Abstract
Pseudoxanthoma elasticum (PXE), a heritable multi-system disorder manifesting with ectopic mineralization of soft connective tissues, is caused by mutations in the ABCC6/MRP6 gene/protein system, but the mechanisms how the ABCC6 mutations lead to aberrant mineralization are currently unknown. In this study, we utilized a transgenic mouse model, Abcc6−/−, to examine the mineralization processes. We focused on matrix gla protein (MGP) which has been shown to be critical, when activated by γ-carboxylation of glutamyl residues, for prevention of unwanted mineralization. The concentration of MGP in the serum of Abcc6−/− mice was significantly reduced when compared to wild-type controls (p<0.004). More importantly, MGP isolated from the liver of Abcc6−/− mice was largely under-carboxylated, and therefore possesses no activity. Finally, examination of the Abcc6−/− mice revealed association of total and under-carboxylated forms of MGP with ectopic mineralization while the γ-carboxylated form was essentially absent. These results suggest that MGP in Abcc6−/− mice is largely in inactive form and is unable to prevent the unwanted mineralization of connective tissues in PXE.
Keywords: Pseudoxanthoma elasticum, connective tissue mineralization, matrix gla protein, γ-glutamyl carboxylation, ABC transporters
Introduction
Pseudoxanthoma elasticum (PXE; OMIM 264800,177850), an autosomal recessive multi-system disorder, is characterized by dystrophic mineralization of soft connective tissues in a number of organs, including the skin, the eyes, and the arterial blood vessels, with considerable morbidity and mortality [1,2]. PXE is caused by mutations in the ABCC6 gene which encodes a putative transmembrane protein, MRP6, a member of the family of ATP-binding cassette (ABC) multi-drug resistance-associated proteins [3]. The ABCC6 gene is primarily expressed in the liver, to a lesser extent in the proximal tubules of kidneys, and at very low level, if at all, in tissues affected in PXE [4,5]. While the mineral deposits in the affected tissues are known to consist of calcium and phosphate, the precise mechanisms leading to aberrrant mineralization, and specifically, the substrate specificity of MRP6 are currently unknown. These and related observations have led us to suggest that PXE is a metabolic disease, rather than a primary connective tissue disorder, and that in the absence of MRP6 functional activity in the liver, alterations in serum concentrations of critical components that regulate the mineralization processes may occur [6].
A number of proteins has been suggested to play a role in prevention of dystrophic mineralization of connective tissues; these include matrix gla protein (MGP), fetuin-A, and ankylosis protein (Ank). The critical role of these proteins in preventing ectopic, unwanted mineralization is derived from observations that development of knock-out mice by targeted ablation of the corresponding genes results in extensive soft tissue mineralization, including the vascular connective tissues [7–9]. Of particular interest is MGP, which requires vitamin K-dependent γ-carboxylation of glutamyl residues to become biologically active [10–12].
We have recently developed a mouse model for PXE by targeted ablation of the Abcc6 gene [13]. These mice are apparently normal at birth and during the early postnatal period, but they subsequently develop extensive connective tissue mineralization, recapitulating the histopathologic and ultrastructural features of human PXE. A particularly interesting finding in this mouse model is mineralization of the connective tissue capsule surrounding the hair follicles in vibrissae, which can be observed as early as the 5–6th week of life [6,13]. It has been proposed that this pathologic feature serves as an early biomarker of the overall mineralization process in PXE in this mouse model. We have previously utilized this mouse model to demonstrate that fetuin-A, Ank, and MGP as well as alkaline phosphatase activity are strongly associated with the mineralization process, as examined by immunostaining [6]. Furthermore, in situ hybridization demonstrated that genes for MGP and Ank are expressed locally in vibrissae while fetuin-A is expressed highly in the liver. Our results suggested, therefore, that the deposition of these proteins spatially coincides with the mineralization and they actively regulate this process locally and systemically [6]. In this study, we have focused our attention to MGP and particularly examined the presence of the activated, γ-glutamyl carboxylated form of this protein in Abcc6−/− mice.
Materials and methods
Mice
The PXE mouse model, Abcc6−/− mouse (KO), was developed by targeted ablation of the Abcc6 gene [13], and the mice were maintained in the Animal Facility of the Thomas Jefferson University in a temperature- and humidity-controlled environment under 12-h light/dark cycles. Mice were fed a standard rodent diet (Lab Diet 5010; PMI Nutrition, Brentwood, MO) and had free access to water. The experimental protocols were approved by the University’s Institutional Animal Care and Use Committee.
Immunoassay for serum MGP
Serum MGP was measured using a commercially available kit from Biomedica (Vienna, Austria). It is a competitive enzyme-linked immunosorbent assay (ELISA) in which microwell plates are coated with mouse monoclonal antibodies raised against the N-terminal 3–15 sequence of human MGP.
Expression of the MGP gene in the liver
The expression of the MGP gene in the liver of WT and KO mice was examined by RT-PCR using RNA isolated by extraction with the Trizol reagent (Invitrogen, Carlsbad, CA) followed by RNeasy Mini Kit (Qiagen, Valencia, CA). Random-primed reverse transcription of RNA was performed with the Superscript First-Strand Synthesis System (Invitrogen), using 1 μg of RNA for each reaction. The primers used for amplification of MGP sequences were: Forward: 5′-TGCGCTGGCCGTGGCAACCCT-3′; Reverse: 5′-CCTCTCTGTTGATCTCGTAGGCA-3. The PCR products were examined on 3% agarose gels, and the bands were quantitated by densitometry. For reference, Gapdh mRNA was amplified and examined in parallel [14].
Identification of total and γ-carboxylated form of MGP in the liver
Protein isolation
Mouse liver was snap-frozen in liquid nitrogen and homogenized in RIPA buffer (Sigma, St. Louis, MO) containing protease inhibitors (Roche Diagnostics, Indianapolis, IN). The extracts were centrifuged at 10,000 g for 10 min to remove cell debris. Protein concentration in the supernatant was measured with a bicinchoninic acid reagent kit (Pierce, Rockford, IL).
Western blot
Proteins isolated from KO or WT mouse liver were subjected to SDS/PAGE (4–20% gel) in the presence of a reducing agent (200 mM dithiothreitol). Protein was electrotransferred to polyvinylidene fluoride membrane, and nonspecific binding sites on the membrane were blocked by incubation in 5% milk for 2 hrs at room temperature. The blot was incubated with poAb-anti-tMGP (recognizing all forms of MGP; tMGP) (1:10,000) or moAb-anti-cMGP (recognizing γ-glutamyl carboxylated MGP; cMGP) (1:20,000) [15] overnight at 4°C, and then incubated with HRP-conjugated goat anti-rabbit antibody (1:5,000) or HRP-conjugated horse anti-mouse antibody (1:2,000) (Cell Signaling Technology, Danvers, MA), respectively, for 1 hr at room temperature. After three 10-min washings, the signal was determined with ECL plus Western Blotting Detection System (Amersham Biosciences, Piscataway, NJ) with nonsaturating exposures. Equal loading of liver proteins was verified by incubation with an anti-β-actin antibody (1:7,500) (Calbiochem, San Diego, CA) for 1 hr at 4°C overnight, followed by incubation with horse anti-mouse HRP-conjugated antibody (1:2,000) for 1 hr at room temperature. Bands were visualized using the same chemiluminescent technique. The films were scanned and the signals were quantified with a Kodak Image Station 440 CF using Kodak 1D 3 imaging analysis software (Eastman Kodak, Rochester, NY).
Immunofluorescence of MGP in the mouse muzzle skin containing vibrissae
Immunofluorescence of the mouse muzzle skin was performed as described previously [6]. The primary antibodies utilized were [15]: poAb-anti-tMGP (1:200), poAb-anti-ucMGP (recognizing under-carboxylated MGP; ucMGP) (1:250) and moAb-anti-cMGP (1:250). Muzzle skin containing vibrissae from both WT and KO mice were examined. The secondary antibody, Texas Red conjugated anti-rabbit IgG (1:1,000) (Molecular Probes, Eugene, OR) or anti-mouse IgG (1:250) (Santa Cruz Biotechnology, Santa Cruz, CA) were applied. Parallel sections were stained for mineral deposits with Alizarin Red [6].
Statistical analysis
Mann-Whitney test or Student’s two-tailed t-test was used to evaluate statistical significance of differences, as appropriate.
Results
MGP concentration in serum is reduced in Abcc6−/− mice
The Abcc6−/− mice serve as a model system recapitulating the histopathologic and ultrastructural features of human PXE, including soft tissue mineralization [13]. This mouse model was utilized in the present study to examine the role of MGP in the mineralization process. Serum concentrations of MGP were determined by an ELISA in mice of three months of age. The results indicated significant (47.8%; p<0.004) reduction of MGP in the serum of KO mice, as compared with WT controls (Fig. 1). Specifically, statistically significant (p<0.04) reduction of 62.5% was noted in male mice, and similar, but somewhat less pronounced, decrease was noted in female mice (Fig. 1).
Figure 1.
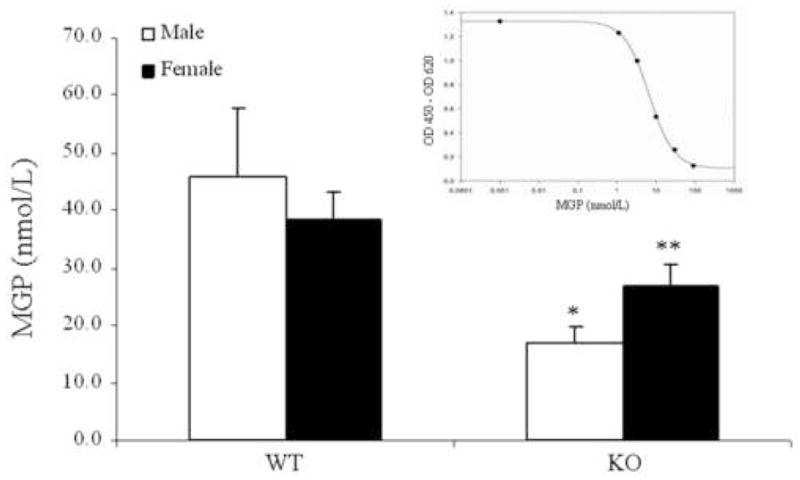
Concentration of serum MGP of wild-type (WT) and Abcc6−/− knock-out (KO) mice, as determined by an ELISA using an antibody recognizing the N-terminal 3–15 amino acids of MGP. The standard curve with human recombinant MGP, provided with the ELISA kit, is shown in the inset. The antibody shows 29% cross reactivity with a corresponding mouse protein [22], and the values are corrected accordingly. The values are expressed as mean ± SE; n = 3–6 animals per group; *p<0.04 (Mann-Whitney); **p<0.03 (Student’s t-test).
Matrix gla protein is expressed in the liver
MGP is synthesized by a variety of tissues and cell types, including fibroblasts, smooth muscle cells and chondrocytes [10]. Since the Abcc6 gene harboring mutations in PXE is expressed primarily in the liver [4,5], we wanted to examine whether MGP is also expressed in the liver, by RT-PCR. Amplification of MGP mRNA revealed the presence of this transcript both in WT and KO mice (Fig. 2a). Quantitation by densitometry, after correction for the levels of Gapdh mRNA in the same samples, suggested that there is no difference in the MGP mRNA in WT and KO mice, the relative abundance being 100.0 ± 37.2 and 106.6 ± 26.2, respectively (mean ± SE; n=3; p=0.8).
Figure 2.
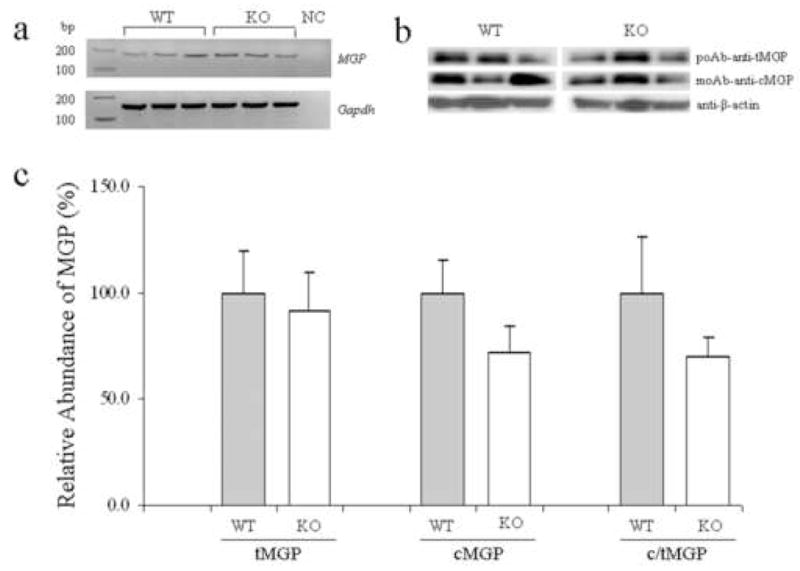
Demonstration of MGP mRNA and assay of total (tMGP) and carboxylated (cMGP) forms of MGP in the liver in WT and KO mice. RT-PCR demonstrated the presence of MGP mRNA both in WT and KO liver RNA samples (panel a; NC, negative control). Protein extracts were examined by Western analysis using anti-tMGP and anti-cMGP antibodies. The equal protein loading was verified by anti-β-actin antibody (panel b). There was no difference in the relative amount of tMGP, while the amount of cMGP and consequently the c/tMGP ratio were reduced in the knock-out mice. The values are mean ± SE; n=3 (panel c).
The γ-glutamyl carboxylated form of MGP is reduced in the liver of Abcc6−/− mice
For activation of MGP, certain glutamyl residues need to be carboxylated by an enzymatic reaction. Thus, both total and the γ-glutamyl carboxylated forms of MGP protein in the liver of WT and Abcc6−/− mice were determined by Western analysis utilizing antibodies recognizing tMGP and cMGP. The results shown in Fig. 2b revealed the presence of the MGP protein of ~10 kD in liver extracts from both WT and KO mice, and no significant difference in the relative quantity of this protein was noted when normalized for β-actin. In contrast, similar analysis of the cMGP revealed ~30% reduction in the Abcc6−/− mice, and calculation of the ratio of c/tMGP revealed similar reduction (Fig. 2c).
Reduced γ-glutamyl carboxylated form of MGP in association of mineral deposits in Abcc6−/− mice
As indicated above, MGP serves as a protein able to prevent ectopic soft connective tissue mineralization, but only the γ-glutamyl carboxylated form of MGP (cMGP) is biologically active, while the under-carboxylated form (ucMGP) is inactive. We have previously demonstrated the presence of MGP in association of the mineralization taking place in connective tissue capsule of the vibrissae in Abcc6−/− mice, however, the previous studies utilized an antibody which recognizes the total MGP [6]. To examine the specific role of the cMGP, immunostaining experiments were performed by using three different antibodies which recognize tMGP, ucMGP and cMGP, respectively [15]. The specificity of these antibodies has been tested by Western blot analysis with synthetic peptides [16], and we showed cross-reactivity with the mouse protein. Staining of mineralized vibrissae in Abcc6−/−mice with an antibody recognizing tMGP revealed close association of tMGP with the mineral deposits, as visualized by Alizarin Red stain (Fig. 3a,d). Similarly, staining of the vibrissae with an antibody that specifically recognizes only the ucMGP form of the protein revealed co-localization with the mineral deposits (Fig. 3b,e). In contrast, staining with an antibody recognizing the γ-glutamyl carboxylated form, cMGP, was essentially negative in the areas of mineralization in the KO mice (Fig. 3c,f). Staining of vibrissae from the wild-type littermates of the Abcc6−/− mice, who do not demonstrate mineralization, was negative with all three antibodies (not shown). These observations suggest that MGP is clearly present in the area of mineralization of the connective tissue capsule of vibrissae in Abcc6−/− mice, but essentially all of the protein is in inactive, under-carboxylated form.
Figure 3.
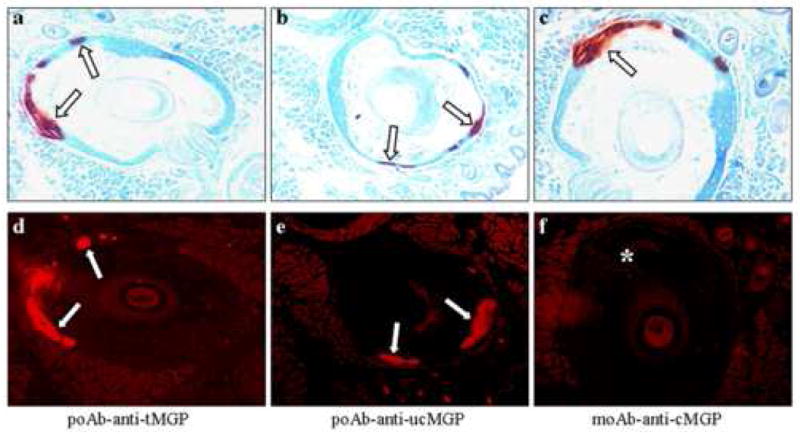
Localization of total (tMGP), under-carboxylated (ucMGP) and carboxylated (cMGP) forms of the protein detected by using specific antibodies in the vibrissae of Abcc6−/−mice. Muzzle skin containing the vibrissae was prepared for histopathology and stained for mineral deposits with Alizarin Red. Distinct foci of mineralization were noted in the connective tissue capsule surrounding the vibrissae (open arrows, panels a–c). Adjacent sections were stained with specific antibodies and the antibody localization was visualized by Texas Red-conjugated secondary antibodies. Co-localization of tMGP and ucMGP with the mineral deposits was noted (white arrows; panels d and e) while staining for cMGP was entirely negative (*) (panel f).
Discussion
Matrix gla protein (MGP) has been shown to be critical for prevention of ectopic mineralization under normal physiologic conditions with normal calcium and phosphate homeostasis [10]. This conclusion is based, in part, on the observation that targeted ablation of the corresponding gene in mice results in extensive mineralization of soft connective tissues, primarily in the vasculature [7]. Matrix gla protein, ~10 kD, contains five glutamine residues that are γ-carboxylated by an enzymatic reaction that is vitamin K dependent [11]. While MGP has been detected in a number of tissues and cell types, liver has been shown to express MGP only at the mRNA level [10]. This was confirmed in our study which demonstrated the presence of MGP mRNA both in WT and KO mice, but there was no difference in the level of expression (Fig. 2a). However, critical for the function of MGP is the γ-carboxylation of glutamyl residues, and this activated form of MGP is then detected in circulation as well as in a number of peripheral tissues undergoing ectopic mineralization [see ref. 6]. Since human ABCC6, the gene harboring mutations in PXE, is expressed primarily in the liver, we examined the degree of γ-glutamyl carboxylation of MGP in the liver of mice recapitulating histopathologic and ultrastructural features of human PXE, including ectopic mineralization of soft connective tissues. The results indicated that while total MGP concentration in the liver of these mice was not altered, this protein, when isolated from the liver, was clearly under-carboxylated, as examined by specific antibodies that distinguish the carboxylated and under-carboxylated forms. These observations suggest then that MGP in these mice, and by inference in human PXE, is partially in inactive form and therefore lacks the full capacity to prevent ectopic mineralization. This conclusion is also supported by recent immuno cytochemical demonstrations on ultrathin sections of skin that calcified elastic fibers in PXE patients’ skin show intense co-localization of ucMGP with mineral precipitates, whereas cMGP precisely localized to the mineralization front [17].0 Furthermore, normal fibroblasts cultured from the skin of healthy individuals were positive for both forms of MGP, while PXE fibroblasts were positive for ucMGP and only barely detectable level of cMGP was found [17].
The role of γ-glutamyl carboxylation in development of PXE is further suggested by recent observations on patients with late-onset, progressive PXE-like cutaneous changes, i.e., loose and sagging skin with ultrastructural demonstration of mineralization of elastic fibers, associated with multiple coagulation factor deficiency [18]. In these patients, homozygous or compound heterozygous mutations in the GGCX gene, which encodes a vitamin K-dependent γ-glutamyl carboxylase responsible for activation of the coagulation factors, was demonstrated. Further, it has been shown that this carboxylase can also utilize MGP as substrate [19]. Observations on these patients then raise the intriguing possibility which would explain soft tissue mineralization in patients with PXE as a result of loss-of-function mutations in the ABCC6 gene. Specifically, MRP6, a baso-lateral transporter protein in the liver, may be involved in transmembrane transport and/or compartmentalization of vitamin K or other co-factors of the carboxylase, thus reducing the activity of the enzyme responsible for γ-glutamyl carboxylation of MGP. As a result, MGP remains largely in inactive, under-carboxylated form, unable to prevent slow, yet progressive, mineralization of soft connective tissues, the pathologic hallmark of PXE.
In addition to γ-glutamyl carboxylation of MGP, other factors, both genetic and environmental, can modulate the mineralization process in PXE. Such factors have been shown to include reduced levels of fetuin-A, a mineralization-associated protein also required for prevention of abnormal, ectopic mineralization [6,20]. Furthermore, other factors, such as diet rich in calcium and vitamin D during childhood or adolescence have been suggested to exacerbate the clinical severity of PXE [21]. Confirmation of these factors might explain the considerable, both intra- and interfamilial phenotypic heterogeneity encountered in PXE.
In summary, we have demonstrated, by utilizing a mouse model of PXE, that MGP, a critical protein preventing ectopic mineralization under physiologic conditions, exists predominantly in inactive, under-carboxylated form. Identification of pathways resulting in γ-glutamyl carboxylation of this critical molecule may provide novel means to activate this protein and thus counteract the ectopic mineralization of soft connective tissues in PXE.
Acknowledgments
The authors thank Carol Kelly for assistance. Jennifer LaRusso and Noriko Udagawa assisted in genotyping and maintenance of the mice. This study was supported by the NIH/NIAMS grants R01 AR28450 and R01 AR52627. Dr. Jiang is supported by a Research Career Development Award from Dermatology Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ringpfeil F, Pulkkinen L, Uitto J. Molecular genetics of pseudoxanthoma elasticum. Exp Dermatol. 2001;10:221–228. doi: 10.1034/j.1600-0625.2001.100401.x. [DOI] [PubMed] [Google Scholar]
- 2.Miksch S, Lumsden A, Guenther UP, Foernzler D, Christen-Zäch S, Daugherty C, Ramesar RK, Lebwohl M, Hohl D, Neldner KH, Lindpaintner K, Richards RI, Struk B. Molecular genetics of pseudoxanthoma elasticum: Type and frequency of mutations in ABCC6. Hum Mutat. 2005;26:235–248. doi: 10.1002/humu.20206. [DOI] [PubMed] [Google Scholar]
- 3.Pfendner EG, Vanakker O, Terry SF, Vourthis S, Mcandrew PE, McClain MR, Fratta S, Marais AS, Hariri S, Coucke PJ, Ramsay M, Viljoen D, Terry PF, De Paepe A, Uitto J, Bercovitch LG. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007 doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belinsky MG, Kruh GD. MOAT-E (ARA) is a full length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: Systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–1402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- 7.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 8.Jahnen-Dechent W, Schinke T, Trindl A, Müller-Esterl W, Sablitzky F, Kaiser S, Blessing M. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–31503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Xu J, Du B, Kirsch T. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol Cell Biol. 2005;25:312–323. doi: 10.1128/MCB.25.1.312-323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 1988;263:11033–11036. [PubMed] [Google Scholar]
- 11.Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care. 2000;3:433–438. doi: 10.1097/00075197-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990:625–636. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, Uitto J. Targeted ablation of the ABCC6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Jiang Q, LaRusso J, Klement JF, Sartorelli AC, Belinsky MG, Kruh GD, Uitto J. Targeted ablation of Abcc1 or Abcc3 in Abcc6−/− mice does not modify the ectopic mineralization process. Exp Dermatol. 2007;16:853–859. doi: 10.1111/j.1600-0625.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 15.Schurgers LJ, Spronk HMH, Soute BAM, Schiffers PM, DeMey JGR, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- 16.Schurgers LJ, Teunissen KJF, Knapen MHJ, Kwaitjtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JPM, Vermeer C. Novel conformation-specific antibodies against matrix γ-carboxyglutamic acid (gla) protein: undercarboxylated matrix gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–1633. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- 17.Gheduzzi D, Boraldi F, Annovi G, Devincenzi CP, Schurgers LJ, Vermeer C, Quaglino D, Pasquali-Ronchetti I. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest. 2007 doi: 10.1038/labinvest.3700667. [DOI] [PubMed] [Google Scholar]
- 18.Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, De Paepe A. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:507–510. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 19.Engelke JA, Hale JE, Suttie JW, Price PA. Vitamin K-dependent carboxylase: utilization of decarboxylated bone gla protein and matrix gla protein as substrates. Biochem Biophys Acta. 1991;1078:31–34. doi: 10.1016/0167-4838(91)90088-h. [DOI] [PubMed] [Google Scholar]
- 20.Hendig D, Schulz V, Arndt M, Szliska C, Kleesiek K, Gotting C. Role of serum fetuin-A, a major inhibitor of systemic calcification, in pseudoxanthoma elasticum. Clin Chem. 2006;52:227–234. doi: 10.1373/clinchem.2005.059253. [DOI] [PubMed] [Google Scholar]
- 21.Renie WA, Pyeritz RE, Combs J, Fine SL. Pseudoxanthoma elasticum: High calcium intake in early life correlates with severity. Am J Med Genet. 1984;19:235–244. doi: 10.1002/ajmg.1320190205. [DOI] [PubMed] [Google Scholar]
- 22.Schurgers LJ, Teunissen KJF, Knapen MHJ, Geusens P, van der Heijde D, Kwaijtaal M, van Diest R, Ketteler M, Vermeer C. Characteristics and performance of an immunosorbent assay for human matrix Gla-protein. Clinica Chimica Acta. 2005;351:131–138. doi: 10.1016/j.cccn.2004.08.003. [DOI] [PubMed] [Google Scholar]


