Abstract
Polybrominated diphenyl ethers (PBDEs) have been incorporated into many consumer products as flame retardants. Due to their persistence and ability to bioaccumulate, PBDEs are ubiquitous in human blood and breast milk samples from industrialized nations. Although there exists a potential for environmental pollutants such as PBDEs to adversely impact birth outcomes and perinatal health, reports of PBDE levels in human reproductive tissues are limited. The aim of the current study is to evaluate the total levels and congener-specific profiles of PBDEs from human extraplacental gestational membranes. Gestational membranes from five term pregnancies were obtained from non-laboring caesarian deliveries at the University of Michigan Women's Hospital Birth Center. Duplicate samples were extracted and analyzed by GC-MS for twenty-one PBDE congeners. Total PBDE loading was 17.4 ± 3.9 pg/g tissue (5.62 ± 1.28 ng/g lipid). Seventy-eight percent of the total measurable PBDE loading was due to BDEs 47, 49, 99, 100, and 153, with measured values of 3.63, 3.15, 3.05, 1.74, and 1.90 pg/g tissue(1170, 1018, 983, 561, and 612 pg/g lipid), respectively. The remaining 28% was comprised of BDEs 17, 28, 66, 71, 85, and 154. No octa-, nona- or deca- BDEs were identified. Although previously unreported in the human gestational compartment, BDE 49 comprised 17% of the total PBDE level. This work establishes baseline accumulated levels of PBDEs in gestational membranes of women in Southeast Michigan.
Keywords: Polybrominated diphenyl ethers (PBDEs), extraplacental membranes, human exposure, pregnancy
Introduction
Polybrominated diphenyl ethers (PBDEs) are commercially produced synthetic flame retardants consisting of two phenyl rings linked by an ether bond with variable hydrogen to bromine substitutions. Mixtures of PBDEs have been used in textiles, plastics, building materials and insulation. Because of their chemical structure, several of the 209 PBDE congeners tend to be environmentally persistent and bioaccumulative (1). In human measurement studies, congeners BDE-47, 99, 100 and 153 are most often detected and comprise the majority of total PBDE loading (2-4). Since 1970, over 50 reports have identified PBDEs in human adipose, liver, breast, whole blood, serum, breast milk, fetal cord blood and placenta (reviewed by Hites) (1). Furthermore, several studies have begun to address gestational compartment dynamics through paired sampling of maternal blood, fetal blood and placenta (3, 5-8), although none to date has addressed the extra-placental maternal/fetal gestational membranes.
In animal studies, PBDEs exhibit neurodevelopmental(9, 10), hepatic (11, 12), immunological (13, 14) and thyroid toxicities (12). Rabbits orally exposed to PBDEs show decreased gestation length (15). Because of their environmental persistence and toxicity, the US EPA has identified PBDEs as a priority human health concern (16). Production of penta-and octa-substituted congeners ceased in 2005 in the USA, and the European Union passed legislation to prohibit all PBDE use after July 2006 (a short-term extension for deca-BDE was granted in October 2005). Although U.S. and European production of PBDEs has ceased except for BDE 209, many PBDEs remain a pertinent risk to human health due to production in other regions, import/export of goods containing PBDEs, the wide stock of PBDE-containing materials in-use, and environmental persistence (17).
Extraplacental gestational membranes are composed of the amniotic, chorionic and decidual layers that surround the fetus and create a protective barrier during gestation. Minimally vascularized and containing dense collagen, the extraplacental membranes are distinctly different from the highly vascularized placental disk used to transfer oxygen, nutrients and waste products between maternal and fetal blood. Additionally, the extraplacental membranes play an important role in parturition, producing both cytokines and prostaglandins in all three layers during labor (18, 19). Cytokines and prostaglandins have been closely linked to the biological processes of birth including dynamic cervical remodeling (20), uterine contractility (21), and gestational membrane rupture (22). Rupture of these membranes plays a key role in term and preterm parturition. Recent increases in the rate of preterm premature rupture of membranes (PPROM) has made it a leading cause of preterm birth, particularly in African-American women, and often results in fetal morbidity or mortality (23). The causes of PPROM are still to be determined, and the role of environmental pollutants such as PBDEs has yet to be addressed.
Americans are ubiquitously exposed to PBDEs, primarily through dust and diet (24). The 2003−2004 National Health and Nutrition Examination Survey (NHANES) identified measurable PBDE levels in 89% (2040 of 2305) of randomly selected blood serum samples. Once in the maternal blood circulation, PBDEs may access the gestational membranes directly or indirectly. The spiral arteries of the uterus supply blood to the decidua, providing a direct route of transfer to the membranes. In addition, previous studies have shown that PBDEs can enter the gestational compartment and fetal blood circulation, presumably by crossing the placental blood interface (25, 26). It is also possible that PBDEs reach the gestational membranes indirectly from the fetal compartment through the amniotic fluid. Although no studies to date have assessed PBDE levels in the amniotic fluid, studies of structurally similar compounds (polychlorinated biphenyls) have shown high levels in human amniotic fluid (27). Because of the likely PBDE exposure of gestational membranes and their critical role in parturition, an assessment of PBDE deposition in the human gestational membranes was undertaken.
The aim of this study was to determine the total PBDE concentrations and congener-specific deposition profiles in human extra-placental gestational membranes from women in southeast Michigan. Twenty-one PBDE congeners (BDEs 17, 28, 47, 49, 66, 71, 75, 85, 99, 100, 138, 153, 154, 166, 183, 190, 203, 206, 207, 208 and 209 [deca-]) were measured in the present study.
Methods and Materials
Sample Collection
From November 2007 through January 2008 duplicate extraplacental membranes samples were obtained from five healthy non-laboring women undergoing scheduled caesarean section deliveries at 37−39 completed weeks gestation at the University of Michigan Women's Hospital Birth Center in Ann Arbor, Michigan. Exclusion criteria included cigarette smoking, prescription of antibiotics in the past two weeks, collagen vascular disease, immunocompromised conditions, bacterial vaginosis or clinical chorioamnionitis (as noted in the chart or suspected by attending physician), cervical cerclage, third trimester bleeding, major maternal medical conditions (e.g., chronic renal disease, sarcoidosis, hepatitis, HIV), pre-eclampsia, diabetes, multifetal pregnancy, or any other condition which would require the tissue to be sent to pathology. Personal identifiable information was not collected, in compliance with the University of Michigan Institutional Review Board requirements. The investigators had no direct interaction with the human subjects and the tissues collected would have been otherwise discarded.
Following delivery, placentae with attached membranes were transported to the laboratory. Full thickness membranes were separated from the placental disk, allowing a 3-cm margin to prevent sampling from the transitional zone. Each 1-g sample was compiled from 5−7 random collections of full-thickness membranes and was placed into a glass sample vials with Teflon® lined caps. To prevent contamination of samples, all stainless steel instruments and glassware used for collection were baked at 500 °C and rinsed with homogenization solvent immediately prior to use. Laboratory practices followed universal safety precautions for handling human tissue (e.g., personnel vaccination for hepatitis B and wearing of laboratory safety glasses, gloves, face mask and lab coat when handling tissues).
Analysis of lipid content
Replicate 1-g tissue samples were homogenized using a Polytron® PT2100 tissue homogenizer (Kinematica, Bohemia, NY) in 25 ml hexane/ethyl acetate (9:1 v/v), or for comparison, in 25 ml hexane/carbon tetrachloride (4:1 v/v). HPLC grade hexane, carbon tetrachloride and ethyl acetate were purchased from Fisher Scientific (Fair Lawn, NJ), Acros Organics (Geel, Belgium) and Sigma (St. Louis, MO), respectively. Samples were centrifuged and the organic fraction was transferred to pre-weighed glass beakers. The solvent was volatilized under a N2 (Cryogenic Gasses, Detroit, MI) stream, and the remaining lipid was maintained overnight at 100 °C to remove any remaining water. The glass beakers were then reweighed to determine the lipid content of the initial sample, which was expressed as percent of total tissue weight.
PBDE analysis
Replicate 1-g samples from each caesarean delivery were homogenized using a Polytron® PT2100 tissue homogenizer in 25 ml hexane/ethyl acetate (9:1 v/v). The organic fractions were transferred to volumetric test tubes and evaporated to 1 ml under a N2 stream. Samples were spiked with internal standards (CB IUPAC Nos 136 and 204). Each sample (2 μl injection) was analyzed for 21 PBDE congeners by GC-MS (Agilent 6890/5973, Palo Alto, CA, USA) using negative chemical ionization mode and a DB-5 column (30 m, 0.25 mm id, 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA). The carrier gas was helium (flow rate of 0.7 mL min−1, inlet pressure of 5.43 psi, average velocity of 31 cm s−1), and methane was the reagent gas. In all runs, the injector was set at 280 °C. The oven temperature started at 80 °C, held for 2 min, then ramped at 10 °C min−1 to 300 °C, and held for 46 min. A separate run was made for BDE-209 using a temperature program that avoided fragmentation. In the latter case, the initial temperature again was 80 °C, held for 2 min, then ramped at 50 °C min−1 to 300 °C and held for 40 min. Calibration standards included BDEs 17, 28, 47, 49, 66, 71, 75, 85, 99, 100, 138, 153, 154, 166, 183, 190, 203, 206, 207, 208 and 209. Method detection limits (MDLs) were estimated based on three times the standard deviation of seven low concentration standards and then dividing by the collected mass (g) of the samples. The estimated MDLs were 0.010−0.020 ng/g for tri-through hexa-BDEs, 0.020−0.040 ng/g for hepta- through nona-BDEs, and 2.5 ng/g for deca-BDE (assuming a 1 g tissue sample).
Data analysis
Data for BDEs 17, 28, 47, 49, 66, 71, 85, 99, 100, 153, and 154 are represented as the mean ± SEM. Two replicate sample measures of BDE congeners were averaged and a grand mean was calculated for each BDE congener (N=5). Measurements below the MDL were assigned a value of one-half the MDL. Congener concentration as a percent of total PBDE loading was calculated by averaging the percent each congener constituted from each independent sample. BDEs 75, 138, 166, 183, 190, 203, 206, 207, 208 and 209 (Deca) were not detected in any samples and have been excluded from subsequent data analysis.
Results and Discussion
The lipid content of 1-g gestational membrane samples using the hexane/carbon tetrachloride solvent averaged 0.30%. Comparable results (0.31%) were obtained using hexane/ethyl acetate. Based on these results, hexane/ethyl acetate was used for tissue extractions prior to PBDE analysis. The lipid content of the human gestational membranes appears lower than the lipid content reported for the human placenta (6, 28).
On a tissue weight basis, the mean total PBDE concentration in the gestational membranes was 17.41 ±3.98 pg/g (n=5; Table 1). Values for each sample are the average of two replicate measures. From this a grand mean was calculated based on the sample means (N=5). On a lipid weight basis, the total PBDE level was 5.62 ± 1.28 ng/g (range from 3.06 to 9.53 ng/g) in the gestational membranes (Table 1). Lipid contents for all human gestational membranes measured were 0.31%.
Table 1.
Total PBDE loading in human fetal membranes. Total measured concentrations of PBDEs represented as pg/g tissue and ng/g lipid (mean±SEM). All process blanks were below the limit of detection.
| Sample: | ||||||||
|---|---|---|---|---|---|---|---|---|
| PBDE Congener | MDL | 1 | 2 | 3 | 4 | 5 | Mean (SEM) | |
| 17 | 0.01 | 0.91 | 0.86 | 0.55 | <0.01a | <0.01a | 0.46 | (0.21) |
| 28 | 0.01 | 1.07 | 0.89 | 0.17 | <0.01a | <0.01a | 0.43 | (0.17) |
| 49 | 0.01 | 5.07 | 4.16 | 2.71 | 1.34 | 2.50 | 3.15 | (0.68) |
| 71 | 0.02 | 1.23 | 1.46 | 0.49 | 0.97 | 0.83 | 0.99 | (0.16) |
| 47 | 0.02 | 5.49 | 5.50 | 2.28 | 2.30 | 2.58 | 3.63 | (0.54) |
| 66 | 0.02 | 0.53 | 0.35 | <0.02a | <0.02a | <0.02a | 0.18 | (0.12) |
| 100 | 0.02 | 2.88 | 1.86 | 1.43 | 1.05 | 1.48 | 1.74 | (0.22) |
| 99 | 0.02 | 4.17 | 3.26 | 2.77 | 2.62 | 2.41 | 3.05 | (0.32) |
| 85 | 0.02 | 1.68 | 2.11 | <0.02a | <0.02a | <0.02a | 0.76 | (0.32) |
| 154 | 0.01 | 3.83 | 1.38 | 0.31 | 0.01 | 0.01 | 1.11 | (0.53) |
| 153 | 0.01 | 2.68 |
2.32 |
1.58 |
1.16 |
1.75 |
1.90 | (0.36) |
| ΣPBDE | 29.56 | 24.14 | 12.29 | 9.47 | 11.58 | 17.41 | (3.98) | |
| ng/g LWb | 9.53 | 7.79 | 3.97 | 3.06 | 3.73 | 5.62 | (1.28) | |
values falling below the MDL were assigned a value one-half the MDL.
LW= lipid weight
Lipid content for all membranes measured were 0.31%. N=5
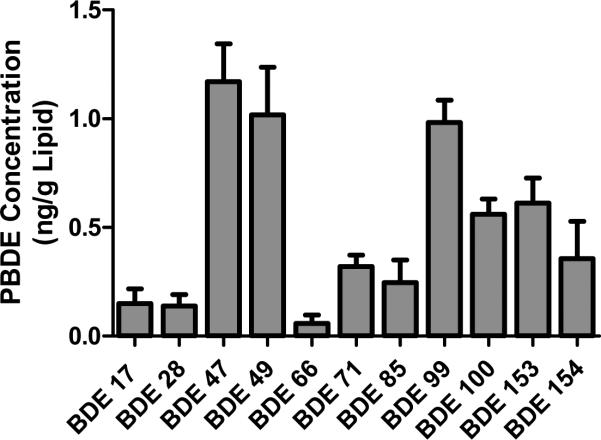
The individual congener profile is shown in Figure 1. Congeners exceeding 0.5 ng/g lipid were BDEs 47 (1.17 ng/g), 49 (1.02 ng/g), 99 (0.98 ng/g), 100 (0.56 ng/g) and 153 (0.61 ng/g). Congeners detected at lower concentrations were BDEs 17 (0.15 ng/g), 28 (0.14 ng/g), 66 (0.06 ng/g), 71 (0.32 ng/g), 85 (0.25 ng/g) and 154 (0.36 ng/g). BDEs 75, 138, 166, 183, 190, 203, 206, 207, 208 and 209 (Deca) were not identified in any samples.
FIGURE 1.
PBDE congener concentrations in human gestational membranes. Data are presented as mean±SEM. N=5
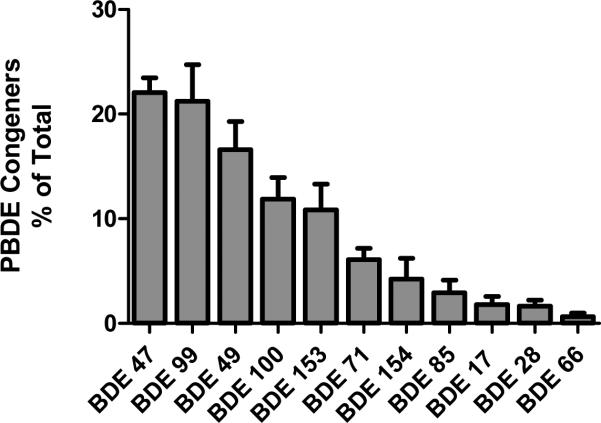
Individual congener contributions to total PBDEs were assessed for each sample. Percent of total (mean±SEM; N=5) is shown in Figure 2. Of the total 5.62 ng/g lipid PBDE loading, BDEs 47, 99, 49, 100, and 153 comprised 22, 21, 17, 12 and 11 %, respectively, for a total of 83%. Additional congeners identified and contributing to the total tissue concentration include BDEs 17(2%), 28(2%), 66(<1%), 71(6%), 85(3%) and 154(4%). BDEs 75, 138, 166, 183, 190, 203, 206, 207, 208 and deca-BDE were below the limit of detection in all samples tested and were therefore excluded from this analysis. When comparing the major contributing congeners analyzed in various studies, this composition is similar to the profiles observed in fish(29), birds (30), air (31), and sediment (32-34) in the great lakes region.
FIGURE 2.
PBDE congener concentration as percent of total PBDE loading. Data are presented as mean±SEM. N=5
This is the first report of PBDE levels in human extraplacental gestational membranes. These PBDE levels are several orders of magnitude lower than those reported by Doucet et al. in placenta proper from North America (35). Doucet et al. report highly variable levels ranging from >20,000 ng/g lipid in 2004 to ∼200 ng/g lipid in 2005. Increased vascularization may lead to increased burdens in the placenta, although the relative PBDEs in the placental tissue versus blood remaining in the placental sample has yet to be addressed. The impact of lipid weight adjustment from tissue weight could not be addressed because placental lipid content was not reported. A similar study of European women showed significant variation in placental lipid content between Denmark and Finland (8). When compared with placenta proper from European women (reviewed by Frederiksen et al.) the PBDE levels reported in this study are 3−5 fold higher those previously measured (1.18−1.9 ng/g lipid) (24). This is not unexpected as previous studies suggest biotic and abiotic PBDE concentrations in North America may be several orders of magnitude higher than those found in Europe (1, 24) and levels in gestational membranes appear to be several orders of magnitude lower than placenta. It should also be noted that BDE congeners selected for measurement vary between studies and may contribute to the variation seen in total PBDE levels between studies.
Previous research has suggested BDEs with greater numbers of bromine atoms have shorter biological half lives (36). Thuresson et al. have estimated the biological half-lives of these large BDEs to range from 15 days (deca-BDE) to 97 days (BDE 183) in human serum. Relatively short biological half-lives in combination with minimal vascularization and potential steric hindrances of infiltration into the dense gestational membranes may contribute to our failure to identify higher order congeners in this study.
The tetra-brominated congener BDE-49 has been identified as a major contributor to PBDE accumulation in fish (37, 38), including one report from the Great Lakes that found it to be the most abundant congener (39). Interestingly, our data also show high levels of BDE-49, which has not been previously reported in the gestational compartment. BDE-49 comprised 17% of the total PBDE concentration. Because BDE-49 is often unreported (and presumably unmeasured) in the vast majority of human research, our results suggest that PBDE levels may be being underestimated by as much as 14−19%.
Oral exposure to PBDEs decreases gestational length in rabbits (15), but a mechanism explaining this finding is lacking. Because the extra-placental gestational membranes play critical roles in pregnancy and parturition (18-22), they may be important targets of PBDE activity. Current research suggests a possible mechanism by which PBDE stimulation of the gestational membranes may activate parturition pathways via reactive oxygen species generation. Penta-BDE increases reactive oxygen species in neutrophil granulocytes in vitro (40). Reactive oxygen species are potent activators of the nuclear factor-kappa B (NF-κB) nuclear transcription pathway, which activates cytokine and prostaglandin production (reviewed by Schoonbroodt and Piette) (41). Because cytokines and prostaglandins are important stimulants of parturition (42-44), premature activation of this pathway by PBDEs could contribute to preterm birth. Further research is needed to investigate this hypothesis, and to assess whether a link can be made between PBDE exposure and preterm birth in women. The identification of total and congener-specific PBDE levels within the human gestational membranes in the present report is an important first step for further research.
This research provides evidence of accumulation for individual PBDE congeners in human gestational membranes. These initial data indicate a need for further investigation into the partitioning of specific PBDE congeners within the fetal membranes and the greater gestational compartment. Furthermore, these data suggest that evaluation of PBDE effects on gestational membranes may be warranted. The levels reported here for human tissue accumulation will allow assessment of the relevance of PBDE concentrations on gestational membrane responses in future experiments.
Supplementary Material
Acknowledgments
We gratefully acknowledge support of this work by the Michigan Institute for Clinical and Health Research of the University of Michigan (NIH UL1-RR-024986). Additional support for Mark Miller was provided by a NIEHS Institutional Training Grant (T32 ES07062), NIH Institutional Training Grant (T32 HD 007048) and the Department of Environmental Health Sciences of the University of Michigan. We thank the staff of the University of Michigan Women's Hospital Birth Center for assisting in the acquisition of tissues, especially Dr. Mark Chames.
Footnotes
Tetrabromobisphenol A was also measured in the current study. Results and discussion can be found in Supporting Information. This information is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 2.Choi JW, Fujimaki TS, Kitamura K, Hashimoto S, Ito H, Suzuki N, Sakai S, Morita M. Polybrominated dibenzo-p-dioxins, dibenzofurans, and diphenyl ethers in Japanese human adipose tissue. Environ Sci Technol. 2003;37(5):817–21. doi: 10.1021/es0258780. [DOI] [PubMed] [Google Scholar]
- 3.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111(9):1249–52. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meironyte Guvenius D, Bergman A, Noren K. Polybrominated diphenyl ethers in Swedish human liver and adipose tissue. Arch Environ Contam Toxicol. 2001;40(4):564–70. doi: 10.1007/s002440010211. [DOI] [PubMed] [Google Scholar]
- 5.Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D, Yu L, Fu J. Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ Pollut. 2006;144(3):1024–30. doi: 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 6.Gomara B, Herrero L, Ramos JJ, Mateo JR, Fernandez MA, Garcia JF, Gonzalez MJ. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007;41(20):6961–8. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- 7.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect. 2007;115(12):1794–800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115(10):1519–26. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24(3):449–62. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 10.Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci. 2006;92(1):211–8. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- 11.Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61(1):76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- 12.Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66(1):105–16. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology. 1994;86(1−2):49–61. doi: 10.1016/0300-483x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 14.Thuvander A, Darnerud PO. Effects of polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) on some immunological parameters after oral exposure in rats and mice. Toxicological and Environmental Chemistry. 1999;79:229–242. [Google Scholar]
- 15.Breslin WJ, Kirk HD, Zimmer MA. Teratogenic evaluation of a polybromodiphenyl oxide mixture in New Zealand white rabbits following oral exposure. Fundam Appl Toxicol. 1989;12(1):151–7. doi: 10.1016/0272-0590(89)90070-5. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Environmental Protection Agency Polybrominated diphenyl ethers (PBDES) project plan. 2006.
- 17.Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112(1):9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen WR, Keelan JA, Skinner SJ, Mitchell MD. Key enzymes of prostaglandin biosynthesis and metabolism. Coordinate regulation of expression by cytokines in gestational tissues: a review. Prostaglandins Other Lipid Mediat. 1999;57(4):243–57. doi: 10.1016/s0090-6980(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Khan AH, Carson RJ, Nelson SM. Prostaglandins in labor--a translational approach. Front Biosci. 2008;13:5794–809. doi: 10.2741/3117. [DOI] [PubMed] [Google Scholar]
- 20.Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3(3):121–6. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- 22.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 23.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008121(4):788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 24.Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs - A review of levels and sources. Int J Hyg Environ Health. 2008 doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111(9):1235–41. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J Toxicol Environ Health A. 2007;70(1):1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- 27.Polishuk ZW, Wassermann D, Wassermann M, Cucos S, Ron M. Organochlorine compounds in mother and fetus during labor. Environ Res. 1977;13(2):278–84. doi: 10.1016/0013-9351(77)90104-9. [DOI] [PubMed] [Google Scholar]
- 28.Bitsanis D, Crawford MA, Moodley T, Holmsen H, Ghebremeskel K, Djahanbakhch O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J Nutr. 2005;135(11):2566–71. doi: 10.1093/jn/135.11.2566. [DOI] [PubMed] [Google Scholar]
- 29.Batterman S, Chernyak S, Gwynn E, Cantonwine D, Jia C, Begnoche L, Hickey JP. Trends of brominated diphenyl ethers in fresh and archived Great Lakes fish (1979−2005). Chemosphere. 2007;69(3):444–457. doi: 10.1016/j.chemosphere.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 30.Norstrom RJ, Simon M, Moisey J, Wakeford B, Weseloh DV. Geographical distribution (2000) and temporal trends (1981−2000) of brominated diphenyl ethers in Great Lakes hewing gull eggs. Environ Sci Technol. 2002;36(22):4783–9. doi: 10.1021/es025831e. [DOI] [PubMed] [Google Scholar]
- 31.Strandberg B, Dodder NG, Basu I, Hites RA. Concentrations and spatial variations of polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air. Environ Sci Technol. 2001;35(6):1078–83. doi: 10.1021/es001819f. [DOI] [PubMed] [Google Scholar]
- 32.Song W, Ford JC, Li A, Mills WJ, Buckley DR, Rockne KJ. Polybrominated diphenyl ethers in the sediments of the Great Lakes. 1. Lake Superior. Environ Sci Technol. 2004;38(12):3286–93. doi: 10.1021/es035297q. [DOI] [PubMed] [Google Scholar]
- 33.Song W, Ford JC, Li A, Sturchio NC, Rockne KJ, Buckley DR, Mills WJ. Polybrominated diphenyl ethers in the sediments of the Great Lakes. 3. Lakes Ontario and Erie. Environ Sci Technol. 2005;39(15):5600–5. doi: 10.1021/es050631z. [DOI] [PubMed] [Google Scholar]
- 34.Song W, Li A, Ford JC, Sturchio NC, Rockne KJ, Buckley DR, Mills WJ. Polybrominated diphenyl ethers in the sediments of the Great Lakes. 2. Lakes Michigan and Huron. Environ Sci Technol. 2005;39(10):3474–9. doi: 10.1021/es048291p. [DOI] [PubMed] [Google Scholar]
- 35.Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent Organic Pollutant Residues in Human Fetal Liver and Placenta from Greater Montreal, Quebec: A Longitudinal Study from 1998−2006. Environ Health Perspect. 2008 doi: 10.1289/ehp.0800205. doi:10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114(2):176–81. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariottini M, Corsi I, Della Torre C, Caruso T, Bianchini A, Nesi I, Focardi S. Biomonitoring of polybrominated diphenyl ether (PBDE) pollution: a field study. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148(1):80–6. doi: 10.1016/j.cbpc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Roosens L, Dirtu AC, Goemans G, Belpaire C, Gheorghe A, Neels H, Blust R, Covaci A. Brominated flame retardants and polychlorinated biphenyls in fish from the river Scheldt, Belgium. Environ Int. 2008;34(7):976–83. doi: 10.1016/j.envint.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Manchester-Neesvig JB, Valters K, Sonzogni WC. Comparison of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in Lake Michigan salmonids. Environ Sci Technol. 2001;35(6):1072–7. doi: 10.1021/es001422b. [DOI] [PubMed] [Google Scholar]
- 40.Reistad T, Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (DE-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicol Sci. 2005;87(1):57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- 41.Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000;60(8):1075–83. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 42.Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, Muglia LJ. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol. 2003;17(8):1454–1469. doi: 10.1210/me.2003-0007. [DOI] [PubMed] [Google Scholar]
- 43.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67(2):668–73. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27(3−4):117–23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.