Summary
Dendritic cell (DC) differentiation is regulated by stroma via a network of soluble and cell-bound factors. Notch is one of the major elements of this network. Its role in DC differentiation, however, is controversial. Here, we demonstrate that activation of Notch signaling in hematopoietic progenitor cells (HPC) up-regulates differentiation of conventional DCs via activation of the canonical Wnt pathway. Inhibition of the Wnt pathway abrogated the effect of Notch on DC differentiation. Activation of the Wnt pathway in Notch-1-deficient embryonic stem cells restored DC differentiation, which indicates that Wnt signaling is downstream of the Notch pathway in regulating DC differentiation. Notch signaling activated the Wnt pathway in HPCs via up-regulated expression of multiple members of the Frizzled family of Wnt receptors. That expression was directly regulated by the CSL/RPB-Jκ transcription factor. Thus, these data suggest a new model of DC differentiation via cooperation between Wnt and Notch pathways.
Introduction
Dendritic cells (DCs) are antigen presenting cells with critically important functions in innate and adaptive immunity. Elucidation of the mechanism of DC differentiation in the bone marrow microenvironment is very important for better understanding the biology of these cells. DC differentiation from hematopoietic progenitor cells (HPC) is controlled by bone marrow stroma (BMS) via a complex network of soluble cytokines and cell-bound molecules. Recent studies have implicated the Notch pathway in differentiation and function of these cells (rev in (Cheng and Gabrilovich, 2008)). According to the conventional model, Notch signaling is initiated upon the binding of the extracellular domain of HPC-expressed Notch receptors to Notch ligands: Jagged and Delta. Proteolytic cleavage within the transmembrane subunit of the Notch receptor results in translocation of the intracellular domain of Notch (ICN) to the nucleus where it interacts with the transcriptional repressor CSL, also known as CBF-1 or RBP-Jκ. Binding of ICN displaces co-repressor complexes, thereby activating transcription by promoters with CSL binding elements (Allman et al., 2002; Artavanis-Tsakonas S, 1999; Curry et al., 2006; Osborne and Minter, 2007).
The effect of Notch on DC differentiation is highly controversial. A number of groups have described a direct role of Notch in promoting DC differentiation. Expression of Delta-1 in conjunction with GM-CSF induced differentiation of bone marrow cells to DCs at the expense of other lineages (Mizutani et al., 2000). Delta-1 also promoted DC and Langerhans cell differentiation from peripheral blood monocyte precursors (Hoshino et al., 2005; Ohishi et al., 2001). Consistent with these observations differentiation of DC was significantly impaired in Notch-1 anti-sense mice that have about half of the normal level of Notch-1 in HPC (Cheng et al., 2001). These findings were further confirmed in an experimental model of DC differentiation of Notch-1−/− embryonic stem (ES) cells (Cheng et al., 2003). Conditional deletion of RBP- Jκ in BM cells and DCs resulted in a substantial reduction of the presence of conventional DCs in spleens of the knockout mice. This decrease affected primarily the CD8− DC subset in the marginal zone of spleen (Caton et al., 2007). The observed increase in PU.1 RNA levels (Schroeder et al., 2003) and IL-4 production (Amsen et al., 2004) by activated Notch-1 may help to explain the shift in DC differentiation induced by Delta-1 and was consistent with another report that Notch-induced generation of interstitial-type DCs was associated with PU.1 up-regulation (Heinz et al., 2006).
A number of studies, however, have described different role of Notch signaling in DC differentiation. Radtke et al. generated Notch-1 conditional knockout mice using the Lox-Cre system and demonstrated that the number of thymic DCs, conventional DCs, and Langerhans cells were normal (Radtke et al., 2000). Inhibition of Notch signaling with γ-secretase inhibitor shifted differentiation into non-T cells including monocyte/dendritic cells (De Smedt et al., 2005). Notch signaling was also found to play a prominent role in inhibiting macrophages, DC, and NK cell differentiation (Garcia-Peydro et al., 2006).
Similar contradictory data exists in respect to the effect of Notch signaling on plasmacytoid DCs (pDC). Oliver et al. reported that Notch signaling via Delta-1 promoted differentiation of pDC (Olivier et al., 2006). In a different study, stromal cells expressing Delta-1 blocked pDC development (Dontje et al., 2006). Inactivation of Notch-1 (Ferrero et al., 2002; Radtke et al., 2000) through Mx-Cre recombinase did not affect the development of pDC. In contrast, RBP-J-deficient mice have shown an increased level of pDC (Caton et al., 2007) suggesting that Notch signaling may play an inhibitory role in the development of these cells.
In an attempt to clarify the role of Notch signaling in DC differentiation we cultured bone marrow HPCs on a monolayer of fibroblasts expressing Delta-1 and evaluated the genes associated with the Notch pathway using focused oligo GEArray (Superarray Bioscience). We observed consistent up-regulation of several members of Frizzled family of Wnt receptors. These data suggested that Wnt might be involved in Notch ligand-mediated effects.
The Wnt signaling pathway is involved at different stages of development including the self-renewal of normal hematopoietic stem cells (HSC) and the determination of HPC cell fate (Staal et al., 2008). HPC responds to Wnt proteins secreted either by surrounding stroma or by HPCs themselves in paracrine or autocrine fashion. Interaction of Wnt with receptors activates several pathways. Canonical Wnt signaling is the most well-known pathway, which is centered around β-cateinin. In the absence of Wnt ligand binding to its receptor complex, β-catenin is targeted for degradation by the proteasome through the action of the destruction complex involving adenomatous polyposis coli, axis inhibition protein 1, the serine/threonine kinases casein kinase 1, and glycogen synthase kinase 3β (GSK3β). At the cell membrane, Wnt binds to Frizzled receptors and co-receptors such as low density lipoprotein receptor-related proteins (LRP). This results in stabilization of β-catenin through disheveled-mediated inhibition of the destruction complex. Soluble β-catenin then translocates to the nuclei and displaces groucho-related repressor from T cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factor. TCF/LEF is then able to regulate expression of target genes (Katoh and Katoh, 2007; Mikels and Nusse, 2006; Reya and Clevers, 2005).
There is now emerging evidence of cross-talk between Notch and Wnt pathways. Wnt signaling may regulate Notch activity and somite formation and pattering via regulated transcription of the Notch ligand Delta-1 in the presomitic mesoderm (Hofmann et al., 2004). Inhibition of Notch signaling can lead to accelerated differentiation of HSCs in vitro and intact Notch signaling was required for Wnt-mediated maintenance of undifferentiated HSCs (Duncan et al., 2005). Regulation of Notch signaling by the Wnt pathway plays a critical role in differentiation of precursors along T or NK differentiation pathways (Aoyama et al., 2007).
Very little is known about the potential role of Wnt signaling in DC differentiation. One study has shown that in vitro differentiation of human monocytes into either DCs or macrophages was associated with Wnt5A expression (Lehtonen et al., 2007). Maturation of DCs under steady-state conditions was caused by alterations in E-cadherin-mediated adhesion. These events were triggered at least in part by activation of the β-catenin pathway (Jiang et al., 2007).
In this study we address the question of how Notch signaling can regulate DC differentiation and report novel observations that DC differentiation requires close cooperation between Notch and Wnt pathways.
Results
Notch ligand Delta-1 activates Wnt signaling in HPC by up-regulation of Frizzled family of Wnt receptors
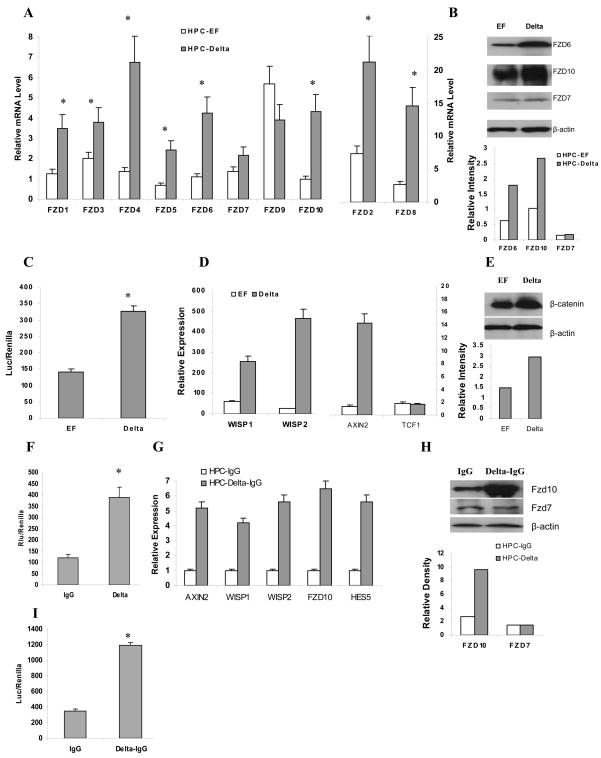
To confirm and expand our preliminary observations we evaluated the effect of Notch ligand Delta-1 on the Frizzled gene family (fzd) of Wnt receptors in HPCs using qRT-PCR. Enriched bone marrow HPCs were incubated for 18 hr on a monolayer of NIH-3T3 fibroblasts expressing either Delta-1 or corresponding control vector EF. Delta-1 fibroblasts induced significant up-regulation of 8 different fzd genes in HPC (Fig. 1A). The protein levels of three representative members of the Fzd family were evaluated by Western blot. Consistent with the results of gene expression Delta-1 cells up-regulated Fzd6 and Fzd10 but did not affect Fzd7 (Fig. 1B). We asked whether Delta-1-inducible up-regulation of Wnt receptors would result in activation of the Wnt pathway. To evaluate the level of Wnt signaling HPCs were transfected with TCF/LEF-luciferase reporter construct and cultured on Delta-1 cells. Forty eight hours incubation of HPC on Delta-1 fibroblasts resulted in more than a three-fold increase in the activity of this transcription factor (Fig. 1C). The effect on Wnt signaling was confirmed further by evaluating the expression of several Wnt-targeted genes: wisp1, wisp2, and axin2. Delta-1 cells significantly up-regulated these genes but did not affect mRNA for tcf-1, which is not transcriptionally regulated by Wnt signaling (Fig. 1D). Delta-1 fibroblasts also increased the level of β-catenin in HPCs (Fig. 1E).
Figure 1. Delta-1 activates Wnt signaling in HPC.
A–E. Enriched HPCs from bone marrow were incubated on a monolayer of NIH3T3 fibroblasts expressing either Delta-1 or control EF vectors in CCM containing 20 ng/mL GM-CSF. A. Expression of Frizzled gene family (fzd) in HPCs was evaluated in triplicates by qRT-PCR after 18 hr culture. Relative gene expression was calculated by normalizing to internal control (cyclophilin). Mean ± SE from 5 performed experiments are shown. * - statistically significant difference (p<0.05) between EF and Delta-1 groups calculated using a two sided t-test. B. Selected Frizzled members were further evaluated by Western blotting using indicated antibodies after 3 days of culture. Relative intensity of bands normalized to β-actin is shown in the bottom panel. C. HPCs were transfected with TCF/LEF reporter plasmid and then cultured on fibroblast cells for 48 hr. Reporter activity was measured using a dual-luciferase reporter assay. * - statistically significant differences from controls (p<0.05). Cumulative results of 3 performed experiments are shown. D. Expression of Wnt target genes in HPCs were measured in triplicates by qRT-PCR after 18 hr culture. Cumulative results of 4 experiments are shown. E. Expression of β-catenin was measured by Western blot after 3 day culture. HPCs were transferred onto new fibroblasts after 2 days. Relative intensity of bands normalized to β-actin is shown in the bottom panel. F–I. Enriched HPCs were cultured on plates coated with immobilized Delta-1 protein. F. Enriched HPCs were transfected with CBF-1-luciferase reporter construct and cultured in wells of a 24-well plate coated with 7.5 ng/ml Delta1-IgG or control IgG protein in the presence of 100 ng/ml recombinant Wnt3A. Reporter activity was measured using a dual-luciferase reporter assay. * - indicates statistically significant differences from controls (p<0.05). Cumulative results of 3 performed experiments are shown. G. Enriched HPCs were cultured in wells of a 24-well plate coated with Delta1-IgG or control IgG protein in the presence of 100 ng/ml recombinant Wnt3A. Expression of fzd genes and Wnt-targeted genes was evaluated using qRT-PCR after 24 hr culture. Cumulative results of three performed experiments are shown. H. Selected Frizzled proteins were further evaluated by Western blotting using specific antibodies after 3 days of culture. Relative intensity of bands normalized to β-actin is shown in the bottom panel. I. HPCs were transfected with TCF/LEF reporter plasmid before culture on a ligand-coated plate. Forty eight hours later reporter activity was measured. Mean ± SE from 3 experiments are shown. * - indicates statistically significant differences from controls (p<0.05).
Since activation of the Wnt pathway may depend on the amount of ligand available, we compared the expression of different Wnt family members in control and Delta-1 cells using qRT-PCR. Both cell lines had no detectable Wnt3a and showed similar levels of expression of Wnt 1, 10b, 2, and 4. Expression of Wnt 5 was significantly lower in Delta-1 expressing cells than in control fibroblasts (Fig. S1A) suggesting that variations in the level of Wnt are not directly responsible for the effect of Delta-1 fibroblasts on HPC. Activation of the Notch pathway by Delta-1 in HPC also did not change the expression of Wnt ligands (Fig. S1B,C). To exclude the possibility that other members of the Wnt family or factors released by fibroblasts may influence these results, we used an experimental system where Notch signaling was activated by Delta-1 immobilized on plastic, thereby removing the involvement of stromal cells. HPCs were cultured for 18 hr in wells coated with ether control IgG or Delta-1-IgG. An equal amount of recombinant Wnt3a was added to all cultures. Delta-1 substantially activated the Notch pathway (p<0.01), determined by activation of CBF-1 transcription factor (Fig. 1F) and by the increased expression of the Notch-targeted gene Hes 5 (Fig. 1G). Delta-1 did not affect the expression of Wnt ligands in HPC (Fig. S1B,C) but induced dramatic up-regulation of the expression of fzd10 as well as Wnt-targeted genes: axin2, wisp1, wisp2 (Fig. 1G). The effect of Delta-1 on Wnt receptors was confirmed by the analysis of protein expression (Fig. 1H). Delta-1 also substantially increased the transcriptional activity of TCF/LEF in HPC (Fig. 1I). These results were very similar to the data obtained from experiments with Delta-1 fibroblasts and indicated that Notch ligand induced up-regulation of Wnt receptors and signaling in HPCs.
Wnt signaling promotes DC differentiation
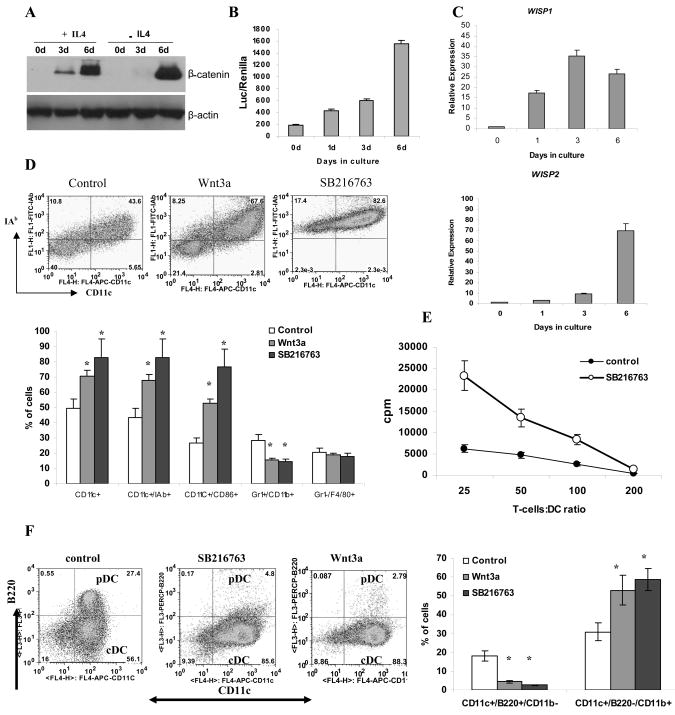
We then went on to explore how changes in Wnt signaling may affect DC differentiation. We first investigated the activity of the Wnt pathway during a 6-day period of DC differentiation from HPC in the presence of GM-CSF with or without IL-4. DC differentiation was associated with dramatic up-regulation of β-catenin (Fig. 2A), which was observed within 24 hr after start of the culture (Fig. S2). This was associated with activation of the TCF/LEF transcription factor (Fig. 2B) and the expression of Wnt-targeted genes (Fig. 2C).
Figure 2. Effect of Wnt signaling on DC differentiation.
A–C. Enriched HPCs were cultured in CCM with 20 ng/ml GM-CSF with or without 10 ng/ml IL-4. Cells were collected at the indicated time points. A. Accumulation of β-catenin was measured by Western blot. B. HPCs were transfected with TCF/LEF reporter construct on days 0, 1, 3 and 6 after the start of the culture and luciferase activity was measured 24 h after transfection. C. Expression of Wnt-targeted genes was measured at different time points using qRT-PCR. D. HPCs were cultured with 20 ng/ml GM-CSF for 5 days. Recombinant Wnt3a (100 ng/ml) or SB216763 (10 nM) were added at the start of the cultures. Top panel – typical example of one experiment. Bottom panel - cumulative results of three performed experiments are shown. E. Allogeneic MLR. Cells from experiments in panel D were co-cultured with T cells isolated from allogeneic BALB/c mice for 4 days in different ratios. Cell proliferation was measured in triplicate by [3H]-thymidine uptake. Values are the mean ± SE. F. Bone marrow cells were cultured with 100 ng/ml FLT3-ligand for 10 days with Wnt3a or SB216763. Left panel – typical example of one experiment. Right panel –Cumulative results of three performed experiments are shown.
Next, we asked whether activation of the Wnt pathway could regulate DC differentiation. At the start of a 5-day HPC culture in the presence of GM-CSF, the Wnt pathway was stimulated with either a specific ligand (Wnt3a) or with the selective GSK3β inhibitor SB216763. Activation of the Wnt pathway resulted in a significant (p<0.05) increase in the proportion of of CD11c+ DCs (Fig. 2D). All CD11c+ DCs generated in Wnt-activating conditions expressed MHC class II or CD86 molecules indicating their fully mature state. Activation of the Wnt pathway did not affect the proportion of F4/80+Gr-1− macrophages and substantially decreased the proportion of Gr-1+CD11b+ immature myeloid cells (IMC) (Fig. 2D). Stimulation of allogeneic T cells is a hallmark of DC activity. Cells differentiated in the presence of SB216763 were much more potent stimulators of allogeneic T cell proliferation than control cells (Fig. 2E).
To test the effect of the Wnt pathway on DC activation, DC cells were generated from enriched HPC using GM-CSF and IL-4 and then cultured for 24 hr with SB216763 or LPS. Activation of the Wnt pathway resulted in a dramatic up-regulation of MHC class II and co-stimulatory molecules on DCs as well as a significant increase in their ability to stimulate allogeneic T-cells. These effects were comparable or even stronger than those of LPS (Fig. S3).
DCs generated in the presence of GM-CSF are CD11c+CD11b+ conventional (myeloid) DCs. To evaluate the effect of the Wnt pathway on differentiation of plasmacytoid DCs (pDC), bone marrow cells were cultured with Flt3L for 10 days with Wnt3a or SB216763. As was the case during DC differentiation in the presence of GM-CSF activation of Wnt signaling during FLT3L-induced DC differentiation resulted in a significant increase in the proportion of conventional CD11c+CD11b+B220− DCs. In striking contrast, the presence of CD11c+CD11b−B220+ pDCs was dramatically reduced (Fig. 2F).
To clarify the effect of the Wnt pathway on macrophage differentiation, enriched HPCs were cultured for 6 days with M-CSF. SB216763 was added at the start of the culture. Activation of the Wnt pathway did not affect the proportion of F4/80+CD11b+Gr-1− macrophages generated from HPCs (Fig. S4).
Canonical Wnt pathway is required for DC differentiation
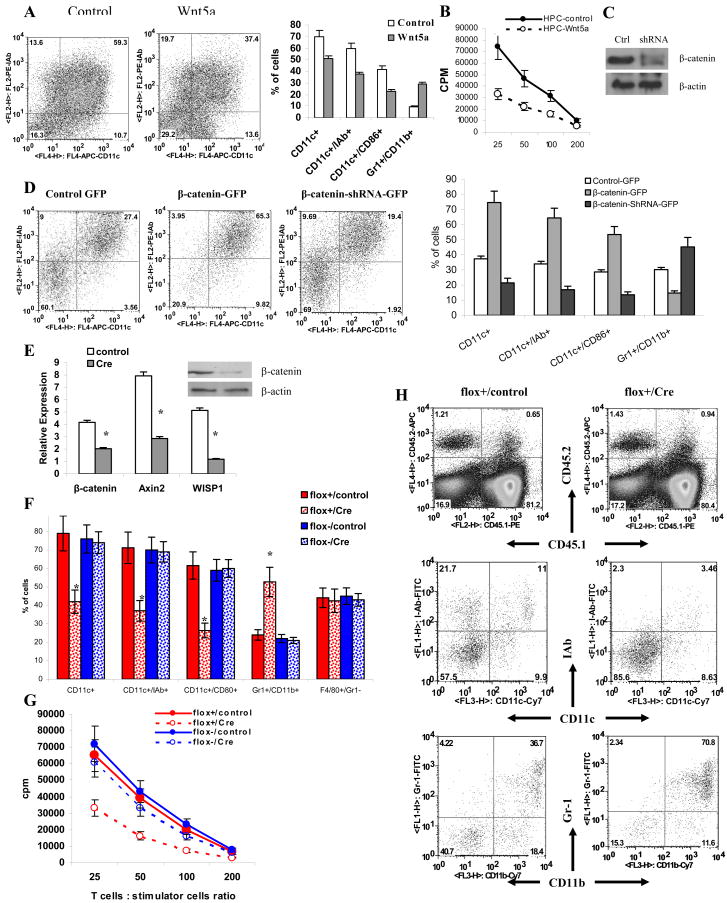
We investigated a possible role of the non-canonical Wnt pathway in DC differentiation. Wnt5a is a main activator of this pathway. Enriched HPCs were cultured with GM-CSF with or without 500ng/ml Wnt5a for 5 days and the phenotype of cells was evaluated. In contrast to Wnt3a, Wnt5a decreased DC differentiation, which manifested in a decreased proportion of CD11c+ cells (Fig. 3A) and a lower ability of DC generated in the presence of Wnt5a to stimulate allogeneic T cells (Fig. 3B).
Figure 3. Regulation of DC differentiation by Wnt pathway.
A. Enriched HPCs were cultured with GM-CSF with or without 500ng/ml Wnt5a recombinant protein for 5 days and the phenotype of cells was evaluated by flow cytometry. Left panel – typical example of one experiment. Right panel –Cumulative results of three performed experiments are shown. B. Allogeneic MLR. Cells from experiments in panel A were co-cultured with T cells isolated from allogeneic BALB/c mice for 4 days at different ratios. Cell proliferation was measured in triplicate by [3H]-thymidine uptake. Values are the mean ± SE. C. NIH-3T3 cells were transfected with control vector or β-catenin shRNA. Whole cell lysates were prepared 72 h after transfection and proteins were analyzed in Western blot. D. Enriched HPCs were transfected with GFP, β-catenin-GFP, or β-catenin-shRNA-GFP vectors. Cells were cultured in CCM with 20 ng/ml GM-CSF for 5 days and evaluated by flow cytometry. CD45+GFP+ cells were gated for further analysis. Left: Typical example of one experiment. Right: Cumulative results of three performed experiments. E–H. Lin− c-kit+ myeloid progenitor cells were sorted from bone marrow of β-cateninflox+ and wild-type β-cateninflox− mice. Cells were then infected with either control retrovirus or retrovirus containing a Cre construct and cultured with a cocktail of cytokines to generate mature myeloid cells. E. The level of β-catenin in transduced cells was evaluated 3 days after infection using qRT-PCR or Western blotting (inset). F. The proportion of DCs was evaluated by flow cytometry in myeloid progenitors 5 days after infection with Cre-retrovirus. G. Allogeneic MLR. Cells 5 days after Cre-retrovirus were co-cultured with T cells isolated from allogeneic BALB/c mice for 4 days in different ratios. Cell proliferation was measured in triplicate by [3H]-thymidine uptake. Values are the mean ± SE. H. Myeloid progenitor cells (2×104 β-cateninflox+, CD45.2+ cells) infected with control or Cre retroviruses were transferred into lethally irradiated congenic (CD45.1+) mice together with 106 syngenic (CD45.1+) bone marrow cells. Cells were evaluated in spleens 3 weeks later. CD45.2+CD45.1− donors cells were gated and the phenotype was evaluated by flow cytometry.
Experiments described above were performed using enriched HPC. We asked whether Wnt signaling affects early stages of myeloid cell differentiation. To address this question Lin−c-kit+Sca-1+CD34− HSC or Lin−c-kit+Sca-1−CD34+ CMP cells were sorted from bone marrow of naïve mice. Cells were cultured in the presence of SB216763, Wnt3a, or Wnt5a in myeloid long-term culture medium with a cocktail of cytokines for 5 days followed by 5-day culture in RPMI-1640 medium supplemented with GM-CSF. Activation of the Wnt pathway with SB216763 or Wnt3a promoted DC differentiation, whereas Wnt5a inhibited it (Fig. S5). We asked whether activation of Wnt signaling may also affect cells at later stages of myeloid differentiation. Gr-1+CD11b+ from spleens of tumor-bearing mice consist of precursors of myeloid cells at different stages of differentiation (Gabrilovich, 2004). We isolated these cells from EL-4 tumor-bearing mouse spleens and cultured them for 5 days with GM-CSF in either medium alone or in the presence of Wnt3a or SB216763. Activation of the Wnt pathway resulted in significant increase in the proportion of DCs and decreased presence of IMC (Fig. S6).
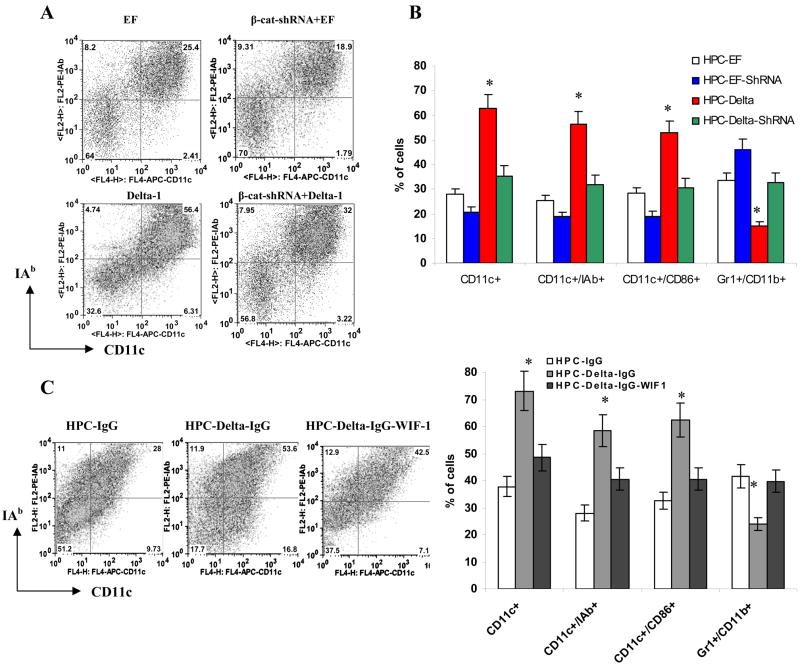
To evaluate the role of the canonical Wnt pathway in DC differentiation, we regulated the level β-catenin in HPC using two GFP-expressing vectors: β-catenin shRNA to block the expression of β-catenin (Fig. 3C); and a vector containing mutant β-catenin, which is resistant to ubiquitination and degradation and thus induces activation of the Wnt pathway (Jiang et al., 2007). As a control we used a GFP-only-expressing vector. HPCs were transfected with these vectors (Fig. S7) and cultured for 6 days with GM-CSF. The proportion of DCs was calculated among GFP positive cells. Constitutively active β-cateinin did not affect the total number of cells generated from HPC (data not shown) but significantly (p<0.05) increased the proportion of CD11c+ DCs as well as mature CD11c+IAb+ or CD86+ DCs. Inhibition of β-catenin resulted in down-regulation of DC differentiation (Fig. 3D). The opposite effect was seen in the proportion of Gr-1+CD11b+ IMC. Decreased β-catenin levels resulted in an increased production of IMC, whereas up-regulation of β-catenin led to decreased presence of IMC (Fig. 3D). No effect on macrophage differentiation was observed (data not shown).
To verify the effect of the canonical Wnt pathway on DC differentiation we used experimental system with a conditional knockout of β-catenin. Lin−c-kit+ myeloid progenitor cells were sorted from bone marrow of β-cateninflox+ and wild-type β-cateninflox− mice. Cells were then infected with retroviruses containing either a Cre construct or control retrovirus, and cultured with cocktail of cytokines to generate mature myeloid cells. The level of β-catenin in transduced cells was evaluated 5 days after infection using qRT-PCR and Western Blotting. As shown in Figure 3E infection of β-cateninflox+ cells with Cre retrovirus substantially reduced the expression of β-catenin. The proportion of DCs generated from β-cateninflox+ myeloid progenitors infected with Cre-retrovirus was substantially (p<0.05) lower than that generated from β-cateninflox+ cells infected with control retrovirus or β-cateninflox− cells infected with either retroviruses (Fig. 3F). The ability of these cells to stimulate allogeneic T cells was also reduced (Fig. 3G).
Next, 2×104 β-cateninflox+ (CD45.2+) myeloid progenitor cells infected with control or Cre retroviruses were transferred into lethally irradiated congenic (CD45.1+) mice together with 106 syngenic (CD45.1+) bone marrow cells. Cells were evaluated in spleens 3 weeks later. The proportion of DCs among the CD45.2+ population in mice transfused with myeloid cells infected with Cre virus was substantially lower than that in mice transfused with myeloid progenitors infected with the control virus (Fig. 3H). In contrast, the proportion of Gr-1+CD11b+ IMC was higher in CD45.2+ cells that were infected with Cre-retrovirus than in cells infected with control virus (Fig. 3H). No differences in the proportion of DCs or IMC were observed among CD45.1+ cells that were not infected with virus (data not shown).
We also investigated the effect of Wnt signaling on the differentiation of human DCs. CD34+ progenitor cells were cultured in the presence of GM-CSF and SCF for 6 days with the Wnt inhibitory factor-1 (Wif-1) or the Wnt activator SB216763. We specifically avoided using IL-4 in the culture medium to prevent selection of DCs during differentiation. Presence of Wif-1 decreased the proportion of Lin- HLA-DR+ DCs from 31.1% to 22.7%, whereas SB216763 increased proportion of DCs to 52.8% (Fig. S8A). Cells generated in the presence of Wif-1 had substantially reduced ability to stimulate allogeneic T cells. In contrast, cells generated in the presence of SB216763 had a significantly greater ability to stimulate allogeneic T cells (Fig. S8B) confirming that activation of the Wnt pathway leads to increased differentiation of human DCs. Thus, in both mice and humans activation of the Wnt pathway promoted DC differentiation, whereas its inhibition resulted in repression of DC differentiation and accumulation of IMC.
Cooperation between Notch and Wnt signaling in differentiation of DC
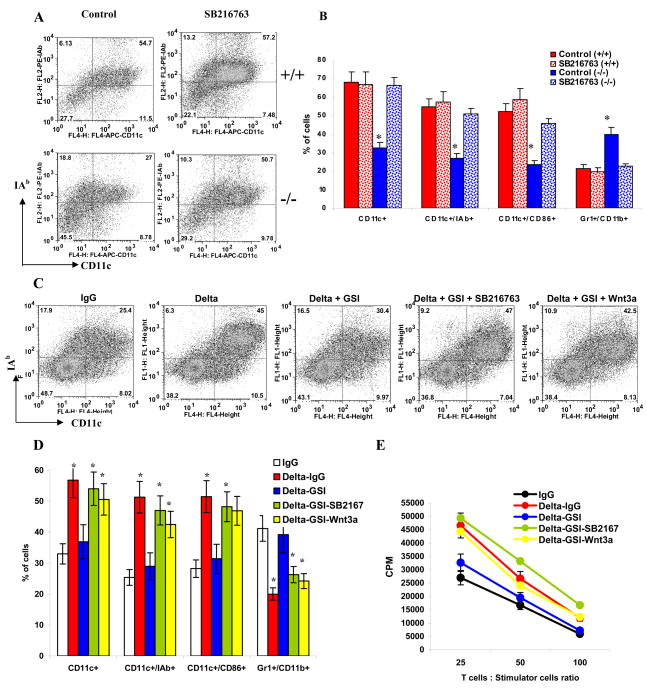
ES cells lacking Notch-1 have impaired ability to differentiate into DCs (Cheng et al., 2003). We asked whether activation of Wnt signaling can overcome that defect. To address this question we used a previously described experimental system where DCs were generated from ES cells (Cheng et al., 2003). We observed that differentiation of DCs from Notch-1−/− ES cells was significantly reduced and the proportion of IMC was significantly increased (p<0.05) consistent with previous observations (Cheng et al., 2003). Wnt signaling was activated with SB216763 at the late post-embryonic bodies stage of DC differentiation from ES cells (to avoid toxicity of the compound). This may explain the fact that in Notch+/+ wild-type cells it did not lead to an increase in the proportion of CD11c+ DCs (Fig. 4A–B). However, activation of Wnt signaling restored the proportion of DCs generated from Notch-1−/− ES cells to the level of wild type ES cells and decreased the presence of IMC (Fig. 4A–B). These data suggests that during regulation of DC differentiation, Wnt signaling is downstream of Notch.
Figure 4. The role of Wnt signaling in Delta-1-mediated DC differentiation.
A, B DCs were differentiated from wild-type R1 ES cells and Notch-1−/− ES cells as described in Experimental Procedures. Cells were treated with 10 nM SB216763 or vehicle for 5 days prior to analysis. A. Typical example of one experiment. B. Cumulative results of three performed experiments. * - statistically significant (p<0.05) differences from control.
C,D. Enriched HPCs were cultured on plates coated with control IgG or Delta-IgG with or without GSI, SB216763, or Wnt3a as indicated. Cells were harvested and phenotype was evaluated by flow cytometry. C. Typical example of one experiment. D. Cumulative results of three performed experiments. * - statistically significant differences from IgG control (p<0.05). E. Allogeneic MLR. Cells were cultured with T cells isolated from allogeneic BALB/c mice for 4 days at different ratios. Cell proliferation was measured in triplicate by [3H]-thymidine uptake. Values are the mean ± SE.
To confirm these observations, we used an alternative experimental system where HPCs were cultured with Delta-1 immobilized on plastic. In this system, Delta-1 significantly increased differentiation of DCs from enriched bone marrow HPC (Fig. 4C,D). To verify that the Delta-1 effect on DC differentiation was mediated via Notch signaling, HPCs were transfected with either control or CBF-1 shRNA and then placed on plastic coated with Delta-1-IgG. Delta-1 induced a significant increase in the proportion of DCs differentiated from HPC. This effect was absent when HPCs were transfected with CBF-1 shRNA (Fig. S9). In a different experimental system Notch signaling in HPC was blocked with γ-secretase inhibitor XII (GSI). This GSI prevents proteolytic cleavage of Notch receptors after their binding to ligands and thus prevents release of intracellular Notch and activation of the Notch pathway (Nefedova et al., 2008). GSI abrogated the effect of Delta-1 on DC differentiation (Fig. 4C–E). However, if GSI was added to the cells cultured together with Wnt3a or SB216763, the proportion of DCs increased to the level observed in cells cultured on Delta-1 without GSI (Fig. 4C–E). These data were consistent with results of experiments with ES cells and indicate that Wnt pathway is downstream of Notch signaling in DC differentiation.
To establish the role of Wnt signaling in the Delta-1-mediated effects on DC differentiation, we inhibited the Wnt pathway in HPC using β-catenin shRNA. Enriched bone marrow HPCs were transfected with control GFP or a β-catenin shRNA-GFP plasmid and then placed on a monolayer of Delta-1-expressing fibroblasts. Cells were cultured with GM-CSF for 5 days. Consistent with previous results, Delta-1 cells substantially enhanced DC differentiation. Inhibition of β-cateinin completely abrogated this effect (Fig. 5A,B). To confirm these observations, we used an alternative experimental system where HPCs were cultured with Delta-1 immobilized on plastic. Furthemore, inhibition of Wnt with Wif-1, substantially reduced DC differentiation induced by Delta-1 (Fig. 5C). These data confirm the important role of Wnt signaling in Delta-1 mediated up-regulation of DC differentiation.
Figure 5. Cooperation between Notch and Wnt signaling in DC differentiation.
A, B. Enriched HPCs were transfected with β-catenin-shRNA-GFP construct or GFP vector, and then cultured on a monolayer of fibroblasts expressing Delta-1 or control vector (EF) for 5 days in CCM with 20 ng/ml GM-CSF. Cells were labeled with a cocktail of indicated antibodies and evaluated by flow cytometry. CD45+GFP+ cells were gated and the proportion of DCs or IMC was analyzed within this group of cells. A. Typical example of one experiment. B. Cumulative results of three performed experiments. * indicates statistically significant differences from EF control (p<0.05).
C. HPCs were incubated in CCM supplemented with 20 ng/ml GM-CSF for 5 days on a 24-well plate coated with Delta-1 protein or control IgG. Wif1 (750 ng/ml) was added at the start of the culture. Cell phenotype was evaluated by flow cytometry. Left – typical example of one experiment. Right - cumulative results of three performed experiments. * - statistically significant differences from IgG control (p<0.05).
Mechanism of Delta-1 effects on Wnt signaling
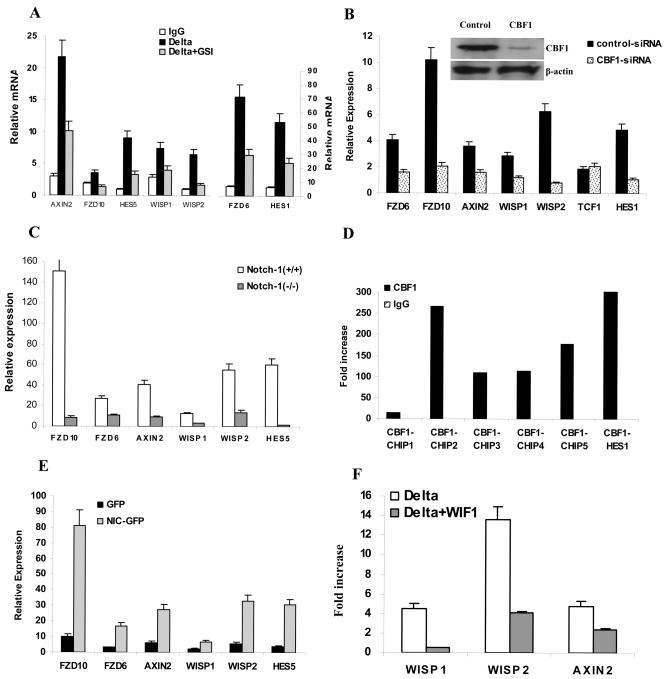
We then set out to determine the mechanism of Delta-1-induced up-regulation of Wnt signaling. First, we investigated whether this effect is dependent on Notch signaling. Notch signaling in HPC was blocked with GSI and inhibition of the Notch pathway was confirmed by measuring expression of the Notch-targeted gene hes-1 (Fig. 6A). Treatment of HPC with GSI completely abrogated the effect of immobilized Delta-1 on the expression of fzd genes and its associated activation of the Wnt pathway (Fig. 6A). To verify the role of RBP-Jκ (CBF-1; the main transcription factor involved in Notch signaling) in the regulation of the Wnt pathway, we blocked CBF-1 expression in the 32D murine hematopoietic cells line using siRNA. Inhibition of CBF-1 resulted in a dramatic down-regulation of fzd gene expression and the Wnt pathway-targeted genes axin2, wisp1 and wisp2 but not a tcf1 gene (Fig. 6B). To investigate the specific role of Notch-1 in regulation of fzd gene expression and Wnt signaling, we used embryonic stem (ES) cells with a targeted deletion of the notch-1 gene (Cheng et al., 2003). Notch-1-deficient ES cells had a dramatically reduced expression of the Notch-targeted gene Hes5. In the absence of Notch-1, expression of fzd and Wnt-targeted genes was significantly reduced (Fig. 6C).
Figure 6. Delta-1 up-regulates expression of Wnt receptors via Notch signaling.
A. Enriched HPCs were cultured in wells of a 24-well plate coated with Delta-IgG or control IgG with or without GSI (5nM) for 24 h. Expression of fzd receptors and Wnt-targeted genes were evaluated in quadruplicates by qRT-PCR. Hes1 was included as a positive control. B. 32D cells were transfected with a control non-target pool of siRNAs or CBF-1 siRNA. Inhibition of CBF1 expression was confirmed by Western-blotting 48 hr after transfection (inset). Expression of fzd and Wnt-targeted genes was measured in quadruplicates by qRT-PCR. C. qRT-PCR. was used to determine the expression of fzd and Wnt-targeted genes in wild type or Notch-1-deficient ES cells. In panels A–C mean ± SE from 3 experiments are shown. D. ChIP assay was performed in 32D cells using antibodies against CBF-1 or control IgG as described in Experimental Procedures. Recruitment of potential CBF-1 binding sites within the fzd10 promoter by CBF-1 protein was evaluated by qPCR and presented as fold increase over input DNA. Two experiments with the same results were performed. E. HPCs were transduced with GFP or Notch-IC-GFP vectors and cultured in complete medium containing 20 ng/ml GM-CSF for 24 h. GPF-positive cells were sorted and the expression of fzd and Wnt-targeted genes was evaluated in quadruplicates by qRT-PCR. Two experiments with the same results were performed. F. HPCs were cultured in wells of a 24-well plate coated with 7.5 ng/ml control IgG or Delta-1 with or without Wnt inhibitor WIF-1 (750 ng/ml) for 24h. Expression of Wnt-targeted genes was measured in quadruplicates by qRT-PCR. Fold increase over background (gene expression in HPC cultured with control IgG) is shown.
Promoter regions of all fzd genes contain multiple RBP-Jκ binding sites (Table S1). To test the ability of this transcription factor to bind to the fzd promoter we selected fzd10 as one representative member of the Frizzled family. A ChIP assay was performed targeting a 3kb sequence upstream of the fzd10 gene transcription initiation site. Five different sets of primers spanning potential CBF-1 binding sites were used. As a positive control we used primers that amplify the promoter region of hes-1. Anti-CBF-1 antibody immunoprecipitated DNA that was amplified by primers specific for hes-1, as well as with 4 out of the 5 sets of primers specific for fzd10. No amplification was detected when control IgG was used for immunoprecipitation (Fig. 6D). To verify the regulation of the Wnt pathway by Notch-1, 32D cells were transfected with a constitutively active Notch-1(NIC)-GFP construct (Cheng et al., 2007). As a control we used a vector containing only GFP. GFP-positive cells were sorted and gene expression was evaluated by qRT-PCR. Over-expression of Notch-1 resulted in a significant up-regulation of fzd6 and fzd10 expression as well as several Wnt-targeted genes (Fig. 6E). Collectively these data indicate that Delta-1-induced up-regulation of Wnt receptors and activation of Wnt signaling is mediated by the RBP-Jκ transcription factor via activation of the Notch pathway. To verify the role of Wnt receptors in Delta-1-mediated activation of Wnt signaling, HPCs were cultured with Delta-1-expressing fibroblasts in the presence of Wif-1, an antagonist that binds Wnt protein and prevent its interaction with the receptors (He et al., 2005; Hsieh et al., 1999). If Delta-1 affects the Wnt pathway downstream of the receptors then blockade of extracellular Wnt interacting with its receptors would have a very limited effect on Wnt signaling. The presence of Wif-1 in culture medium, however, dramatically reduced the effect of Delta-1 on activation of the Wnt pathway (Fig. 6F).
Spleen stroma activates Wnt signaling leading to DC differentiation
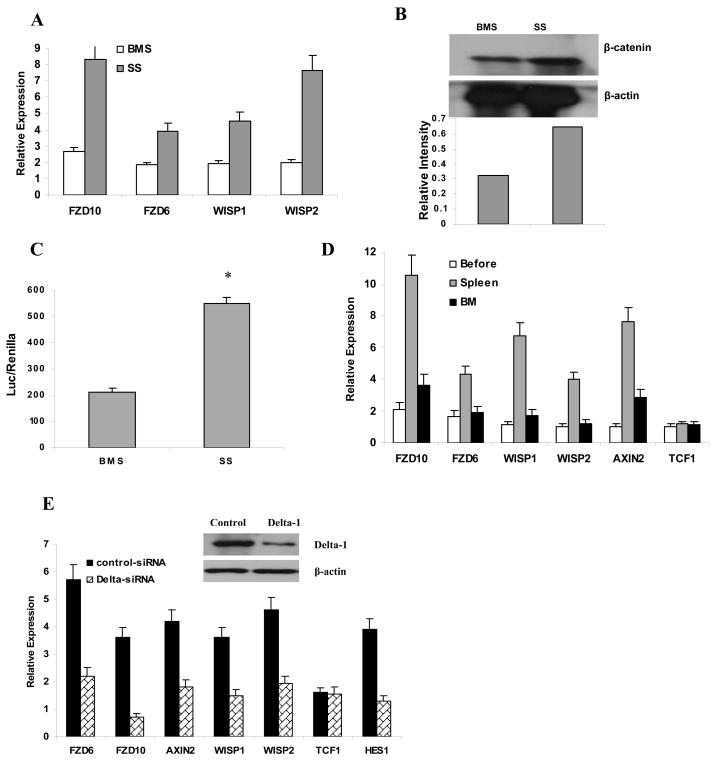
We have previously shown that spleen stroma (SS) promoted DC differentiation, whereas bone marrow stroma (BMS) promoted accumulation of immature DC precursors. We also demonstrated that this effect was associated with different levels of Delta-1 in these cells. Expression of Delta-1 on SS was much higher than that in BMS (Cheng et al., 2007). To investigate a potential effect of different stroma on Wnt signaling in HPC, a monolayer of BMS and SS was prepared and HPCs were cultured on top of these cells for 48 h with GM-CSF. Expression of fzd genes, Wnt pathway-targeted genes (Fig. 7A), as well as β-catenin levels (Fig. 7B) and TCF/LEF transcriptional activity (Fig. 7C) were significantly higher in HPCs cultured on SS than on BMS. To evaluate the possible effects of BMS and SS on Wnt signaling in HPC in vivo, enriched HPCs were isolated from bone marrow of C57BL/6 (CD45.2+) mice and 106 cells were transferred i.v. into irradiated congenic (CD45.1+) recipients. Thirty-six hours after transfer, donor CD45.2+ cells were isolated from spleens and bone marrow and RNA was extracted. HPCs prior to transfer served as a background control. During this procedure, we typically recovered 1.3–1.5 ×104 donor cells from bone marrow and 0.9–1.1 ×104 donor cells from the spleen. Donor cells isolated from the spleen demonstrated a significant increase in the expression of fzd6 and fzd10 as well as Wnt pathway-targeted genes wisp1, wisp2, and axin2, whereas only slight and mostly non-significant changes were observed in cells isolated from bone marrow (Fig. 7D). These data were consistent with in vitro observations of the effects of Delta-1 on Wnt signaling. To verify the potential role of Delta-1 in the SS effect on Wnt signaling in HPC, SS cells were transfected with Delta-1 siRNA. HPCs cultured on a monolayer of SS with a reduced level of Delta-1 demonstrated a substantially lower level of expression of Wnt receptors and Wnt-targeted genes than HPCs cultured on SS transfected with control non-target pools of siRNAs (Fig. 7E).
Figure 7. Effect of bone marrow and spleen stroma on Wnt signaling in HPC.
A–C. BMS and SS were generated from naive mice and enriched HPCs were cultured on a BMS or SS monolayer for 18 h. After that time CD45-positive cells were isolated and expression of fzd and Wnt-targeted genes was measured in triplicates by qRT-PCR. Mean ± SE from 3 independent experiments are shown. B. The level of β-catenin was measured by Western blot after 5 days of culture. Relative intensity of bands was normalized to β-actin and is shown in the bottom panel. C. HPCs were transfected with TCF/LEF reporter plasmid before culture on BMS or SS monolayer for 48 h. Luciferase activity was measured as described in Experimental Procedures. Each measurement was performed in duplicate and the results of three independent experiments are shown. D. Naive C57BL/6 recipient mice were irradiated and injected intravenously with 1.5×106 enriched HPCs from CD45.1+ congenic mice. Spleen and bone marrow were harvested 36 hr after injection and CD45.1+ cells were isolated using magnetic beads. Expression of Wnt-targeted genes was evaluated in quadruplicates by qRT-PCR. Values are the mean ± SE from 2 mice. E. Spleen stroma cells were transfected with Delta-1 siRNA or control non-target pool siRNA. Inhibition of Delta-1 expression was confirmed by Western blot (inset) 48 hr after transfection. Expression of fzd and Wnt-targeted genes was evaluated by qRT-PCR. Each measurement was performed in triplicate and the results of three independent experiments are shown.
Discussion
Our results demonstrate the existence of a close cooperation between Notch and Wnt pathways in the regulation of DC differentiation. Notch signaling was previously implicated in the regulation of DC differentiation. However, reported results are controversial. On one hand, a number of groups primarily using “gain of function” experiments, where Notch signaling was activated by Delta-1, demonstrated the induction of differentiation of conventional DCs (Caton et al., 2007; Cheng et al., 2003; Cheng et al., 2001; Hoshino et al., 2005; Mizutani et al., 2000; Ohishi et al., 2001). On the other hand, a number of studies primarily utilizing experiments with “loss of function” based on knockout mice showed that Notch signaling either did not have an effect or that it inhibited DC development (De Smedt et al., 2005; Garcia-Peydro et al., 2006; Radtke et al., 2000). Similar contradictory data exists in respect to the effect of Notch signaling on pDCs (Caton et al., 2007; Dontje et al., 2006; Ferrero et al., 2002; Olivier et al., 2006; Radtke et al., 2000). Our study may help to resolve some of those contradictions and clarify the role of Notch signaling in DC differentiation.
We have shown that Delta-1 may exert its effect on DC differentiation primarily via activation the Wnt pathway. Our results demonstrate, for the first time, a critical role of the canonical Wnt pathway for differentiation of myeloid DCs. DC differentiation from HPCs was associated with gradual activation of the Wnt pathway. Down-regulation of β-catenin in HPC inhibited DC differentiation, whereas degradation-resistant β-catenin promoted DC differentiation. Similar results were obtained when Wnt signaling was activated by Wnt3a or inhibition of GSK3β. Importantly, activation of the Wnt pathway had the opposite effect on pDC and did not affect differentiation of macrophages.
Our results are consistent with the recent observation that disruption of E-cadherin-mediated interactions between DCs and adjacent cells increase the amount of β-catenin in the cytoplasm. E-cadherin disruption resulted in the phenotypic maturation of DCs, as shown by the increased expression of co-stimulatory molecules, MHC class II molecules and chemokine receptors (Jiang et al., 2007). It is important to point out that our study did not address the role of β-catenin and Wnt signaling in DC function. It is possible that regulation of the Wnt pathway in DCs may affect the ability of these cells to stimulate immune responses. Recent information that DC-specific stabilization of β-catenin in mice prevented the efficient generation of antigen-specific immune responses (Staal et al., 2008) might support such a possibility.
Wnt proteins are produced by many types of cells including HPC, stroma and macrophages. (Blumenthal et al., 2006; Lobov et al., 2005). During DC differentiation in vitro or in vivo, therefore, HPCs are constantly in contact with ligands. This may explain the fact that blockade of Wnt signaling affects DC differentiation even without presence of stroma cells. It appears that Notch ligands may affect Wnt signaling in HPC by regulating the level of expression of the Frizzled family of Wnt receptors. Several lines of evidence have demonstrated that Delta-1 regulates the expression of Frizzled via activation of the Notch pathway: inhibition of Notch pathways with either GSI or down-regulation of CBF-1 resulted in abrogation of a Delta-1 effect on the Wnt pathway; Notch-1 deficient ES cells had substantially reduced expression of fzd and Wnt-targeted genes; there is a direct physical interaction between CBF-1 and the promoter of fzd10; constitutively active Notch-1 directly up-regulated fzd expression.
Our data suggest that Delta-1 activates Wnt signaling in HPCs primarily via up-regulation of Frizzled receptors. Delta-1 up-regulated expression of at least 8 different members of Frizzled family. Because of functional overlap between them ablation of individual receptor would not help to clarify their role in Delta-1 mediated activation of Wnt signaling. Stroma cells and HPCs also express different members of the Wnt family. Therefore, we blocked Wnt ligands with the soluble inhibitor Wif-1 and observed abrogation of Delta-1-mediated activation of Wnt signaling. We argue that if Delta-1 targeted downstream molecules in the Wnt pathway Wif-1 should have a minimal effect on Wnt signaling.
Our data indicate that the Notch-Wnt axis may have a specific role in regulation of DC but not macrophage differentiation. These data are in agreement with observations from one group that conditional deletion of β-catenin in HPC resulted in normal development of granulocytes and macrophages (Cobas et al., 2004; Koch et al., 2008). However, it remains to be seen whether DC differentiation is affected in those cells and whether our data on specific regulation of DCs by Notch-Wnt pathways could be reproduced in different experimental systems. Our data suggest that the canonical and non-canonical pathways of Wnt signaling have opposite effect on DC differentiation. Activation of the non-canonical pathway with Wnt5a in contrast to canonical pathway resulted in inhibition of DC differentiation. It has been previously demonstrated that there is competition between non-canonical and canonical Wnt pathways. Non-canonical Wnt ligands can inhibit canonical Wnt/β-catenin signaling in transformed cell lines and in Xenopus and mouse embryos. It was also reported that Wnt5a treatment could inhibit canonical Wnt signaling in hematopoietic stem cells (Malhotra and Kincade, 2009). Wnt5a has been shown to antagonize Wnt3a signals via GSK-3-independent β-catenin degradation (Topol et al., 2003). Other potential mechanisms for the non-canonical Wnt mediated inhibition of canonical pathways would involve competition for molecules like Dishevelled that are shared by the two pathways.
The presented data provides a new model of regulation of DC differentiation in various tissue compartments. Notch ligands expressed on adjacent stroma cells induce up-regulation of members of the Frizzled family of Wnt receptors in HPCs. Since Wnt ligands are produced by stroma cells this results in activation of the Wnt pathway and differentiation of DCs. Thus, DC differentiation is regulated by the amount of available Wnt proteins and by the level of Notch ligand expressed on adjacent cells. Our data suggest that the Wnt pathway can be downstream of Notch in the regulation of DC differentiation. This model may also explain the discrepancy in reports of Notch effects on DCs. Activation of the Notch pathway by itself is not sufficient for DC differentiation and requires Wnt, the level of which depends on the presence and the nature of the surrounding stromal cells. On the other hand, activation of the Wnt pathway may overcome the absence of Notch signaling. This may explain the normal level of DCs in Notch knockout mice reported previously (Radtke et al., 2000; Radtke et al., 1999). This cooperation between Notch and Wnt signaling may provide a DC system with the flexibility it needs to adequately respond to various pathological and physiological stimuli.
Experimental Procedures
Mice and reagents
All mouse experiments were approved by the University of South Florida Institutional Animal Care and Use Committee. Female C57BL/6 mice (aged 6–8 weeks) were obtained from the National Cancer Institute. β-cateninflox+ mice (mouse strain # 004152) and CD45.1+ congenic mice (B6.SJL-PtrcaPep3b/BoyJ) were purchased from Jackson Laboratories. Detailed description of reagents used in the study is provided in the Supplementary Materials.
Cell lines, HPC-3T3 co-culture, and flow cytometric analysis
The NIH 3T3 cell lines transfected with Delta-1 or control plasmid were described previously (Cheng et al., 2007). Confluent fibroblasts were cultured overnight in DMEM with 10% FBS. Mouse HPCs from mouse bone marrow were enriched using lineage cell depletion kit from Miltenyi Biotec Inc (Auburn, CA). Enriched HPC cells from naïve bone marrow were placed onto 3T3 cells the following day and cultured in RPMI medium supplemented with 10% FBS and 20 ng/ml GM-CSF for various time periods. HPCs were transferred to new wells of coated with corresponding 3T3 cells every 2–3 days. Cell phenotype was analyzed by flow cytometry on a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA) and data analyzed using the CellQuest program (Becton Dickinson, Mountain View, CA). To distinguish hematopoietic cells from contaminated stroma CD45+ cells were gated. For the analysis of gene and protein expression hematopoietic cells were isolated using biotin-conjugated anti-CD45 antibody and magnetic beads (Miltenyi Biotec Inc.). 32D cells (murine hematopoietic cells line) were cultured in RPMI medium with 10% FBS and 20% conditioned medium containing IL-3 (WEHI-3B cell line).
Immobilization of Notch ligands on plastic was performed as described previously (Ohishi et al., 2000). Briefly, 20 μg/ml of antibody against human IgG was placed into each well of a 24-well plate and incubated for 30 minutes at 37°C. Wells were blocked with CCM for 30 minutes followed by incubation for 2 hr at 37°C with 7.5 ng/ml Delta-1 or IgG in CCM.
Differentiation of human DCs
After obtaining informed consent, bone marrow cells were collected from disposed filters used for bone marrow transplantation. CD34+ cells were isolated using magnetic beads (Miltenyi Biotec Inc.) and cultured in complete culture medium supplemented with 100 ng/ml hGM-CSF, 25 ng/ml hSCF, and 2.5 ng/ml TNFα for 6 days. Ten nM SB216763 or 750 ng/ml recombinant human Wif-1 were added at the start of the cultures.
Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting
RNA was extracted with an RNase Mini kit and cDNA was synthesized using SuperScript III Reverse Transcriptase kit (QIAGEN, Valencia, CA). PCR was performed as described earlier (Nefedova et al., 2004) with 2.5 μl cDNA, 12.5 μl SYBR Master Mixture (Applied Biosystems, Foster City, CA), and target gene-specific primers (Table S2). Amplification of endogenous cyclophilin was used as an internal control. The analysis of gene expression by Western blotting was performed as previously described (Cheng et al., 2007). Cell lysate was subjected to electrophoresis in 8% sodium dodecyl sulfate–polyacrylamide gels followed by transfer to a polyvinylidene difluoride membrane and probing with specific antibodies. Equal loading was determined by β-actin levels. The specific bands were visualized by an enhanced chemiluminescence (ECL) detection kit (Amersham Life Sciences, Arlington Heights, IL).
HPC cell transfection and Luciferase reporter assay
HPCs were transfected using mouse macrophage buffer and Y01 program at AMAXA nucleofector (AMAXA, Gaithersburg, MD). GFP-positive cells were gated for further analysis by flow cytometry or sorted using a FACSAria cell sorter. Transfection efficacy was 5–10% (Fig. S4). For the reporter activity assay, enriched HPCs were transfected with either TCF/LEF or CBF-1 reporter plasmids (a mixture of inducible TCF/LEF or CBF-1 responsive firefly luciferase and Renilla luciferase constructs) from SABiosciences (Frederick, MD). Relative light units (RLU) of luminescence were measured 48 hr after transfection using Dual-luciferase reporter assay system (Promega).
Bone marrow stroma (BMS) and spleen stroma (SS) culture
BMS and SS cells were generated as described previously (Cheng et al., 2007).
Embryonic stem (ES) cell culture
Differentiation of DCs from ES cells was performed as described earlier (Cheng et al., 2003). Briefly, ES cells were grown on gelatinized plates for three days, transferred to bacterial-grade Petri dishes without leukocyte inhibitory factor to induce formation of embryonic bodies. Two weeks later, individual bodies were picked and transferred to a 24-well plate with 20 ng/mL GM-CSF and 10 ng/mL IL-3 and cultured for 14 days. DC cells were plated in a new 24-well plate and treated with SB216763 for 5 days before analysis.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed using the reagents and protocol from UPSTATE. Briefly, 2×106 32D cells were fixed by adding formaldehyde into culture media to a final concentration of 1%. Cells were then lysed and sonicated to shear the chromatin DNA. Cell lysate was pre-cleared with protein G-agarose before immunoprecipitation overnight at 4°C with 2 μg of anti-CBF1 antibody or normal mouse IgG. The precipitated antibody/chromatin complex was collected by incubation with protein G-agarose for 1 hr at 4°C, and was then washed and eluted in elution buffer. DNA in the precipitated samples was recovered after reverse cross-linkage at 65°C for 4 hr. Ten percent of lysate before antibody precipitation was used as the input. Real-time PCR was carried out with fzd10-specific primers encompassing each of the potential CBF1 binding sites. Hes1-specific primers that encompassed the CBF1 binding site were used as a positive control. Amplification of cyclophilin from the input was used as the loading control.
DC differentiation from HSC and CMP
HSC (Lin−c-kit+Sca-1+CD34−) and CMP (Lin−c-kit+Sca-1−CD34+) were sorted from mouse bone marrow. Cells were then cultured in myeloid long-term culture medium with a cocktail of cytokines (20 ng/ml of IL-3, IL-11, SCF, Flt3L and GM-CSF) for 5 days followed by 5-day culture in RPMI-1640 medium supplemented with GM-CSF in the presence of SB216763, Wnt3a, or Wnt5a to induce DC differentiation.
Adoptive cell transfer
Naive C57BL/6 (CD45.2+) recipient mice were irridiated (500 Rad, twice with a 3hr interval) and 1.5×106 isolated HPCs from CD45.1+ congenic mice were then injected intravenously (i.v.). Spleen and bone marrow cells were harvested 36 hr after injection and CD45.1+ cells were sorted using a FACSAria.
Allogeneic mixed leukocyte reaction (MLR)
HPCs were isolated from bone marrow of naïve C57BL/6 mice and were co-cultured with T cells (105/well) from allogeneic BALB/c mice for 4 days in triplicate in U-bottom 96-well plates at different ratios. [3H]-thymidine (1 μCi [0.037 MBq]) was added to each well 18 hr prior to cell harvest. T-cell proliferation was measured by [3H]-thymidine incorporation using a liquid scintillation counter (Packard Instrument, Meriden, CT). For the human MLR assay, cells differentiated from donor CD34+ cells were cultured with T cells isolated from peripheral blood of a different healthy donor for 5 days. T-cell proliferation was then measured by [3H]-thymidine incorporation as described above.
Retrovirus preparation and infection of myeloid progenitor cells
The HR-MMPCreGFP retroviral construct (Harvard Institute of Proteomics, Massachusetts) carrying an bioactive Cre-GFP fusion protein with a lox 511 in the 30 LTR U3 region were transfected into Phoenix-Ampho cells with GenePORTER2 (GTS, San Diego) as described by the supplier. Supernatants were harvested at 48 and 72 hr after transfection, and concentrated by ultracentrifugation at 16,600 rpm at 4°C for 90 min. The pellet was resuspended overnight at 4°C in 400 μl RPMI media and kept frozen at −80 °C. Lin-/c-kit+ myeloid progenitor cells were sorted from mouse bone marrow and were cultured in myeloid long-term culture medium supplemented with cytokine cocktail (20 ng/ml of IL-3, IL-11, SCF, Flt3L and GM-CSF) for 1 day. Cells were then infected with concentrated Cre- or control retrovirus twice with a 24 hr interval as described earlier (Cheng et al., 2007). These cells were then used for adoptive transfer into CD45.2+ congenicmice or cultured in 20ng/ml GM-CSF for 5 days to induce DC differentiation.
In adoptive transfer experiments 2×104 CD45.2+ myeloid progenitor cells infected with retroviruses were injected i.v. into irradiated (500 rad twice with a 3 hr interval) CD45.1+ congeneic mice together with 106 bone marrow cells from CD45.1+ mice. Spleens were harvested and evaluated 3 weeks after the transfer.
Statistical methods
Most of the data were analyzed using two-tailed Mann-Whitney test. Where warranted two-tailed parametric t-test was used. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thanks Drs. I. Mellman and I. Bernstein for providing us with reagents. This work has been supported in part by the Analytic Microscopy and Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center.
Abbreviations
- BMS
bone marrow stroma
- SS
spleen stroma
- TCF
T cell factor
- LEF
lymphoid enhancer factor
- GSK3β
glycogen synthase kinase 3β
- HPC
hematopoietic progenitor cells
- Wif-1
Wnt inhibitory factor-1
- HSC
hematopoietic stem cells
- CMP
common myeloid progenitors
- CCM
complete culture medium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allman D, Punt J, Izon DJ, Aster JC, Pear WS. An invitation to T and more: Notch signaling in lymphopoiesis. Cell. 2002;109:S1–S11. doi: 10.1016/s0092-8674(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Delaney C, Varnum-Finney B, Kohn AD, Moon RT, Bernstein ID. The interaction of the Wnt and Notch pathways modulates natural killer versus T cell differentiation. Stem Cells. 2007;25:2488–2497. doi: 10.1634/stemcells.2007-0102. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas SRM, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Gabrilovich D. Notch signaling in differentiation and function of dendritic cells. Immunol Res. 2008;41:1–14. doi: 10.1007/s12026-007-8011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic cell differentiation by bone marrow stroma via different Notch ligands. Blood. 2007;109:507–515. doi: 10.1182/blood-2006-05-025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Nefedova Y, Miele L, Osborne BA, Gabrilovich D. Notch signaling is necessary but not sufficient for differentiation of dendritic cells. Blood. 2003;102:3980–3988. doi: 10.1182/blood-2003-04-1034. [DOI] [PubMed] [Google Scholar]
- Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Miele L, Gabrilovich DI. Notch-1 regulates NF-kappa B activity in hematopoietic progenitor cells. J Immunol. 2001;167:4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 2006;86:842–852. doi: 10.1038/labinvest.3700442. [DOI] [PubMed] [Google Scholar]
- De Smedt M, Hoebeke I, Reynvoet K, Leclercq G, Plum J. Different thresholds of Notch signaling bias human precursor cells toward B-, NK-, monocytic/dendritic-, or T-cell lineage in thymus microenvironment. Blood. 2005;106:3498–3506. doi: 10.1182/blood-2005-02-0496. [DOI] [PubMed] [Google Scholar]
- Dontje W, Schotte R, Cupedo T, Nagasawa M, Scheeren F, Gimeno R, Spits H, Blom B. Delta-like1-induced Notch1 signaling regulates the human plasmacytoid dendritic cell versus T-cell lineage decision through control of GATA-3 and Spi-B. Blood. 2006;107:2446–2452. doi: 10.1182/blood-2005-05-2090. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Ferrero I, Held W, Wilson A, Tacchini-Cottier F, Radtke F, MacDonald HR. Mouse CD11c+ B220+ Gr1+ plasmacytoid dendritic cells develop independently of the T-cell lineage. Blood. 2002;100:2852–2857. doi: 10.1182/blood-2002-01-0214. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Garcia-Peydro M, de Yebenes VG, Toribio ML. Notch1 and IL-7 receptor interplay maintains proliferation of human thymic progenitors while suppressing non-T cell fates. J Immunol. 2006;177:3711–3720. doi: 10.4049/jimmunol.177.6.3711. [DOI] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- Heinz LX, Platzer B, Reisner PM, Jorgl A, Taschner S, Gobel F, Strobl H. Differential involvement of PU.1 and Id2 downstream of TGF-beta1 during Langerhans-cell commitment. Blood. 2006;107:1445–1453. doi: 10.1182/blood-2005-04-1721. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Schuster-Gossler K, Watabe-Rudolph M, Aulehla A, Herrmann BG, Gossler A. WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev. 2004;18:2712–2717. doi: 10.1101/gad.1248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino N, Katayama N, Shibasaki T, Ohishi K, Nishioka J, Masuya M, Miyahara Y, Hayashida M, Shimomura D, Kato T, et al. A novel role for Notch ligand Delta-1 as a regulator of human Langerhans cell development from blood monocytes. J Leukoc Biol. 2005;78:921–929. doi: 10.1189/jlb.1204746. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. WNT Signaling Pathway and Stem Cell Signaling Network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell stem cell. 2009;4:27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Matsubayashi T, Iwase S, Doi T, Kasai K, Yazaki M, Wada M, Takahashi T, Obata Y. Murine Delta homologue, mDelta1, expressed on feeder cells control cellular differentiation. Cell Struct Funct. 2000;25:21–31. doi: 10.1247/csf.25.21. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103:3503–3510. doi: 10.1182/blood-2003-07-2340. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–2229. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- Ohishi K, Varnum-Finney B, Flowers D, Anasetti C, Myerson D, Bernstein ID. Monocytes express high amounts of Notch and undergo cytokine specific apoptosis following interaction with the Notch ligand, Delta-1. Blood. 2000;95:2847–2854. [PubMed] [Google Scholar]
- Ohishi K, Varnum-Finney B, Serda RE, Anasetti C, Bernstein ID. The Notch ligand, Delta-1, inhibits the differentiation of monocytes into macrophages but permits their differentiation into dendritic cells. Blood. 2001;98:1402–1407. doi: 10.1182/blood.v98.5.1402. [DOI] [PubMed] [Google Scholar]
- Olivier A, Lauret E, Gonin P, Galy A. The Notch ligand delta-1 is a hematopoietic development cofactor for plasmacytoid dendritic cells. Blood. 2006;107:2694–2701. doi: 10.1182/blood-2005-03-0970. [DOI] [PubMed] [Google Scholar]
- Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- Radtke F, Ferrero I, Wilson A, Lees R, Aguet M, MacDonald HR. Notch1 deficiency dissociate intrathymic development of dendritic cells and T cells. J Exp Med. 2000;191:1085–1093. doi: 10.1084/jem.191.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Schroeder T, Kohlhof H, Rieber N, Just U. Notch signaling induces multilineage myeloid differentiation and up-regulates PU.1 expression. J Immunol. 2003;170:5538–5548. doi: 10.4049/jimmunol.170.11.5538. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.