Abstract
Background
A systematic review was conducted to evaluate the literature regarding the impact of follow-up on colorectal cancer patient survival and, in a second phase, recommendations were developed.
Methods
The MEDLINE, CANCERLIT, and Cochrane Library databases, and abstracts published in the 1997 to 2002 proceedings of the annual meeting of the American Society of Clinical Oncology were systematically searched for evidence. Study selection was limited to randomized trials and meta-analyses that examined different programs of follow-up after curative resection of colorectal cancer where five-year overall survival was reported. External review by Ontario practitioners was obtained through a mailed survey. Final approval of the practice guideline report was obtained from the Practice Guidelines Coordinating Committee.
Results
Six randomized trials and two published meta-analyses of follow-up were obtained. Of six randomized trials comparing one follow-up program to a more intense program, only two individual trials detected a statistically significant survival benefit favouring the more intense follow-up program. Pooling of all six randomized trials demonstrated a significant improvement in survival favouring more intense follow-up (Relative Risk Ratio 0.80 (95%CI, 0.70 to 0.91; p = 0.0008). Although the rate of recurrence was similar in both of the follow-up groups compared, asymptomatic recurrences and re-operations for cure of recurrences were more common in patients with more intensive follow-up. Trials including CEA monitoring and liver imaging also had significant results, whereas trials not including these tests did not.
Conclusion
Follow-up programs for patients with curatively resected colorectal cancer do improve survival. These follow-up programs include frequent visits and performance of blood CEA, chest x-rays, liver imaging and colonoscopy, however, it is not clear which tests or frequency of visits is optimal. There is a suggestion that improved survival is due to diagnosis of recurrence at an earlier, asymptomatic stage which allows for more curative resection of recurrence.
Based on this evidence and consideration of the biology of colorectal cancer and present practices, a guideline was developed.
Patients should be made aware of the risk of disease recurrence or second bowel cancer, the potential benefits of follow-up and the uncertainties requiring further clinical trials. For patients at high-risk of recurrence (stages IIb and III) clinical assessment is recommended when symptoms occur or at least every 6 months the first 3 years and yearly for at least 5 years. At the time of those visits, patients may have blood CEA, chest x-ray and liver imaging. For patients at lower risk of recurrence (stages I and Ia) or those with co-morbidities impairing future surgery, only visits yearly or when symptoms occur. All patients should have a colonoscopy before or within 6 months of initial surgery, and repeated yearly if villous or tubular adenomas >1 cm are found; otherwise repeat every 3 to 5 years. All patients having recurrences should be assessed by a multidisciplinary team in a cancer centre.
Background
Colorectal cancer is one of the most common malignancies with an estimated age-adjusted annual incidence in North America of 75 cases per 100,000 population [1-3]. About 75% of newly diagnosed cases have the tumour confined to a portion of the bowel and regional lymph nodes. Complete removal of the tumour en-bloc with a portion of normal bowel along with mesenteric and regional lymph nodes is considered a curative resection. In spite of this curative resection approximately half the patients develop recurrent disease and their median survival does not exceed two years [1-4]. Most of these recurrences occur in patients who, at initial staging, had a tumour invading across the bowel wall causing perforation of the bowel, adhesion, invasion of neighbouring organs (stage IIb disease), or had lymph node metastases (stage III disease). Beside disease recurrence, patients with colorectal cancer are considered to be at a higher risk for developing a second or metachronous bowel cancer [5-10], particularly if they are 60 years of age or younger [8,9].
The principal aim of follow-up programs after curative resection of colorectal cancer is to improve survival. To achieve this goal, patients are screened for early recurrent disease and second colorectal cancer with the intent of a second curative surgery. Common sites of recurrence and the screening tests used to detect early disease in those sites are shown in Table 1. As no single screening test is best for all sites of recurrent disease and second colorectal cancer, a combination or package of tests is commonly used. The screening tests are directed to areas of potential disease and conducted at pre-established intervals. Since the incidence of recurrent disease occurs at an exponential rate over the first three to four years after surgery but then plateaus [1-3], the screening tests for recurrent disease are conducted frequently during the first three years and infrequently afterwards. Screening tests for second colorectal cancer, on the other hand, must be done at equally spaced intervals for life because the incidence of second colorectal cancer occurs at a constant cumulative rate of 3% every six years [6,9]. An example of this strategy is noted in a 1994 survey of members of the American Society of Colon and Rectal Surgeons [11]; it showed that most patients are assessed every three months for three years, and every six to twelve months thereafter. During those visits patients have clinical assessment, blood tests including CEA (carcinoembryonic antigen), x-rays of the chest and abdomen, and colorectal endoscopy.
Table 1.
Sites of recurrent disease and screening tests for colorectal cancer.
| Site of Recurrence | Percent of Patients with Recurrence at 5 Years by Site of Initial Tumoura | Screening Tests | |
| Colon | Rectum | ||
| Liver | 35 | 30 | CEA, US or CT, RIS?, Sx |
| Lung | 20 | 30 | Chest x-ray, CEA, Sx |
| Peritoneal | 20 | 20 | CEA, Sx, CT, RIS? |
| Retroperitoneal | 15 | 5b | CEA, CT, RIS?, Sx |
| Peripheral Lymph Nodes | 2 | 7b | Physical exam, CEA |
| Other (brain, bones) | <5 | <5 | Sx, scans |
| Loco-regional | 15 | 35b | CT pelvis, CEA, RIS? Sx, endoscopy? FOB? |
| Second or metachronous colorectal cancer | 3 | 3 | Colonoscopy, FOB? |
Note: ?, questionable test; CEA, carcinoembrionic antigen; CT, computerized tomography; FOB, fecal occult blood; RIS, radioimmunoscintigraphy; Sx, symptoms; US, ultrasound. a Data modified from Galandiuk et al (4). The median time to recurrence is significantly shorter for stage C versus B and for lesions that originally had perforation or adhesion/invasion of surrounding structures (p < 0.01). b Indicates significant differences (p < 0.05).
A debate has developed as to the effectiveness and expense of intensive follow-up programs. A program of colorectal cancer follow-up similar to those described by Vernava et al [11], and using U.S. costs per test given by Nelson [12], would have an approximate five-year cost per patient of $10,000, half of it being due to colonoscopy. This cost does not include surgical procedures ($8,000 per operation) for asymptomatic disease, most of which cannot be curatively resected. Several other investigators have also emphasized these high economic costs [13,14]. Similar costs will occur in the Canadian context. There is also the added potential for possible harms that may result from such intensive follow-up programs [15-18]. This debate has resulted from the varied and likely biased results of non-randomized studies [19-28] and meta-analyses based mostly on these studies [29,30]. The purpose of the present systematic overview is to critically evaluate the results of available randomized trials and corresponding meta-analyses to obtain more definitive evidence regarding the impact of colorectal cancer follow-up on patient survival. Further, we investigate attitudes of Ontario physicians regarding follow-up practices to develop appropriate recommendations.
Methods
This practice guideline report was developed by the Practice Guidelines Initiative (PGI), using the methodology of the Practice Guidelines Development Cycle [31]. The practice guideline report is a convenient and up-to-date source of the best available evidence on follow-up of patients with curatively resected colorectal cancer, developed through systematic reviews, evidence synthesis, and input from practitioners in Ontario. The report is intended to enable evidence-based practice. The Practice Guidelines Initiative is editorially independent of Cancer Care Ontario and the Ontario Ministry of Health and Long-Term Care.
Evidence was selected and reviewed by one member of the PGI's Gastrointestinal Cancer Disease Site Group (DSG) and methodologists. Members of the Gastrointestinal Cancer DSG disclosed potential conflict of interest information, reviewed the analysis of the evidence and prepared draft recommendations. External review was obtained from a random sample of a registered list of Ontario practitioners involved in cancer patient care. The mailed survey consisted of items that addressed the quality of the draft practice guideline report and recommendations, and whether the recommendations should serve as a practice guideline. Final approval of the original guideline report modified by practitioners' feedback was obtained from the Practice Guidelines Coordinating Committee (PGCC).
The PGI has a formal standardized process to ensure the currency of each guideline report. This consists of periodic review and evaluation of the scientific literature and, where appropriate, integration of this literature with the original guideline information.
Examination Of The Evidence
Literature Search Strategy
MEDLINE (1966 to May 2003), CANCERLIT (1983 to May 2003) and the Cochrane Library (2003, issue 1) were searched with no language restrictions. "Colonic neoplasms" (Medical subject heading [MeSH]), "rectal neoplasms" (MeSH) and "colorectal neoplasms" (MeSH) were combined with "recurrence" (MeSH), "prognosis" (MeSH), "compliance" (MeSH), "survival analysis" (MeSH) and the following phrases used as text words: "follow-up" and "surveillance." These terms were then combined with the search terms for the following study designs or publication types: randomized controlled trials, systematic reviews or meta-analyses, and clinical guidelines. Details of the literature search strategy appear in Appendix 3 - see Additional file: 3. In addition, investigators personal files and reference lists from retrieved papers were searched for additional trials.
Study Selection Criteria
Articles were selected for inclusion in this systematic review of the evidence if they were fully published reports or published abstracts of:
1. Randomized trials comparing groups of patients receiving different follow-up programs after curative resection of colorectal cancer, and overall patient survival was reported.
2. Meta-analyses of these randomized trials.
Excluded were non-randomized studies or randomized trials without at least 5-year survival data.
Although survival was the main outcome of interest, results of trials were also searched for recurrence rates, time to recurrence, asymptomatic recurrences, re-operation rates for recurrences, complications, and compliance with follow-up programs.
Synthesizing the Evidence
Only published data has been used for this systematic review. Due to the multiple factors that can affect survival results (e.g., variety and frequency of screening tests, compliance with tests and interventions, co-morbidity), both clinical and statistical heterogeneity among study results was expected. Prior to the estimation of risk reduction, each study was appraised individually by two authors (AF, BR) using the Detsky instrument [32]. Mortality rates for both groups were assessed for heterogeneity using scatter plots according to the methodology described by L'Abbe et al [33], and visual impressions were confirmed by calculating heterogeneity coefficients with significance levels set at 0.10 as recommended in the statistical literature [33,34]. Mortality rates were pooled using Review Manager 4.1 (Metaview© Update Software), which is available through the Cochrane Collaboration. The numbers used for data pooling were those reported or those calculated from survival rates in published data. Results were reported as relative risk ratios (RR) with 95% confidence intervals (CI) obtained by the random effects model of DerSimonian and Laird [35]. An RR less than one favours the more intense follow-up and an RR more than one favours less intense follow-up. Inverted funnel plots were done to investigate publication bias [34].
It was planned, a priori, to conduct a subgroup analysis to examine the pooled results of studies using or not using blood CEA testing and liver imaging. This was based on the premise that these screening tests were widely used, and was therefore considered important for analysis.
Survival rates from the randomized trials were pooled and the results were used to develop recommendations for follow-up programs.
Results
Literature Search Results
The literature search identified eight randomized trials [36-43] and two published meta-analyses of randomized trials [44,45]. Two of the randomized trials are excluded. The trial by Lennon et al [42] investigated patients only after the development of an elevated CEA determination and, therefore, is not comparable with the other trials where patients were randomized shortly after surgery. The trial by Barillari et al [43] combined results of randomized with non-randomized patients. Therefore, these two trials are excluded from the analysis. The remaining six randomized trials were classified according to the intensity of follow-up programs compared and use of blood CEA testing and liver imaging (Table 2).
Table 2.
Classification of randomized trials of colorectal cancer follow-up
| Trials [Reference] | Follow-up programs compared | CEA testing used | Liver Imaging testing used |
| Makela [36] | Intense vs. Conventional | Yes | Yes |
| Ohlsson [37] | Intense vs. Minimal | Yes | No |
| Kjeldsen [38] | Intense vs. Minimal | No | No |
| Schoemaker [39] | Intense vs. Minimal | No* | Yes |
| Pietra [40] | Intense vs. Conventional | Yes | Yes |
| Secco [41] | Intense vs. Minimal | Yes | Yes |
* Isolated increase in CEA levels did not trigger further investigations.
Quality of trial reporting
Study evaluation with the Detsky instrument [32] detected significant deficits in the reporting of trial design and performance. Although all trials randomized patients after surgery, the description of the randomization process and stratification for prognostic factors was rarely mentioned [39]. It was impossible to assess bias in assignment except for the trial by Schoemaker et al [39]. Criteria for patient inclusion and exclusion were described but co-morbidity was not mentioned. Criteria to measure outcomes were described and seemed objective but blind assessment was mentioned in only the trial by Schoemaker et al [39]: radiologists unaware of follow-up assignment. Except for the Schoemaker et al study [39] it is not known how many patients were excluded from randomization due to refusal, co-morbidity or other factors. The follow-up regimens were well described. The statistical analysis was appropriate. Sample size justification and confidence intervals for negative trials were mentioned in only two trials [38,39]. Overall scores ranged from 0.57 to 0.86, median 0.67.
Compliance with the programs of follow-up were described in three trials [37,39,41]. The description was about drop-outs in all three and compliance with testing in two [37,39]. Most patients were followed for five years except one study in which reported only 52% of patients doing so [38] and another indicating only median follow-up time [41].
Population investigated
In all randomized trials, the study population was patients who underwent curative resection for colorectal cancer. There were 1679 patients, with a male to female ratio of 1.17, a median age in the mid-sixties and an age range of 30 to 87 years; one trial had a cut-off age of 76. Tumours involved the colon (n = 837) and rectum (n = 505). All trials included patients with Dukes' or modified Dukes' stages A, B and C. Patients with stage Dukes' C comprised only 33% of the populations studied. Only one trial had a risk-adapted follow-up for patients at higher and lower risk of recurrence [41]. Patient characteristics were reported in tabular form and appeared comparable. Formal statistical tests were rarely performed.
Outcomes
Randomized Trials
The six randomized trials [36-41] reported the survival of patients who received different follow-up programs following curative surgery. Four trials compared intensive follow-up (intervention) to minimal follow-up (control) [and two trials compared intensive follow-up (intervention) to conventional follow-up (control)] (Table 2). Blood CEA testing was used in the management of patients in four trials [36,37,40,41], and liver imaging was used in four trials [36,39-41] (Table 2).
The follow-up programs for each of the trials are shown in Table 3. Results of individual trials are presented in Table 4.
Table 3.
Description of randomized trials of follow-up of colorectal cancer after resection.
| Study [Reference] | Location (Years) | Follow-up Program | |
| Control | Intervention | ||
| Makela [36] | Finland (1988–90) | Regular (n = 54): Clinical assessment, blood counts and CEA, chest x-ray, and fecal occult blood (FOB) every 3 months for 2 years, then every 6 months for next 3 years; rigid sigmoidoscopy for rectosigmoid tumours at each visit, and yearly barium enema for all patients. | Intensive (n = 52): Clinical assessment, blood counts and CEA, chest x-ray and FOB as in regular follow-up program. In addition, colonoscopy at 3 months if not performed preoperatively and then yearly thereafter on all patients, flexible sigmoidoscopy for rectosigmoid tumors every 3 months, liver ultrasound every 6 months, and yearly CT of liver and site of operation. |
| Ohlsson [37] | Sweden (1983–86) | Minimal (n = 54): FOB every 3 months for 2 years, then yearly, and to consult for a list of symptoms. | Regular (n = 53): Clinical assessments, blood CEA and liver enzyme, chest x-ray, FOB and rigid sigmoidoscopy every 3 months for 2 years, then every 6 months; endoscopy control of anastomosis by flexible endoscopy at 9, 21, and 42 months; complete colonoscopy at 3, 15, 30, and 60 months; CT of pelvis (if they had abdominoperineal resection) at 3,6,12, 18, and 24 months. |
| Kjeldsen [38] | Denmark (1985–94) | Minimal (n = 307): Clinical assessment, blood hemoglobin, sedimentation rate and liver enzymes, chest x-ray, FOB, and colonoscopy (if incomplete, double contrast barium enema) at 5, 10, and 15 years. | Regular (n = 290): Same tests as minimal follow-up program, but tests were conducted every 6 months for 3 years, and then at 4, 5, 7.5, 10, 12.5, and 15 years. |
| Schoemaker [39] | Australia (1984–90) | Minimal (n = 158): Clinical assessment, blood counts, CEA, liver function tests and FOB every 3 months for 2 years, then every 6 months for 5 years; chest x-rays, liver CT scan and colonoscopy at 5 years. | Regular (n = 167): Clinical assessment, blood counts, CEA, liver function tests and FOB as in regular follow-up program. In addition, chest x-rays, liver CT scan and colonoscopy annually. Isolated increase in CEA levels did not trigger further investigations. |
| Pietra [40] | Italy (1987–90) | Regular (n = 103) Clinical assessment, CEA, and liver ultrasound every 6 months for one year, then yearly; chest x-ray and colonoscopy yearly. | Intensive (n = 104) Clinical assessment, CEA, and liver ultrasound as regular follow-up program, but tests conducted every 3 months for 2 years, then every 6 months for 3 years, and yearly thereafter. In addition, chest x-ray, abdominal CT and colonoscopy yearly. |
| Secco [41] | Italy (1988–96) | Minimal (n = 145) Patients to phone the surgical team every 6 months. Clinical assessment by family physician at least once a year or when suggestive symptoms of recurrence occurred. | Intensive (n= 192) High Risk Patients: Clinical assessment and CEA every 3 months for 2 years, every 4 months in the third year and every 6 months in years 4 and 5. Abdominal and pelvic ultrasound performed every 6 months the first 3 years and yearly in years 4 and 5. Rigid recto-sigmoidoscopy and chest x-ray yearly for patients with rectal cancer. Low Risk Patients: Clinical assessment and CEA every 6 months for 2 years, then yearly; abdominal and pelvic ultrasound every 6 months for 2 years, then once a year. Rigid recto-sigmoidoscopy for rectal cancer yearly twice, then every 2 years and chest x-ray yearly. |
Note: CEA, carcinoembryonic antigen; FOB, fecal occult blood; CT, computerized tomography.
Table 4.
Results of randomized trials of follow-up after resection of colorectal cancer.
| Study, Year [Reference] | Follow-up Program intensity | Number of Patients Randomized | Median Observation (months) | Overall Recurrence Rate (%) | Number of Second Bowel Cancers | Radical Reoperation Rate (%) | 5-year Survival Rate (%) |
| Makela 1995 [36] |
Less More |
54 52 |
>60 | 39 42 |
NR | 14 23 |
54 59 |
| Ohlsson 1995 [37] |
Less More |
54 53 |
82 | 33 32 |
NR | 17 29 |
67 75 |
| Kjeldsen 1997 [38] |
Less More |
307 290 |
>60 | 26 26 |
3 7 |
NR | 68 70 |
| Schoemaker 1998 [39] |
Less More |
158 167 |
>60 | NR | 5 3 |
NR | 70 76 |
| Pietra 1998 [40] |
Less More |
103 104 |
>60 | 19 25a |
1 0 |
10 65 |
58 73b |
| Secco 2002 [41] |
Less More |
145 192 |
>60 | 53 57 |
NR | 16 31 |
48 63 |
Note: NR, not reported. a p < 0.01 b p < 0.05
Overall survival is significantly improved for patients in the more intensive programs of follow-up. This improvement amounts to a risk difference of 7% (95%CI, 3% to 12%; p = 0.002) in five year survival. The number of all recurrences was similar in programs of more and less intense follow. The incidence of asymptomatic recurrences was, however, significantly more common on patients on the more intense follow-up. The latter may explain the more common re-operation for cure of recurrences in the group of patients undergoing the more intense follow-up.
Overall survival was also investigated for the group of trials according to the use of CEA and liver imaging screening. Trials using blood CEA screening demonstrate a significant impact on survival whereas those not using CEA do not (Figure 2). A similar finding is that whereas trials using liver imaging show a significant improvement in survival, and trials not using liver imaging do not (Figure 3).
Figure 2.
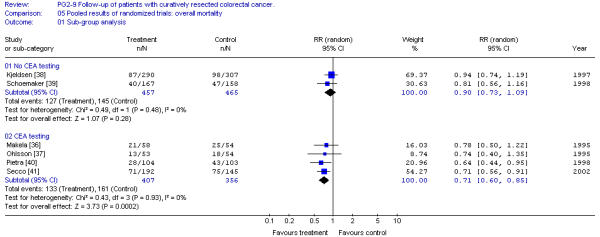
Pooled results of randomized trials: overall survival sub-group analysis. [No CEA testing; CEA testing]. Overall relative risk ratio, no CEA testing = 0.90 (95% CI, 0.73 to 1.09; p = 0.28)Overall relative risk ratio, CEA testing = 0.71 (95% CI, 0.60 to 0.85; p = 0.0002)
Figure 3.
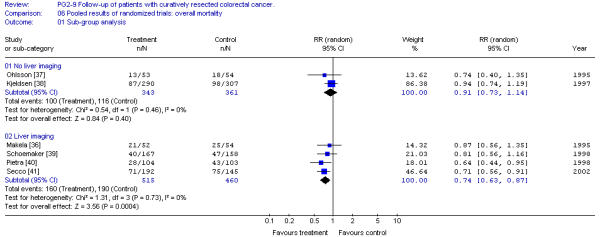
Pooled results of randomized trials: overall survival sub-group analysis. [No liver imaging; liver imaging] Overall relative risk ratio, no liver imaging = 0.91 (95%CI, 0.73 to 1.14; p = 0.40)Overall relative risk ratio, liver imaging = 0.74 (95%CI, 0.63 to 0.87; p = 0.0004)
Pooled results of all randomized trials revealed no statistically significant heterogeneity (X2 = 3.95; p > 0.10). There was no evidence of publication bias as the relative risk ratios of the individual trials distributed symmetrically about the pooled risk ratio.
Meta-analyses
Two meta-analyses of randomized trials have been published [44,45]. Both studies are of excellent methodological quality with clear description of bibliographical search, inclusion/exclusion criteria, standardized collection of data, consideration of quality of trial reporting, concerns with possibility of biases in publication and reporting, and high level statistical management of the data. In both studies, further details not reported in the published trials were obtained by contacting investigators involved with the trials; however, which data was acquired in this manner is not reported.
Both studies analyzed the same five randomized trials [36-40]. The analysis by Renehan et al [44] found a significant improvement in overall survival, with a larger effect in four trials using abdominal CT scans and frequent CEA determinations. In attempting to explain the survival benefits, the authors note the similar recurrence rates regardless of follow-up plan but the earlier diagnosis (by 8.5 months; 95% CI; 7.6 to 9.4) and the more frequent finding of isolated local recurrences with the more intense follow-up. The study by Jeffery et al [45] had similar findings: improved survival, no difference in recurrence rates, earlier diagnosis of recurrences and more frequent curative resection on patients undergoing more intense follow-up. Further, this occurred because of performing more tests, especially liver imaging. These latter results, when expressed as risk differences, were not significant. Both studies noted the low rate of metachronous cancer, which was not different for the compared follow-up programs. Also noted in these overviews are the paucity of data on complications and quality of life: colonoscopy complication rate by Schoemaker et al [39] and the limited quality of life study by Kjeldsen et al [46]. The conclusions of these systematic reviews are that intensifying programs of follow-up improve survival but that there is no data to recommend tests or frequency of visits.
Discussion
We must start by acknowledging that results of only two individual studies [40,41] showed a statistically significant benefit in survival for organized programs of follow-up of patients with curatively resected colorectal cancer. However, some of these studies [36,37] lacked the power to detect statistically significant differences in survival associated with two follow-up programs of different intensities. Meta-analysis, by pooling the results of under-powered studies, may detect small but clinically significant differences. Indeed, meta-analyses by Renehan et al [44] and Jeffery et al [45] have shown significant improvements in survival for patients on more intense follow-up. The results obtained in our pooled analysis of the six randomized trials comparing two intensities of follow-up also demonstrated that patients on more intensive programs of follow-up have improved survival compared with patients on minimal or no follow-up (Relative Risk Ratio 0.80 (95% CI; 0.70 to 0.91; p = 0.0008).
The finding of decreased mortality with more intensive follow-up does not permit us to recommend a specific program of follow-up. To be more specific, we investigated the role of CEA monitoring and use of liver imaging. Our results demonstrate that only trials including CEA testing and/or liver imaging give significant improvements in survival (Figure 2 and 3). It must be stressed that all studies including liver imaging also used blood CEA monitoring. CEA testing alone was investigated in a randomized trial by Lennon et al [42] but unfortunately other screening tests were not controlled. Patients were randomized only after a period of follow-up and when the CEA level was significantly elevated over several weeks and then to either an aggressive surgical approach to search and resect recurrence or a more conventional approach. Preliminary results indicate no difference in post-randomization survival. The lack of survival benefit of an aggressive surgical approach to CEA elevation may be due to several causes: 1) More than 60% of patients with elevated CEA had symptoms suggestive of disease. 2) There was no control for other tests which may render the CEA screening ineffective. 3) The criteria for CEA elevation required two values over 20 ng/ml or two values over 10 ng/ml but with a rise of greater than seven units. This conservative estimate of elevated CEA will decrease the diagnostic sensitivity of the test and may delay the diagnosis by two or three months, which may have an impact on patient survival. 4) This trial required an "aggressive" pursuit of the diagnosis of recurrent disease that might include a second-look laparotomy. This "aggressive" approach may cause some harm, reducing the benefit of CEA monitoring. Therefore, the negative results of this trial do not negate a potential benefit for CEA testing. Our finding of a CEA effect may simply represent confounding factors. CEA testing has been combined with chest and abdominal imaging and endoscopic examination. These factors as well as CEA testing may have an impact on survival results.
In regard to liver imaging, three studies used computerized tomography (CT) [36,39,40] while one study used ultrasonography (US) [41]. Computerized tomography has been shown to be more sensitive than US in detecting liver metastases [47,48]. In a cohort study of 100 patients with resected colorectal cancer (mostly Dukes' stage C) who had normal livers as determined by CT, US, and intra-operative palpation of the liver [49], several imaging tests and CEA were performed after a median follow-up of 41 months (range 36 to 48). Sensitivity for the detection of liver metastases was: for CT 0.67 (95% CI; 0.43 to 0.91), for US 0.43 (95% CI; 0.17 to 0.69), and for CEA 0.33 (95% CI; 0.09 to 0.57). The addition of CEA to CT and US increased the sensitivity up to 0.53 and 0.73, respectively. This study did not address the question of whether detection of resectable liver lesions was better by any of the screening methods.
Imaging of the chest by plain radiographs has been included in all intensive follow-up programs. Lung metastases occur in 25% of patients with resected colorectal cancer (Table 1); localized lesions are less common but resection led to 30% long-term survival [50]. In a large cohort study of 1247 patients with resected colon cancer [51], recurrences occurred in 548 after a median follow-up of 7 years. There were 22 patients with resectable lung metastases, all detected by plain chest radiographs, and 6 were long-term survivors. In the same study, only 49 patients had hepatic resections and 32% survived more than 5 years. Thus, although plain radiographs detect very few patients with localized lung metastases, the situation is very similar to that of liver metastases. Computerized tomography of the chest has not been used as a screening test in colorectal cancer.
In regard to the incidence of second bowel cancer, no definite comments can be made based on the evidence reviewed. Only three randomized trials reported on the incidence of such tumors and the rates were similar for both follow-ups. Most studies had median observation periods around five years. Therefore, the expected number of metachronous cancers in these patients is <3% [6,7].
Patient compliance with the follow-up plans is described in three trials [37,39,41]. Overall, it appears that patients are quite willing to undergo frequent visits and tests.
The improvement in patient survival receiving intensive follow-up programs is achieved at the cost of frequent visits, extensive testing, earlier knowledge of disease recurrence, and increased number of further testing and surgical interventions. Harmful consequences of such extra testing and intervention have rarely been measured in randomized trials. One trial noted two perforations and two episodes of bleeding after polypectomy in 731 colonoscopies, a complication rate of 0.55% [39]. This complication rate is comparable to that of other colonoscopy series [52]. The quality of life and attitudes of patients participating in follow-up programs were initially investigated in a pilot study by Stiggelbout et al [53]. Results indicated that regular contact with a physician reassured patients and that visits and tests caused only slight anticipatory anxiety and other minor inconveniences. Kjeldsen et al [46] confirmed these findings in a subgroup of patients participating in a randomized trial comparing minimal to regular follow-up and which demonstrated similar survival for both follow-ups [38]. Quality of life (Nottingham Health Profiles) and patient attitudes toward follow-up were investigated on 350 of 597 patients who were alive after closure of the randomized trial. Patients were mailed the questionnaires to complete at home. Ninety-one percent of patients returned completed questionnaires. Quality of life measures and attitudes were almost the same for patients on the minimal and intensive follow-up indicating the extra tests or inconveniences were balanced by the more frequent reassurance of health. These results are also consistent with those of one of the randomized trials in resected breast cancer follow-up where there was no impact of follow-up on the patients' quality of life, even after knowing that the follow-up program had not improved their survival [54].
In summary, follow-up programs for patients with curatively resected colorectal cancer do improve survival. These follow-up programs include frequent visits and performance of blood CEA, chest x-rays, liver imaging and colonoscopy. It is not clear which tests or frequency of visits is optimal. There is a suggestion that improved survival is due to diagnosis of recurrence at an early and asymptomatic stage which allows for more curative resection of recurrence. There is almost no data on complications from testing and therapies. Patients' quality of life does not appear to be affected.
Development Of The Clinical Practice Guideline
Gastrointestinal Cancer Disease Site Group Consensus
Intense debate occurred during several sessions around the interpretation of the presented evidence as well as the consideration of common practices and our role in guiding other physicians as to what is an acceptable follow-up program. Further, there are other goals for follow-up than to increase survival, including psychosocial support, documentation of disease course and close contact with patients to test new therapies. The evidence presented clearly demonstrates a survival benefit for patients receiving programs of more intense follow-up. The evidence for the schedule of visits and screening tests to detect disease recurrence is soft or non-existent. The evidence for the use of colonoscopy to detect second colorectal cancer and its precursors must derive from other investigations such as the Polyp Surveillance Study in the United States [55]. Common practice has been to follow patients at high risk of recurrence (stages IIb and III) with clinical assessment and blood tests including CEA every three to four months for the first two or three years, and every six to 12 months to complete five years following resection. Blood CEA monitoring seems to uncover resectable liver metastases, is relatively inexpensive and causes minimal inconvenience. Patients also have colonoscopy in the perioperative period, and if adenomatous polyps are present, colonoscopy is repeated yearly or, if no polyps are detected, every three to five years. This practice was recommended in a document prepared by the Gastrointestinal Cancer DSG in January 1997 (see Appendix 2 - see Additional file: 2) and a group of experts of the American Society of Clinical Oncology recently supported similar views [56]. These recommendations encompass the available evidence from clinical trials and what is known about the clinical biology of colorectal cancer recurrences and second tumours, and should serve as a guide to other physicians. These recommendations and the reviewed evidence were distributed to Ontario physicians caring for patients with colorectal cancer. The Gastrointestinal Cancer DSG also emphasized that further trials are needed to determine which tests lead to the detection of resectable recurrent disease and whether patients' quality of life is also improved.
External Review
Practitioner feedback was obtained through a mailed survey. The survey consisted of items evaluating the methods, results, and interpretive summary used to inform the draft recommendations and whether the draft recommendations should be approved as a practice guideline. Written comments were invited. Follow-up reminders were sent at two weeks (post card) and four weeks (complete package mailed again). The Gastrointestinal Cancer DSG reviewed the results of the survey.
1. Number surveyed: 153 practitioners in Ontario involved in the care of patients with cancer (9 medical oncologists, 20 radiation oncologists, and 104 surgeons).
2. Return rate: 62%
3. Written comments attached: 44%
4. Agreement with the summary of evidence: 88%
5. Agreement with the recommendation: 76%
6. Approval of the recommendation as a practice guideline: 73%
Summary of Main Findings
Written comments provided by practitioners varied. One practitioner believed that liver and lung imaging should be included in the recommendations, since the only positive study included liver ultrasound. Another practitioner thought that the lack of specificity of CEA testing, its cost, and the poor results with resection of intraperitoneal recurrences argued against its routine use. Based on the studies that were reviewed, it is not clear how CEA testing every four months was recommended, and this practitioner suggested adding the following recommendation: "If a patient would not be considered fit for resection of liver, lung, or intraperitoneal metastases, there is no value to CEA monitoring." Another practitioner thought that clinical exam every four months would not be effective as no resectable disease can be diagnosed on exam. Another practitioner thought that if several randomized controlled studies showed survival benefits for yearly colonoscopy on an intensive follow-up program, then routine annual colonoscopy should be recommended as suggested by the literature review, but that the lack of evidence for the schedule of visits should be emphasized.
Gastrointestinal Cancer Disease Site Group Modifications and Actions
Although 80% of the respondents were favourable to the draft recommendations, 20% were not in full agreement and wrote specific comments. Major concerns were low sensitivity of clinical assessment and blood CEA, and the more specific value in detecting resectable solitary metastases by liver and chest imaging. These concerns are reflected in a recent survey of Canadian oncologists regarding frequency of visits and tests performed in the follow-up of curatively resected colorectal cancer: Of oncologists surveyed, 35% recommend liver ultrasound and 50% recommend chest x-rays (Grunfeld et al, unpublished results). In the randomized trials reviewed, the more intensive follow-up programs which showed an increase in survival did indeed use liver and chest imaging. Therefore, we suggested the use of chest radiographs and liver imaging with CT or US. Although CT is more sensitive than US, availability and cost of CT are significant problems. Similarly, modifications were made to address the importance of an optimal decision regarding treatment of disease recurrence. In regard to colonoscopy, we advise the recent American Gastroenterology Association Guidelines recommendations [57]. Other comments were also considered, including colonoscopy for patients with stage I disease and more intense follow-up of patients who are fit and willing to undergo investigations and potential intervention for recurrence, regardless of age. An Information Sheet to be given to the patient at the start of the follow-up (Appendix 1 - see Additional file: 1) was added.
Several recent practice guidelines for surveillance after colorectal cancer resection were reviewed [58-61]. Two of these practice guidelines discussed levels of evidence for the recommendations [58,59] but only one or two of the RCTs analyzed in this paper were considered. The recommendations are not consistent between practice guidelines, partly because of the use of biased data from cohort and non-randomized studies. Clearly, even after considering only randomized studies neither of the overviews provided definite answers to the tests required, and further research with support from sources other than those dedicated to patient care is required [12,18,47].
Patients should be made aware of the importance of these research trials, and should be encouraged to participate in them. These clinical studies should be randomized to prevent biases and should be directed to homogeneous groups of patients stratified according to risks. Patients should be randomized to specific screening procedures (i.e., abdominal ultrasound or CT, PET scanning), and should measure quality of life and survival. Sufficiently long observation periods will be important to achieve reliable differential rates of risk, and sufficiently large sample sizes are necessary to obtain conclusive results. In planning such trials, a cost-benefit analysis must be performed to assess the economic costs of potential improvements in survival and quality of life [13,14,48]. Several such trials are under way [44,62].
Conclusions
Practice Guideline
This practice guideline applies to adult patients with curatively resected colorectal cancer, defined as patients who have had all apparent disease removed by surgery.
• Patients should be alerted to the future risks of disease recurrence, which is related to tumour stage, and to the development of a second colorectal cancer.
• There is evidence from six randomized trials and two meta-analyses of a small but significant survival benefit with more intensive follow-up compared to less intensive follow-up. This benefit is due to the early diagnosis and resection of limited recurrent disease in the liver, lungs, or local sites. It seems that this diagnosis of resectable recurrences is due to early assessment of symptoms and the use of screening tests (blood carcinoembryonic antigen, chest x-ray, liver imaging, or colonoscopy). There is insufficient evidence on which to base a recommendation for specific screening tests and frequency of visits.
• In light of the uncertainty of the schedule of visits and screening tests to be recommended, and based on the rate of recurrent disease and second neoplasms and on current practices, we advise:
1. In patients who are at high risk of relapse (stages IIb and III disease) and are fit and willing to undergo investigations and treatment of recurrence:
- Prompt assessment for symptoms of potential disease relapse (see Appendix 1);
- Clinical assessment at least every six months for three years, and then annually for an additional three years;
- During those visits patients may have blood CEA, chest x-rays, and liver ultrasound or CT;
- When recurrences of disease are detected, patients should be assessed by a multi-disciplinary oncology team including surgical, radiation, and medical oncologists to determine the best treatment options.
2. In patients at high risk of relapse but who have co-morbidities which may interfere with prescribed tests or potential treatment for recurrence, or who are unwilling to undergo prescribed tests or potential treatment for recurrence:
- Clinical assessments yearly or for suggestive symptoms of relapse.
3. In all patients with resected colorectal cancer (stages I, II, and III) and based on the American Gastroenterological Association recent guideline:
Colonoscopy postoperatively if not yet done;
- if high-risk polyps (villous or tubular >1 cm) are present, excise as they are potential precursors of colorectal cancer; and repeat colonoscopy yearly as long as polyps are found.
- If there are low-risk or no polyps, repeat colonoscopy in three to five years.
4. Patients should be encouraged to participate in clinical trials investigating screening tests added on to their clinical assessment. These trials of follow-up need to target patients with resectable recurrent disease and who are fit for required surgery.
List of abbreviations
In order of appearance:
CEA, carcinoembryonic antigen; PGI, Practice Guidelines Initiative; DSG, Disease Site Group; PGCC, Practice Guidelines Coordinating Committee; MeSH, medical subject heading; RR, relative risk ratio; CI, confidence interval; CT, computerized tomography; PET, positron emission tomography.
Competing interests
None declared.
Authors' contributions
AF performed the original evidence review, participated in the statistical analysis, and participated in the writing of all drafts and the final version of the manuscript. BR, LZ, and CZ participated in the statistical analysis, writing of drafts, and providing review and commentary. Final editing and preparation of the manuscript was performed by BR and AF. Along with AF, JM, CE, BC, and RM made up a team of experts that determined the scope, content, and interpretation of this systematic review.
Figure 1.
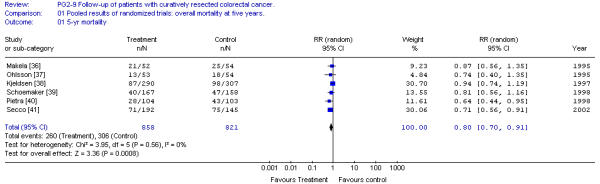
Pooled results of randomized trials: overall mortality at five years. Overall relative risk ratio = 0.80 (95% CI, 0.70 to 0.91; p = 0.0008)
Note
Mean return rate for Gastrointestinal Cancer DSG Practice Guidelines = 60.2%; Range = 51% – 84%
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Acknowledgments
Acknowledgements
Additional members of Cancer Care Ontario's Program in Evidence-based Care's Gastrointestinal Cancer Disease Site Group include: O. Agboola MD, M. Citron, F.G. DeNardi MD, S. Fine MD, B. Fisher MD, C. Germond MD, D. Jonker MD, K. Khoo MD, W. Kocha MD, M. Lethbridge, W. Lofters MD, R. Malthaner MD, M. Moore MD, V. Tandan MD, and R. Wong MD. Please see the Practice Guidelines Initiative (PGI) web site http://www.cancercare.on.ca/ for a complete list of current Disease Site Group members.
The Gastrointestinal Cancer DSG would like to thank the reviewers, Dr. Andrew Renehan and Dr. Rick Nelson, for their input and suggestions.
Contributor Information
Alvaro Figueredo, Email: alvaro.figueredo@hrcc.on.ca.
R Bryan Rumble, Email: rumbleb@mcmaster.ca.
Jean Maroun, Email: Jean.Maroun@orcc.on.ca.
Craig C Earle, Email: craig_earle@dfci.harvard.edu.
Bernard Cummings, Email: bernard.cummings@rmp.uhn.on.ca.
Robin McLeod, Email: rmcleod@mtsinai.on.ca.
Lisa Zuraw, Email: zurawl@mcmaster.ca.
Caroline Zwaal, Email: zwaalc@post.queensu.ca.
the members of the Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care, Email: rumbleb@mcmaster.ca.
References
- Griffin MR, Bergtralh EJ, Coffey RJ, Beart RW, Melton LJ. Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer. 1987;60:2318–2324. doi: 10.1002/1097-0142(19871101)60:9<2318::aid-cncr2820600934>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Berge T, Ekelund G, Mellner C, Wenckert A. Carcinoma of the colon and rectum in a defined population. Acta Chir Scand. 1973;438 Suppl:1–86. [PubMed] [Google Scholar]
- Weinerman BH, Orr KB. Colorectal cancer: total provincial experience with survival analysis. Can J Gastroenterol. 1989;3:126–130. [Google Scholar]
- Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ, Jr, Pemberton JH, Wolff BG. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32. [PubMed] [Google Scholar]
- Enblad P, Adami H-O, Glimelius B, Krusemo U, Pahlman L. The risk of subsequent primary malignant diseases after cancers of the colon and rectum. A nationwide cohort study. Cancer. 1990;65:2091–2100. doi: 10.1002/1097-0142(19900501)65:9<2091::aid-cncr2820650934>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bulow S, Svendsen LB, Mellemgaard A. Metachronous colorectal carcinoma. Br J Surg. 1990;77:502–505. doi: 10.1002/bjs.1800770509. [DOI] [PubMed] [Google Scholar]
- Cali RL, Pitsch RM, Thorson AG, Watson P, Tapia P, Blatchford GJ, Christensen MA. Cumulative incidence of metachronous colorectal cancer. Dis Colon Rectum. 1993;36:388–398. doi: 10.1007/BF02053945. [DOI] [PubMed] [Google Scholar]
- Evans HS, Moller H, Robinson D, Lewis CM, Bell CMJ, Hodgson SV. The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut. 2001;50:647–652. doi: 10.1136/gut.50.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shureiqi I, Coaksley CD, Morris J, Soliman AS, Levin B, Lippman SM. Effect of age on risk of second primary colorectal cancer. J Natl Cancer Inst. 2001;93:1264–1266. doi: 10.1093/jnci/93.16.1264. [DOI] [PubMed] [Google Scholar]
- Green RJ, Metlay JP, Propert K, Catalano PJ, Mayer RJ, Haller DG. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Int Med. 2002;136:261–269. doi: 10.7326/0003-4819-136-4-200202190-00005. [DOI] [PubMed] [Google Scholar]
- Vernava AM, Longo WE, Virgo KS, Coplin MA, Wade TP, Johnson FE. Current follow-up strategies after resection of colon cancer. Results of a survey of members of the American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 1994;37:573–583. doi: 10.1007/BF02050993. [DOI] [PubMed] [Google Scholar]
- Nelson RL. Postoperative evaluation of patients with colorectal cancer. Semin Oncol. 1995;22:488–493. [PubMed] [Google Scholar]
- Audisio RA, Robertson C. Colorectal cancer follow-up: perspectives for future studies. Eur J Surg Oncol. 2000;26:329–337. doi: 10.1053/ejso.1999.0894. [DOI] [PubMed] [Google Scholar]
- van der Hout WB, van den Brink M, Stiggelbout AM, van de Velde CJH, Kievit J. Cost-effectiveness analysis of colorectal cancer treatment. Eur J Cancer. 2002;38:953–963. doi: 10.1016/S0959-8049(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Safi F, Link KH, Beger HG. Is follow-up of colorectal cancer patients worthwhile? Dis Colon Rectum. 1993;36:636–644. doi: 10.1007/BF02238589. [DOI] [PubMed] [Google Scholar]
- Bohm B, Schwenk W, Hucke HP, Stock W. Does methodic long-term follow-up affect survival after curative resection of colorectal carcinoma? Dis Colon Rectum. 1993;36:280–286. doi: 10.1007/BF02053511. [DOI] [PubMed] [Google Scholar]
- Kronborg O. Optimal follow-up in colorectal cancer patients: what tests, how often? Semin Surg Oncol. 1994;10:217–224. doi: 10.1002/ssu.2980100310. [DOI] [PubMed] [Google Scholar]
- Vignati PV, Roberts PL. Preoperative evaluation and postoperative surveillance for patients with colorectal carcinoma. Surg Clin North Am. 1993;73:67–84. doi: 10.1016/s0039-6109(16)45929-3. [DOI] [PubMed] [Google Scholar]
- Ekman CA, Gustavson J, Henning A. Value of a follow-up study of recurrent carcinoma of the colon and rectum. Surg Gyncol Obstet. 1977;145:895–897. [PubMed] [Google Scholar]
- Tornqvist A, Ekelund G, Leandoer L. The value of intensive follow-up after curative resection for colorectal carcinoma. Br J Surg. 1982;69:725–728. doi: 10.1002/bjs.1800691213. [DOI] [PubMed] [Google Scholar]
- Mentges B. Kontroversen in der Nashsorge des Kolonkarzinoms [Controversies on after-care of colon carcinoma] Zent bl Chir. 1988;113:1087–1092. [PubMed] [Google Scholar]
- Fucini C, Rosi S, Herd-Smith A, Malatantis G, Panichi S. Significato e limiti del follow up intensivo dopo intervento radicale per cancro del colon-retto [Value and limitations of intensive follow-up after radical surgery for rectocolonic cancer. Minerva Chir. 1985;40:783–786. [PubMed] [Google Scholar]
- Ovaska J, Jarvinen H, Kujari H, Perttila I, Mecklin J. Follow-up of patients operated on for colorectal carcinoma. Am J Surg. 1990;159:593–596. doi: 10.1016/s0002-9610(06)80074-7. [DOI] [PubMed] [Google Scholar]
- Bergamaschi R, Arnaud JP. Routine compared with nonscheduled follow-up of patients with "curative" surgery for colorectal cancer. Ann Surg Oncol. 1996;3:464–469. doi: 10.1007/BF02305764. [DOI] [PubMed] [Google Scholar]
- Secco GB, Fardelli R, Rovida S, Gianquinto D, Baldi E, Bonfante P, Derchi L, Ferraris R. Is intensive follow-up really able to improve prognosis of patients with local recurrence after curative surgery for rectal cancer? Ann Surg Oncol. 2000;7:32–37. doi: 10.1007/s10434-000-0032-y. [DOI] [PubMed] [Google Scholar]
- Pugliese V, Aste H, Saccomanno S, Bruzzi P, Bonelli L, Santi L. Outcome of follow-up programs in patients previously resected for colorectal cancer. Tumori. 1984;70:203–208. doi: 10.1177/030089168407000216. [DOI] [PubMed] [Google Scholar]
- Eckardt VF, Stamm H, Kanzler G, Bernhard G. Improved survival in patients complying with a postoperative endoscopic surveillance program. Endoscopy. 1994;26:523–527. doi: 10.1055/s-2007-1009027. [DOI] [PubMed] [Google Scholar]
- Castells A, Bessa X, Daniels M, Ascaso C, Lacy AM, Garcia-Valdecasas JC, Gargallo L, Novell F, Astudillo E, Filella X, Piqué JM. Value of postoperative surveillance after radical surgery for colorectal cancer: results of a cohort study. Dis Colon Rectum. 1998;41:714–723. doi: 10.1007/BF02236257. [DOI] [PubMed] [Google Scholar]
- Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema DF, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg. 1994;219:174–182. doi: 10.1097/00000658-199402000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M, Chan L, Beart RW, Jr, Vucasin P, Anthone G. Follow-up of colorectal cancer. A meta-analysis. Dis Colon Rectum. 1998;41:1116–1126. doi: 10.1007/BF02239433. [DOI] [PubMed] [Google Scholar]
- Browman GP, Levine MN, Mohide EA, Hayward RSA, Pritchard KI, Gafni A, Laupacis A. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–512. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- Detsky AS, Naylor D, O'Rourke K, McGeer AJ, L'Abbe AL. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45:255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Int Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. Ann Int Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Makela JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg. 1995;130:1062–1067. doi: 10.1001/archsurg.1995.01430100040009. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Breland U, Ekberg H, Graffner H, Trannberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum. 1995;38:619–626. doi: 10.1007/BF02054122. [DOI] [PubMed] [Google Scholar]
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–669. doi: 10.1046/j.1365-2168.1997.02733.x. [DOI] [PubMed] [Google Scholar]
- Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7–14. doi: 10.1016/s0016-5085(98)70626-2. [DOI] [PubMed] [Google Scholar]
- Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer. Dis Colon Rectum. 1998;41:1127–1133. doi: 10.1007/BF02239434. [DOI] [PubMed] [Google Scholar]
- Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, Derchi L, Ferraris R. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol. 2002;28:418–423. doi: 10.1053/ejso.2001.1250. [DOI] [PubMed] [Google Scholar]
- Lennon T, Houghton J, Northover J. What is the value of clinical follow-up for colorectal cancer patients? The experience of the CRC/NIH CEA second-look trial [abstract] In the proceedings of the Nottingham International Colorectal Cancer Symposium, Nottingham. 1995.
- Barillari P, Ramacciato G, Manetti G, Borino A, Sammartino P, Stipa V. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Eur J Surg Oncol. 2000;26:329–337. doi: 10.1053/ejso.1999.0894. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intense follow-up after curative resection of colorectal cancer: systematic review and meta-analysis of randomized trials. Brit Med J. 2002;324:1–8. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery GM, Hickey BE, Hider P. The Cochrane Library. Oxford; Update Software; 2003. Follow-up strategies for patients treated for non-metastatic colorectal cancer (Cochrane review) [DOI] [PubMed] [Google Scholar]
- Kjeldsen BJ, Thorsen H, Whalley D, Kronborg O. Influence of follow-up on health-related quality of life after radical surgery for colorectal cancer. Scand J Gastroenterol. 1999;34:509–515. doi: 10.1080/003655299750026254. [DOI] [PubMed] [Google Scholar]
- Kievit J, Bruinvels DJ. Detection of recurrence after surgery for colorectal cancer. Eur J Cancer. 1995;31A:1222–1225. doi: 10.1016/0959-8049(95)00155-C. [DOI] [PubMed] [Google Scholar]
- Kievit J. Follow-up of patients with colorectal cancer: numbers needed to test and treat. Eur J Cancer. 2002;38:986–999. doi: 10.1016/S0959-8049(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Glover C, Douse P, Kane P, Karani J, Meire H, Mohammadtaghi S, Allen-Mersh TG. Accuracy of investigations for asymptomatic colorectal liver metastases. Dis Colon Rectum. 2002;45:476–484. doi: 10.1007/s10350-004-6224-y. [DOI] [PubMed] [Google Scholar]
- McCormack PM, Ginsberg RJ. Current management of colorectal metastases of the lung. Chest Surg Clin North Am. 1998;8:119–126. [PubMed] [Google Scholar]
- Goldberg RM, Fleming TR, Tangen CM, Moertel CG, Macdonald JS, Haller DG, Laurie JA. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Int Med. 1998;129:27–35. doi: 10.7326/0003-4819-129-1-199807010-00007. [DOI] [PubMed] [Google Scholar]
- American Society for Gastrointestinal Endoscopy Complications of colonoscopy. Gastrointest Endosc. 2003;57:441–445. doi: 10.1016/s0016-5107(03)80005-6. [DOI] [PubMed] [Google Scholar]
- Stiggelbout AM, de Haes JCJM, Vree R, van de Velde CJ, Bruijninckx CM, van Groningen K, Kievit J. Follow-up of colorectal cancer patients: quality of life and attitudes towards follow-up. Br J Cancer. 1997;75:914–920. doi: 10.1038/bjc.1997.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The GIVIO Investigators Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized study. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Zauber AG, O'Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET, Panish J, Ackroyd F, Kurtz RC, Shike M, the National Polp Study Workgroup Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901–906. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- Bensen AB, III, Desch CE, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Petrelli NJ, Pfister DG, Smith TJ, Somerfield MR. 2000 Update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18:3586–3588. doi: 10.1200/JCO.2000.18.20.3586. [DOI] [PubMed] [Google Scholar]
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C. American Gastroenterological Association: Colorectal Cancer Screening and Surveillance: Clinical Guidelines and Rationale. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- Desch CE, Benson AB, 3rd, Smith TJ, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Petrelli NJ, Pfister DG, Somerfield MR. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999;17:1312–21. doi: 10.1200/JCO.1999.17.4.1312. [DOI] [PubMed] [Google Scholar]
- Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal resection. Lancet. 2000;355:395–399. doi: 10.1016/S0140-6736(99)06552-6. [DOI] [PubMed] [Google Scholar]
- O'Dwyer PJ, Stevenson JP, Haller DG, Rosman N, Giantonio BJ. Follow-up of stage B and C colorectal cancer in the United States and France. Sem Oncol. 2001;28:45–49. doi: 10.1053/sonc.2001.19729. [DOI] [PubMed] [Google Scholar]
- Scholefield JH, Steele RJ. Guidelines for follow-up after resection of colorectal cancer. Gut. 2002;51:x3–x5. doi: 10.1136/gut.51.suppl_5.v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi P, Andreoni C, Confalonieri R, Fossati R, Labianca R, G Martignoni G, for Gruppo Italiano Lavoro per la Diagnosi Anticipata (GILDA) A randomized trial of intensive versus minimalist follow-up of patients with resected Dukes B2-C colorectal carcinoma. The pilot phase [abstract 1063] Proc Am Soc Clin Oncol. 1998;17:276a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


