Abstract
Programmed death one (PD-1) is an inducible molecule belonging to the immunoglobulin superfamily. It is expressed on activated T and B lymphocytes and plays pivotal roles in the negative regulation of adaptive immune responses. We report here an unexpected finding: that PD-1 could also be induced on splenic dendritic cells (DCs) by various inflammatory stimuli. Adoptive transfer of PD-1–deficient DCs demonstrates their superior capacity to wild-type DCs in innate protection of mice against lethal infection by Listeria monocytogenes. Furthermore, PD-1–deficient mice are also more resistant to the infection than wild-type controls, even in the absence of T and B cells, accompanied by elevated production of DC-derived interleukin-12 and tumor necrosis factor-α. Our results reveal a novel role of PD-1 in the negative regulation of DC function during innate immune response.
Introduction
Innate immune response constitutes early host defense against infection by intracellular pathogens, such as Listeria monocytogenes (LM), and is essential for host survival.1,2 An effective innate immune response allows the host to develop antigen-specific adaptive immune responses in which T and B cells are responsible for the clearance of the infection.3 At the cellular level, neutrophils and macrophages are thought to be the major innate cells killing LM.2,4 In recent years, dendritic cells (DCs) have also been implicated in playing a critical role in innate immunity.5 A subset of DCs (TipDC) have been shown to produce tumor necrosis factor-α (TNF-α) and iNOS, which, contributes to bacteria inhibition.6 In addition, DCs are an important in vivo source of interleukin-12 (IL-12), which feeds into an IL-12–interferon-γ (IFN-γ)–protective feedback mechanism.7 IL-12, generated by LM-infected macrophages and DCs, stimulates natural killer–cell (NK) activity, including IFN-γ production, which in turn further activates macrophages, DCs, and neutrophils.8 The importance of DC-derived IL-12 has also been implicated in an intracellular parasite Toxoplasma gondii infection mouse model. The temporary depletion of DCs abolished IL-12 production and increased early mortality.9 Furthermore, CD8α+ DCs were found to be essential for efficient LM entry into the spleen.10 With the importance of DCs for early host defense against pathogens demonstrated, the molecular mechanisms regulating activated DCs during an innate response, however, still remain largely unknown.
Programmed death one (PD-1), a member of the immunoglobulin superfamily, is an inducible molecule on activated T and B lymphocytes and is present on double-negative thymocytes during T-cell development.11,12 The critical roles of PD-1 to control lymphocyte activation and to maintain peripheral tolerance have been validated in PD-1–deficient mice, which develop spontaneous autoimmune diseases in different genetic backgrounds.13,14 B7-H1 (PD-L1) and B7-DC (PD-L2) are 2 counterreceptors for PD-1.15,16 B7-H1 is broadly inducible in various tissues and cell types,17 whereas B7-DC expression is limited to DCs and macrophages.18 Studies using B7-H1 knockout mice support that B7-H1 is the primary regulatory counterreceptor for the inhibitory function of PD-1 in the peripheral tissues.19 The interaction between B7-H1 and PD-1 also plays a critical role in the dysfunction of T cells during chronic viral infections20–23 and serves as a checkpoint molecule in determining the fate of T-cell activation and tolerance during T-cell priming.24,25
Although ample evidence supports the roles of PD-1 in negative regulation of adaptive immune responses, its expression and function in innate cells and immunity remain unexplored. In this report, we show that PD-1 could be induced to be expressed on splenic DCs (sDCs) during LM infection and subsequently inhibits innate immune responses.
Methods
Animals, cell lines, and reagents
C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). B6 Rag-1KO breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 PD-1KO breeding pairs were generous gifts from Dr Tasuku Honjo, Kyoto University (Kyoto, Japan). B7-H1KO mice in B6 background were generated and described previously.19 B6.FVB-Tg.Itgax−DTR/EGFP.57Lan/J transgenic mice were purchased from The Jackson Laboratory. Rag-1 PD-1 double knockout (DKO) mice in B6 background were generated by backcrossing B6 PD-1KO with B6 Rag-1KO mice. B6 Rag-1KO mice were bred and kept in the same facility as controls. Age-matched 6- to 8-week-old mice were used for all experiments. All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Anti–mouse PD-1 monoclonal antibody (mAb; clone G4) and anti–mouse B7-H1 mAb (clone 10B5) were generated in our laboratory and described previously.26,27 For PD-1 staining, FMAT-blue–conjugated (Applied Biosystems, Foster City, CA) or biotin-labeled G4 was used. Biotin-labeled 10B5 was used for B7-H1 staining. All other antibodies used in flow cytometry analysis were purchased from BD Biosciences (San Jose, CA) or eBioscience (San Diego, CA). Control hamster IgG was purchased from Sigma-Aldrich (St Louis, MO). The Toll-like receptor (TLR) ligands were purchased from Invivogen (San Diego, CA).
LM infection
Mice were infected intravenously with 105 colony-forming units (CFUs) of a virulent strain of LM (DP-L4056), which was kindly provided by Thomas W. Dubensky Jr from Cerus (Concord, CA). Mouse survival was monitored up to 3 months. For serum cytokine analysis, blood was collected through the tail vein at indicated time points after infection. For some experiments, liver and spleen were harvested 1, 2, or 3 days after infection for further analysis. Bacterial titers were measured by plating of homogenized organs on Difco Listeria plates (BD Biosciences).
Preparation of spleen DCs
The method to purify mouse sDCs was described previously.28 Briefly, spleens were cut into small pieces (∼ 1 mm3) and incubated at 37°C in collagenase buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2) containing 1 mg/mL collagenase D (Roche Diagnostics, Indianapolis, IN) for 45 minutes. Cell suspension was obtained by vigorous pipetting and washing with complete RPMI 1640 (RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). CD11c+ DCs were isolated using CD11c magnetic-activated cell sorting (MACS) beads according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Purified cells contained approximately 95% CD11c+ cells.
DC and NK-cell adoptive transfer
Splenic DCs were purified using CD11c MACS beads from Rag-1KO and Rag-1 PD-1 DKO mice. NK cells were purified using a NK negative selection kit (Miltenyi Biotec) from Rag-1KO and Rag-1 PD-1 DKO mice. A total of 6 million DCs or 10 million NK cells were intravenously injected into each Rag-1KO host. One hour later, the mice were intravenously infected with 105 CFU LM. Spleens were harvested 3 days after infection. Bacterial titers were measured by plating of homogenized spleens and livers on Difco Listeria plates (BD Biosciences).
Intracellular cytokine staining and ELISA
Each mouse spleen was first digested with collagenase D and resuspended in a 6-well plate. Spleen cells were then stimulated in vitro with phosphate-buffered saline (PBS) or 108 heat-killed LM (HKLM) per milliliter in the presence of brefeldin A (eBioscience) for 4 hours. In some experiments, 20 μg/mL 10B5 was included in the culture. Intracellular stainings for IFN-γ, IL-12 p70, and TNF-α were performed according to the manufacturer's protocol (Cytofix/Cytoperm; BD Biosciences).
Mouse serum IFN-γ, IL-12p70, and TNF-α were detected by sandwich enzyme-linked immunosorbent assay (ELISA) methods according to the manufacturer's instructions (BD Biosciences PharMingen, San Diego, CA; or eBioscience).
Reverse-transcription–polymerase chain reaction
Naive T cells were collected from B6 and B6 PD-1KO mice and activated by plate-bound anti–mouse CD3 (2C11) for 24 hours. Naive spleen CD11c+ cells were purified from B6 Rag-1KO and B6 Rag-1 PD-1 DKO mice, and then activated by LM in culture for 48 hours. Messenger RNAs were extracted by the QIAGEN RNeasy mini kit (QIAGEN, Valencia, CA). First-strand cDNAs were synthesized by the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA) and PD-1–specific polymerase chain reactions (PCRs) were performed. Reverse-transcription–polymerase chain reaction (RT-PCR) specific for mouse β-actin was used as control. PD-1 primers: 5′ ATGTGGGTCCGGCAGGTACCCTGG, 3′ TCAAAGAGGCCAAGAACAATGTCC. β-actin primers: 5′ GTCCCTCACCCTCCCAAAAG, 3′ GCTGCCTCAACACCTCAACCC.
Statistics
Liver or spleen LM CFU counts and serum cytokine levels were compared between groups using the 2-tailed Student t test. Survival experiments were analyzed using the log-rank test. P values less than .05 were considered statistically significant. The error bars in the figures represent SD.
Results
PD-1 deficiency increases innate resistance of mice to LM infection
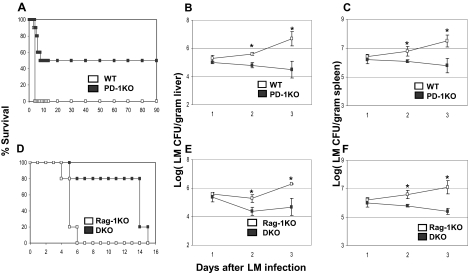
To explore the role of B7-H1-PD-1 pathway in early innate defense, we used a mouse model of LM infection, in which LM replication is controlled largely by innate immunity at an early stage.1 PD-1–deficient (KO) mice were challenged with 105 CFU of LM intravenously, which is a lethal dose for 100% wild-type (WT) C57BL/6 (B6) mice within 4 days. However, PD-1KO mice survived much longer than their WT littermates. Up to 50% of PD-1KO mice cleared the bacteria and achieved long-term survival (> 90 days after the challenge; Figure 1A). This survival advantage was correlated with decreased bacteria titer in the liver (Figure 1B) and spleen (Figure 1C) of PD-1KO mice on day 2 and day 3 after LM infections, compared with WT control. Our results suggest that PD-1 plays a negative role in the control of early-stage LM infection.
Figure 1.
PD-1 deficiency renders resistance to listeriosis. (A) WT B6 (n = 10) or B6 PD-1KO (n = 10) mice were infected with 105 LM by intravenous injection. Their survival was monitored daily. A total of 50% of PD-1KO mice survived more than 90 days. P < .01 between the 2 groups. With the same infection procedure as in panel A, liver (B), and spleen (C) LM count (CFU per gram tissue) was measured daily for 3 days after infection. Data shown are the average of 5 mice. (D) B6 Rag-1KO (n = 10) or B6 Rag-1 PD-1 DKO (n = 10) mice were infected with 105 LM by intravenous injection, and their survival was monitored for 15 days. P < .01 between the 2 groups. With the same infection procedure as in panel D, liver (E) and spleen (F) LM count (CFU per gram tissue) was measured daily for 3 days after infection. Data shown are the average of 4 mice. *P < .05.
We next backcrossed PD-1KO to Rag-1KO background, which eliminates all T and B lymphocytes and allows us to exclusively evaluate the role of PD-1 in innate cellular components. Phenotypic analysis of cells from 7- to 8-week-old PD-1 Rag-1 DKO mice by flow cytometry did not display any significant differences in numbers of NK cells, neutrophils, macrophages, and DCs in the spleen, liver, or peripheral blood mononuclear cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Therefore, PD-1 does not seem to affect the development of these cell types in DKO mice.
DKO mice were challenged with a lethal dose of LM to evaluate their innate resistance. Consistent with previous findings in PD-1KO mice in B6 background, DKO mice had prolonged survival compared with control Rag-1KO littermates, which all died within 6 days of infection. The majority of DKO mice survived up to 14 days (Figure 1D). Therefore, the lack of PD-1 enhances innate resistance to LM infection, even in the absence of adaptive immunity, although the eventual elimination of bacteria and long-term protection requires adaptive immune responses. Consistent with prolonged survival, DKO mice had significantly less bacteria titer in their livers and spleens on day 2 and day 3 than Rag-1KO controls (Figure 1E,F). Enhanced resistance to LM infection was also observed in Rag-1KO mice by infusing an anti–B7-H1 antibody (Figure S2), which blocks B7-H1 and PD-1 interaction.26 Our results thus indicate a previously unappreciated inhibitory function of B7-H1-PD-1 interaction in innate immunity against LM infection. Importantly, our data suggest that PD-1 on cells other than T and B lymphocytes inhibits the innate function.
PD-1 is up-regulated on sDCs but not on other innate immune cells
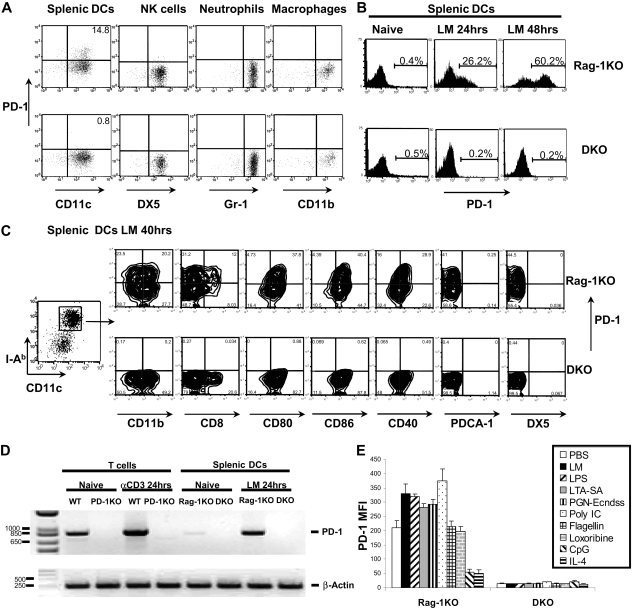
We first examined PD-1 surface expression on cells from spleens and lymph nodes of WT B6 and B6 Rag-1KO mice by flow cytometry using anti–PD-1 antibody. Although there were small fractions of cells from spleens and lymph nodes of WT B6 mice expressing PD-1, no PD-1 expression was found on cells from Rag-1KO mice (data not shown). In contrast, 16 hours after LM infection in vivo, CD11chigh and major histocompatibility complex (MHC) class II (I-Ab)high sDCs were found to express PD-1. As shown, 14.8% of sDCs from Rag-1KO were PD-1+, whereas only 0.8%, which represented background staining, of DKO sDCs were PD-1+ (Figure 2A). Meanwhile, we were unable to detect PD-1 on NK cells, neutrophils, or peritoneal macrophages after LM infection (Figure 2A). These results revealed that PD-1 is selectively induced on a subset of DCs on LM challenge.
Figure 2.
Expression of PD-1 on activated spleen DC. (A) Rag-1KO and Rag-1 PD-1 DKO mice were infected with 105 LM by intravenous injection. Spleens were harvested 16 hours after infection. CD11c+ cells were purified using CD11c MACS beads and staining with MHC II (I-Ab), NK (DX5), PD-1, and DC (CD11c) markers. A total of 14.8% of CD11c+I-Ab+DX5− DCs from Rag-1KO mice were PD-1+, whereas NK cells (CD11b+I-Ab− DX5+), neutrophils (I-Ab− Gr-1+ CD11b+), and peritoneal macrophages (I-Ab+CD11b+) from Rag-1KO mice stained negative for PD-1. (B) Splenic CD11c+ cells were purified using CD11c MACS beads from Rag-1KO and DKO mice, incubated in vitro for 48 hours with 5 × 104 LM/mL, and stained for PD-1 expression. (C) Same procedure as in panel B. Conventional sDCs were further gated as CD11chighI-Ab high population and stained for PD-1 and various other surface markers as indicated. (D) Semiquantitative RT-PCR analysis of PD-1 transcripts was performed with mRNA extracted from naive and anti-CD3–activated T cells from B6 WT and PD-1KO mice, followed by native and LM-activated splenic CD11c+ cells from B6 Rag-1KO and B6 DKO mice. RT-PCR specific for mouse β-actin was used as the loading control. (E) Splenic CD11c+ cells were purified using CD11c MACS beads from Rag-1KO and DKO mice, incubated in vitro for 48 hours with different TLR ligands and cytokines. Expression of PD-1 was measured as mean fluorescence intensity (MFI).
To validate the expression of PD-1 on DCs, DCs were enriched from spleens of Rag-1KO and DKO mice by positive selection with CD11c MACS beads. These positively selected cells had more than 95% CD11c+ cells, among which 85% of them were CD11chigh MHC IIhigh conventional DCs. The remaining 15% of the CD11c+ cells were a mixture of CD11cmedium MHC IImedium PDCA-1+ plasmacytoid DCs and recently identified CD11cmedium MHC IImedium DX5+ IFN-producing killer DCs.29,30 Freshly isolated sDCs showed a naive phenotype with low expression levels of costimulatory molecules CD80, CD86, and CD40, and did not have detectable PD-1. When incubated in vitro with LM, conventional DCs up-regulated PD-1: approximately 20% to 26% of CD11chigh MHC IIhigh DCs became PD-1+ in 24 hours (Figure 2B), and 50% to 60% DCs became PD-1+ after 40 hours (Figure 2B,C). Increased expression of PD-1 was accompanied by significant up-regulation of CD80, CD86, and CD40 by 40 hours (data not shown). There was no detectable cell-surface PD-1 in purified sDCs from DKO mice. We also purified peritoneal macrophages and spleen NK cells from Rag-1KO and DKO mice and stimulated them with TLR ligands (lipopolysaccharide [LPS], polyinosinic-polycytidylic acid [Poly I:C], and cytosine-phosphate-guanine [CpG]) or cytokines (IL-2, IL-12, and IFN-γ) in vitro for 48 hours. No PD-1 surface expression was observed on macrophages or NK cells after in vitro stimulation (data not shown).
Further characterization of PD-1+ cells after LM stimulation indicated that PD-1 was mainly expressed on CD11chighMHC IIhighCD11b+CD40highCD80highCD86high cells, a subset of myeloid DCs with an activated phenotype.31 Within this subset, PD-1 expression was higher on CD8+ DCs (MFI = 199) than CD8− DCs (MFI = 95; Figure 2C). The PD-1+ DC subset was also negative for PDCA-1 (plasmacytoid DC marker), DX5 (NK marker), Gr-1, CD3, and CD19 (Figure 2C; and data not shown). In addition, PD-1 expression was not detectable on plasmacytoid DCs after a 2-day culture with LM (Figure S3). Surprisingly, there was no significant surface PD-1 expression on naive or activated bone marrow–derived DCs (data not shown). In summary, our results indicate that PD-1 expression in T cell– and B cell–deficient Rag-1KO mice is highly selective and could only be found on a subset of DCs. Therefore, ablation of PD-1 on DCs may contribute to the innate resistance against LM infection.
Consistent with flow cytometric analysis, RT-PCR experiments confirmed that PD-1–specific mRNA was present at low levels from freshly purified sDCs from Rag-1KO mice, whereas after culture with LM for 48 hours DCs greatly up-regulated PD-1 transcripts. As a positive control, naive T cells were shown to constitutively express PD-1 mRNA, additional activation by plate-bound anti-CD3 antibody up-regulated its expression (Figure 2D).
In contrast to the limited expression pattern of PD-1, B7-H1 could be broadly up-regulated on virtually all splenic cells, including NK cells, neutrophils, macrophages, and DCs after LM infection (Figure S4). This broad expression pattern of B7-H1 provides ample opportunity for PD-1 engagement on sDCs.
PD-1 surface expression on sDCs is modulated by TLR ligands
As a Gram-positive bacterium, Listeria lacks LPS, but possesses various other Toll-like receptor (TLR) ligands, among which TLR2 ligands have been indicated as the most important for host recognition.2,32 Up-regulation of PD-1 by LM infection implies that expression of PD-1 may be a consequence of TLR and/or cytokine signaling. To test this, we next stimulated sDCs with various cytokines and TLR ligands in vitro to assess their ability to modulate PD-1 surface expression. Interestingly, we observed up-regulation of PD-1 on the cell surface in 20% to 40% of sDCs during 48 hours of culturing without any additional reagents. This correlates with in vitro partial maturation of sDCs in culture.28 Compared with this “spontaneous” up-regulation, purified lipoteichoic acid (LTA-SA; TLR2 ligand), Poly I:C (TLR3 ligand), LPS (TLR4 ligand), and soluble sonicated peptidoglycan (PGN-Ecndss; NOD1/2 ligand) further up-regulated PD-1 expression, whereas IL-4 and CpG (TLR9 ligand) significantly inhibited PD-1 surface expression (Figures 2E, S5). Flagellin (TLR5 ligand) and Loxoribine (TLR7/8 ligand) had no effect on PD-1 expression (Figure 2E). Similarly, addition of IL-2, IL-6, IL-10, IFN-γ, IL-12, and TNF-α also did not affect the expression of PD-1 (data not shown). Our results thus indicate PD-1 on sDCs could be selectively induced by ligation of TLR2, TLR3, TLR4, and nucleotide-binding oligomerization domain (NOD) proteins, and its expression could be inhibited by IL-4 and TLR9 ligation.
PD-1 inhibits sDC-derived IL-12 and TNF-α response to LM infection
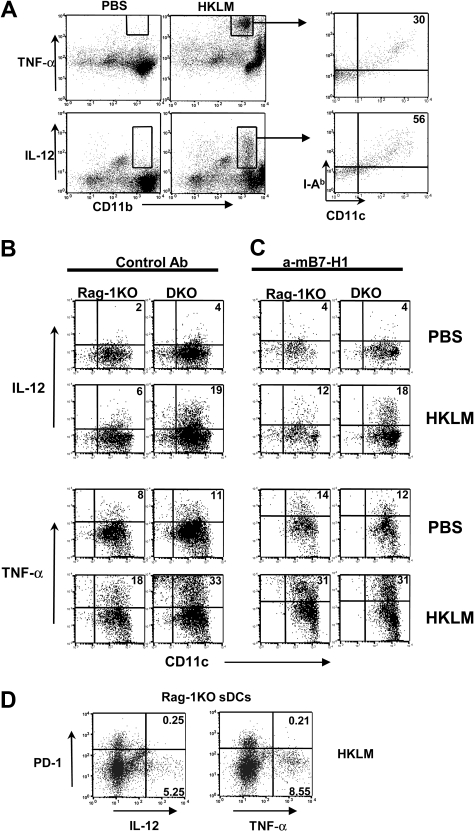
Cytokines play pivotal roles in modulating innate immune responses against infection. DCs are an abundant source for IL-12 and TNF-α, which have been shown to be important in controlling bacterial infection.6,33 We first determined whether LM infection stimulates DC-derived IL-12 and TNF-α production. To facilitate this analysis, we adopted an ex vivo culture system in which spleen cells were harvested 24 hours after LM infection, and stimulated with or without HKLM for 4 hours in the presence of Golgi blocker. Intracellular IL-12p70 and TNF-α in DCs and other spleen subsets were then determined by flow cytometry using specific mAbs. Consistent with previous observations, CD11b+ myeloid cells in the spleen were the major source for IL-12p70 and TNF-α by HKLM stimulation.7,8 Among the TNF-α–producing myeloid cells, 30% were MHC class II (I-Ab)+CD11c+ DCs, whereas 56% of IL-12p70–producing cells were I-Ab+CD11c+ DCs (Figure 3A). Therefore, sDCs are a significant source of IL-12 and contribute a large amount of TNF-α after LM stimulation.
Figure 3.
PD-1 negatively regulates DC IL-12 and TNF-α production. (A) Total splenocytes from Rag-1KO or DKO mice were incubated with PBS control or 106 CFU/mL HKLM for 4 hours in the presence of Golgi blocker brefeldin A. Intracellular staining of TNF-α and IL-12p70 revealed that CD11b+ myeloid cells were the main producer of both cytokines on LM stimulation. Cytokine-producing cells were further gated and stained with MHC II (I-Ab) and CD11c markers. (B) Rag-1KO or DKO mice were intravenously infected with 105 CFU LM. At 24 hours later, splenocytes were purified and restimulated with PBS or HKLM in the presence of brefeldin A for 4 hours. DCs were purified using CD11c MACS beads. I-Ab+CD11c+ gated DC population was stained for intracellular IL-12p70 and TNF-α. (C) Same condition as in panel B: anti-B7-H1 mAb 10B5 was added during in vitro restimulation. (D) Same condition as in panel B: CD11c+ gated Rag-1KO sDCs population was costained for cell-surface PD-1 and intracellular IL-12p70 and TNF-α. Percentage of quadrants was marked on the plots.
The roles of PD-1 in the regulation of IL-12 and TNF-α production were evaluated in sDCs from Rag-1KO and DKO mice. Whereas DCs from LM-infected Rag-1KO and DKO mice produced negligible IL-12 p70 and a moderate amount of TNF-α without in vitro restimulation, HKLM induced higher production of these cytokines. A more significant increase in cytokine production was observed in DKO sDCs than Rag-1KO sDCs mice after HKLM restimulation (Figure 3B). This enhanced response to HLKM was not caused by the increase of intrinsic ability of DKO DCs to release these cytokines because naive sDCs from Rag-1KO and DKO mice produced similar amounts of IL-12p70 and TNF-α on HKLM stimulation in vitro (Figure S6). Inclusion of B7-H1–blocking mAb in the culture after LM infection significantly increased the percentage of IL-12p70 and TNF-α–positive Rag-1KO DCs (Figure 3C), but not the percentage of IL-12p70 and TNF-α–positive DKO DCs (Figure 3C), indicating B7-H1-PD-1 pathway negatively modulates DC cytokine response to LM. This result suggests that B7-H1 is a major counterreceptor for the inhibitory effect of PD-1 on DC-derived cytokine prodution. In addition, in Rag-1KO sDCs, the PD-1–positive population largely lost their capacity to produce IL-12 and TNF compared with the PD-1–negative population (Figure 3D), indicating that up-regulation of PD-1 on DCs directly inhibits DC cytokine production.
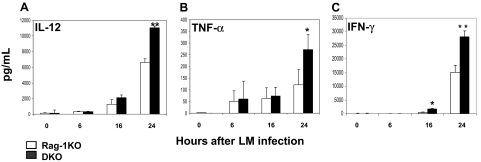
We further examined levels of IL-12 and TNF-α in the mouse sera after LM infection to determine whether our findings ex vivo are correlated with those in vivo. Serum samples were collected from Rag-1KO and DKO mice at several time points up to 24 hours after LM infection. Cytokine levels of the samples were measured by sandwich ELISAs. Without LM infection, IL-12 and TNF-α were undetectable in Rag-1KO and DKO mice, indicating that that PD-1 is not involved in the regulation of these cytokines at basal levels. After LM infection, however, DKO mice produced significantly higher levels of these cytokines than Rag-1KO (Figure 4). Our results thus demonstrate a correlation of our findings in vitro with in vivo infection and implicate a role of PD-1 in the regulation of these cytokines from DCs in vivo.
Figure 4.
Increased inflammatory cytokines in Rag-1 PD-1 DKO mice. B6 Rag-1KO (n = 3) and DKO (n = 3) mice were intravenously infected with 105 CFU LM. Sera were collected 0, 6, 12, and 24 hours after infection and pooled within each group. Serum (A) IL-12p70, (B) TNF-α, and (C) IFN-γ were determined by sandwich ELISA. Data shown are representative of 3 experiments. Error bars represent SD. *P < .05; **P < .01.
Transfer of PD-1–deficient DCs augments innate resistance
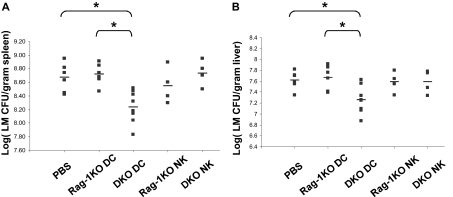
To provide direct evidence that lack of PD-1 on DCs is responsible for the early resistance against LM infection in PD-1KO mice, we initially used B6.FVB-Tg.Itgax−DTR/EGFP.57Lan/J transgenic mice, in which CD11c+ cells could be transiently deleted in vivo after injection of diphtheria toxin.34 Unfortunately, diphtheria toxin injection in these mice greatly enhances early innate resistance to LM infection, perhaps because of activation of macrophages, which mediate clearance of dying DCs. We therefore failed to establish a workable model using this strain. As an alternative approach, we transferred purified sDCs (6 × 106/mouse) from DKO mice into Rag-1KO hosts to determine whether this will convey augmented resistance to LM infection. As a control, identical numbers of sDCs from Rag-1KO mice were also transferred. As additional controls, NK cells (107/mouse) from Rag-1KO and DKO mice were also purified and transferred into Rag-1KO hosts. Only the mice receiving DKO DCs showed significantly augmented resistance to LM infection: the spleen and liver LM titer was significantly lower in DKO DC-transferred mice than that of the control mice on day 3 after LM infection (Figure 5). No significant differences were found by transfer of NK cells from either Rag-1KO or DKO mice into Rag-1KO hosts. Accordingly, Rag-1KO mice receiving DKO DCs had higher levels of serum IL-12 than controls at 24 hours after infection (Figure S7). In conclusion, adoptive transfer of PD-1–deficient DCs enhances innate immune function against subsequent LM infection in vivo.
Figure 5.
Adoptive transfer of PD-1–deficient DC provides innate protection against LM infection. Rag-1KO hosts received PBS control or 6 × 106 purified sDCs or 107 purified NK cells from Rag-1KO or DKO mice, and then intravenously challenged with 105 CFU LM the same day. Mice were killed at day 3 after infection. Spleen (A) and liver (B) LM counts (CFU per gram tissue) were measured. *P < .05.
Discussion
Our study reveals that, in addition to T and B lymphocytes, PD-1 can be induced on sDCs by LM infection or TLR2, TLR3, TLR4, and NOD engagements. PD-1 thus may have a much broader distribution pattern than previously thought. We further demonstrate that PD-1 is a negative regulator of DC-mediated innate immunity by using genetic knockout mice and specific neutralizing mAb. Interaction of PD-1 with its counterreceptor B7-H1 is required for its negative effect. Our findings also shed light on potential suppression mechanisms of PD-1 on DC function: inhibition of IL-12 and TNF-α response to infection. These findings thus reveal a new aspect in negative regulation of DC function during innate immunity.
PD-1KO mice in B6 background showed strong resistance to LM challenge in the first week of infection when a de novo adaptive immune response has yet to be established. Although our data support an enhanced innate rather than adaptive immunity in PD-1KO mice, T cells with effector/memory phenotypes are found to accumulate in the peripheral and lymphoid organs of PD-1KO mice.13 These effector/memory T cells could release IFN-γ quickly on LM infection in an antigen nonspecific fashion to active innate immune cells.35 This possibility, however, was excluded by our experiments using Rag-1 PD-1 DKO mice: the DKO mice still showed strong innate resistance to LM compared with Rag-1KO mice, although longer-term protection to LM infection was clearly dependent on adaptive immunity. Virtually no DKO mice could survive more than 15 days after LM infection (Figure 1C). In contrast, 50% PD-1 KO/B6 mice achieved long-term survival (Figure 1A).
In innate immune components, expression of PD-1 appears to be highly restricted to DCs but not on NK cells, neutrophils, or macrophages after infection by LM in vivo or in vitro. PD-1 expression could also be up-regulated by LTA-SA, Poly I:C, LPS, and PGN-ECndss, which engage TLR2, TLR3, TLR4, and NOD1/2, respectively. Although these data demonstrate that the regulation of PD-1 expression by LM infection is possibly through TLRs and cytosolic NOD recognition and signaling, the mechanism underlying selective up-regulation of PD-1 on DCs in vivo remains unclear. Thus far, the most reliable method described to induce PD-1 on T cells is through TCR engagement, which triggers multiple downstream signaling pathways. This suggests that PD-1 expression in each cell subset may be controlled by distinct pathways rather than one universal pathway.
Interaction with B7-H1 appears to be required for PD-1–mediated suppression of DC function. After LM infection, B7-H1 was up-regulated in various tissues and cells. Therefore, there is plenty of B7-H1 available for interaction with PD-1 on DCs. Interestingly, DCs also express high levels of B7-H1 on cell surface. Therefore, a B7-H1-PD-1 binding during DC-DC interaction could not be excluded.
The biochemical mechanism underlying PD-1–mediated inhibition is yet to be clarified. It has been proposed that PD-1 may suppress T-cell responses via the immunoreceptor tyrosine inhibitory motif and/or the immunoreceptor tyrosine switch motif in its cytoplasmic tail.36 In a B-cell model, cross-linking of PD-1 along with the B-cell receptor leads to tyrosine phosphorylation of the PD-1 cytoplasmic domain, recruitment of SHP-2 phosphatase, and reduced phosphorylation of B-cell receptor proximal kinases.37 A recent study using site-directed mutagenesis, however, showed that the immunoreceptor tyrosine switch motif, but not immunoreceptor tyrosine inhibitory motif, is essential for the inhibitory function of PD-1 in T cells.38 The precise pathway that is responsible for PD-1–mediated suppression of DCs is yet to be determined.
Although the initial signaling pathway leading to PD-1 suppression is yet to be explored, our experiments may shed light on the mechanism. It is possible that PD-1, induced by engagement of selective TLRs, down-regulates TLR signaling as a negative feedback mechanism to tune down innate response and reduce tissue damage. It appears that PD-1 can directly inhibit DC production of IL-12 and TNF-α. This observation is confirmed in LM infection models both in vitro and in vivo. Increased release of cytokines from PD-1–deficient DCs in vitro after LM stimulation correlated with significantly elevated levels of these cytokines in DKO mice sera after LM infection. It is important to point out that DCs are not the only source for these cytokines. Macrophages can also release large amounts of TNF-α and IL-12 after LM infection. Macrophages, however, do not express PD-1. Herein the changes in cytokine production from macrophages, if any, will not be a direct effect of PD-1. In addition to IL-12 and TNF-α, we also observed significant increases of other cytokines in DKO sera, including IFN-γ (Figure 4C), which is probably produced by NK cells.2 Further analysis demonstrates that NK cells, but not DCs, are the major producer of IFN-γ after LM infection (data not shown). This implicates a cytokine cascade in LM infection involving other innate immune cells and that PD-1–mediated negative regulation is an important player in the control of infection.
Selective deletion of PD-1 on DCs represents an ultimate approach for precise evaluation of the role of PD-1 in vivo. However, a DC-specific PD-1 knockout is not yet available. Our adoptive transfer experiments, nevertheless, clearly demonstrate that DCs from DKO mice are superior to those from Rag1-KO mice in providing innate resistance. On the other hand, Rag-1KO mice receiving DKO DCs could not reach the same resistant level as seen in DKO mice to LM infection. Several potential reasons could account for this discrepancy. First, adoptive transferred sDCs could not completely functionally replace splenic-resident DCs in DKO mice. Alternatively, PD-1 might also exert a negative role in other antigen-presenting cells or innate cells during LM infection.
In conclusion, our findings reveal a new molecular mechanism through which DC-mediated innate immunity is regulated. Interaction of B7-H1 with PD-1 thus serves as a checkpoint to regulate mature DC function in vivo.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Acknowledgments
The authors thank Drs Drew M. Pardoll and Suzanne Topalian for helpful discussions, Dr Thomas W. Dubensky Jr for Listeria strains, Dr Tasuku Honjo for PD-1KO mice, and Miss Jennifer Osborne for editing.
This work was supported in part by the National Institutes of Health (grants CA97085 and CA113341). S.Y. was supported by the National Institutes of Health (training grant T32HL07525).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.Y. designed and performed the research, analyzed the data, and wrote the manuscript; S.W., Y.Z., L.L., G.Z., S.F., H.X., W.R., M.B., I.-H.C., and K.T. performed some experiments; and L.C. supervised the research project, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lieping Chen, Johns Hopkins Medical Institutions, 2M07 David H. Koch Cancer Research Bldg, 1550 Orleans St, Baltimore, MD 21231; e-mail: lchen42@jhmi.edu.
References
- 1.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 2.Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 2007;9:1208–1215. doi: 10.10110/2/076/j.micinf.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGregor DD, Koster FT, Mackaness GB. The short lived small lymphocyte as a mediator of cellular immunity. Nature. 1970;228:855–856. doi: 10.1038/228855a0. [DOI] [PubMed] [Google Scholar]
- 4.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 6.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 7.Tam MA, Wick MJ. Differential expansion, activation and effector functions of conventional and plasmacytoid dendritic cells in mouse tissues transiently infected with Listeria monocytogenes. Cell Microbiol. 2006;8:1172–1187. doi: 10.1111/j.1462-5822.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 8.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CH, Fan YT, Dias A, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Neuenhahn M, Kerksiek KM, Nauerth M, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura H, Agata Y, Kawasaki A, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 18.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 20.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 21.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 22.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 23.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsushima F, Yao S, Shin T, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 27.Tamura H, Dong H, Zhu G, et al. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–1816. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 28.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 29.Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 30.Taieb J, Chaput N, Menard C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 31.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 32.Ozoren N, Masumoto J, Franchi L, et al. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 33.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 34.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 37.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 on primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.
Supplementary PDF file available online.