Abstract
The dorsal, action-related, visual stream has been thought to have little or no memory. This hypothesis has seemed credible because functions related to the dorsal stream have been generally unsusceptible to priming from previous experience. Tests of this claim have yielded inconsistent results, however. We argue that these inconsistencies may be due to methodological differences in the time between primes and test stimuli. In this study we sought to clarify the effect of time between primes and test stimuli by having participants complete a visually-guided manual obstacle avoidance task with varying times between trials. Consistent with a previous study using this task, we found that hand path curvature depended on the presence or absence of an obstacle in the previous trial. This hand path priming effect decayed quickly as the time between trials increased, however, with the effect being almost, though not entirely, eliminated when 1,000 ms separated successive trials. The results are consistent with the hypothesis that the dorsal stream can be primed but that this priming attenuates rapidly. We suggest that this outcome may indicate that the period over which the dorsal stream retains information may be related to the sequential statistics of action.
INTRODUCTION
A fundamental features of any information processing module is how long it retains information. For example, long-term memory maintains information that can be repeatedly accessed over a prolonged period of time, whereas short-term memory maintains information that can only be accessed for a brief time after the information is stored.
Similar differences in retention time have been proposed for the modules responsible for visual processing. It has long been known (Goodale & Milner, 1992) that separate neural streams process visual information for perceptual identification (the ventral stream) and for the control of action (the dorsal stream). Besides differing in the behavioral functions they perform and their neuroanatomic bases, these two streams also differ in the time span over which they retain information. Ventral stream processing required for picture naming (e.g., van Turennout, Bielamowicz, & Martin, 2003) shows evidence of information retention in the form of priming over a 48 week delay (Cave, 1997). By contrast, dorsal stream processing shows little evidence of long-term retention (Goodale & Westwood, 2004), which may be advantageous when the relationship between the actor and the target of action changes rapidly. Under these condition, computing movement parameters immediately before movement initiation may be more efficient than relying on stored movement parameters that may no longer be appropriate (Cant, Westwood, Valyear, & Goodale, 2005).
To check whether the dorsal stream fails to retain information, Garofeanu, Kroliczak, Goodale, and Humphrey (2004) asked whether grasping or naming an object would be primed by previous experience with either type of response involving the same object. The authors found that naming was primed by both grasping and by naming primes, consistent with priming within the ventral stream. However, grasping responses were not primed by prior naming or by prior grasping, consistent with a lack of priming within the dorsal stream (see also Experiment 3 of Cant et al., 2005).
In contrast to the foregoing studies, other studies have suggested that the dorsal stream may in fact be susceptible to priming. There are reports that the orientation of a preceding visual stimulus can prime a subsequent grasp of a remembered target, a result consistent with dorsal stream priming (Craighero et al., 2002; Vogt et al., 2003). One can argue back, however, that the control of movements to remembered targets may differ from the control of movements to visible targets, with movements made to remembered targets being more reliant on target representations in the ventral stream (Hu & Goodale, 2000; Milner, Paulignan, Dijkerman, Michel, & Jeannorod, 1999). Consistent with this hypothesis, the orientation of a visual stimulus does not prime the grasp of a visible target (Cant et al., 2005) though it can prime the grasp of a remembered target (Craighero et al., 2002; Vogt et al., 2003). Therefore, the priming of grasping movements to remembered targets may occur within the ventral stream.
Grasping has also been shown to be primed by the observation of another’s grasp (Castiello, Luscher, Mari, Edwards, & Humphreys, 2002; Edwards, Humphreys, & Castiello, 2003), but again this outcome may have stemmed from non-dorsal-stream areas such as the mirror-neuron system (Rizzolatti, Fogassi, & Gallese, 2001), which occupies the ventral premotor and inferior parietal lobes (an area between the dorsal and ventral streams; Buxbaum, 2001; Pisella, Binkofski, Lasek, Toni, & Rossetti, 2006; Rizzolatti & Matelli, 2003).
Owing to the uncertainty about whether dorsal stream function is primable, Jax and Rosenbaum (2007) conducted an experiment designed to test for priming without the contaminations associated the studies mentioned above. In Jax and Rosenbaum’s study, participants completed a visually-guided manual obstacle avoidance task. When obstacle-present and obstacle-absent trials were mixed within a block, the hand paths that participants produced were strongly influenced by the presence or absence of an obstacle on the previous trial. The authors called this effect of the previous trial type on behavior hand path priming.
The nature of the hand path priming effect was that when obstacles were likely but did not appear, participants made much more curved hand movements to targets that stood “behind” the likely position of the obstacles than when obstacles never appeared. Of critical importance for the present argument, these needlessly curved hand paths were obtained even where obstacles had never occurred or had occurred many trials ago. This made it unlikely that the hand path priming effect was merely due to memory for obstacles. Similarly, hand paths in obstacle-present trials were less curved if preceded by obstacle-absent trials than if preceded by obstacle-present trials. Jax and Rosenbaum argued that the hand path priming effect was most likely due to priming within the dorsal stream because this stream is known to play a critical role in visually-guided direct movements and visually-guided obstacle avoidance movements (Culham & Kanwisher, 2001; Goodale & Westwood, 2004; McIntosh, McClements, Dijkerman, Birchall, & Milner, 2004; Schindler et al., 2004).
Why did Jax and Rosenbaum (2007) obtain evidence for dorsal stream priming while other studies (Cant et al., 2005, Garofeanu et al., 2004) failed to do so? One possibility is that the delay between primes and test stimuli varied across these studies. Such delays are known to influence priming (Roediger & McDermott, 1993). In the study of Jax and Rosenbaum, the delay between the primes (completion of a movement) and the test stimuli (presentation of the next target) was relatively short (250 ms). By contrast, the delays were longer in the studies that failed to support dorsal stream priming, ranging from 1,250 ms (Cant et al., 2005) to several minutes (Garofeanu et al., 2004). Thus, it is possible that Jax and Rosenbaum obtained the findings they did because their prime-target delays were shorter than in the other studies.
If this interpretation is correct, hand path priming should be reduced or even eliminated when longer times between primes and test stimuli are used. We tested this hypothesis in the present experiment by lengthening the time between successive movements from 250 ms, the value used before, to 600 ms in one condition and 1000 ms in another condition. We predicted that the hand path priming effect would be reduced as the time between movements increased. Such an outcome would be consistent with the view that the dorsal stream is subject to memory decay just like other established neural storage systems.
METHOD
Participants
Sixty-eight undergraduate students at Pennsylvania State University (32 males, 36 females) served as participants. All were right handed, received course credit for their participation, and gave informed consent to complete the study.
Materials, Design, and Procedure
The method was identical to that used by Jax and Rosenbaum (2007) except that two additional response-stimulus intervals (RSIs) were tested: 600 ms and 1000 ms. RSI was defined as the time between completion of one movement and presentation of the next target.
The task was completed using a feedback system consisting of a motion tracking system, computer, and TV monitor. Targets and obstacles were presented on a TV monitor chosen for its large size (80 cm screen diagonal) and adequate refresh rate (60 Hz). To move between targets and to avoid the obstacle, participants made arm movements along a horizontal table top. An OPTOTRAK 3020 motion tracking system was used to track the position of the arm using infrared light–emitting diodes attached to the participant’s left and right shoulders, right elbow, right wrist, and the top of a hand-held slider. The positions of the diodes were used to display the arm movements in real time as a stick-figure arm on the TV monitor. If the stick-figure arm on the monitor contacted the obstacle, the stick-figure turned red to indicate that a collision occurred. This feedback system made it possible to control the timing and appearance of the targets and obstacles more precisely, inconspicuously, and safely than would have been possible with physical objects.
Participants completed a center-out reaching task in which they moved back and forth between a central starting circle (2.4 cm diameter) and twelve randomly presented target circles (2.4 cm diameter). Target circles were arranged in an equally-spaced circular array (16 cm radius) around the starting circle. On some trials, an obstacle (2.4 cm diameter) appeared midway between the start and target circles and remained on for both the outward and return portions of the movement. More details about the method can be found in Jax and Rosenbaum (2007).
Four groups of 17 participants completed the study. Each group completed 10 blocks of 48 trials. The first two groups completed all blocks with obstacle-present and obstacle-absent trials randomly intermixed (with equal frequency). The RSI was 600 ms for one group and was 1000 ms for the other. The remaining two groups completed five blocks of only obstacle-absent trials and then five blocks of only obstacle-present trials. These two groups were also distinguished by RSI (600 ms and 1000 ms). For each RSI, there were four possible trial types: (1) Trials in which an obstacle appeared when obstacles always appeared in the block (herewith A trials); (2) Trials in which an obstacle appeared when obstacles sometimes appeared in the block (herewith + trials); (3) Trials in which an obstacle did not appear though obstacles sometimes appeared in the block (herewith − trials); and (4) Trials in which an obstacle did not appear and obstacles never appeared in the block (herewith N trials). The + and − trials were the trials of primary interest, whereas the A and N trials were the comparison, or control, trials.
RESULTS
Trials in which the motion-tracking system failed to track the arm due to an obscured view of the markers were removed from analysis (approximately 2.6% of trials), as were trials in which the stick-figure arm collided with an obstacle (approximately 0.9% of obstacle-present trials).
To quantify hand path curvature, we calculated two measures for each movement path. The first was the initial angular offset, defined as the absolute value of the angular deviation between the position of the hand 150 ms after movement initiation and the straight line connecting the starting and end points of the movement. We used a time of 150 ms for the initial angular offset measure because movement properties up to this time were likely to be preprogrammed whereas movement properties after this time were more likely to have been altered based on feedback (Elliot, Helson, & Chua, 2001). The second measure was the curvature index, defined as the maximum perpendicular distance of the hand from the straight line connecting the starting and ending points of the movement, divided by that straight-line distance, multiplied by 100.
Data from two 250 ms RSI conditions (one with obstacle-present and obstacle-absent trials mixed, and one with the trial types blocked) were included in the analyses to facilitate evaluation of RSI effects. Those data were previously reported in Experiment 1 of Jax and Rosenbaum (2007). The 4 groups of participants tested in the 600 ms and 1000 ms RSI conditions were tested in the same lab and in the same semester as the 2 groups of participants tested in the 250 ms RSI conditions. In fact, in every way, the 600 ms and 1000 ms groups were tested in the same way as the 250 ms groups except for the length of the RSI.
For all dependent measures, we used two sets of inferential statistics. The first was a set of mixed-factor 3 (RSI: 250 ms, 600 ms, or 1000 ms) × 2 (trial type: + or −) × 2 (previous trial type: + or −) ANOVAs designed to compare data within the mixed-trial blocks. In these ANOVAs, all factors were manipulated within participants except for RSI. The second set of inferential statistics compared data in the mixed-trial blocks with their respective blocked-trial control conditions (e.g., comparing the + conditions to the A conditions or comparing the − conditions to the N conditions) using independent-samples t-tests with Bonferroni correction for multiple comparisons (overall α of .05).
Outward Movements
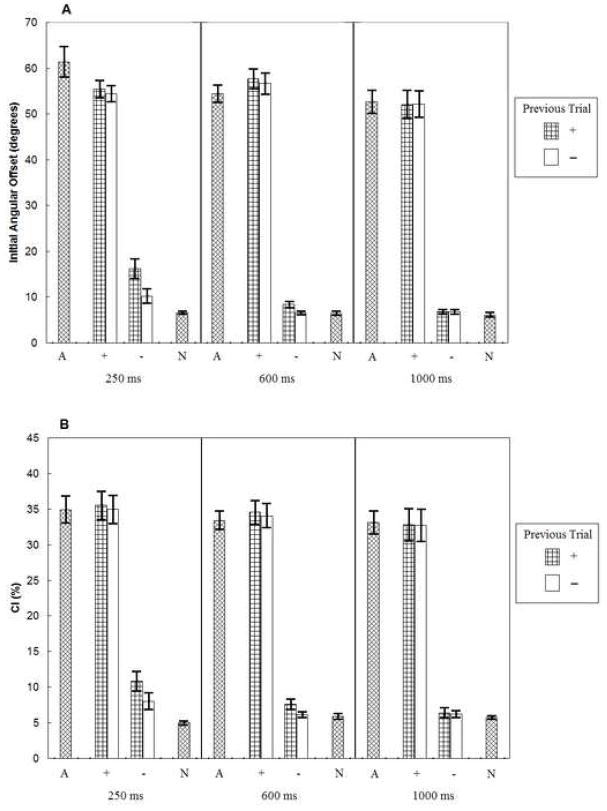
The effects of RSI and previous trial type on initial angular offset and curvature index are shown in Figure 1a and 1b, respectively. As seen in Figure 1a, initial angular offsets were greater in + trials than in − trials (main effect of trial type, p < .001). Movements also exhibited priming from the previous trial, as indicated by the greater initial angular offsets when the previous trial was a + trial than a − trial (main effect of previous trial type, p < .001). Initial angular offset also decreased as RSI increased (main effect of RSI, p < .001). The most important finding was the significant interaction between RSI and previous trial type (p < .01), such that the effect of previous trial type decreased as RSI increased. No other effects were statistically significant (p > .46).
Figure 1.
Mean (± 1SE) initial angular offsets (panel a) and curvature indices (panel b) for outward movements when an obstacle always appeared (A), when an obstacle could sometimes appear and did (+), when an obstacle could sometimes appear and did not (−), and when an obstacle never appeared (N). The values of 250 ms, 600 ms, and 1000 ms refer to the RSIs.
Comparing mixed- and blocked-trial conditions, all obstacle-present conditions in mixed-trial blocks (+ conditions) had lower initial angular offset values than the corresponding blocked-trial conditions (A conditions, p < .05) except for the + trials preceded by + trials in the 600 ms RSI condition (p > .92). Initial angular offsets in all obstacle-absent trials in the mixed-trial conditions (− conditions) were higher than those in their corresponding blocked-trial conditions (N conditions, p < .05) except for − trials preceded by − trials in the 1000 ms RSI mixed-trial condition (p > .64).
A similar pattern of results was obtained for the curvature index measure (Figure 1b). All main effects and interactions were reliable, p < .03. The one difference between the initial angular offset and curvature index measures was that there was an effect of previous trial type for − trials (p < .005) but not + trials (p > .45). Thus, there was a significant three-way interaction (p < .008), such that the effect of previous trial type decreased as RSI increased, but this was only true for the − trials.
Comparing mixed- and blocked-trial conditions, the curvature index values on obstacle-present trials did not differ between mixed-trial and blocked-trial conditions (p > .20). However, the curvature index values in the obstacle-absent trials were higher in all of the mixed-trial conditions than in the blocked-trial conditions (p < .05).
Inward Movements
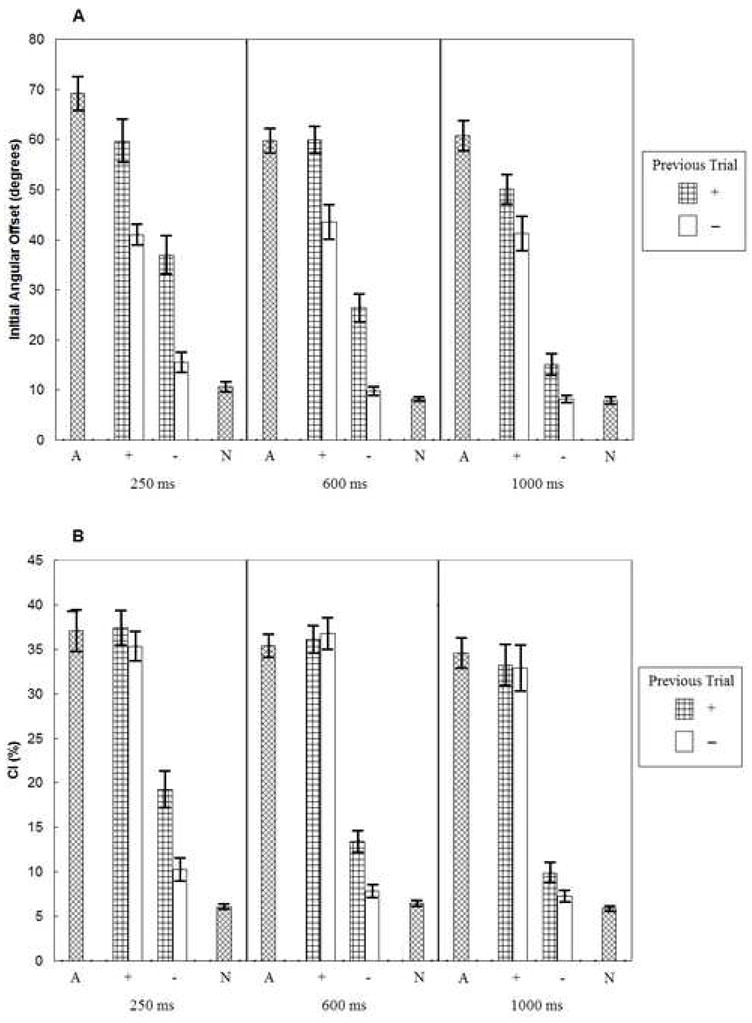
Priming from previous trials was also attenuated in the inward, return, movements as RSI increased. These effects are shown in Figure 2a and 2b for the initial angular offset and curvature index measures, respectively. For the initial angular offset measure, all main effects and interactions were reliable, p < .05. In the three way interaction, the effect of previous trial type decreased as the RSI increased, but only for the − trials (p < .017; p > .79 for the + trials).
Figure 2.
Mean (± 1SE) initial angular offsets (panel a) and curvature indices (panel b) for inward movements when an obstacle always appeared (A), when an obstacle could sometimes appear and did (+), when an obstacle could sometimes appear and did not (−), and when an obstacle never appeared (N). The values of 250 ms, 600 ms, and 1000 ms refer to the RSIs.
Comparing mixed- and blocked-trial conditions showed that all + conditions had initial angular offset values that were not different from those in the A condition (p > .35) except the + trials of the 250 ms RSI condition (p < .05). Initial angular offsets on all − trials were higher than those in the N condition (p < .05) except for − trials preceded by − trials in the 600 and 1000 ms RSI conditions (p > .45 and p > .21, respectively) and − trials preceded by + trials in the 1000 ms RSI condition (p > .26).
Analysis of the curvature index measure yielded similar results. All main effects and interactions were reliable, p < .05, except the interaction between RSI and previous trial type, p > .75. The three way interaction (p < .013) again showed that the effect of previous trial type decreased with the increase in RSI, but only for the − trials (p < .029; p > .94 for the + trials).
Comparing the mixed- and blocked-trial conditions showed that all + conditions had CI values that were not reliably different from those in the A condition (p > .35). CI values on all − trials were higher than those in the N condition (p < .05) except for − trials preceded by − trials in the 600 and 1000 ms RSI conditions (p > .54 and p > .25, respectively) and − trials preceded by + trials of the 1000 ms RSI condition (p > .19).
DISCUSSION
The goal of this study was to ask why Jax and Rosenbaum (2007) observed priming of visually-guided actions whereas others did not (Cant et al., 2005; Garofeanu et al., 2004). We hypothesized that the cause of this difference across studies was that our earlier study used a shorter time between primes and test stimuli. The results of the present study support this hypothesis. The hand path priming effect discovered by Jax and Rosenbaum was significantly reduced as the prime-test interval increased from 250 ms to 600 ms and to 1,000 ms. Hand path priming was at least partially maintained for 1,000 ms (see right side of Figure 1a and 1b). The pattern of data we obtained are consistent with the hypothesis that fairly rapid memory decay accounts for the failure of previous studies to find priming of visually guided actions when those studies had times between primes and test stimuli ranging from 1250 ms (Cant et al., 2005) to minutes (Garofeanu et al., 2004).
Our discovery of a time-dependent decrease of priming was observed within individual trials of the present study. The clearest evidence for priming came in the early part of the outward movement, the part of the trial that most immediately followed the previous trial. As measured by the initial angular offset measure, priming effects of previous trial type were observed in all obstacle-present and obstacle-absent trials. However, priming was often reduced in the later portions of the outward movement (as quantified using the curvature index measure), especially in obstacle-present trials, and was further reduced in the subsequent return trips. These within-trial effects provide additional support for the claim that hand path priming declines with the passage of time.
A critical remaining issue is why priming within the dorsal stream is so evanescent. A motor system that only maintains information for a very short time would seem to lose information that could promote planning efficiency. As we and our colleagues have argued elsewhere, programming of upcoming movements could be more efficient by changing just those features that distinguish upcoming movements from recent movements rather than starting “from scratch” each time a movement is required (Rosenbaum, Cohen, Jax, Van Der Wel, & Weiss, 2007; Rosenbaum, Meulenbroek, Vaughan, & Jansen, 2001; Van Der Wel, Fleckenstein, Jax, & Rosenbaum, 2007).
The results of the present study suggest that this potential gain in planning efficiency may come at a behavioral cost. For example, obstacle-absent movements were unnecessarily curved when preceded by obstacle-present trials, leading to a greater expenditure of time and energy during movement execution than if a more direct movement were made. Of potentially greater behavioral significance is the opposite form of priming, in which obstacle-present trials were initially less curved when preceded by obstacle-absent trials, leading to a greater chance of colliding with an obstacle. Although obstacle collisions in the present study were rare and had negligible consequences, the increased risk of obstacle collisions may be more consequential in other contexts (e.g., industrial work environments).
A difficult, and unanswered, question is how the motor system balances the minimization of these movement planning and movement production costs. Although computational frameworks exist to address this question (for recent review, see Shadmehr & Krakauer, 2008), evidence for movement priming suggests that current instantiations of many of these optimal feedback control theories would need to be extended to consider optimization of costs over a timescale that is longer than a single trial (Shadmehr & Krakauer, 2008). The present results indicate that this timescale is relatively short given that the priming effects from previous trials were almost completely eliminated after 1000 ms between movements.
We hypothesize that this limited retention of dorsal stream information may relate to the statistics of movement. One could imagine that the shorter the delay between successive movements, the more features those movement have in common. Although this is speculative for perceptual-motor control in general, there is evidence in the data of Jax and Rosenbaum (2007) as well as the present data that hand path priming depends not just on the similarity of the last movement performed and the time since it was performed, but also on the similarity of all movements performed prior to the current movement, weighted by their recency. By this line of thinking, the critical delay for the maintenance of information about recent performance reflects the likelihood that the maintenance of such information will be useful. We propose that the dorsal stream, being a neural system involved in the visual guidance of action, is sensitive to such statistics. If this analysis is correct, it helps join two of the most influential lines of research on perception and action in the past 15 years -- research on separate visual streams for perception and action (Milner & Goodale, 1992), and research on the sensitivity of action planning to the statistics of recent experience (Körding, & Wolpert, 2004; Trommershäuser, Landy, & Maloney, 2006; Trommershäuser, Maloney, & Landy, 2003; Wolpert & Flanagan, 2001). Memory systems are tuned to the statistics of the events they are meant to record (Anderson & Milsom, 1989), and it makes sense that the dorsal stream, which is related to action, should store information over a period for which those events are likely to recur.
Acknowledgments
The work was supported by grant F31 NS 047784-01 from the National Institutes of Health to SAJ, and by grant SBR-94-96290 from the National Science Foundation, grants KO2- MH0097701A1 and R15 NS41887-01 from the National Institute of Mental Health, and grants from the Research and Graduate Studies Office of The College of Liberal Arts, Pennsylvania State University, to DAR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JR, Milsom R. Human memory: An adaptive perspective. Psychological Review. 1989;96:703–719. [Google Scholar]
- Buxbaum LJ. Ideomotor Apraxia: A call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Cant JS, Westwood DA, Valyear KF, Goodale MA. No evidence for visuomotor priming in a visually guided action task. Neuropsychologia. 2005;43:216–226. doi: 10.1016/j.neuropsychologia.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Castiello U, Lusher D, Mari M, Edwards MG, Humphreys GW. Observing a human or robotic hand grasping an object: Differential motor priming effects. In: Prinz W, Hommel B, editors. Attention and performance XIX. Cambridge, MA: MIT Press; 2002. pp. 314–334. [Google Scholar]
- Cave BC. Very long-lasting priming in picture naming. Psychological Science. 1997;8:322–325. [Google Scholar]
- Craighero L, Bello A, Fadiga L, Rizzolatti G. Hand action preparation influences the responses to hand pictures. Neuropsychologia. 2002;40:492–502. doi: 10.1016/s0028-3932(01)00134-8. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Edwards MG, Humphreys GW, Castiello U. Motor facilitation following action observation: A behavioural study in prehensile action. Brain and Cognition. 2003;53:495–502. doi: 10.1016/s0278-2626(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Elliot D, Helsen WF, Chua R. A century later: Woodworth’s (1899) two component model of goal-directed aiming. Psychological Bulletin. 2001;127:342–357. doi: 10.1037/0033-2909.127.3.342. [DOI] [PubMed] [Google Scholar]
- Garofeanu C, Kroliczak G, Goodale MA, Humphrey GK. Naming and grasping common objects: A priming study. Experimental Brain Research. 2004;159:55–64. doi: 10.1007/s00221-004-1932-z. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neuroscience. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Current Opinion in Neurobiology. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Hu Y, Goodale MA. Grasping after a delay shifts size-scaling from absolute to relative metrics. Journal of Cognitive Neuroscience. 2000;12:856–868. doi: 10.1162/089892900562462. [DOI] [PubMed] [Google Scholar]
- Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: Evidence that the dorsal stream does not only control visually guided actions in real time. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:425–441. doi: 10.1037/0096-1523.33.2.425. [DOI] [PubMed] [Google Scholar]
- Körding KP, Wolpert DM. Bayesian integration in sensorimotor learning. Nature. 2004;437:244–247. doi: 10.1038/nature02169. [DOI] [PubMed] [Google Scholar]
- McIntosh RD, McClements KI, Dijkerman HC, Birchall D, Milner AD. Preserved obstacle avoidance during reaching in patients with left visual neglect. Neuropsychologia. 2004;42:1107–1117. doi: 10.1016/j.neuropsychologia.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Milner AD, Paulignan Y, Dijkerman HC, Michel F, Jeannerod M. A paradoxical improvement of misreaching in optic ataxia: New evidence for two separate neural systems for visual localization. Proceedings of the Royal Society of London Series B Biological Sciences. 1999;266:2225–2229. doi: 10.1098/rspb.1999.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisella L, Binkofski F, Lasek K, Toni I, Rossetti Y. No double-dissociation between optic ataxia and visual agnosia: Multiple sub-streams for multiple visuo-manual integrations. Neuropsychologia. 2006;44:2734–2748. doi: 10.1016/j.neuropsychologia.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neuropsychological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Experimental Brain Research. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Implicit memory in normal human subjects. In: Spinnler H, Boller F, editors. Handbook of neuropsychology. Vol. 8. Amsterdam: Elsevier; 1993. pp. 63–131. [Google Scholar]
- Rosenbaum DA, Meulenbroek RGJ, Vaughan J, Jansen C. Posture-based motion planning: Applications to grasping. Psychological Review. 2001;10:709–734. doi: 10.1037/0033-295x.108.4.709. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Cohen RG, Jax SA, Van Der Wel R, Weiss DJ. The problem of serial order in behavior: Lashley’s legacy. Human Movement Science. 2007;26:525–554. doi: 10.1016/j.humov.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Schindler I, Rice NJ, McIntosh RD, Rossetti Y, Vighetto A, Milner AD. Automatic avoidance of obstacles is a dorsal stream function: Evidence from optic ataxia. Nature Neuroscience. 2004;7:779–784. doi: 10.1038/nn1273. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Experimental Brain Research. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommershäuser J, Landy MS, Maloney LT. Humans rapidly estimate expected gain in movement planning. Psychological Science. 2006;11:981–988. doi: 10.1111/j.1467-9280.2006.01816.x. [DOI] [PubMed] [Google Scholar]
- Trommershäuser J, Maloney LT, Landy MS. Statistical decision theory and tradeoffs in motor response. Spatial Vision. 2003;16:255–275. doi: 10.1163/156856803322467527. [DOI] [PubMed] [Google Scholar]
- Van Der Wel PR, Fleckenstein R, Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: Evidence for abstract spatio-temporal forms in human motor control. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:1117–1126. doi: 10.1037/0096-1523.33.5.1117. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Bielamowicz L, Martin A. Modulation of neural activity during object naming: Effects of time and practice. Cerebral Cortex. 2003;13:381–391. doi: 10.1093/cercor/13.4.381. [DOI] [PubMed] [Google Scholar]
- Vogt S, Taylor P, Hopkins B. Visuomotor priming by pictures of hand postures: Perspective matters. Neuropsychologia. 2003;41:941–951. doi: 10.1016/s0028-3932(02)00319-6. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Current Biology. 2001;11:729–732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]