of male circumcision does not reach the levels of condom use, but it requires little ongoing adherence [97]. While surgery is rarely used for public health and prevention, circumcision of men, male adolescents, or male infants may represent exactly such an opportunity. We believe that STI services and male circumcision are both vital tools for control of HIV worldwide.
Acknowledgments
Supported in part by NIH grants 1U01AI068619-01 (HIV Prevention Trials Network Coordinating and Operations Center); 5D43TW000010-19 and 5D43TW001035-08 (Johns Hopkins and Vanderbilt-UAB AIDS International Training and Research Programs).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS website. [Accessed February 18, 2007];UNAIDS/WHO AIDS Epidemic Update: December 2006. 2006 Available online at: http://www.unaids.org.
- 2.The United States President’s Emergency Plan for AIDS Relief (PEPFAR) website. [Accessed February 18, 2007]; Available online at: http://www.pepfar.gov.
- 3.The Global Fund for AIDS, Tuberculosis and Malaria website. [Accessed February 18, 2007]; Available online at: http://www.theglobalfund.org.
- 4.Global HIV Prevention Working Group. New approaches to HIV prevention: Accelerating research and ensuring future access. Bill and Melinda Gates Foundation; Henry. J. Kaiser Family Foundation; 2006. [Accessed February 18, 2007]. Available online: http://www.kff.org/hivaids/hivghpwgpackage.cfm. [Google Scholar]
- 5.Stover J, Bertozzi S, Gutierrez JP, et al. The global impact of scaling up HIV/AIDS prevention programs in low- and middle-income countries. Science. 2006;311:1474–6. doi: 10.1126/science.1121176. [DOI] [PubMed] [Google Scholar]
- 6.Aral SO, Padian NS, Holmes KK. Advances in multilevel approaches to understanding the epidemiology and prevention of sexually transmitted infections and HIV: an overview. J Infect Dis. 2005;191 Suppl 1:S1–6. doi: 10.1086/425290. [DOI] [PubMed] [Google Scholar]
- 7.Manhart LE, Holmes KK. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked? J Infect Dis. 2005;191 Suppl 1:S7–24. doi: 10.1086/425275. [DOI] [PubMed] [Google Scholar]
- 8.Salomon JA, Hogan DR, Stover J, et al. Integrating HIV prevention and treatment: from slogans to impact. PLoS Med. 2005;2(1):e16. doi: 10.1371/journal.pmed.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maticka-Tyndale E, Brouillard-Coylea C. The effectiveness of community interventions targeting HIV and AIDS prevention at young people in developing countries. World Health Organ Tech Rep Ser. 2006;938:243–85. [PubMed] [Google Scholar]
- 10.Kirby D, Obasi A, Laris BA. The effectiveness of sex education and HIV education interventions in schools in developing countries. World Health Organ Tech Rep Ser. 2006;938:103–50. [PubMed] [Google Scholar]
- 11.Stoneburner R, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science. 2004;302:714–18. doi: 10.1126/science.1093166. [DOI] [PubMed] [Google Scholar]
- 12.Cleland J, Ali MM. Sexual abstinence, contraception, and condom use by young African women: a secondary analysis of survey data. Lancet. 2006;368:1788–93. doi: 10.1016/S0140-6736(06)69738-9. [DOI] [PubMed] [Google Scholar]
- 13.Sangani P, Rutherford G, Wilkinson D. Population-based interventions for reducing sexually transmitted infections, including HIV infection. Cochrane Database Syst Rev. 2004;(2):CD001220. doi: 10.1002/14651858.CD001220.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth M, Beyrer C, Soucat A. AIDS and public policy: the lessons and challenges of “success” in Thailand. Health Policy. 2003;64:13–37. doi: 10.1016/s0168-8510(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 15.Phoolcharoen W. HIV/AIDS prevention in Thailand: success and challenges. Science. 1998;280:1873–4. doi: 10.1126/science.280.5371.1873. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. Asia--the next frontier for HIV/AIDS. Two hard-hit countries offer rare success stories: Thailand and Cambodia. Science. 2003;301:1658–62. doi: 10.1126/science.301.5640.1658. [DOI] [PubMed] [Google Scholar]
- 17.USAID. [Accessed February 18, 2007];USAID, Senegal country profile. Available online at: http://www.usaid.gov/locations/sub-saharan_africa/countries/Senegal.
- 18.Pisani E, Garnett GP, Grassly NC, et al. Back to basics in HIV prevention: focus on exposure. BMJ. 2003;326:1384–7. doi: 10.1136/bmj.326.7403.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegbreit J, Bertozzi S, DeMaria LM, et al. Effectiveness of HIV prevention strategies in resource-poor countries: tailoring the intervention to the context. AIDS. 2006;20:1217–35. doi: 10.1097/01.aids.0000232229.96134.56. [DOI] [PubMed] [Google Scholar]
- 20.Rekart ML. Sex-work harm reduction. Lancet. 2005;366:2123–34. doi: 10.1016/S0140-6736(05)67732-X. [DOI] [PubMed] [Google Scholar]
- 21.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–6. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 22.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353:525–35. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 23.Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomized trial. Lancet. 2003;361:645–52. doi: 10.1016/s0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- 24.Korenromp EL, White RG, Orroth KK, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J Infect Dis. 2005;191 Suppl 1:S168–78. doi: 10.1086/425274. [DOI] [PubMed] [Google Scholar]
- 25.White RG, Orroth KK, Korenromp EL, et al. Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: A modeling study. J Acquir Immune Defic Syndr. 2004;37:1500–13. doi: 10.1097/01.qai.0000127062.94627.31. [DOI] [PubMed] [Google Scholar]
- 26.Grosskurth H, Gray R, Hayes R, et al. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet. 2000;355:1981–7. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 27.Mayaud P, Mosha F, Todd J, et al. Improved treatment services significantly reduce the prevalence of sexually transmitted diseases in rural Tanzania: results of a randomized controlled trial. AIDS. 1997;11:1873–80. doi: 10.1097/00002030-199715000-00013. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed February 18, 2007];NIAID press release, 13 December 2006. Available online at: http://www3.niaid.nih.gov/news/newsreleases/2006/AMC12_06press.htm.
- 29.CDC Fact sheet on Male Circumcision, December 2006. [Accessed February 18, 2007]; Available online: http://www.cdc.gov/hiv/resources/factsheets/circumcision.htm.
- 30.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. Erratum in: PLoS Med 2006;3(5):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss HA, Thomas SL, Munabi SK, et al. Male circumcision and risk of syphilis, chancroid, and genital herpes: A systematic review and meta-analysis. Sex Transm Infect. 2006;82:101–9. doi: 10.1136/sti.2005.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS. 2000;14:2361–70. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- 33.Siegfried N, Muller M, Volmink J, et al. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2003;(3):CD003362. doi: 10.1002/14651858.CD003362. [DOI] [PubMed] [Google Scholar]
- 34.Siegfried N, Muller M, Deeks J, et al. HIV and male circumcision--a systematic review with assessment of the quality of studies. Lancet Infect Dis. 2005;5:165–73. doi: 10.1016/S1473-3099(05)01309-5. [DOI] [PubMed] [Google Scholar]
- 35.Gray RH, Kiwanuka N, Quinn TC, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team AIDS. 2000;14:2371–81. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 36.Kahn JG, Marseille E, Auvert B. Cost-effectiveness of male circumcision for HIV prevention in a South African setting. PLoS Med. 2006;3:e517. doi: 10.1371/journal.pmed.0030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams BG, Lloyd-Smith JO, Gouws E, et al. The potential impact of male circumcision on HIV in Sub-Saharan Africa. PLoS Med. 2006;3:e262. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett EL, Makamure B, Cheung YB, et al. HIV incidence during a cluster-randomized trial of two strategies providing voluntary counselling and testing at the workplace, Zimbabwe. AIDS. 2007;21:483–489. doi: 10.1097/QAD.0b013e3280115402. [DOI] [PubMed] [Google Scholar]
- 39.Thielman NM, Chu HY, Ostermann J, et al. Cost-effectiveness of free HIV voluntary counseling and testing through a community-based AIDS service organization in Northern Tanzania. Am J Public Health. 2006;96:114–9. doi: 10.2105/AJPH.2004.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middelkoop K, Myer L, Smit J, et al. Design and evaluation of a drama-based intervention to promote voluntary counseling and HIV testing in a South African community. Sex Transm Dis. 2006;33:524–6. doi: 10.1097/01.olq.0000219295.50291.1d. [DOI] [PubMed] [Google Scholar]
- 41.Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356:113–21. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 42.Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Lancet. 2000;356:103–12. [No authors listed] [PubMed] [Google Scholar]
- 43.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Trop Med Int Health. 2004;9:566–72. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 44.Stringer JS, Sinkala M, Stout JP, et al. Comparison of two strategies for administering nevirapine to prevent perinatal HIV transmission in high-prevalence, resource-poor settings. J Acquir Immune Defic Syndr. 2003;32:506–13. doi: 10.1097/00126334-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grinstead OA, Gregorich SE, Choi KH, et al. Positive and negative life events after counselling and testing: the Voluntary HIV-1 Counselling and Testing Efficacy Study. AIDS. 2001;15:1045–52. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 46.Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17 Suppl 1:S102–13. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- 47.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 48.Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191:1391–3. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 49.Pronyk PM, Hargreaves JR, Kim JC, et al. Effect of a structural intervention for the prevention of intimate-partner violence and HIV in rural South Africa: a cluster randomised trial. Lancet. 2006;368:1973–83. doi: 10.1016/S0140-6736(06)69744-4. [DOI] [PubMed] [Google Scholar]
- 50.Hogan DR, Baltussen R, Hayashi C, et al. Cost effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ. 2005;331:1431–7. doi: 10.1136/bmj.38643.368692.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dayton JM, Merson MH. Global dimensions of the AIDS epidemic: implications for prevention and care. Infect Dis Clin North Am. 2000;14:791–808. doi: 10.1016/s0891-5520(05)70134-3. [DOI] [PubMed] [Google Scholar]
- 52.Green E. Rethinking AIDS prevention: Learning from successes in developing countries. Westport (Connecticut): Praeger Publishers; 2003. p. 374. [Google Scholar]
- 53.Dworkin SL, Ehrhardt AA. Going beyond “ABC” to include “GEM”: critical reflections on progress in the HIV/AIDS epidemic. Am J Public Health. 2007;97:13–8. doi: 10.2105/AJPH.2005.074591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elsey H, Tolhurst R, Theobald S. Mainstreaming HIV/AIDS in development sectors: Have we learnt the lessons from gender mainstreaming? AIDS Care. 2005;17:988–98. doi: 10.1080/09540120500102250. [DOI] [PubMed] [Google Scholar]
- 55.Elliott R, Csete J, Wood E, et al. Harm reduction, HIV/AIDS, and the human rights challenge to global drug control policy. Health Hum Rights. 2005;8:104–38. [PubMed] [Google Scholar]
- 56.Ayres JR, Paiva V, Franca I, Jr, et al. Vulnerability, Human Rights, and Comprehensive Health Care Needs of Young People Living With HIV/AIDS. Am J Public Health. 2006;96:1001–6. doi: 10.2105/AJPH.2004.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. [Accessed on February 18, 2007];Prevention and control of sexually transmitted infections: draft global strategy. 2006 May; Available online at: http://www.who.int/gb/ebwha/pdf_files/WHA59/A59_11-en.pdf.
- 58.Holmes KK, DeLay PR, Cohen MS. STD control: A public health priority. In: Dallabetta GA, Laga M, Lamptey PL, editors. Control of Sexually Transmitted Diseases: A Handbook for the Design and Management of Programs. Airlington, VA: AIDSCAP/Family Health International; 1996. pp. v–xii. [Google Scholar]
- 59.May RM, Anderson RM. The transmission dynamics of human immunodeficiency virus (HIV) Philos Trans R Soc Lond B Biol Sci. 1988;321:565–607. doi: 10.1098/rstb.1988.0108. [DOI] [PubMed] [Google Scholar]
- 60.Hogan DR, Salomon JA. Prevention and treatment of human immunodeficiency virus/acquired immunodeficiency syndrome in resource-limited settings. Bull World Health Organ. 2005;83:135–43. [PMC free article] [PubMed] [Google Scholar]
- 61.Shelton J, Halperin D, Nantulya V, et al. Partner reduction is crucial for balanced “ABC” approach to HIV prevention. BMJ. 2004;328:891–893. doi: 10.1136/bmj.328.7444.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halperin D, Steiner M, Cassell M, et al. The time has come for common ground on preventing sexual transmission of HIV. Lancet. 2004;364:1913–1915. doi: 10.1016/S0140-6736(04)17487-4. [DOI] [PubMed] [Google Scholar]
- 63.Laga M, Alary M, Nzila N, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344(8917):246–8. doi: 10.1016/s0140-6736(94)93005-8. [DOI] [PubMed] [Google Scholar]
- 64.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7(1):95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 66.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349(9069):1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 67.Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med. 2004;12:104–7. [PubMed] [Google Scholar]
- 68.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 69.Kalichman SC, Rompa D, Cage M. Sexually transmitted infections among HIV seropositive men and women. Sex Transm Infect. 2000;76:350–4. doi: 10.1136/sti.76.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilleece Y, Sullivan A. Management of sexually transmitted infections in HIV positive individuals. Curr Opin Infect Dis. 2005;18:43–7. doi: 10.1097/00001432-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Dallabetta G, Serwadda D, Mugrditchian D. Controlling Other Sexually Transmitted Diseases. In: Gibney L, DiClemente R, Vermund S, editors. Preventing HIV in Developing Countries: Biomedical and Behavioral Approaches. Kluwer Academic/Plenum Publishers; New York: 1999. [Google Scholar]
- 72.World Health Organization. (WHO) [Accessed February 18, 2007];Training Modules for the Syndromic Management of Sexually Transmitted Infections. (2). Available online at: http://www.who.int/reproductive-health/stis/training.htm.
- 73.Hylton-Kong T, Brathwaite AR, Del Rosario GR, et al. Marginal validity of syndromic management for reproductive tract infections among pregnant women in Jamaica. Int J STD AIDS. 2004;15:371–5. doi: 10.1258/095646204774195209. [DOI] [PubMed] [Google Scholar]
- 74.Cheluget B, Joesoef MR, Marum LH, et al. Changing patterns in sexually transmitted disease syndromes in Kenya after the introduction of a syndromic management program. Sex Transm Dis. 2004;31(9):522–5. doi: 10.1097/01.olq.0000137896.40790.7d. [DOI] [PubMed] [Google Scholar]
- 75.Behets FM, Williams Y, Brathwaite A, et al. Management of vaginal discharge in women treated at a Jamaican sexually transmitted disease clinic: use of diagnostic algorithms versus laboratory testing. Clin Infect Dis. 1995;21:1450–5. doi: 10.1093/clinids/21.6.1450. [DOI] [PubMed] [Google Scholar]
- 76.Wolday D, Gebremariam Z, Mohammed Z, et al. The impact of syndromic treatment of sexually transmitted diseases on genital shedding of HIV-1. AIDS. 2004;18:781–5. doi: 10.1097/00002030-200403260-00009. [DOI] [PubMed] [Google Scholar]
- 77.Jenniskens F, Obwaka E, Kirisuah S, et al. Syphilis control in pregnancy: decentralization of screening facilities to primary care level, a demonstration project in Nairobi, Kenya. Int J Gynaecol Obstet. 1995;48 Suppl:S121–8. doi: 10.1016/0020-7292(95)02326-8. [DOI] [PubMed] [Google Scholar]
- 78.Sahin-Hodoglugil NN, Woods R, Pettifor A, et al. A comparison of cost-effectiveness of three protocols for diagnosis and treatment of gonococcal and chlamydial infections in women in Africa. Sex Transm Dis. 2003;30:455–69. doi: 10.1097/00007435-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 79.Geisler WM, Yu S, Venglarik M, et al. Vaginal leucocyte counts in women with bacterial vaginosis: relation to vaginal and cervical infections. Sex Transm Infect. 2004;80(5):401–5. doi: 10.1136/sti.2003.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams EJ, Garcia PJ, Garnett GP, et al. The cost-effectiveness of syndromic management in pharmacies in Lima, Peru. Sex Transm Dis. 2003;30:379–87. doi: 10.1097/00007435-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Crabbe F, Tchupo JP, Manchester T, et al. Prepackaged therapy for urethritis: the “MSTOP” experience in Cameroon. Sex Transm Infect. 1998;74:249–52. doi: 10.1136/sti.74.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gangopadhyay DN, Chanda M, Sarkar K, et al. Evaluation of sexually transmitted diseases/human immunodeficiency virus intervention programs for sex workers in Calcutta, India. Sex Transm Dis. 2005;32:680–4. doi: 10.1097/01.olq.0000175399.43457.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanenberg RS, Rojanapithayakorn W, Kunasol P, et al. Impact of Thailand’s HIV-control programme as indicated by the decline of sexually transmitted diseases. Lancet. 1994;344:243–5. doi: 10.1016/s0140-6736(94)93004-x. [DOI] [PubMed] [Google Scholar]
- 84.Tuladhar SM, Mills S, Acharya S, et al. The role of pharmacists in HIV/STD prevention: evaluation of an STD syndromic management intervention in Nepal. AIDS. 1998;12 Suppl 2:S81–7. [PubMed] [Google Scholar]
- 85.Behets FM, Genece E, Narcisse M, et al. Approaches to control sexually transmitted diseases in Haiti, 1992–95. Bull World Health Organ. 1998;76:189–94. [PMC free article] [PubMed] [Google Scholar]
- 86.Sanchez J, Gotuzzo E, Escamilla J, et al. Sexually transmitted infections in female sex workers: reduced by condom use but not by a limited periodic examination program. Sex Transm Dis. 1998;25:82–9. doi: 10.1097/00007435-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Moherdaui F, Vuylsteke B, Siqueira LF, et al. Validation of national algorithms for the diagnosis of sexually transmitted diseases in Brazil: results from a multicentre study. Sex Transm Infect. 1998;74 Suppl 1:S38–43. [PubMed] [Google Scholar]
- 88.Green M, Hoffman IF, Brathwaite A, et al. Improving sexually transmitted disease management in the private sector: the Jamaica experience. AIDS. 1998;12 Suppl 2:S67–72. [PubMed] [Google Scholar]
- 89.Patterson BK, Landay A, Siegel JN, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–73. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szabo R, Short RV. How does male circumcision protect against HIV infection? BMJ. 2000;320(7249):1592–4. doi: 10.1136/bmj.320.7249.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weiss HA. Male circumcision as a preventive measure against HIV and other sexually transmitted diseases. Curr Opin Infect Dis. 2007;20:66–72. doi: 10.1097/QCO.0b013e328011ab73. [DOI] [PubMed] [Google Scholar]
- 92.Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect. 2004;80:174–82. doi: 10.1136/sti.2002.004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.USAID. [Accessed February 18, 2007];Issue Brief: Male Circumcision. 2007 Available online at: http://www.usaid.gov/our_work/global_health/aids/TechAreas/research/issue_mc.pdf.
- 94.Halperin DT, Bailey RC. Male circumcision and HIV infection: 10 years and counting. Lancet. 1999;354:1813–5. doi: 10.1016/S0140-6736(99)03421-2. [DOI] [PubMed] [Google Scholar]
- 95.Wise J. Demand for male circumcision rises in a bid to prevent HIV. Bull World Health Organ. 2006;84:509–511. [PMC free article] [PubMed] [Google Scholar]
- 96.Hankins C, Williams BG, Schmid G, et al. Male circumcision and the HIV epidemic: The kindest cut? (abstract). Canadian Journal of Infectious Diseases and Medical Microbiology; Abstracts of the 15th Annual Canadian Conference on HIV/AIDS Research; Quebec. 2006. [Google Scholar]
- 97.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004;82:454–61. [PMC free article] [PubMed] [Google Scholar]
- 98.O’Reilly KR, Higgins DL. AIDS Community Demonstration Projects for HIV prevention among hard-to-reach groups. Public Health Rep. 1991;106:714–20. [PMC free article] [PubMed] [Google Scholar]
- 99.Martin DJ, Arns PG, Batterham PJ, et al. Workforce reentry for people with HIV/AIDS: intervention effects and predictors of success. Work. 2006;27:221–33. [PubMed] [Google Scholar]
- 100.Walker D. Cost and cost-effectiveness of HIV/AIDS prevention strategies in developing countries: is there an evidence base? Health Policy Plan. 2003;18:4–17. doi: 10.1093/heapol/18.1.4. [DOI] [PubMed] [Google Scholar]
- 101.Vassall A, Compernolle P. Estimating the resource needs of scaling-up HIV/AIDS and tuberculosis interventions in sub-Saharan Africa: a systematic review for national policy makers and planners. Health Policy. 2006;79:1–15. doi: 10.1016/j.healthpol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Janssen RS, Holtgrave DR, Valdiserri RO, et al. Serostatus Approach to Fighting the HIV Epidemic: prevention strategies for infected individuals. Am J Public Health. 2001;91:1019–24. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liechty CA. The evolving role of HIV counseling and testing in resource-limited settings: HIV prevention and linkage to expanding HIV care access. Curr HIV/AIDS Rep. 2004;1:181–5. doi: 10.1007/s11904-004-0028-5. [DOI] [PubMed] [Google Scholar]
- 104.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 105.Bessinger R, Akwara P, Halperin D. Sexual behavior, HIV and fertility trends: A comparative analysis of six countries. [Accessed February 17, 2007];Phase I of the ABC study. Measure Evaluation, USAID. 2003 Available: http://www.cpc.unc.edu/measure/publications/pdf/sr-03-21b.pdf.
- 106.Mantell JE, Harrison A, Hoffman S, et al. The Mpondombili Project: Preventing HIV/AIDS and Unintended Pregnancy among Rural South African School-Going Adolescents. Reprod Health Matters. 2006;14:113–22. doi: 10.1016/S0968-8080(06)28269-7. [DOI] [PubMed] [Google Scholar]
- 107.Anyangwe SC, Mtonga C, Chirwa B. Health inequities, environmental insecurity and the attainment of the millennium development goals in sub-Saharan Africa: the case study of Zambia. Int J Environ Res Public Health. 2006;3:217–27. doi: 10.3390/ijerph2006030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watkins S. Navigating the AIDS Epidemic in Rural Malawi. Popul Dev Rev. 2004;4:673–705. [Google Scholar]
- 109.Kirungi WL, Musinguzi J, Madraa E, et al. Trends in antenatal HIV prevalence in urban Uganda associated with uptake of preventive sexual behaviour. Sex Transm Infect. 2006;82(Suppl 1):i36–i41. doi: 10.1136/sti.2005.017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sales JM, Milhausen RR, Diclemente RJ. A decade in review: building on the experiences of past adolescent STI/HIV interventions to optimise future prevention efforts. Sex Transm Infect. 2006;82:431–6. doi: 10.1136/sti.2005.018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DiClemente RJ, Milhausen R, Sales JM, et al. A programmatic and methodologic review and synthesis of clinic-based risk-reduction interventions for sexually transmitted infections: research and practice implications. Semin Pediatr Infect Dis. 2005;16:199–218. doi: 10.1053/j.spid.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Corbett EL, Makamure B, Cheung YB, et al. HIV incidence during a cluster-randomized trial of two strategies providing voluntary counselling and testing at the workplace, Zimbabwe. AIDS. 2007;21:483–489. doi: 10.1097/QAD.0b013e3280115402. [DOI] [PubMed] [Google Scholar]
- 113.Mast TC, Kigozi G, Wabwire-Mangen F, et al. Immunisation coverage among children born to HIV-infected women in Rakai district, Uganda: Effect of voluntary testing and counselling (VCT) AIDS Care. 2006;18:755–63. doi: 10.1080/09540120500521053. [DOI] [PubMed] [Google Scholar]
- 114.Rasch V, Yambesi F, Massawe S. Post-abortion care and voluntary HIV counselling and testing--an example of integrating HIV prevention into reproductive health services. Trop Med Int Health. 2006;11:697–704. doi: 10.1111/j.1365-3156.2006.01607.x. [DOI] [PubMed] [Google Scholar]
- 115.Thielman NM, Chu HY, Ostermann J, et al. Cost-effectiveness of free HIV voluntary counseling and testing through a community-based AIDS service organization in Northern Tanzania. Am J Public Health. 2006;96:114–9. doi: 10.2105/AJPH.2004.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]


