Abstract
Invariant natural killer T cells (iNKT cells) have pivotal roles in graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) effects. iNKT cells are activated through their T-cell receptors by glycolipid moieties (typically the α-galactosylceramide [α-GalCer] derivative KRN7000) presented within CD1d. We investigated the ability of modified α-GalCer molecules to differentially modulate alloreactivity and GVL. KRN7000 and the N-acyl variant, C20:2, were administered in multiple well-established murine models of allogeneic stem cell transplantation. The highly potent and specific activation of all type I NKT cells with C20:2 failed to exacerbate and in most settings inhibited GVHD late after transplantation, whereas effects on GVL were variable. In contrast, the administration of KRN7000 induced hyperacute GVHD and early mortality in all models tested. Administration of KRN7000, but not C20:2, was found to result in downstream interleukin (IL)-12 and dendritic cell (DC)–dependent natural killer (NK)– and conventional T-cell activation. Specific depletion of host DCs, IL-12, or donor NK cells prevented this pathogenic response and the induction of hyperacute GVHD. These data demonstrate the ability of profound iNKT activation to modulate both the innate and adaptive immune response via the DC–NK-cell interaction and raise concern for the use of α-GalCer therapeutically to modulate GVHD and GVL effects.
Introduction
Immune-mediated graft-versus-leukemia (GVL) effects after allogeneic hemopoietic stem cell transplantation (SCT) can effectively induce immunologic tumor clearance. The reciprocal destructive immune reaction, however, may result in destruction of normal tissues and graft-versus-host disease (GVHD). Separation of these closely related, but mechanistically distinct, immunologic processes remains the major barrier to advancement of allogeneic SCT.1
Invariant natural killer T (iNKT) cells are a unique subset of αβ-T cells that express highly conserved semi-invariant T-cell receptors (TCRs), and recognize glycolipid antigens in association with the major histocompatibility complex (MHC) class I–like molecule, CD1d. Stem cell mobilization with potent granulocyte colony-stimulating factor (G-CSF) analogues effectively separates GVHD and GVL in mouse models, and maximal GVL effects are dependent on the presence of iNKT cells.2 In the absence of leukemic challenge, however, GVHD severity is reduced in recipients of iNKT-cell–deficient (Jα18−/−) grafts, demonstrating that donor iNKT cells also contribute to the pathogenesis of GVHD.2,3
Much interest has focused on the therapeutic potential of direct iNKT-cell activation with the synthetic α-galactosylceramide (α-GalCer), KRN7000, which characteristically induces rapid production of interferon (IFN)-γ and interleukin (IL)-4. Despite the expression of semi-invariant TCRs, stimulation with modified glycolipid antigens may result in strikingly different patterns of iNKT-cell stimulation. Of particular interest is the molecule C20:2, which has a shorter N-acyl chain than KRN7000, with cis-unsaturations at positions 11 and 14. Importantly, unlike other analogues, the potency of C20:2 and the population of iNKT cells stimulated are very similar to KRN7000.4 We recently reported that direct iNKT-cell activation after allogeneic SCT with C20:2 results in enhanced antihost cellular cytotoxicity.2 We have now examined the differential effects of iNKT-cell activation with KRN7000 and C20:2 on both GVHD and GVL after allogeneic SCT.
Methods
Mice
Female C57BL/6 wild-type (B6.WT, H-2b, CD45.2+), B6.Ptprca (H-2b, CD45.1+), B6D2F1 (H-2b/d, CD45.2+), BALB/c (H-2d, CD45.2+), and B10.D2 (H-2d, CD45.2+) mice were purchased from the Animal Resources Center (Perth, Australia). BALB/c.Ptprca (H-2d, CD45.1+), B6.CD11c.DTR, B6, and BALB/c.Jα18−/− mice were supplied by the Peter MacCallum Cancer Center (Melbourne, Australia). B6.IL-12p40−/− mice were supplied by the Herston Medical Research Centre, Brisbane, Australia, and B6.IFN-γ receptor (R)−/− mice were supplied by The Jackson Laboratory (Bar Harbor, ME). Mice were housed in sterilized microisolator cages and received acidified autoclaved water (pH 2.5) after transplantation. All animal experiments were approved by the Queensland Institute of Medical Research's institutional ethics committee.
Stem cell transplantation
Mice were transplanted as described previously.5 On day −1, total body irradiation (TBI; 137Cs source at 108 cGy/min) was administered to B6D2F1 mice (1100 cGy), wild-type/knockout mice on a B6 background (1000 cGy), or BALB/c mice (900 cGy). TBI was split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity. Chimeric mice were generated by injection of 107 T-cell–depleted (TCD) bone marrow from B6.IFN-γR−/− mice into irradiated (1000 cGy) B6.WT mice. These chimeric mice were left to reconstitute for 2 months, before subsequent allogeneic transplantation (900 cGy). Splenocytes from G-CSF–pretreated (2 μg/mouse/day for 6 days) C57BL/6, or (10 μg/mouse/day for 6 days) BALB/c and B10.D2 donors were injected intravenously at doses of 107 and 2.5 × 107 per graft, respectively. KRN7000 and C20:2 were produced in house by G.S.B. and S.A.P., reconstituted to 500 μM with 2% dimethylsulfoxide (DMSO) plus 0.5% Tween 20 in phosphate-buffered saline (PBS), and further diluted in saline for administration by intraperitoneal (IP) injection on days +1 and +4 at a dose of 2 μg/mouse, or where specified, 0.5 μg or 5 μg per mouse, respectively. Control mice were given an equivalent volume of 2% DMSO plus 0.5% Tween 20 in PBS, diluted in saline. The degree of systemic GVHD was assessed by scoring, as previously described (maximum index = 10).6 For survival experiments, transplanted mice were monitored daily, and those with GVHD clinical scores of 6 or higher were killed and the date of death registered as the next day, in accordance with institutional animal ethics committee guidelines.
Cellular depletion
Splenocytes were depleted of T cells in vitro by antibody and complement, as previously described.2 Natural killer (NK) cells were depleted from donors in vivo by intravenous administration of 20 μL (approximately 560 μg) of anti-asialo GM1 per mouse, as per manufacturer's instructions (Cedarlane Laboratories, Burlington, ON). In experiments using B6.CD11c.DTR mice, recipients were injected IP with diphtheria toxin (Sigma-Aldrich, Castle Hill, Australia) just before transplantation, at a dose of 100 ng/mouse.
Leukemia challenge
The luciferase-transfected leukemia cell lines, P815 (H-2d, CD45.2+ derived from DBA/2 mice) or EL4 (H-2b, CD45.2+ derived from B6 mice), were injected intravenously (2.5 × 103 or 106 per mouse, respectively) into B6D2F1 or B6 recipients on day 0 of transplantation. Survival and clinical scores were monitored daily, and the cause of death (determined by postmortem examination) was established as GVHD or leukemia, as previously described.2 Leukemic death was defined by the occurrence of positive Xenogen imaging (Xenogen Ivis 100; Caliper Life Sciences, Hopkinton, MA), macroscopic tumor nodules in the liver or spleen, or hind-limb paralysis. GVHD death was defined by the absence of leukemia (both by biphotonic imaging and at postmortem) and the presence of clinical signs of GVHD, assessed by the clinical scoring system. In vivo imaging was performed using an IVIS Imaging System (Xenogen). Light emission is presented as photons/second per cm2 per steradian (photons/s/cm2/sr).
Hepatic lymphocyte and splenic DC isolation
At various time points after transplant, recipient mice were killed and livers were perfused in situ with 5 to 10 mL of cold PBS via the hepatic portal vein. Livers were then removed and homogenized, and lymphocytes were isolated by room temperature isotonic Percoll density centrifugation (GE Biosciences, Uppsala, Sweden), as per manufacturer's instructions. Dendritic cell (DC) purification was undertaken, as previously described.2 Briefly, low-density cells known to contain all DC populations were selected from digested and pooled splenocyte preperations by nycodenz density gradient (1.077 g/L) centrifugation early after transplant and then stained for various markers, as described.7
Fluorescence-activated cell sorter analysis
The following antibodies were purchased from BioLegend (San Diego, CA): fluorescein isothiocyanate (FITC)–conjugated CD49b (DX5), IA/IE (M5/114.15.1), and CD45.1 (A20); phycoerythrin (PE)–conjugated CD3 (2C11), NK1.1 (PK136), CD45.2 (104), H-2Dd (34-2-12), and rat IgG2b iostype control (RTK4530); and allophycocyanin-conjugated CD11c (N418), NK1.1 (PK136), and rat IgG2b isotype control (RTK4530). FITC-conjugated CD3 (KT31.1), rat IgG2a isotype control (Mac4), and IgG2b isotype control (Mac5) were generated in house. Allophycocyanin-conjugated mouse IFN-γ secretion assay detection kit was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). FITC-conjugated CD229.1/Ly9.1 (30C7) was purchased from BD Pharmingen (San Jose, CA). PE-conjugated, α-GalCer (KRN7000)-loaded or unloaded (control) mCD1d (University of Melbourne, Melbourne, Australia) tetramers were generated by Daniel G. Pellicci.
Cytokine analysis
Serum cytokine levels were determined using the BD cytometric bead array system (BD Pharmingen, San Jose, CA). All assays were performed according to the manufacturer's protocol. IFN-γ capture assay (Miltenyi Biotec) was performed per manufacturer's instructions.
CFSE labeling
Carboxy fluoresceine diacetate succinimidyl ester (CFSE; Sigma-Aldrich) labeling of spleen cells was performed, as described. Cells were resuspended at 30 × 106 cells/mL in serum-free media with 1 μM CFSE, incubated at 37°C for 10 minutes, then washed before injection. Cells were analyzed by FACSCalibur (BD Biosciences, San Jose, CA) and processed using ModFit LT cell cycle analysis software (Verity Software House, Topsham, ME).
Polymerase chain reaction analysis
RNA was extracted from sort-purified cells using RNeasy Micro Kits (QIAGEN, Doncaster, Australia), according to the manufacturer's protocol. cDNA was reverse transcribed using avian myeloblastosis virus reverse transcriptase (AM-VRT; Promega, Madison, WI), according to the manufacturer's protocol, and cDNA was stored at −20°C. Real-time polymerase chain reaction (PCR) was undertaken using Taqman Gene 13 Expression assays for murine I12a (IL-12 p35; Mm00434165_m1; Applied Biosystems, Foster City, CA), carried out on a Rotor-Gene3000 (Corbett Life Science, Doncaster, Australia), and data were analyzed using Rotor-Gene V-5.0 (Corbett Life Science). IL-12 cDNA copy numbers were then normalized for variations in the efficiency of RNA extraction and cDNA transcription against the β2m housekeeping gene using Taqman Gene Expression assay for murine β2m (Mm00437762_m1; Applied Biosystems).
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. The Mann Whitney U test was used for the statistical analysis of cytokine data, cellular data, and clinical scores. A P value less than .05 was considered statistically significant. Data are presented as mean plus or minus SEM.
Results
KRN7000 and C20:2 have differential effects on GVHD and GVL
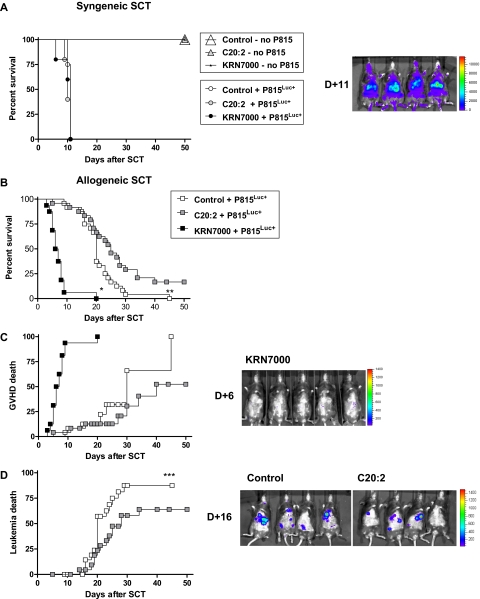
To determine the immunomodulatory effects of KRN7000 and C20:2, we administered glycolipid or control diluent to recipients after SCT. After syngeneic SCT, in the absence of leukemic challenge, all recipients survived long-term regardless of administration of control diluent, C20:2, or KRN7000. After challenge with the host-type mastocytoma cell line, P815 (H-2d), all recipients required sacrifice by day +11 due to rapid leukemic progression (Figure 1A). Thus, in the absence of alloreactivity, neither glycolipid induced significant toxicity nor inhibition of tumor growth.
Figure 1.
Differential effects of KRN7000 and C20:2 on GVHD and GVL. (A) Irradiated B6D2F1 mice received whole spleen from G-CSF–mobilized syngeneic donors, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. Cohorts of mice received leukemic challenge with P815Luc+ or no challenge (control + P815Luc+, n = 8; C20:2 + P815Luc+, n = 5; KRN7000 + P815Luc+, n = 5; no leukemic challenge, n = 4-6). Overall survival by Kaplan-Meier analysis. Images are representative biphotonic bioluminescence images captured using the Xenogen system. Color scale represents signal intensity code (photons/s/cm2/sr). (B) Irradiated B6D2F1 mice received whole spleen from G-CSF–mobilized B6 allogeneic donors plus P815Luc+, followed by control diluent, C20:2, or KRN7000 on days +1 and +4 (control and C20:2, n = 24 per group; KRN7000, n = 16). Overall survival by Kaplan-Meier analysis; combined data from 3 identical experiments. *P < .001 KRN7000 vs control or C20:2. **P = .02 control vs C20:2. (C) GVHD mortality by Kaplan-Meier analysis for the experiments in panel B. (D) Leukemic mortality by Kaplan-Meier analysis for the experiments in panel B. ***P = .0379 (control vs C20:2).
In the context of allogeneic SCT, significantly different results were obtained. KRN7000 resulted in the rapid death of all mice (median survival 7 days; P < .001 vs control or C20:2; Figure 1B). All deaths were determined to be due to GVHD (Figure 1C) based on elevated clinical scores (data not shown) and the absence of leukemia by bioluminescent imaging and postmortem examination. In contrast, C20:2 modestly improved overall survival (Figure 1B, median survival control diluent 20 days; median survival C20:2 25 days; P = .02). GVHD was not increased in recipients of C20:2 (P = .2 control vs C20:2; Figure 1C), and this was confirmed in further experiments in the absence of leukemic challenge (median survival control 35 days vs C20:2 34 days, P = .48; data not shown). Improved overall survival after administration of C20:2 was found to be due to a modest delay in leukemic progression (P = .038 vs control), consistent with enhanced GVL effects (Figure 1D).
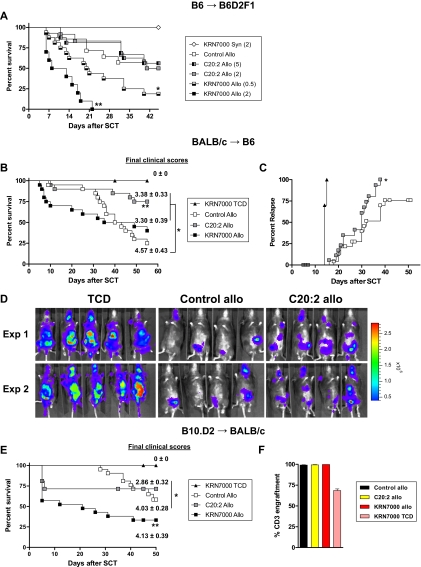
To study whether these differential effects of KRN7000 and C20:2 could be influenced by dose reduction and/or escalation, respectively, dose-response studies were undertaken. These studies demonstrate that KRN7000 results in enhanced GVHD relative to C20:2, even when the C20:2 dose is escalated 10-fold (Figure 2A), arguing that the molecules exert qualitatively, rather than just quantitatively, different immunologic responses.
Figure 2.
KRN7000 induces hyperacute GVHD in multiple models. (A) Irradiated B6D2F1 received whole spleen from G-CSF–mobilized syngeneic B6D2F1 or allogeneic B6 donors, followed by control diluent, C20:2 at 2 μg or 5 μg per mouse, or KRN7000 at 0.5 μg or 2 μg per mouse IP on days +1 and +4. Overall survival by Kaplan-Meier analysis of combined data from 2 experiments (control allo, n = 14; C20:2 2 μg allo, n = 12; C20:2 5 μg allo, n = 16; KRN7000 2 μg allo, n = 10; KRN7000 0.5 μg allo, n = 16; KRN7000 syn, n = 8). **P < .001 for KRN7000 2 μg allo vs C20:2 2 μg and 5 μg allo. *P < .04 for KRN7000 0.5 μg allo vs C20:2 2 μg and 5 μg allo. (B) Irradiated B6 mice received whole or TCD spleen from G-CSF–mobilized BALB/c donors, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. Overall survival by Kaplan-Meier analysis and mean ± SE of clinical scores in surviving mice at the end of each experiment (allo groups, n = 20 each; TCD group, n = 12). Combined data from 3 identical experiments. **P < .02 for C20:2 allo vs control and KRN7000 allo. *P < .05 for control allo clinical scores vs C20:2 and KRN7000 allo. (C) Leukemic relapse in irradiated B6 mice receiving whole or TCD spleen from G-CSF–mobilized BALB/c donors with the addition of host-type luciferase expressing EL4 leukemia, followed by control diluent or C20:2 IP on days +1 and +4. Relapse by Kaplan-Meier analysis (allo groups, n = 24 each; TCD group, n = 10). Combined data from 3 identical experiments. *P = .019 for C20:2 allo vs control allo. (D) Representative bioluminescence images of recipients shown in panel C at day 14 after transplant from 2 separate experiments. (E) Irradiated BALB/c mice received whole or TCD spleen from G-CSF–mobilized B10.D2 donors, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. Overall survival by Kaplan-Meier analysis and mean (± SE) of clinical scores in surviving mice at the end of each experiment (allo groups, n = 21 each; TCD group, n = 13). Combined data from 3 identical experiments. **P < .02 for KRN7000 allo vs control and C20:2 allo. *P < .05 for C20:2 allo clinical scores vs control allo. (F) Donor T-cell engraftment (Ly9.1neg) at day 50 in transplant recipients from panel E. n = 4-5 in allo groups; n = 3 in the TCD group.
Finally, we repeated these studies in models of bone marrow transplantation (as opposed to stem cell transplantation). Hyperacute GVHD after KRN7000 was less after bone marrow transplantation than SCT, but at day 30 mortality was still 50% in animals receiving KRN7000 and only 14% in animals receiving either C20:2 or control diluent (P < .05; data not shown).
The induction of hyperacute GVHD after KRN7000 is seen in multiple models and is associated with proinflammatory cytokine generation
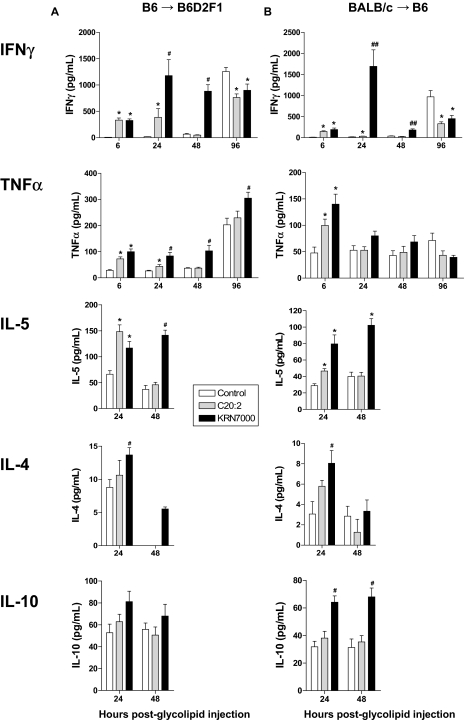
We next studied the effect of glycolipid administration in additional models of GVHD. In the BALB/c→B6 model of acute GVHD directed to MHC, the administration of KRN7000 again induced rapid GVHD mortality, whereas C20:2 paradoxically resulted in a significant protective effect (Figure 2B). Interestingly, those mice that did survive the first 2 weeks after KRN7000 administration also had reduced GVHD clinical scores relative to recipients of diluent (Figure 2B). To study whether the inhibition of GVHD after C20:2 would influence GVL, we performed leukemia experiments using a second host-type leukemia of lymphoid origin (EL4). In this system, the attenuation of GVHD seen after C20:2 did indeed inhibit GVL (Figure 2C,D). Finally, we used a third model in which chronic GVHD is produced in response to multiple minor histocompatibility antigens only. In this system, KRN7000 resulted in early GVHD mortality and severe long-term disease (Figure 2E). Long-term survivors of allogeneic SCT that received C20:2 were again protected from GVHD, again in contrast to those that received KRN7000. This effect was not due to any impairment in engraftment in glycolipid-treated recipients (Figure 2F). Thus, in all models tested, KRN7000 induced an initial hyperacute period of GVHD of varying severity that was largely absent after C20:2 administration. In contrast, C20:2 was neutral or mediated long-term protection from GVHD, although the latter came at the cost of the eventual attenuation of GVL. To define the differential effects of these glycolipids on early posttransplant outcome, we next determined cytokine generation posttransplantation. Stimulation with KRN7000 characteristically results in rapid iNKT cell activation with secretion of large amounts of cytokines, including IFN-γ and IL-4.8 As shown in Figure 3, 24 hours after administration both KRN7000 and C20:2 increased serum IFN-γ and tumor necrosis factor (TNF); however, KRN7000 induced a prolonged and exaggerated secretion of IFN-γ in both the B6→B6D2F1 and B6→BALB/c models, but was most severe in the former system, in which GVHD resulted in uniform early mortality. KRN7000 also resulted in a persistent increase in TNF-α after transplantation, most dominant in the B6→B6D2F1 system, again consistent with the early hyperacute GVHD and mortality. In contrast, IFN-γ generation was inhibited later following transplantation after glycolipid administration, suggesting donor T-cell contraction or inhibition of cytokine secretion. KRN7000 administration also induced, to various degrees, the secretion of IL-4, IL-5, and IL-10 within 48 hours of administration. The levels of these cytokines induced by C20:2 administration were consistently similar or lower than that induced by KRN7000 (Figure 3), indicating that the improved survival of C20:2-treated mice was unlikely to be the result of T helper 2 (Th2) differentiation.
Figure 3.
KRN7000 promotes proinflammatory cytokine secretion after allogeneic SCT. (A) Irradiated B6D2F1 mice received whole spleen from G-CSF–mobilized B6 donors, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. IFN-γ, TNF, IL-5, IL-4, and IL-10 were determined in the sera at various times thereafter by cytometric bead array (n = 8-12 per group and time point, combined from a minimum of 2 experiments, except for IL-5 and IL-4 at 48 hours and IL-10, where n = 3-4). *P < .05 vs control diluent; #P < .03 vs C20:2 and control diluent. (B) Irradiated B6 mice received whole spleen from G-CSF–mobilized BALB/c donors, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. IFN-γ, TNF, IL-5, IL-4, and IL-10 were determined in the sera at various times thereafter by cytometric bead array (n = 8-12 per group and time point combined from a minimum of 2 experiments, except IL-5 and IL-4 at 48 hours and IL-10, where n = 4). *P < .05 vs control diluent; #P < .03 vs C20:2 and control diluent; ##P < .001 vs C20:2 and control diluent.
The induction of hyperacute GVHD after KRN7000 is the result of enhanced NK- and T-cell activation
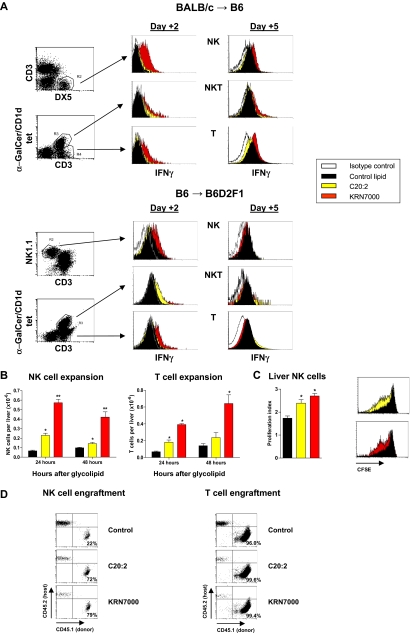
Because both C20:2 and KRN7000 induce similar levels of early iNKT-cell IFN-γ secretion,4 we next sought to determine the cellular source of the prolonged IFN-γ secretion seen after KRN7000 administration. As shown in Figure 4A, only administration of KRN7000 post-SCT resulted in increased IFN-γ production from NK-cell and T-cell compartments relative to control diluent or C20:2. Although both glycolipids resulted in early NK- and T-cell expansion (Figure 4B), this was far greater and more prolonged after KRN7000 administration. The increase in hepatic NK cells after glycolipid administraton was at least in part due to increased proliferation as determined by CFSE dilution (Figure 4C). However, this did not fully explain the difference between C20:2 and KRN7000, suggesting that preferential NK-cell migration to the liver was also occurring in the latter group. In contrast, the proliferation of T cells in the liver was similar across all groups (data not shown) and did not reflect the relative numbers at this site, suggesting that migration to the liver by conventional T cells is the main determinant of the differential expansion after glycolipid administration. In addition, both glycolipids increased the rate of donor NK- and T-cell engraftment (Figure 4D), whereas NKT cells were more than 96% donor in both B6→B6D2F1 and BALB/c→B6 systems at this time (data not shown). Interestingly, both glycolipids resulted in an eventual contraction of the donor T-cell pool, most clearly in the BALB/c→B6 system (data not shown), consistent with the reduction in GVHD and GVL seen after C20:2 delivery in this model.
Figure 4.
KRN7000 promotes NK- and T-cell activation after SCT. (A) Irradiated B6 or B6D2F1 mice received whole spleen from G-CSF–mobilized BALB/c or B6 allogeneic donors, respectively, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. Hepatic lymphocytes were pooled from 4 to 5 mice per group at days 2 and 5 after transplant, gradient-purified, and IFN-γ secretion by cell subsets determined by cytokine secretion assay. Representative plots from 3 experiments in each strain. (B) Irradiated B6D2F1 mice received whole spleen from G-CSF–mobilized B6 allogeneic donors, followed by control diluent, C20:2, or KRN7000 IP on day +1 and hepatic NK (NK1.1+CD3neg)- and T-cell (CD3posNK1.1neg) subsets enumerated 24 and 48 hours after glycolipid injection. Mean ± SE from 5 to 7 mice per group. *P < .05 vs control diluent; **P < .05 vs control diluent and C20:2. (C) Irradiated B6D2F1 mice received whole CFSE-labeled spleen from G-CSF–mobilized B6 allogeneic donors, followed by control diluent, C20:2, or KRN7000 IP on day +1. Proliferation of liver NK cells was determined by CFSE dilution on day +3. Proliferation indices of individual mice were determined by Modfit analysis and are shown as mean ± SE (n = 5 per group). Representative CFSE fluorescence-activated cell sorter (FACS) plots are also shown. *P < .02 vs control diluent. (D) Irradiated CD45.2+ B6D2F1 mice received whole spleen from CD45.1+ G-CSF–mobilized B6 allogeneic donors, followed by control diluent, C20:2, or KRN7000 IP on days +1 and +4. Donor engraftment (CD45.1+CD45.2neg) in NK- and T-cell subsets (gated as above) was determined at day +5 in hepatic lymphocytes pooled from 4 to 5 mice per group.
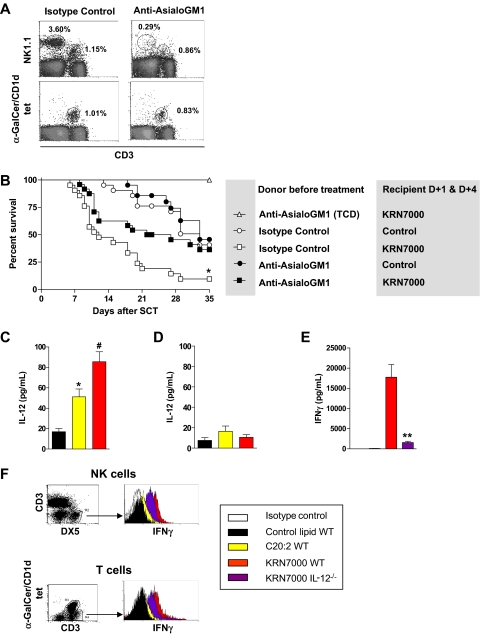
To confirm a role for donor NK cells in KRN7000-mediated GVHD, we performed further transplants in which groups of donors were specifically depleted of NK cells using anti-asialo GM1. In these studies, we focused only on KRN7000 because of its differential effects on NK-cell activation and ability to induce GVHD. As shown in Figure 5A, anti-asialo GM1 effectively depleted donor NK cells, with minimal effects on iNKT cells. In recipients of control diluent, GVHD was not affected by NK-cell depletion of the graft (P = .61; Figure 5B), confirming that, in these systems, a pathogenic role for donor NK cells is only seen after downstream activation of NKT cells by KRN7000. In contrast, after administration of KRN7000, GVHD severity was significantly reduced in recipients of NK-cell–depleted grafts (P = .01). Thus, KRN7000 induced the expansion of IFN-γ–secreting NK cells, and NK-cell–dependent GVHD.
Figure 5.
GVHD induction by KRN7000 is NK cell–dependent. (A) B6 donors received isotype control or anti-asialo GM1, as described in “Cellular depletion,” and specific NK-cell depletion of the graft confirmed before transplantation by FACS. (B) Irradiated B6D2F1 mice received G-CSF–mobilized spleen from B6 donors pretreated with isotype control or anti-asialo GM1 (corrected to transfer equivalent numbers of T cells to each group) or T-cell–depleted spleen, followed by control diluent or KRN7000 IP on days +1 and +4. (TCD, n = 5; all other groups, n = 21). Combined data from 3 experiments shown. Overall survival by Kaplan-Meier analysis. *P < .05 vs all other groups. Differences between all other T-cell–replete groups are not significant. (C) Irradiated B6 mice received whole spleen from G-CSF–mobilized BALB/c allogeneic donors, followed by control diluent, C20:2, or KRN7000 IP on day +1, and IL-12 levels were measured in sera 6 hours later (n = 8-12 per group combined from 2 experiments). Data expressed as mean ± SE; *P = .002 vs control; #P = .02 vs C20:2. (D) Irradiated B6.Jα18−/− mice received whole spleen from G-CSF–mobilized BALB/c.Jα18−/− donors, followed by control diluent, C20:2, or KRN7000 IP on day +1. IL-12 levels were measured in sera 6 hours later (n = 5 per group). Data are expressed as mean ± SE. (E) Irradiated B6.WT or B6.IL-12−/− mice received whole spleen from G-CSF–mobilized BALB/c allogeneic donors, followed by control diluent, C20:2, or KRN7000 IP on day +1, and IFN-γ levels were measured in sera 24 hours later (n = 8-14 per group, combined from 3 experiments). Data are expressed as mean ± SE; **P = .001, KRN7000 B6.WT vs B6.IL-12−/− recipients. (F) IFN-γ secretion assay on hepatic NK- and T-cell populations 24 hours after glycolipid administration in transplants, as described in panel E, one of 3 representative experiments shown.
Enhanced NK- and T-cell activation after KRN7000 is IL-12 dependent
To study the mechanisms by which type I NKT activation by KRN7000 resulted in NK-cell IFN-γ secretion, we determined levels of IL-12 in the serum 6 hours after glycolipid administration. Both KRN7000 and C20:2 induced IL-12 secretion, but this was most marked after KRN7000 (Figure 5C). Furthermore, the production of IL-12 was entirely dependent on the presence of iNKT cells (Figure 5D). To confirm that host IL-12 was important in this trans-activation of NK cells by KRN7000 administration, we determined IFN-γ generation in B6.IL-12−/− recipients. As shown in Figure 5E, serum IFN-γ levels were reduced by 90% in B6.IL-12−/− recipients receiving KRN7000, and consistent with this, IFN-γ secretion was reduced in both NK- and T-cell subsets (Figure 5F).
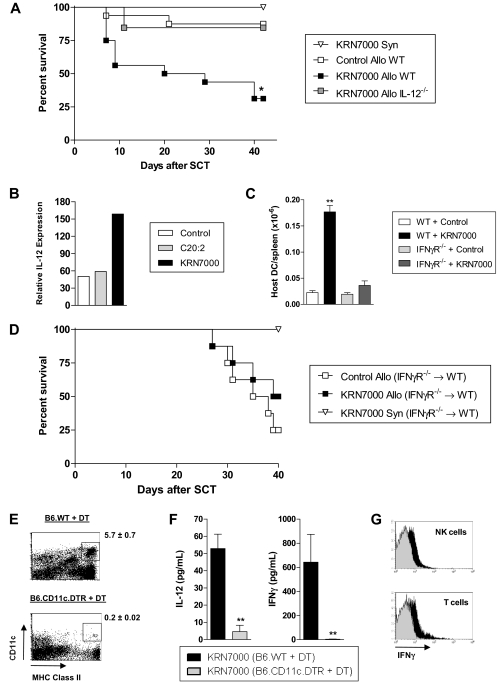
To study whether IL-12 was involved in the hyperacute GVHD seen after KRN7000 administration, we next administered KRN7000 to B6.IL-12−/− SCT recipients. This confirmed that the acute GVHD induced by KRN7000 in wild-type recipients was completely lost in the absence of host-derived IL-12 (Figure 6A). Because KRN7000 requires endosomal processing by professional antigen-presenting cells (APCs; ie, DCs) to be presented within CD1d,4 we next analyzed IL-12 levels in host DCs. This confirmed the preferential induction of IL-12 (p35) mRNA in host DCs after KRN7000 administration (Figure 6B). Surprisingly, this induction of IL-12 in host DCs by KRN7000 was also associated with their enhanced survival, and this effect was IFN-γ–dependent (Figure 6C). Unfortunately, GVHD experiments using IFN-γR−/− mice are not possible because all animals succumb very early to idiopathic pneumonia syndrome.9 This is due to the protective effect of IFN-γ signaling on cell migration into the lung via effects on nonhematopoietic cells. Because of this, and the fact that we hypothesized that IFN-γ signaling of host APC played a pathogenic role after KRN7000 administration, we generated B6 chimeras that lack the IFN-γR only on hematopoietic cells. When these animals were used as secondary allogeneic transplant recipients, KRN7000 administration had no effect on GVHD (Figure 6D), confirming that IFN-γ signaling through residual host hematopoietic tissue (putatively DCs) is indeed involved in the pathogenic cascade after glycolipid administration.
Figure 6.
Host DCs, IL-12, and IFN-γ are critical for the hyperacute GVHD induced by KRN7000. (A) Irradiated B6.WT or B6.IL-12−/− mice received whole spleen from G-CSF–mobilized syngeneic B6 or allogeneic BALB/c donors, followed by control diluent or KRN7000 IP on days +1 and +4. Overall survival by Kaplan-Meier analysis, combined data from 2 experiments (n = 4, syn KRN7000; n = 16, allo B6.WT groups; n = 13, allo KRN7000 B6.IL-12−/− group). *P < .005 allo KRN7000 B6.WT vs allo KRN7000 B6.IL-12−/− and allo control B6.WT. (B) Irradiated B6 mice received whole spleen from G-CSF–mobilized BALB/c donors, followed by control diluent, C20:2, or KRN7000 IP on day +1. DCs were sort-purified from spleens pooled from 4 mice per group 6 hours after glycolipid injection, and their IL12a expression relative to β2m was determined by real-time PCR. (C) Irradiated B6.WT or B6.IFN-γR−/− recipients were transplanted with G-CSF–mobilized BALB/c grafts and KRN7000 or control diluent administered at day +1 (n = 6 per group). Numbers of host (H-2Dd−) DCs remaining in the spleen were elucidated 3 days after transplant. **P < .01 vs other groups. Combined data from 2 replicate experiments. (D) B6.IFN-γR−/−→B6.WT chimeras were transplanted with whole spleen from G-CSF–mobilized syngeneic B6 or allogeneic BALB/c donors, followed by control diluent or KRN7000 on days +1 and +4. Overall survival by Kaplan-Meier analysis (n = 4, syn group; n = 8, allo groups). P = .33 between allo groups. (E) Irradiated B6.WT or B6.CD11c.DTR mice were injected with diphtheria toxin IP, transplanted with whole spleen from G-CSF–mobilized BALB/c donors 1 hour later, and given KRN7000 IP on day +1 (24 hours later). Effective depletion of splenic DCs was confirmed 24 hours after KRN7000 administration. Representative FACS plots are shown, with the mean ± SE of residual DC percentages given alongside (n = 9-10). (F) Serum IL-12 was determined at 6 hours, and serum IFN-γ at 24 hours, after KRN7000 injection, in transplant recipients from panel E. Data show mean ± SE of combined data from 2 identical experiments (n = 8-10). **P < .001. (G) Hepatic lymphocytes from transplants described in panel E were pooled from 5 mice per group at 24 hours after glycolipid injection and gradient-purified, and IFN-γ secretion by cell subsets was determined by cytokine secretion assay. One of 2 representative experiments is shown.
We next analyzed whether host DCs were important in the pathogenic cascade induced by glycolipid administration. In these studies, we used recipient mice in which the diphtheria toxin receptor is driven off the CD11c promoter. The depletion of host DCs by the administration of diphtheria toxin at the time of transplantation (Figure 6E) resulted in the complete loss of IL-12 and IFN-γ production in response to KRN7000 (Figure 6F) and reduced the secretion of IFN-γ by NK and conventional T cells (Figure 6G).
Discussion
iNKT cells have pivotal roles in the coordination of innate and adaptive immune responses, and are thus attractive targets for the modulation of GVHD and GVL effects after allogeneic SCT. Despite the expression of highly restricted, semi-invariant TCRs, alteration of the basic α-GalCer structure can alter the pattern of iNKT-cell stimulation. We report that iNKT-cell stimulation with KRN7000 and the novel N-acyl variant, C20:2, result in different outcomes in models of allogeneic SCT and leukemic challenge. Importantly, these molecules highlight the ability of iNKT cells to shape both the innate and adaptive immune response. Thus, profound iNKT activation by KRN7000 induces IL-12 from host DCs and activates both NK cells and conventional T cells, resulting in rapid and prolonged IFN-γ secretion, which in turn prolongs the period of the DC-T-cell interaction.
The roles of iNKT cells in the regulation of autoimmunity and tumor surveillance are complex, and, whereas administration of KRN7000 may provide protection in some models, it may exacerbate immune-mediated damage in others.10 Activation of host iNKT cells by the administration of α-GalCer significantly reduces GVHD due to Th2 polarization of donor T cells.11 Haraguchi et al, using a MHC disparate model with nonlethal recipient irradiation, demonstrated that specific stimulation of host iNKT cells could induce graft rejection and thereby reduce GVHD, particularly in the absence of donor iNKT cells.3 In our study, the variable of graft rejection was excluded by including a parent to F1 model, by delivering fully myeloablative doses of TBI and by delaying glycolipid administration until days +1 and +4 after SCT, so that predominantly donor iNKT cells were stimulated. The important studies from the Strober group have demonstrated the clear ability of host iNKT cells, enriched and modulated by specific conditioning with total lymphoid radiation and antithymocycte serum, to inhibit GVHD.12 Importantly, suppressive effects appear predominantly mediated by type II rather than type I iNKT cells.13,14 Thus, activated type I iNKT cells have antitumor effects in multiple systems15,16 and appear to be associated with a proinflammatory, IL-12–dominated cytokine response, whereas type II NKT cells suppress antitumor immunity in association with Th2 cytokines.17,18 However, it should be noted that type II NKT cells have not been activated by the strategies in this study because they do not respond to glycolipids.19 Because type I iNKT cells vary in function in different anatomical sites,20 the outcome with regard to alloimmunity may be dependent on the principal site of activation. As such, activation within the liver would be expected to enhance immune responses, whereas activation in the thymus would be more likely to exert a suppressive response.20 Interestingly, a subset of type I iNKT cells, namely the CD4−CD8− fraction, confer the most potent antitumor cytotoxic T lymphocyte (CTL) generation in vivo, whereas the type I CD4+ iNKT fraction has little activity or may be suppressive,2,20 adding further complexity to the functional effects of iNKT cells in vivo.
C20:2 was synthesized to generate a molecule that stimulates the same population of iNKT cells as KRN7000 with the same affinity, but with Th2-biased cytokine responses.4 Although both C20:2 and KRN7000 induced similar early iNKT cell-derived IFN-γ, only KRN7000 induced downstream expansion of IFN-γ–secreting NK cells with subsequent NK-dependent GVHD. Although the promotion of IFN-γ–secreting NK cells would be predicted to greatly enhance antitumor effects,21 beneficial effects are totally effaced by overwhelming GVHD. Alloreactive donor NK cells have been described with the ability to mediate GVL and inhibit GVHD in the setting of highly efficient T-cell depletion, the later due to their ability to clear recipient antigen-presenting cells.22 In contrast, in the setting of T-cell–replete transplantation, alloreactive NK cells demonstrate increased activation (IFN-γ secretion) when in the presence of alloreactive T cells and are associated with enhanced acute GVHD,23,24 consistent with the findings in these studies. NK cells activated after α-GalCer administration in allogeneic recipients (in addition to allogeneic signals delivered by activating receptors and the failure of killer immunoglobulin-related receptor-dependent inhibition) will subsequently be capable of target tissue damage by virtue of their potent cytotoxic machinery. In addition, the large amount of IFN-γ produced by these cells will directly induce GVHD, especially of the gastrointestinal tract.9
Optimal CTL priming by APC requires the combination of class I antigen presentation, adequate costimulation, and provision of a third signal, characteristically attributed to IL-12.25 Consistent with this, we have previously demonstrated that IL-12 secretion is augmented from residual host DCs in response to activated donor iNKT cells.2 These effects are restricted to the host APC and donor iNKT synapse because donor APC have only a minor influence on CD8-dependent GVL effects.26,27 Fujii et al demonstrated that administration of KRN7000 to naive mice induced rapid IFN-γ–dependent surface remodeling of DCs, including increased CD40 and CD86 expression.28 Furthermore, CD40 expression was absolutely required for the induction of effective CTL responses, and they concluded that a distinct CD40/CD40L signal, functioning together with antigen presentation and costimulation, is required for the effective bridging of innate and adaptive immune responses.28 It is thus likely that, after SCT, APC activation is enhanced by donor iNKT-cell–derived IFN-γ and subsequent CD40 ligation by CD40L on both iNKT cells and conventional T cells. These signals induce the secretion of IL-12, which together optimizes priming by APC. It is somewhat surprising that C20:2 largely failed to exacerbate GVHD, as would have been predicted. However, in the transplant setting, it appears that the very intense iNKT activation with glycolipids may induce excessive alloreactive donor T-cell activation and subsequent contraction/deletion with an eventual reduction in GVHD. This is consistent with prior studies demonstrating that very high doses of exogenous IL-12 administration early after transplant could promote tolerance due to the deletion of alloreactive CD4+ T cells.29,30 Obviously in the case of KRN7000, this represents an inherently dangerous means by which to promote long-term tolerance because NK-cell–dependent hyperacute GVHD develops initially.
It remains unclear why C20:2 induces little or no downstream NK-cell activation, as opposed to KRN7000. Yu et al demonstrated that KRN7000 presentation could be impaired by interference with endosomal loading through cell fixation, disruption of endosomal processing, or disruption of molecules required for CD1d localization.4 In contrast, C20:2 had remarkably minimal requirement for endosomal loading, leading to the theory that C20:2 may be preferentially presented by certain APC subsets or that C20:2-loaded CD1d may be less likely to localize to costimulatory molecule-rich lipid rafts.4 These mechanisms remain the focus of investigation, and further understanding will allow specific tailoring of glycolipid molecules to induce desirable immunologic outcomes.
The unexpected and dramatic enhancement of GVHD after administration of KRN7000 postallogeneic hemopoietic transplantation highlights the need for care in the use of iNKT ligands for translation into the clinic. Conversely, better understanding of the mechanisms of action of modified iNKT ligands may allow selection of molecules most appropriate for specific clinical circumstances.
Acknowledgments
We thank Dr Dale Godfrey for critically reviewing the manuscript, and Daniel Pellicci and Dr Mitchel Kronenberg for provision of CD1d-tetramer.
This work was supported by grants from the National Health and Medical Research Council and the Leukemia Foundation of Australia. G.R.H. is a National Health and Medical Research Council Practitioner Fellow. K.P.A.M. is a National Health and Medical Research Council R.D. Wright Fellow. S.A.P. is supported by the National Institutes of Health (AI45889) and the Hirschl Trust. G.S.B. acknowledges support from James Bardrick in the form of a Personal Research Chair.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.D.K. and E.S.M. performed research and wrote the paper; K.P.A.M., K.A.M., H.M.M., N.C.R., T.B., A.L.J.D., V.R., and A.C.B. performed research; A.D.C. performed data interpretation; C.F. provided essential reagents and knockout mice; G.S.B. and P.A.I. generated and provided essential reagents (glycolipids); M.J.S. performed data interpretation and experimental design; S.A.P. provided essential reagents, data interpretation, and experimental design; and G.R.H. performed experimental design and helped write the paper.
Conflict-of-interest disclosure: S.A.P. is a paid consultant for Vaccinex (Rochester, NY), which has licensed commercial rights to pending patent applications that cover the use of α-galactosylceramides as immunomodulators. The remaining authors declare no competing financial interests.
Correspondence: Dr Geoffrey R. Hill, H Floor, CBCRC, Queensland Institute of Medical Research, Bone Marrow Transplant Laboratory, 300 Herston Rd, Brisbane, QLD 4029, Australia; e-mail: Geoff.Hill@qimr.edu.au.
References
- 1.Morris ES, MacDonald KP, Hill GR. Stem cell mobilization with G-CSF analogs: a rational approach to separate GVHD and GVL? Blood. 2006;107:3430–3435. doi: 10.1182/blood-2005-10-4299. [DOI] [PubMed] [Google Scholar]
- 2.Morris ES, MacDonald KP, Rowe V, et al. NKT cell-dependent leukemia eradication following stem cell mobilization with potent G-CSF analogs. J Clin Invest. 2005;115:3093–3103. doi: 10.1172/JCI25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraguchi K, Takahashi T, Matsumoto A, et al. Host-residual invariant NK T cells attenuate graft-versus-host immunity. J Immunol. 2005;175:1320–1328. doi: 10.4049/jimmunol.175.2.1320. [DOI] [PubMed] [Google Scholar]
- 4.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris ES, MacDonald KPA, Rowe V, et al. Donor treatment with pegylated G-CSF augments the generation of IL-10 producing regulatory T cells and promotes transplant tolerance. Blood. 2004;103:3573–3581. doi: 10.1182/blood-2003-08-2864. [DOI] [PubMed] [Google Scholar]
- 6.Hill GR, Crawford JM, Cooke KJ, Brinson YS, Pan L, Ferrara JLM. Total body irradiation and acute graft versus host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 7.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 8.Crowe DL, Yoon E. A common pathway for chemotherapy-induced apoptosis in human squamous cell carcinoma lines distinct from that of receptor-mediated cell death. Anticancer Res. 2003;23:2321–2328. [PubMed] [Google Scholar]
- 9.Burman AC, Banovic T, Kuns RD, et al. IFNγ differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110:1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 10.Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82:315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto D, Asakura S, Miyake S, et al. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J Immunol. 2005;174:551–556. doi: 10.4049/jimmunol.174.1.551. [DOI] [PubMed] [Google Scholar]
- 12.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Choi EY, Chung DH. Donor bone marrow type II (non-Vα14Jα18 CD1d-restricted) NKT-cells suppress graft-versus-host disease by producing IFN-γ and IL-4. J Immunol. 2007;179:6579–6587. doi: 10.4049/jimmunol.179.10.6579. [DOI] [PubMed] [Google Scholar]
- 14.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosino E, Terabe M, Halder RC, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 17.Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Van Kaer L, Brutkiewicz RR. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer. 2006;118:3045–3053. doi: 10.1002/ijc.21764. [DOI] [PubMed] [Google Scholar]
- 18.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 20.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 23.Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JS, Cooley S, Parham P, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 26.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 27.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 28.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dey BR, Yang YG, Szot GL, Pearson DA, Sykes M. Interleukin-12 inhibits graft-versus-host disease through an Fas-mediated mechanism associated with alterations in donor T-cell activation and expansion. Blood. 1998;91:3315–3322. [PubMed] [Google Scholar]
- 30.Yang YG, Sergio JJ, Pearson DA, Szot GL, Shimizu A, Sykes M. Interleukin-12 preserves the graft-versus-leukemia effect of allogeneic CD8 T cells while inhibiting CD4-dependent graft-versus-host disease in mice. Blood. 1997;90:4651–4660. [PubMed] [Google Scholar]