Abstract
Background
Prior to the selection of disinfectants for low, intermediate and high (sterilizing) levels, the decimal reduction time, D-value, for the most common and persistent bacteria identified at a health care facility should be determined.
Methods
The D-value was determined by inoculating 100 mL of disinfecting solution with 1 mL of a bacterial suspension (104 – 105 CFU/mL for vegetative and spore forms). At regular intervals, 1 mL aliquots of this mixture were transferred to 8 mL of growth media containing a neutralizing agent, and incubated at optimal conditions for the microorganism.
Results
The highest D-values for various bacteria were determined for the following solutions: (i) 0.1% sodium dichloroisocyanurate (pH 7.0) – E. coli and A. calcoaceticus (D = 5.9 min); (ii) sodium hypochlorite (pH 7.0) at 0.025% for B. stearothermophilus (D = 24 min), E. coli and E. cloacae (D = 7.5 min); at 0.05% for B. stearothermophilus (D = 9.4 min) and E. coli (D = 6.1 min) and 0.1% for B. stearothermophilus (D = 3.5 min) and B. subtilis (D = 3.2 min); (iii) 2.0% glutaraldehyde (pH 7.4) – B. stearothermophilus, B. subtilis (D = 25 min) and E. coli (D = 7.1 min); (iv) 0.5% formaldehyde (pH 6.5) – B. subtilis (D = 11.8 min), B. stearothermophilus (D = 10.9 min) and A. calcoaceticus (D = 5.2 min); (v) 2.0% chlorhexidine (pH 6.2) – B. stearothermophilus (D = 9.1 min), and at 0.4% for E. cloacae (D = 8.3 min); (vi) 1.0% Minncare® (peracetic acid and hydrogen peroxide, pH 2.3) – B. stearothermophilus (D = 9.1 min) and E. coli (D = 6.7 min).
Conclusions
The suspension studies were an indication of the disinfectant efficacy on a surface. The data in this study reflect the formulations used and may vary from product to product. The expected effectiveness from the studied formulations showed that the tested agents can be recommended for surface disinfection as stated in present guidelines and emphasizes the importance and need to develop routine and novel programs to evaluate product utility.
Keywords: Disinfectants, decimal reduction time (D-value), Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Bacillus stearothermophilus
Background
To render a material or environment safe for handling purposes, a decontamination program must strive to remove organic/inorganic contaminants from a material, being required before any other process intended to inactivate or eliminate microorganisms [1]. A decontamination program begins with a cleaning method that assures a reduction of the natural bioburden, the initial population of viable microorganisms present, on the material to guarantee proper and successful application of a disinfectant, a sanitizer or a sterilant [2] for use in health care settings.
Antimicrobial agents applied to a material under set conditions can be classified in accordance with the level of decontamination provided [3,4] as: (i) antiseptics – chemical agents that inhibit or kill microbial growth and are nontoxic when applied to living tissues, used for hand washing or for treating surface wounds. Under certain circumstances, some antiseptics are also effective disinfectants; (ii) disinfectants – chemical and/or physical agents used to destroy or irreversibly inactivate many or all of the pathogenic microorganisms but not necessarily spores and not all viruses [5]. Under set conditions, the disinfectants may show sterilizing activity; (iii) sanitizers – chemical agents used to reduce, but not necessarily eliminate, bacteria from the inanimate material to levels considered acceptable as determined by public health codes or regulations. The main difference between a sanitizer and a disinfectant is that, at a specified dilution, the disinfectant must have a higher killing capability for pathogenic bacteria compared to that of a sanitizer [6]; (iv) sterilants are high level disinfectants that, under appropriate circumstances, provide sterilization by fully killing or removing all life forms from inanimate objects and surfaces, to specified sterility levels, including the inactivation or removal of spores and viruses. Sterilization is associated with the total absence of viable microorganisms, which refers to an absolute condition and assures the greatest safety margin than any other antimicrobial method [7,8].
Depending on the antimicrobial effectiveness expected from chemical agents under set conditions, disinfection can be classified [9] in health care centers as either "high level" (sterilization activity), "intermediate level" (inactivation of Mycobacterium tuberculosis and the most resistant types of viruses, such as the ones without protein membranes in their structure [5]) or "low level" (reduction of bioburden). Disinfection at intermediate and low levels is not effective against spores [1]. To meet these requirements, it is necessary to standardize the use of disinfectant and select the test organism that is mostly prevalent or common to the material or environment upon which the particular disinfectant is to be applied [10].
The effectiveness of a disinfectant can be affected by: (i) previous cleansing of the material; (ii) concentration of the disinfectant and duration of the application; (iii) concentration of the disinfectant on solution and final pH; (iv) temperature of the disinfecting procedure at the moment it is applied [4].
The Brazilian Legislature [11] provides a list of acceptable chemical agents, recommendations for their appropriate use, the maximum level of toxicity tolerated per agent and those chemical agents prohibited for disinfection purposes. The Legislature also established the Commission of Hospital Infection Control (CCIH) and Hospital Infection Programs (PCIH) and defined their objectives to standardize procedures so as to minimize the incidence of nosocomial infections caused by bioburdens, typical of specific environments. Together with the Pharmacy and Therapeutic Committees, these agencies should define effective policies and procedures for the application of various antimicrobial agents, integrated into the administrative structure of the institution.
Unfortunately, fewer than 20% of Brazilian hospitals have established adequate hospital infection control enforcement and prevention programs. In October 1996, Acinetobacter calcoaceticus was confirmed as the agent responsible for infant deaths in maternity hospitals in Boa Vista (Rondonia). From August to November 1997, Serratia marcescens was the causative organism in the deaths of 15 newborns. It was verified that Enterobacter cloacae infected 10 infants in São Paulo Maternity Hospitals. In a short period of time, from October 1999 to January 2000, the deaths of at least 24 infants from hospital infections were registered in Brazil. The probable cause for these outbreaks was inadequate disinfection programs implemented by these hospitals [12-14].
The selection of test microorganism(s) should be related to the use of the disinfectant, defined by the hospital program, and to the bioburden present in the specific environment or on the material. The selected bacteria should be associated with outbreaks of infections in health care environments. The effectiveness of a chemical agent can be related to the resistance of a specific microbiological species that can be used as a biological indicator (BI), and can be defined in terms of decimal reduction time (D-value). The biological indicator (BI) is a specific microorganism ("MO") suspension (microbiological test system) with a defined resistance to a specified decontamination procedure [7,8]. The suspension can be expected to follow a predictable death rate when exposed to certain physical and chemical parameters. Any reduction in BI bioburden levels, as related to the extent of exposure to the test disinfectant, depends upon the disinfection procedure, the chemical agent used and the application of this disinfectant to a particular environment or material.
To evaluate the efficacy of the chemical agents for hospital use against the bacteria (Staphylococcus aureus, Escherichia coli, Enterobacter cloacae, Serratia marcescens and Acinetobacter calcoaceticus) involved in the outbreak of infections in hospital nurseries in Brazil [14], the resistance of these bacteria to the particular disinfectant was investigated and expressed in decimal reduction time (D-value). The confidence levels were set for 6 to 12 log10 reduction of the initial population of bacterium in order to a predicted probability of a surviving microorganism of 10-1 or better.
Method
Microorganism Cultures
The bacterial strains, obtained from lyophilized culture collection at the Adolfo Lutz Institute (IAL, SP, Brazil), were Enterobacter cloacae IAL 1976; Serratia marcescens IAL 1478; and Acinetobacter calcoaceticus ATCC 19606, IAL 124. The reference bacteria used were Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Bacillus subtilis var. globigii ATCC 9372, and Bacillus stearothermophilus ATCC 7953.
Culturing [14]
Working cultures were kept on Tryptic Soy Agar (TSA, Difco, Detroit, Michigan, USA) at 4°C with weekly transfers. The 24 h cultures, grown on TSA at 22°C for S. marcescens, and at 35–37°C for E. cloacae, A. calcoaceticus, E. coli; S. aureus, were harvested into Tryptic Soy Broth (TSB, Difco), centrifuged (1000 × g/ 15 min/ 4°C) and suspended in saline solution (0.9% NaCl). Bacterial viability was estimated on TSA pour plates by confirming populations ≥ 106 CFU/mL. Spore cultures were developed for a period of six days in a sporulation medium [g/L: D-glucose, 2.5 (Sigma, St. Louis, Missouri, USA); L-glutamic acid, 0.4 (Sigma); yeast extract, 4.0 (Difco); peptone, 5.0 (Difco); sodium chloride, 0.01; manganese sulfate, 0.01; bacteriological agar, 20.0 (Difco)] at 37°C for B. subtilis, and at 62°C for B. stearothermophilus were harvested, centrifuged (4 times at 1935 × g/ 30 min), and kept suspended in chilled 0.02 M calcium acetate solution (pH = 9.7) at 4°C [15]. The viability of heat-shocked (80°C/10 min for B. subtilis and 100°C/ 20 min for B. stearothermophilus) spores was obtained through TSA pour plates by confirming populations ≥ 106 spores/mL. The spore suspensions of B. subtilis and B. stearothermophilus were used for the tests of the D-value determination.
Chemical Agents
Chlorhexidine digluconate (biguanide, 1,6-dichorophenyldiguanido hexane; 40% w/v; Zeneca Farmacêutica, SP, Br); and sodium dichloroisocyanurate (NaDCC, sodium salt 50% w/w in tablets, Johnson and Johnson, J&J, SP, Br), glutaraldehyde (1,5-pentanediol; 2.0% w/v with sodium bicarbonate, pH = 8.3, Aster Produtos Médicos, SP, Br), formaldehyde (monoaldehyde; 37% w/v, Aster Produtos Médicos, SP, Br); sodium hypochlorite (10% w/v, Aster Produtos Médicos, SP, Br); hydrogen peroxide (40%, Laborosa Farmacêutica, SP, Br); a mixture of peracetic acid (4.5% v/v, PAA) and hydrogen peroxide (2.2% v/v, H2O2) plus acetic acid, 10 mg/L (Minncare®, pH = 1.3, Minntech Corporation, Minneapolis, MN, USA) were used. The chemical solutions were prepared in sterile water for injection (WFI) to obtain: 0.4% and 2.0% v/v Chlorhexidine (pH = 6.2); 2.0% w/v glutaraldehyde (pH = 7.4); 0.1% and 0.2% v/v sodium dichloroisocyanurate (pH = 7.0); 0.025%, 0.05% and 0.1% sodium hypochlorite (pH = 7.0); 0.5% v/v formaldehyde (pH = 6.5); 1.0% v/v Minncare® (0.45% of PAA + 2.2% of H2O2, pH between 2.0 and 2.3) and 1.5% v/v and 26.5% hydrogen peroxide (pH = 3.3). The concentration of total available chlorine and hydrogen peroxide was determined by the iodometric method [16]. The dilute solutions, prepared with chlorine-free glassware, were filtered through a 0.22 μm membrane (Millipore, Bedford, MA, USA) for daily use. The solutions of the test chemical agents are approved for hospital disinfection use by the Brazilian Ministry of Health [11].
The agents [17] used to inactivate the test disinfectants in solutions at 1% concentration were, respectively, polysorbate 80 (Tween 80) to quench chlorhexidine, glycine to bind glutaraldehyde and formaldehyde, catalase to degrade peracetic acid plus hydrogen peroxide; sodium thiosulphate to degrade sodium dichloroisocyanurate and hypochlorite. The efficacy of the disinfectant inactivating agents were confirmed as follows: (i) not having an inhibitory effect on the previous bioburden; (ii) completely eliminating the activity of the disinfectant; (iii) and when combined with the disinfectant, the resultant product was non-toxic to the test bacterium.
Assay for D-value
The resistance value (death kinetics) of a BI was characterized in terms of decimal reduction time, D-value, which is the exposure time required, under specified set of conditions, to cause one log10 or 90% reduction of the initial population (N0, bioburden) of viable BI in the suspension [7,8]. The determination of the D-value for the tested agent consisted in the transference of 1.0 mL of a 24 h suspension of the bacterial strain into 100 mL of the disinfectant solution that was kept in constant agitation at a controlled temperature room (at 25°C ± 1.0°C), at time zero. The initial concentration of bacteria (No) exposed to the disinfectant at time zero was around 104 to 105 CFU/mL (colony forming units/mL). At regular intervals (1 min for vegetative forms and 5 min for spore forms), a sample of the 1.0 mL mixture was transferred to 8 mL of TSB containing 1 mL of an inactivating agent at 1% concentration to guarantee a complete inactivation of the disinfectant without interfering with survivor growth. Using TSA pour plates, the survivors were evaluated by dilution (1:10, 1:100, 1:103, 1:104, 1:105) in saline solution. A negative control was made in a tube of 9 mL TSB plus 1 mL of an inactivating agent. A positive control was made adding 0.1 mL organism suspension in 10 mL TSB in a tube. The method for each disinfectant and test bacterium was repeated at least four times, when bacterial cultures were exposed to four different samples of diluted disinfectant, before the determination of D-values by the survivor curves.
Analysis of the Results
Determination of D-Value and Calculation of the Confidence Level
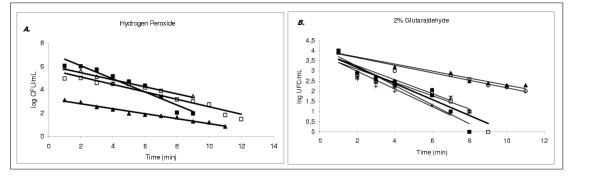
The D-value at 25°C was determined from the negative reciprocal of the slopes of the regression lines, using the linear portions of the survivor curves (log10 CFU/mL versus time of exposure to the disinfectant solution, at constant temperature [7] (Figure 1)). The D-value was determined from the inactivation kinetic curve given by the equation: t = D × (log No-log Nf) = D × n [1], where D = D-value (min) at specified conditions, No = bioburden of the chosen bacterium as the BI; Nf = surviving population after an exposure time, t (min), to the selected disinfectant and n = (log No-log Nf) = log10 reduction of a bioburden.
Figure 1.
A. Survivor curves for the bacteria exposed to hydrogen peroxide solutions, at 1.5% concentration, the respective decimal reduction times (D-values) were: for Enterococcus cloacae D = 1.8 min (■); Escherichia coli D = 3.2 min (△) and Staphylococcus aureus D = 3.4 min (□). At 26.5% hydrogen peroxide concentration, Bacillus stearothermophilus, D = 4.7 min (▲). B. Survivor curves for the bacteria exposed to 2% glutaraldehyde solutions, the respective decimal reduction times (D-values) were: for Acinetobacter calcoaceticus D = 4.7 min (■); Enterococcus cloacae D = 6.7 min (*); Escherichia coli D = 7.1 min (●); Staphylococcus aureus D = 5.9 min (□); Serratia marcescens D = 5.0 min (+);Bacillus stearothermophilus D = 25 min (▲); Bacillus subtilis D = 25 min (○).
The confidence level and the final number (NF) of the surviving population per mL solution was calculated in such a manner to be equivalent to 6log10 and 12log10 reduction in viable bioburden, considering, respectively, low level disinfection and high level disinfection [1,4,9]. Once confirmed the inactivation of a bioburden (No), the extent of the treatment for the disinfection procedure was determined by extrapolation to a predicted probability (safety factor) of a surviving microorganism of Nf = 10-6 or better [7,8]. Taking into consideration the following populations of the chosen bacterium: (i) No = 106 and 10° in the BI; (ii) No = 106 and Nf = 10-6 in the BI, the exposure time to the selected disinfectant set conditions, respectively, for 6 decimal logarithm (6log10) reduction and for an overkill of 12 decimal logarithm (12log10) of reduction in the bacterium population was calculated by the equation [1].
Results and Discussion
The classification of the activity spectrum for each chemical agent according to D-values for test bacteria at set conditions outlines its possible use in infection control programs in the health care environment [3,8,10]. However, there is no disinfectant that can serve all situations and meet all needs because of the existence of different conditions of usage in the health care routine [3,5,6,8,10].
For a better understanding of a disinfectant's effectiveness and standardization of use in hospital sanitation programs, the tested bacteria in solution were considered standard biological indicators (BI). The task demanded from the BI to monitor the disinfection procedure is a function of both the initial BI population (N0) and the D-value [15,18]. The overkill approach to disinfectant agent exposure is based on the premise that the extent of treatment will inactivate the initial bioburden (> 104 CFU/mL) and provide an additional safety factor [7,8]. The D-values and levels of confidence for every disinfecting solution and bacteria tested are shown in Table 1.
Table 1.
Decimal reduction times (D-values) for the bacteria in different chemical agent solutions.
| Bacteria | Disinfectant concentration | Survivors1 | D value | 2t = n × D | 2t = n × D | |
| (%) | (mg/L) | logN | (min) | 3n = 6-log10 | n = 12-log10 | |
| (min) | (min) | |||||
| SODIUM DICHLORO ISOCYANURATE(pH = 7.0) | ||||||
| A. calcoaceticus | 0.1 | 1000 | -0.17t | 5.9 | 35.4 | 70.8 |
| E. cloacae | 0.1 | 1000 | -0.21t | 4.7 | 28.2 | 56.4 |
| E. coli | 0.1 | 1000 | -0.17t | 5.9 | 35.4 | 70.8 |
| S. aureus | 0.1 | 1000 | -0.20t | 5.0 | 30.0 | 60 |
| S. marcescens | 0.1 | 1000 | -0.23t | 4.3 | 25.8 | 51.6 |
| B. stearothermophilus | 0.2 | 2000 | -0.22t | 4.4 | 26.6 | 53.3 |
| B. subtilis | 0.2 | 2000 | -0.26t | 3.8 | 22.5 | 45 |
| SODIUM HYPOCHLORITE (pH = 7.0) | ||||||
| E. coli | 0.05 | 500 | -0.16t | 6.1 | 36.4 | 72.9 |
| B. stearothermophilus | 0.05 | 500 | -0.106t | 9.4 | 56.4 | 112.8 |
| B. subtilis | 0.05 | 500 | -0.097t | 10.3 | 61.8 | 123.6 |
| B. stearothermophilus | 0.1 | 1000 | -0.28t | 3.5 | 20.9 | 41.8 |
| B. subtilis | 0.1 | 1000 | -0.31t | 3.2 | 19.2 | 38.3 |
| SODIUM HYPOCHLORITE(pH = 7.0) | ||||||
| A. calcoaceticus | 0.025 | 250 | -0.16t | 6.2 | 37.3 | 74.6 |
| E. cloacae | 0.025 | 250 | -0.13t | 7.5 | 44.8 | 89.6 |
| E. coli | 0.025 | 250 | -0.13t | 7.5 | 45.1 | 90.1 |
| S. aureus | 0.025 | 250 | -0.21t | 4.7 | 27.9 | 55.8 |
| S. marcescens | 0.025 | 250 | -0.149t | 6.7 | 40.1 | 80.2 |
| B. stearothermophilus | 0.025 | 250 | -0.041t | 24.0 | 144.0 | 288.0 |
| B. subtilis | 0.025 | 250 | -0.048t | 20.6 | 123.6 | 247.2 |
| GLUTARALDEHYDE(pH = 7.4) | ||||||
| A. calcoaceticus | 2.0 | 20000 | -0.21t | 4.7 | 28.2 | 56.4 |
| E. cloacae | 2.0 | 20000 | -0.15t | 6.7 | 40.2 | 80.4 |
| E. coli | 2.0 | 20000 | -0.14t | 7.1 | 42.6 | 85.2 |
| S. aureus | 2.0 | 20000 | -0.17t | 5.9 | 35.4 | 70.8 |
| S. marcescens | 2.0 | 20000 | -0.20t | 5.0 | 30.0 | 60.0 |
| B. stearothermophilus | 2.0 | 20000 | -0.04t | 25.0 | 150.0 | 300.0 |
| B. subtilis | 2.0 | 20000 | -0.04t | 25.0 | 150.0 | 300.0 |
| FORMALDEHYDE(pH = 6.5) | ||||||
| A. calcoaceticus | 0.5 | 5000 | -0.19t | 5.2 | 31.2 | 62.4 |
| E. cloacae | 0.5 | 5000 | -0.22t | 4.5 | 27.0 | 54 |
| S. marcescens | 0.5 | 5000 | -0.48t | 2.1 | 12.6 | 25.2 |
| B. stearothermophilus | 0.5 | 5000 | -0.10t | 10.9 | 65.4 | 130.8 |
| B. subtilis | 0.5 | 5000 | -0.13t | 11.8 | 70.8 | 141.6 |
| CHLORHEXIDINE(pH = 6.2) | ||||||
| A. calcoaceticus | 0.4 | 4000 | -0.24t | 4.1 | 24.6 | 49.2 |
| E. cloacae | 0.4 | 4000 | -0.12t | 8.3 | 49.8 | 99.6 |
| E. coli | 0.4 | 4000 | -0.34t | 3.0 | 18.0 | 36.0 |
| S. aureus | 0.4 | 4000 | -0.17t | 5.9 | 35.4 | 70.8 |
| S. marcescens | 0.4 | 4000 | -0.25t | 4.0 | 24.0 | 48.0 |
| B. stearothermophilus | 2.0 | 20000 | -0.11t | 9.1 | 54.6 | 109.2 |
| B. subtilis | 2.0 | 20000 | -0.15t | 6.7 | 40.2 | 80.4 |
| MINNCARE5 (pH = 2.3) | ||||||
| A. calcoaceticus | 1.0 | 10000 | -0.30t | 3.4 | 20.4 | 40.8 |
| E. cloacae | 1.0 | 10000 | -0.29t | 3.5 | 21.0 | 42.0 |
| E. coli | 1.0 | 10000 | -0.15t | 6.7 | 40.2 | 80.4 |
| S. aureus | 1.0 | 10000 | -0.25t | 4.0 | 24.0 | 48.0 |
| S. marcescens | 1.0 | 10000 | -0.28t | 3.6 | 21.6 | 43.2 |
| B. stearothermophilus | 1.0 | 10000 | -0.11t | 9.1 | 54.6 | 109.2 |
| B. subtilis | 1.0 | 10000 | -0.17t | 5.9 | 35.4 | 70.8 |
| HYDROGEN PEROXIDE(pH = 3.3) | ||||||
| E. cloacae | 1.5 | 15000 | -0.56t | 1.8 | 10.8 | 21.6 |
| E. coli | 1.5 | 15000 | -0.31t | 3.2 | 19.2 | 38.4 |
| S. aureus | 1.5 | 15000 | -0.30t | 3.4 | 20.4 | 40.8 |
| B. stearothermophilus | 26.5 | 265000 | -0.21t | 4.7 | 28.2 | 56.4 |
| B. subtilis | 1.5 | 15000 | -0.02t | 55.2 | 331.2 | 662.4 |
1 Survivor Curve: log Nf = log No-1/D × t; No= bioburden; Nf= survival population; D-value = decimal reduction time; (-1/D) = slope. 2 t = n × D, where: t = total exposure time (min); n = log10 reduced cycles 3 t = n*D and n = 6-log10, t = the exposure time for a 6-log10 reduction in the bioburden (No) with a defined D-value4 t = n*D and n = 12-log10, t = the exposure time for a 12-log10 reduction in the bioburden (No) with a defined D-value 5MINNCARE = 0.45 % peracetic acid + 2.2 % hydrogen peroxide
In general, a disinfectant is expected to be capable of at least a 5-log10 reduction of pathogenic bacteria during a time frame greater than 5 but lower than 10 minutes [6,19]. A chemical disinfecting agent is expected to be capable of a 3-log10 reduction of the infectious bacteria, within 30 seconds [6,19]. However, the elimination of spores, which is carried out by other chemical agents, requires longer exposure time to attain the confidence level established.
Chlorhexidine
The vegetative strains which showed the best resistance to the solution of 0.4% chlorhexidine were E. cloacae and S. aureus, at least 1.5 times greater than the D-values shown by the more sensitive ones, which were A. calcoaceticus, S. marcencens and E. coli. A time interval of 3 to 4 min was enough to reduce 90% of the population of E. coli, S. marcescens and A. calcoaceticus; a 3log10 reduction in bioburden for these species varied between 9 to 12 min.
The spore strains exposed to 2.0% chlorhexidine showed D-values 1.4 times higher for B. stearothermophilus than for B. subtilis.
It was observed that the strongest 2.0% chlorhexidine solution (5x that concentration of 0.4% chlorhexidine used against the other bacteria) showed a positive activity against populations of B. subtilis and B. stearothermophilus. It is unusual to find that alcohol/chlorhexidine mixtures would show any activity against spores, and may reflect log reductions of vegetative cells that remain in the spore population used for testing purposes. Therefore, the decimal reduction times reflected indeed log reductions of mixed populations formed of vegetative cells and spores, and this finding should be confirmed.
Chlorhexidine at 0.4% concentration in water or 70% alcohol is widely applied as a skin low-level disinfectant, becoming a leading hospital antiseptic in recent years due to its confirmed bactericidal (no sporocidal) effect and non-toxic side effects [11,20,21]. Chlorhexidine at 0.2% concentration is a well-known antimicrobial in the dental profession and 2.0% chlorhexidine solution has been applied to a disinfection of orthodontic prosthesis after mechanical cleansing.
In Brazil, 0.4%, 2.0% and 4.0% chlorhexidine solutions [11] are well-known as widely used disinfectants in hospitals. Within its principal applications, chlorhexidine is recommended for sanitizing the hands and the forearms of the surgical team and the patient's skin (pre-operative and invasive procedures), as well as bathing newborns. It was reported that a preparation with 0.5% chlorhexidine in 70% alcohol applied to baby skin before invasive procedures provided a significant delay in the colonization of catheters [20].
The 2.0% and 4.0% chlorhexidine used for surgical hand scrub products achieved a 3log10 reduction in microorganisms from baseline population count, and the 2.0% solution caused less irritation to hands than the 4% preparation [20], considering 20 seconds of hands friction [11,21].
For total immersion of invasive medical devices, a total exposure time for: (i) 6 to 12log10 reductions of vegetative bioburden with 0.4% chlorhexidine solution varied between 49.8 min and 99.6 min in relation to E. cloacae resistance, considering low disinfection level; and (ii) 12log10 of sporulating strains (n = 12) varied between 80.4 min (1 h 20 min) and 109.2 min (1 h 49 min), considering high level disinfection with 2.0% chlorhexidine solution.
The 2.0% chlorhexidine in 70% alcohol will be tested in our laboratory to determine the D-values of the test strains of bacteria, as well as in the hand asepsis and the immersion of various medical devices at a hospital setting in the city of São Paulo.
Formaldehyde (0.5%)
In a solution of 5000 mg/L (0.5%) of formaldehyde, the spore formers B. stearothermophilus and B. subtilis evidenced similar D-values, respectively, showing twice the resistance of A. calcoaceticus and E. cloacae; and five-fold resistance as determined for S. marcescens, the most sensitive species.
Despite its effectiveness in low concentrations (0.5%), formaldehyde is a highly corrosive and toxic compound considered potentially carcinogenic. Its vapors irritate the eyes and lungs. A maximum concentration of 1.0 mg/L in the working environment is recommended by the National Institute for Occupational Safety and Health. Nowadays, the 37% formaldehyde concentrate solution is industrially prepared without any exhaling odor or irritation during handling but with all safeness. In Brazil, formaldehyde is less costly than glutaraldehyde, being selected to be used mainly in public health care centers for multiple purposes, under severe regulations [4,11].
In Brazil, in the hospital environment 4.0% formaldehyde solution is considered a high level disinfectant and is applied to a 24-hour exposure in the disinfection of capillary tubes of dialysis systems. For the semi-critical articles high level disinfection, formaldehyde in alcoholic at 8.0% v/v or aqueous at 10.0% v/v preparations are applied to an 18-hour exposure. Considering the low disinfection level of hospital devices, a 4.0% formaldehyde solution, for a period from 30 minutes to 4-hours, is suggested [11]. The recommended concentration of formaldehyde in preparations is 10 times higher than the concentration of the test formaldehyde solution at 0.5% that showed 12log10 reductions of spores in a shorter time than that suggested by the Brazilian legislation. Both B. subtilis and B. stearothermophilus showed to be adequate BIs in the evaluation of the efficacy of formaldehyde in the immersion of medical devices. Periods from 65.4 min to 141.3 min are needed to reduce 6 and 12log10 of the initial spore population, respectively.
Even for manufacturers of parenteral solutions, a solution of 1.0% formaldehyde has also been historically associated with disinfection of the water purification system. Recently, for the same applications, formaldehyde has been replaced by solutions of peracetic acid, with or without hydrogen peroxide [22].
Glutaraldehyde
In a solution of 2.0% glutaraldehyde, both spore formers B. subtilis and B. stearothermophilus showed the same D-value, D = 25.0 min, which is approximately 3 to 4 times greater than that determined for the most resistant vegetative species of E. coli and E. cloacae. The most sensitive vegetative strains were A. calcoaceticus, S. marcescens, S. aureus and E. cloacae.
The extensive use of the high level disinfectant glutaraldehyde is due to its special characteristics. It is active in the presence of organic material, and unreactive in natural and synthetic materials and detergents. It does not coagulate proteinaceous material, and it has no corrosive effects on metals and rubber. However, glutaraldehyde is a toxic compound, which irritates the skin, mucous and eyes. For this reason, as most chemical solutions, its handling demands the use of equipment for individual protection [4,11].
Glutaraldehyde is currently the most widely used chemical for high and medium levels of disinfection. A solution of glutaraldehyde is widely used in thermo labile and semi-critical articles, such as materials used in respiratory therapy, fiber optic endoscopes, as well as equipment used in dispensing anesthesia gas, metallic articles and suction equipment [23,24]. The Brazilian Legislature recommends the use of 2.0% glutaraldehyde for a period of 8 to 10-hours exposure for sterilization procedures [4,11].
B. subtilis and B. stearothermophilus proved to be BIs appropriate for the evaluation of glutaraldehyde as a sterilizing agent, requiring exposure times of 300 min (5 h), to undergo a 12log10 reduction in viable spore forms. Glutaraldehyde is also appropriate in the disinfection of semi-critical articles, requiring 150 min exposure for a 6log10 reduction, respectively, in populations of B. subtilis and B. stearothermophilus. Comparing the sterilizing effect of 0.5% formaldehyde and 2.0% of glutaraldehyde, the necessary time for decay n = 12 cycles of B. subtilis varied from 141.6 min (2 h 22 min) to 300.0 min (5 h), respectively. The 0.5% formaldehyde solution was more efficient in relation to that of 2.0% glutaraldehyde. The ability of glutaraldehyde to reduce n = 6 cycles of the vegetative strains varied from 28.2 min (A. calcoaceticus) to 42.6 min (E. coli) exposure time. After meticulous cleaning, exposing endoscopes for 120 min, was deemed to be sufficient for their immediate reuse.
Association of peracetic acid (PAA, 0.45%) plus hydrogen peroxide (H2O2 2.2%) (Minncare®) and 1.5% hydrogen peroxide solutions
The most resistant bacteria to the solution of 1.0% Minncare (0.45% peracetic acid plus 2.2% of hydrogen peroxide) were B. stearothermophilus, B. subtilis and E. coli, which showed 2–3 times higher D-values than the more sensitive strains of A. calcoaceticus, E. cloacae and S. aureus, which presented similar resistance.
The D-values evaluated for vegetative bacteria in 1.5% H2O2 (pH = 3.3) were similar to those determined in 1.0% Minncare mixture (pH = 2.3) with 2.2% H2O2. However, the presence of 0.45% peracetic acid provided to the mixture sporocidal activity, reducing 10 times the D-value of B. subtilis (from D = 55.2 min in 1.5% H2O2 solution to 5.9 min in 1% Minncare mixture). Considering a spore suspension of B. stearothermophilus, the D-value was 4.7 min for 26.5% H2O2, the concentration of which was 12 times greater than that present in 1% Minncare mixture. Peracetic acid at concentration variable ≤ 1.0% was confirmed sporocidal [1,25].
Minncare is less malodorous than glutaraldehyde, but it can irritate the eyes and respiratory tract requiring the use of personal protective gear during handling. A balanced mixture of peracetic acid, hydrogen peroxide and water decomposes to acetic acid and water and this is considered safe by most occupational safety guidelines. Its corrosive effects on metallic surfaces in high concentrations is a disadvantage. Minncare should not be used on materials like copper or bronze, but it is well compatible with plastics. Dilution and the addition of an anticorrosive agent convert it to a suitable product for the disinfection of endoscopes [25]. The recommended concentration for high level disinfection is a stabilized mixture of 0.2 to 0.35% peracetic acid and 4–6% hydrogen peroxide [1,25].
The stabilized 1.0% Minncare mixture can be applied to the cleaning of reverse osmosis membranes and continuous deionization apparatus during three hours to obtain purified water and 18-hours to obtain water for injection, which will be used to prepare parenteral solutions, including peritoneal dialysis solutions. According to Vessoni Penna et al. [22], strains of E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 showed the greatest resistance among the test vegetative Gram negative bacteria, when exposed, respectively, to 4.0% hydrogen peroxide and 1.0% Minncare (peracetic acid (0.45%) + hydrogen peroxide (2.2%) solution), suggesting that P. aeruginosa and E. coli should be both required for monitoring disinfection of the systems for water purification purposes.
In the recent work, B. stearothermophilus showed to be a more appropriate BI for the evaluation of peracetic acid and hydrogen peroxide solutions. For the reduction of 6 (n = 6 cycles) and 12 (n = 12 cycles) log10 in viable counts, respectively, the effective exposure times were 54.6 min and 109.2 min. Among the vegetative strains, E. coli showed to be an adequate BI with a logarithm reduction factor of 6 in 40.2 min in comparison to 35.4 minutes observed for B. subtilis, for either disinfection of osmose reverse membranes (in water purification systems) or semi-critical articles in health care environment.
Hydrogen peroxide (H2O2) solution (1%–2% by weight), which has been used as a powerful oxidizing agent of proteins and other organic and inorganic encrustrated soils, is recommended for the low disinfection [11] of drinking water, non critical items, hard and smooth surfaces. A H2O2 solution at 1.5 ± 0.5% was prepared according to Brazilian hospital practices for cleaning medical devices [26], the concentration of which is easy to handle, has low toxicity and brings no damage to fiber materials and metal surfaces.
Sodium dichloroisocyanurate (NaDCC) and sodium hypochlorite (NaOCL)
The most resistant vegetative strains to a 1000 mg/L (0.1%) solution of sodium dichloroisocyanurate (NaDCC) were A. calcoaceticus and E. coli, which showed the same D-value. The most sensitive vegetative strains, with similar D-values, were S. marcescens, E. cloacae and S. aureus. To reduce a logarithmic cycle of B. subtilis and B. stearothermophilus, an exposure time between 3.8 min and 4.4 min to 0.2% NaDCC was required.
The D-values for the vegetative test bacteria in 0.025% (250 mg/L) sodium hypochlorite (NaOCL) solution (pH = 7.0) ranged from 4.7 min for S. aureus to 7.5 min for both E. cloacae and E. coli, the D-values of which were close to those determined for the same strains exposed to 0.1% NaDCC solution. The resistance observed for the spore forms: B. subtilis (D = 20.6 min) and B. stearothermophilus (D = 24.0 min) exposed to 0.025% sodium hypochlorite were close and doubled the D-values achieved for the same spore forms, respectively, D = 10.3 min and D = 9.4 min, exposed to 0.05% sodium hypochlorite solution at the same conditions (pH = 7.0, 25°C).
Chlorine-based disinfectants react readily with organic material, including blood, excrement and tissues. Its antimicrobial activity is proportionally decreased in relation to the amount of the organic material present. Therefore, the concentration of chlorine available at the disinfection process should be sufficiently high to satisfy the expenditure of chlorine (chorine reacted with the organic material) and provide enough chlorine for anti-microbial activity.
The non-dissociated form, hypochlorous acid (HOCL) in water at pH 5–8, is responsible for the microbial inactivation by the chlorine releasing agents (CRAs) [25]. The dissociated forms, hypochlorite ions (OCl-) that predominate in strong alkaline (pH>9.0) solutions, show less antimicrobial activity. At higher concentrations, the sodium hypochlorite (NaOCL) solutions are at pH>8 making the liberation of the active compound difficult [14]. NaOCL solutions, though effective at very low concentrations, as much as 250 mg/L (0.025%), as compared to the concentration of 1000 mg/L (0.1%) of NaDCC solution, have highly corrosive effects on metallic surfaces and irritate the skin [4,11,19].
The organic chlorine (NaDCC) can be used at higher concentrations than inorganic compounds (NaOCL) since NaDCC shows slow decomposition and liberation of active chlorine (HOCL). Its stability at pH 7 provides for exceptional experimental reproducibility. On the other hand, the 0.1% NaDCC solution shows lower toxicity, irritation to eyes and skin, less corrosive effects on metallic surface and less aggressiveness to plastic and rubber items than a solution of 250 mg/L of sodium hypochlorite.
In practice, NaDCC in the form of tablets, which dissolved very slowly and thereby released hypochlorous acid at the same rate it was being used, maintained an appropriate level of available chlorine without affecting the pH of the water [27]. Therefore, 0.1% NaDCC was recommended to be used in opened containers for intermediate disinfection level of semi-critical items; and 0.2% NaDCC solution might be applied to attain a high disinfection level at health care centers.
The recommendation of the Brazilian Ministerial directives [4,11] for the low level disinfection of lactates, inalotherapy, and oxigentherapy is an exposure of 60 min to a solution of sodium hypochlorite (NaOCL) at 250 mg/L (0.025%). Solutions of 0.5% NaOCL, for overnight exposure, are used to provide intermediate disinfection level of oxygen therapy and anesthesia devices, in hospital centers. Solutions of 1% NaOCL are applied to hard-surface disinfection of critical areas in hospital environments (intensive therapy rooms, nurseries, wards, operating theaters, special procedure places).
E. coli and A. calcoaceticus might have been used as appropriate BI for the evaluation of NaDCC as a disinfectant agent (n = 6 cycles) for an interval of 35.4 min. The high level disinfection parameter, n = 12, required exposure times of 53.3 min and 45.0 min for B. stearothermophilus and B. subtilis, respectively. B. stearothermophilus spores were observed to form appropriate BI to monitor sterilizing activity by a 0.2% NaDCC application.
General Comments
A successful disinfection at low and high levels (sterilization aim) depends upon the selection of the correct chemical agent associated with a proper disinfecting procedure. A thorough understanding of the unique characteristics of each chemical agent, including their limitations and appropriate applications, is necessary. It is also essential that the chemicals used in commercial products and in the preparation of the disinfecting solutions meet established quality requirements.
The foregoing work stated that the suspension studies were an indication of the disinfectant efficacy on a surface, and surface testing is widely recommended by regulatory standards [4,9,11,19]. Therefore, the data in this study reflect the formulations used, which may vary from product to product.
Conclusions
For hand disinfection, chlorhexidine can be used. However, due to the low D-values, concentrations higher than 0.4% should be tested to accomplish the set aim.
For instrument intermediate level disinfection, sodium dichloroisocyanurate (NaDCC) was recommended for the stabilized pH value and low corrosiveness to the metal articles.
For critical items, we recommended the stabilized Minncare mixture of 0.2 to 0.35% peracetic acid and 4–6% hydrogen peroxide, pursuant to these guidelines.
Although glutaraldehyde is better accepted to be used in sterilization procedures than formaldehyde, both disinfectants can be applied in a shorter period than that recommended by legislation.
The expected effectiveness of the studied formulations shows that the tested agents can be recommended for surface disinfection purposes, as stated in foregoing guidelines, and emphasizes the importance and need to develop routine and novel programs to evaluate product utility.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgement
We gratefully acknowledge the support of the Brazilian Committees FAPESP and CNPq, which provided us with undergraduate scholarships and funds.
Contributor Information
Priscila Gava Mazzola, Email: pmazzola@usp.br.
Thereza Christina Vessoni Penna, Email: tcvpenna@usp.br.
Alzira M da S Martins, Email: martins_alzira@hotmail.com.
References
- Rutala WA, et al. Principles of Disinfecting Patient-Care Items. In: William A Champlain, editor. Disinfection, Sterilization and Antiseptics in Health Care. Rutala: Polyscience Publishers; 1998. pp. 133–149. [Google Scholar]
- Jacobs PT, Wang JH, Gorham RA, Roberts CG. In: Disinfection, Sterilization and Antiseptics in Health Care. Champlain. William A, editor. Rutala: Polyscience Publishers; 1998. pp. 165–166. [Google Scholar]
- Madigan MT, Martinko JM, Parker J. Brock Biology of Microorganisms. 10. Pearson Education Publishers; 2002. pp. 696–707. [Google Scholar]
- Brazilian Ministry of Health Regulatory Agency – ANVISA-Agência Nacional de Vigilância Sanitária. 2003. http://www.anvisa.gov.br/
- National Air Duct Cleaners Association http://www.nadca.com/
- Regulatory and Environmental Consulting Choosing The Proper Sanitizer Or Disinfectant http://www.schiff-consulting.com/choosing.html
- ISO 14937 – International Standard Sterilization of Health Care Products – General Requirements for Characterization of a Sterilizing Agent and the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. 2000.
- ISO 11134 – International Standard Sterilization of Health Care Products – Requirements for Validation and Routine Control – Industrial Moist Heat Sterilization. 1994.
- Spaulding EH. Disinfection, sterilization and preservation. Philadelphia: Lea & Febiger; 1968. Chemical disinfection of medical and surgical materials; pp. 517–31. [Google Scholar]
- Croshaw B. Disinfectant Testing – with Particular Reference to the Rideal-Walker and Kelsey-Sykes Tests. In: Collins CH, editor. In Disinfectants Their use and evaluation of effectiveness. New York Academic Press; 1981. pp. 1–14. [Google Scholar]
- The Brazilian Ministry of Health Directives n° 15/MS/ANVS n° 211/MS/SNVS 1999, n° 196/MS/AVNS 1983, n° 113/MS/AVNS 1993, n° 122/DTN 1993, n° 211/MS/ANVS 1999, Health Sanitation Code n°12342, 1978, Decree n° 930 of the 27th of August 1992. Disposition concerning the names for the Control of Hospital Infections. Official Newspaper of the Union; Brazillian Section 1. pp. 12279–81. 4th September 1992.
- News Database http://www.estadao.com.br/agestado/
- Brazilian Pharmacy Council PAHO: Program to reduce deaths of babies in Brazil. Pharmacia Brasileira. 2000;3:34. [Google Scholar]
- Penna TC, Mazzola PG, Martins AM. The efficacy of chemical agents in cleaning and disinfection programs. BMC Infect Dis. 2001;1:16. doi: 10.1186/1471-2334-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna TCV, Machoshvili IA, Taqueda MES. Bacillus stearothermophilus sporulation response to different composition media. PDA J Pharm Sci and Techn. 1998;52:198–208. [PubMed] [Google Scholar]
- Penna TCV, Schaffnern D, Abe LE, Machoshvili IA. Inactivation of Brazilian wild type and enterotoxigenic Escherichia coli by chlorine. J Ind Microbiol. 1996;16:57–61. doi: 10.1007/BF01569922. [DOI] [PubMed] [Google Scholar]
- Russell AD. Neutralization Procedures in the Evaluation of Bacterial Activity. In: Collins CH, editor. Desinfectants. Their use and Evaluation of Effectiveness. New York Academic Press; 1981. pp. 45–57. [Google Scholar]
- Graham GS, Boris CA. Chemical and Biologica Indicators. In: Robert F Morrisey and G Briggs Phillipis, editor. Sterilization Technology, A Pratical Guide for Manufacturers and Users of Health Care Products. New York: Van Nostrand Reinhold; 1993. pp. 36–70. [Google Scholar]
- Rutala WA. APIC Guideline for selection and use of disinfectants. AJIC Am J Infect Control. 1995;23:35A–65A. doi: 10.1016/0196-6553(90)90089-b. [DOI] [PubMed] [Google Scholar]
- Gruendeman BJ, Larson EL. Antiseptics in Current Practice. In: William A Champlain, editor. Disinfection, Sterilization and Antiseptics in Health Care. Rutala: Polyscience Publishers; 1998. pp. 183–195. [Google Scholar]
- APIC guide line for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23:251–269. doi: 10.1016/0196-6553(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Penna TCV, Martins AM, Mazzola PG. Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. J BMC Public Health. 2002;2:13. doi: 10.1186/1471-2458-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala WA, Weber DJ. FDA labeling requirements for disinfection of endoscopes: a counterpoint. Infect Control Hosp Epidemiol. 1995;16:231–235. doi: 10.1086/647095. [DOI] [PubMed] [Google Scholar]
- Bond WW. Endoscopes reprocessing. In: William A Champlain, editor. Disinfection, Sterilization and Antiseptics in Health Care. Rutala: Polyscience Publishers; 1998. p. 159. [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiology Review. 1998;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna TCV, et al. Cleaning of Blood-Contaminated Reprocessed Angiographic Catheters and Spinal Needles. Infect Control Hosp Epidemiol. 2000;21:499–504. doi: 10.1086/501793. [DOI] [PubMed] [Google Scholar]
- Warren IC, Hutchinson M, Ridgway JW. Comparative Assment of Swimming Pool Disinfectants. In: CH Collins, editor. Desinfectants. Their use and Evaluation of Effectiveness. New York Academic Press; 1981. pp. 45–57. [Google Scholar]