Abstract
The effect of breastfeeding on asthma is controversial, which may be explained by related and interacting early childhood risk factors. We assessed the joint effects of a risk-triad consisting of maternal smoking during pregnancy, breastfeeding for less than 3 months, and recurrent lower respiratory tract infections (RLRTI) on physician-diagnosed childhood asthma. The association was assessed in the Isle of Wight birth cohort study (1989–1990) using a repeated measurement approach with data collection at birth, and at ages 1, 2, 4, and 10 years. The population consists of 1,456 children recruited between January 1989 and February 1990. Prenatal smoking, breastfeeding for less than 3 months, and recurrent lower respiratory infections (RLRTI) were combined into eight risk-triads. Relative risks (RR) and 95% confidence intervals were estimated with a log-linear model. The risk-triad involving RLRTI in infancy, maternal smoking during pregnancy, and breastfeeding for less than 3 months showed a stronger association with asthma at ages 4 and 10 compared to other risk-triads (RR of 5.79 for any asthma at ages 1, 2, 4, and 10; and 3.1 for asthma at ages 4 and 10). Of the three individual risk factors, RLRTI appeared to be the major driver of the combined effects in the risk-triads. The effect of RLRTI on asthma was modified by breastfeeding. Breastfeeding for ≥3 months also attenuated the effect of prenatal smoking on asthma in children without RLRTI. A high proportion of asthma cases in childhood can be prevented by promoting breastfeeding, by preventing smoking during pregnancy, and by avoidance of recurrent lower respiratory tract infections in early childhood.
Keywords: asthma, cohort study, breastfeeding, smoking, child, lower respiratory tract infections
INTRODUCTION
The prevalence of asthma, a chronic disease of the respiratory system, has increased dramatically within the last three decades. Asthma has become a critical clinical and public health problem that is diagnosed in 7–15% of U.S. children (1–3). In order to prevent asthma, it is important to identify modifiable risk factors. This work focuses on three intertwined risk factors: smoking during pregnancy, breastfeeding for less than 3 months, and lower respiratory tract infections.
There is evidence that maternal smoking is related to diminished initiation and duration of breastfeeding (Table 1) (4–7). In addition, several studies have shown that maternal smoking during pregnancy increases the prevalence of asthma attacks in the offspring (8–17), not only in the first years but also later in childhood (18, 19). Mechanistically, prenatal smoking may alter intrauterine pulmonary development and function (20) or the newborn’s immune system (21).
TABLE 1.
Studies with a long-term follow-up asthma (minimum 10 years) and breastfeeding.
| Author and Year | Definition of asthma | Assessed at ages | Duration of breastfeeding | Breastfeeding (BF) effects | Smoking | Respiratory tract infections (RTI) |
|---|---|---|---|---|---|---|
| Saarinen et al. 19954 | Asthma defined as either: • allergic asthma diagnosed • ≥ 3 episodes of wheezing (at 3–10 years) • wheezing history + ≥3 respirat. Infect, (at 3–5 yrs) • wheezing history + rhinoconjuctival symptoms |
3 years 5 years 10 years 17 years |
≤ 1 months 1–6 months ≥ 6 months |
Protective against infections; Protective against respiratory allergy at 17 years (BF > 1 month) | Controlled for the effect of smoking; smoking for 5 yrs post-delivery is not associated with atopic disease in adolescence. | No association between susceptibility to RTI in infancy and atopic disease in adolescence. |
| Wright et al. 20017 | • Physician diagnosis of asthma + symptoms on ≥2 questionnaires • Recurrent wheeze (≥ 4 episodes during past yr) |
6 years 9 years 11 years 13 years |
0 < 4 months ≥ 4 months |
Children ≤ 3 years: lower prevalence of recurrent wheeze (BF ≥4 months); Children >3 years: higher prevalence of recurrent wheeze if the mother had asthma. | Controlled for the effect of smoking | Predominately infectious wheeze until 6 years included. The authors acknowledge a wheeze-asthma overlap. |
| Takemura et al. 20019 | Questionnaire: • Ever asthma • Attack of wheezing • Physician diagnosis |
Age 6–13 years |
0–3 months | Increased risk of asthma in children who were breastfed. | Smoking at the time of the survey, not in pre- or postnatal. | Not included |
| Sears et al. 20026 | • Questionnaires • Pulmonary function tests • Bronchial challenge tests • Allergy skin tests |
From age 9–26 yrs, every 2–5 yrs |
0 ≥ 1 months |
Increased risk of asthma (BF ≥ 1 month); | Controlled for the effect of smoking | Not included |
| Burgess et al. 20068 | Questionnaire: • Child asthma last 6 mths • Asthma medications • Days missed in school • Asthma-related hospital admission |
14 years | 0 <3 weeks 3–6 weeks 7 weeks-3 months ≥ 4 months |
Increased asthma prevalence in BF children of asthmatic women; no association with asthma at 14 years | Maternal smoking was not included in the multivariate analyses; it was associated with the decision and duration of BF. | Not included |
| Matheson et al. 200710 | Questionnaire: • Attacks of asthma or wheezy breathing last 12 months |
13 years 20 years 31 years |
First 3 months | BF reduced the risk for asthma in children with atopic mothers at 7 years, but increased at >7 years; no effect in children of non-atopic mothers. | Maternal smoking was controlled for; it was related to not exclusive breastfeeding. | Controlled for pleurisy & pneumonia. Exclusive BF was protective against pneumonia or pleurisy and bronchitis. |
There is also evidence that smoking during pregnancy leads to a higher risk of recurrent lower respiratory tract infection (RLRTI) in children (22, 23), in particular in early childhood (12, 22, 24, 25). There are contradictory reports in the literature on whether the protective effect of breastfeeding against asthma persists until late childhood (the first decade of life) (4–7, 26–28). Against that, breastfeeding is considered to protect against lower respiratory tract infections (29, 30). In turn, RLRTI confers an increased risk of airway hyperresponsiveness, bronchial obstruction and asthma later in childhood (24, 31–37). In early childhood, respiratory tract infections (viral or bacterial) resemble asthmatic manifestations (e.g., wheezy bronchitis). As the prevalence of RLRTI declines with age, this overlap diminishes and the diagnosis of asthma becomes more certain.
Previous studies that followed children at least up to age 10 have considered the individual effects of breastfeeding, smoking, and RLRTI on asthma and reported inconsistent results (Table 1) (4–7, 26–28). The studies reviewed enrolled children at different ages and had varying durations of follow-up. Three of these studies found no protective effect of breastfeeding on asthma (4, 6, 28). Wright et al. reported a protective effect of breastfeeding conditional upon the absence of maternal asthma (5); on the other hand, Matheson et al. demonstrated an effect of breastfeeding in early childhood conditional upon the presence of maternal asthma (7). In contrast, Saarinen et al. showed that breastfeeding for more than one month was associated with a decreased prevalence of asthma at 17 years of age, independent of atopic heredity (26).
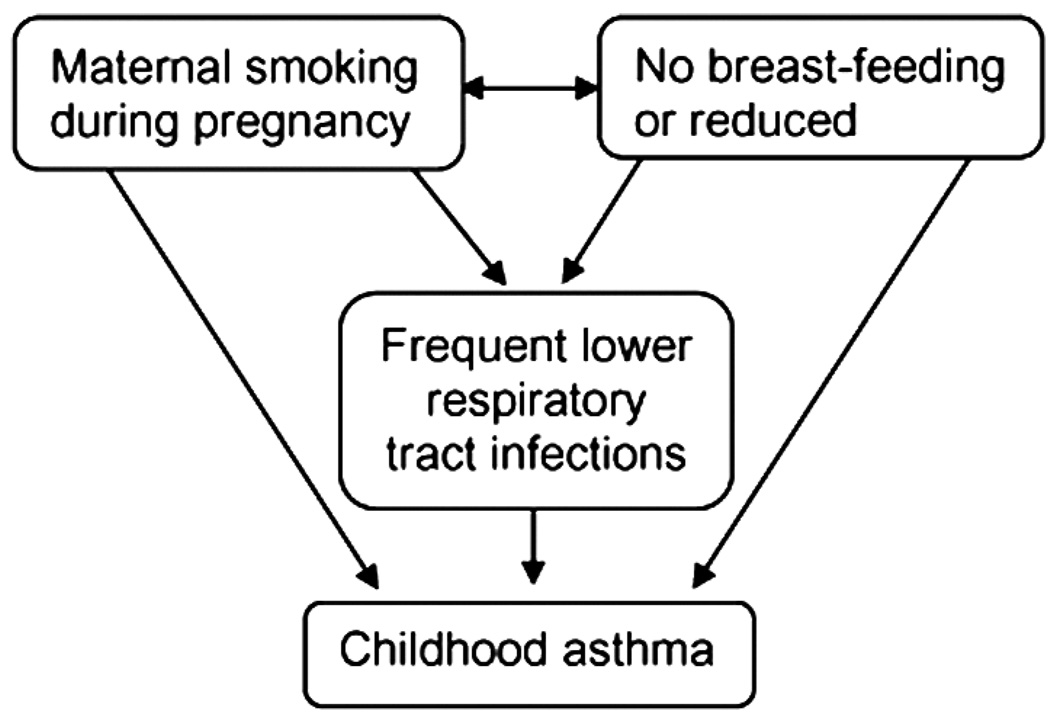
No study has yet investigated the combined effects of maternal smoking, breastfeeding for at least 3 months, and RLRTI. Since the three elements are associated (Figure 1), to some extent as intervening variables, treating them as independent risk factors (as in the reports presented here) may be inappropriate (Table 1). The purpose of this work is to characterize the joint effects of these three risk factors on childhood asthma. The three risk factors were combined into one variable, which we refer to as a risk-triad.
FIGURE 1.
The early childhood asthma risk-triad.
METHODS
Population and Data Collection
The Isle of Wight (IOW) Birth Cohort Study represents an unselected whole population birth cohort based on the Isle of Wight, U.K. The Isle of Wight is an island (13 × 23 miles) just off the South coast of England with a resident population of 130,000. The ethnic background of the island residents is mainly Caucasian. While the population is neither geographically nor genetically isolated, it is stable to the extent that the majority of participants in the cohort have not moved away and are thus available for follow-up. The intention was to prospectively study a whole population cohort for the development of asthma and allergic diseases and identify genetic and environmental risk factors relevant to these conditions. Enrollment took place at birth. Of the 1,536 children born on the isle between January 1, 1989 and February 28, 1990, a cohort of 1,456 consenting children was recruited after approval of the Local Research Ethics Committee. Data were collected after delivery, and at ages 1, 2, 4, and 10 years. Informed written parental consent was obtained from all participants. Details of the study population have been described elsewhere (38–40).
After delivery, maternal smoking, gender of the child, and birth weight were ascertained from birth and obstetric records and by questionnaire. At ages 1, 2, 4, and 10 years, questionnaires were used to obtain information on breastfeeding duration (we dichotomized at 3 months), RLRTI, and asthma. A study physician evaluated symptoms of asthma at ages 1, 2, 4 and 10 years. At age 10, the International Study of Asthma and Allergy in Childhood (ISAAC) written questionnaire was used to assess respiratory and allergy symptoms. At the ages of 4 (n = 981) and 10 years (n = 1036), skin prick tests (SPT) with a standard battery of aeroallergens were administered (41).
Variable Definition
Any active smoking of the mother during pregnancy was coded as prenatal smoking exposure. Low birth weight was defined as less than 2,500 grams. At birth, we obtained maternal history of asthma. Recurrent lower respiratory tract infection (RLRTI) was defined as two or more episodes of parental report of chest infections, based on productive cough lasting for five or more days in the preceding 12 months. Antibiotic usage and wheezing were not prerequisites for the diagnosis of RLRTI. Information on recurrent chest infection was collected at ages 1 and 2 years. Due to the lack of consensus in the literature on the categorization of duration of breastfeeding (Table 1), we dichotomized duration into ≥3 months or <3 months. The rationale for choosing this cutoff is that breastfeeding for at least three months would potentially maximize anti-inflammatory benefits of breast milk (42), in addition to providing comparability to previous publications of this cohort (23, 34, 41, 43–49).
The operational definition for asthma was a history of physician-diagnosed asthma and at least one episode of wheezing in the preceding 12 months. For early childhood, an alternative minimum criterion for asthma diagnosis was a history of three separate episodes of persistent wheezing (>3 days duration), because an asthma diagnosis is rarely made for infants/young children. The presence of asthma was investigator-determined using these criteria at ages 1, 2, 4 and 10 years.
Due to the associations among smoking, breastfeeding for at least 3 months, and RLRTI, and because breastfeeding ≥3 months and chest infection are also likely to represent intervening variables in the path to asthma, these three risk factors were combined into one variable with eight categories, called risk-triads.
STATISTICAL ANALYSES
Cross-tabulation was used to assess the prevalence of asthma in the various subgroups. Because asthma was ascertained at four time periods, repeated measurements analyses were applied to obtain odds ratios and their 95% confidence intervals, controlling for confounders (gender, low birth weight [<2,500 g], child’s age at examination, and maternal history of asthma). The fact that the outcome variable was dichotomous and that the goal was to estimate marginal probabilities controlling for the within-child effect, provided the rationale for utilizing generalized estimated equation (GEE) analysis (50). Using GEE, we estimated the association between the different risk-triads of smoking, breastfeeding for at least 3 months, and RLRTI and the outcome, asthma. Since asthma is not a rare disease, odds ratios are likely to overestimate relative risks. To directly estimate relative risks, we applied a log-binomial model.
Because the risk-triads define mutually exclusive settings, we tested each triad separately, comparing it with the reference (non-smoking, breastfeeding for at least 3 months, and no RLRTI) and controlling for all confounders. Based on previous finding of an overlap in the diagnoses of RLRTI and asthma in early childhood, we first investigated repeated asthma occurrences from ages 1 to 10 and then compared the effect of the risk factors for asthma occurrence in later childhood (4–10 years). Data analysis was performed with the SAS system (51).
RESULTS
Among the 1,456 children in the original cohort, 1,360 were followed to age 10, and 1,336 had information available on the three risk factors and asthma (Table 2). The proportions of smoking, breastfeeding for at least 3 months, and RLRTI were 25.3, 44.7, and 7.4%, respectively. The prevalence of asthma varied with age: 9.6% (128/1336) at age 1; 10.7% (128/1192) at age 2; 15.0% (167/1113) at age 4, and 12.8% (156/1224) at age 10.
TABLE 2.
Population characteristics.
| Variable | Percent | n/N | Missing |
|---|---|---|---|
| Smoking during pregnancy | 25.3 | 384/1521 | 15 |
| Breastfeeding at least 3 months | 44.7 | 600/1342 | 194 |
| Chest infections in infancy | 7.4 | 101/1374 | 162 |
| Information on the three factors described above | 1336 | 200 | |
| Boys | 51.2 | 785/1534 | 2 |
| Low birth weight (≤2500 g) | 4.1 | 61/1494 | 42 |
| Maternal history of asthma | 17.7 | 233/1318 | 218 |
Fourteen percent of the children whose mothers smoked during pregnancy developed RLRTI during infancy compared to 5.2% of the children without prenatal maternal smoking (data not shown). The proportion of breastfeeding ≥3 months was 26.1% in mothers who smoked and 51.1% in non-smokers. Among children breastfed ≥3 months, 5% had RLRTI in infancy whereas 9.2% of those not breastfed ≥3 months developed RLRTI.
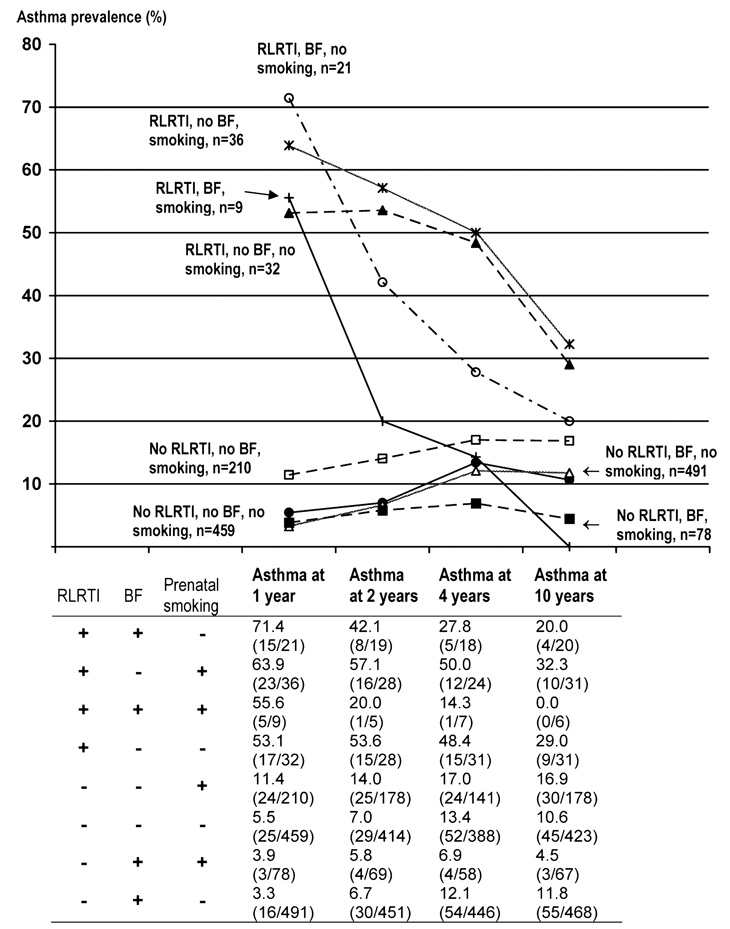
The triad-category of no smoking, breastfeeding for at least 3 months, and no RLRTI comprised 34.4% of the study population (Table 3). Of these, 11.8% had asthma at age 10 (Figure 2). On the other hand, the category of smoking, breastfeeding <3 months, and no RLRTI made up 15.7% of the study population and 16.9% of this triad-category had asthma at age 10 (Figure 2).
TABLE 3.
Risk-ratios for the triad of smoking during pregnancy, breast feeding, and lower respiratory infections and repeated occurrence of asthma from age 1 to 10.
| Repeated measurement of asthma at ages 1, 2, 4, 10 years Number of clusters = 1491 Number of observations used = 4729 |
Repeated measurement of asthma at ages 4 or 10 years Number of clusters = 1426 Number of observations used = 2271 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||||||||
| Model with individual risk factors• | |||||||||||
| RLRTI∏ | 4.16 | 3.33 | 5.19 | 2.50 | 1.81 | 3.44 | |||||
| Breastfeeding ≥3 months | 0.83 | 0.67 | 1.02 | 0.82 | 0.64 | 1.06 | |||||
| Prenatal smoking | 1.17 | 0.94 | 1.46 | 1.03 | 0.77 | 1.37 | |||||
| Model with risk-triads• | |||||||||||
| Label | RLRTI | BF# | Prenatal smoking | n | (%) | ||||||
| 1 | + | + | − | 21 | (1.6) | 4.93 | 3.46 | 7.03 | 2.32 | 1.19 | 4.55 |
| 2 | + | − | + | 36 | (2.7) | 5.79 | 4.14 | 8.11 | 3.10 | 1.84 | 5.23 |
| 3 | + | + | + | 9 | (0.7) | 4.03 | 2.15 | 7.56 | 0.79 | 0.16 | 3.95 |
| 4 | + | − | − | 32 | (2.4) | 5.51 | 3.88 | 7.82 | 3.39 | 2.15 | 5.35 |
| 5 | − | − | + | 210 | (15.7) | 1.71 | 1.24 | 2.36 | 1.41 | 0.98 | 2.02 |
| 6 | − | − | − | 459 | (34.4) | 1.05 | 0.78 | 1.41 | 0.98 | 0.72 | 1.34 |
| 7 | − | + | + | 78 | (5.8) | 0.72 | 0.36 | 1.47 | 0.47 | 0.17 | 1.31 |
| 8 | − | + | − | 491 | (36.8) | 1 | Reference | 1 | Reference | ||
Adjusted for low birth weight, maternal history of asthma, and gender.
RLRTI, recurrent lower respiratory tract infections.
BF, breastfeeding for at least 3 months.
FIGURE 2.
Prevalence of asthma at ages 1, 2, 4, 10, and the various combinations (triads) of smoking, RLRTI and breastfeeding for at least 3 months.
Overall, triad-categories in which the children had RLRTI showed a higher prevalence of asthma at all time periods (Figure 2). Of the children who were characterized by prenatal smoking exposure, breastfeeding <3 months, and RLRTI, 53.1% had asthma at age 1 and 29% had asthma at age 10. Although exposed to prenatal smoking, children breastfed for at least 3 months who did not develop RLRTI, had the lowest prevalence of asthma overall (3.9 at age 1 and 4.5% at age 10).
When analyzing the three risk factors as independent predictors, only RLRTI showed a 4.16-fold increase in the relative risk of asthma in the first decade of life and a 2.5-fold increased risk for asthma at ages 4 and 10 (Table 3).
For the model using the risk-triads, the reference was no smoking, breastfeeding for at least 3 months, and no RLRTI (label 8, Table 3). The following contrasts are based on the principle that variation in one factor while keeping the other two factors constant will provide information on the impact of the one factor.
When comparing children with regard to RLRTI (comparing label 1 to label 8; 2 to 5; 3 to 7; and 4 to 6; Table 3), children with RLRTI had a much higher relative risk of asthma at ages 1–10. Second, regarding breastfeeding ≥3 months and comparing label 4 and 1 (Table 3), breastfeeding for at least 3 months modified the impact of RLRTI in children not exposed to cigarette smoke (RR = 4.93 and RR = 5.51). In addition, contrasting label 2 and 3 indicates (RR = 5.79 and RR = 4.03) that breastfeeding ≥3 months also modifies the relative risk of asthma for recurrent infections if prenatal exposure to cigarette smoke is present.
Third, comparing labels 5 and 7, the presence of breastfeeding for more than three months reduces the adverse effects imposed by cigarette smoking (RR = 1.71 and RR = 0.72) also in the absence of RLRTI. Fourthly, by comparing labels 6 and 8, breastfeeding for at least 3 months is not a protective factor if none of the two other risks are present. Lastly, in children without RLRTI and breastfed for <3 months (label 5 and 6), maternal smoking during pregnancy showed an increased risk for asthma (RR = 1.71 and RR = 1.05).
Comparing the relative risks of asthma for ages 1, 2, 4 and 10 with the relative risk at age 4 and 10, provides insights into long-term effects. In addition, focusing on the relative risk at ages 4 and 10 also diminishes the problem of overlapping diagnoses of asthma and RLRTI, which may be present at ages 1 and 2. The relative risks in labels 1 to 4, indicate that RLRTI is more strongly associated with early onset asthma, but much less so with later onset. Also at ages 4 and 10, breastfeeding for at least 3 months reduces the relative risk of RLRTI (label 1 vs. 4: RR = 2.32 and 3.39) and the negative impact of prenatal smoking in children with RLRTI (label 2 vs. 3: RR = 3.10 and RR = 0.79) and without RLRTI (label 5 vs. 7: RR = 1.41 and RR = 0.47).
DISCUSSION
This study shows that the risk-triad of recurrent lower respiratory tract infections (RLRTI) in infancy, breastfeeding <3 months, and prenatal smoking was a strong risk factor for the occurrence of asthma in the first decade. The presence of breastfeeding ≥3 months attenuates the adverse effect of RLRTI and prenatal smoking. Our findings support the assumption that the protective effect of breastfeeding ≥3 months in children affected by RLRTI or maternal smoking depends on the balance of immune, infectious, and developmental mechanisms related to this triad of risk factors.
Epidemiologic studies have shown that breastfeeding, RLRTI, and smoking are risk factors for asthma; some may have direct effects, whereas others act as intervening variables (7, 13, 23, 24, 52). For instance, smoking during pregnancy is associated with absence of breastfeeding for at least 3 months and also increases the risk of RLRTI (Figure 1). Hence, epidemiologically, it is not appropriate to treat these variables as single independent predictors. This analytical challenge may account for the conflicting findings regarding smoking, breastfeeding, and RLRTI reported in previous studies (4–6, 26). However, there are only a few studies that consider the combined effects of these risk factors. Recently, Guedes et al. reported that maternal smoking reduces the protective effect of breastfeeding (53). We believe that the risk-triad approach in this study provides an appropriate way to take the interrelatedness of these factors into account.
Our findings are not likely a result of a selection bias. The proportion of participation was 80.2% (n = 1167), 80.6% (n = 1,174), 83.7% (n = 1,218), and 94.3% (n = 1,373) at ages 1, 2, 4, and 10, respectively. This analysis is based on 86.4% of the original cohort.
Parental information and assessments by the study physician were collected prospectively for the preceding 12 months at ages 1, 2, 4, and 10. This approach reduces information bias. Repeated assessment of asthma in this sample is a strength since the analysis is not dependent on transitional occurrence of asthma at a specific age. We have previously shown that repeated measurement analysis of asthma (age 1, 2, 4, 10) estimates the risk for persistent asthma since it takes the frequency (persistence) of the occurrence into account (46).
Confounders controlled for include low birth weight, gender, child’s age and maternal history of asthma in the repeated measurement analysis. Previous studies have found significant associations between low birth weight and asthma (54, 55). Additionally, we chose to adjust for gender, knowing that boys appear to be more predisposed to asthma (56) and considering that the relationship between breastfeeding and asthma can differ by gender (52).
A potential limitation is that exposure definitions in our sample were based on parental reports (smoking during pregnancy, RLRTI, and breastfeeding for at least 3 months). Previous studies have shown that maternal reports of smoking were reliable (8, 10, 12). To avoid uncertainties with the time-order of smoking, breastfeeding for at least 3 months, and RLRTI, we choose to use smoking during pregnancy as exposure. Our data shows that all mothers who smoked during pregnancy continued to smoke after delivery.
Another limitation is the fact that the information collected did not differentiate the etiology of RLRTI. Lower respiratory tract infections in childhood may be of viral origin (influenza, parainfluenza, respiratory syncytial virus and rhinovirus) or bacterial (22, 24, 25). Studies based on laboratory analyses have shown that wheezing associated with rhinovirus infections is more likely to progress to asthma in childhood (25, 57–60). Independent of pathogen specificity, RLRTI has been shown to be strongly associated with childhood wheezing and asthma (23, 61). Our findings are consistent with previous studies and sustain the idea that even though not differentiated, RLRTI represent a strong risk factor for asthma development (25, 57–60). On the other hand, one can argue that RLRTI in infancy is not a risk factor, but an indicator of asthma. However, to be an indicator of asthma, we would anticipate that the majority of children with RLRTI at age 1 would develop asthma. Contrary to this expectation, our data show that only 62% of the children with RLRTI at age 1 had asthma at age 1, 51% at age 2, 42% at age 4, and only 25% at age 10 (data not shown).
Interestingly, the prenatal and early childhood risk-triads studied had long-term effects detectable even at age 10. All the component risk factors are modifiable. However, prevention efforts targeted at these factors may require more than a single risk factor approach, namely to improve living conditions and behavior of pregnant women. In addition, however, we also need to determine and reduce risk factors that increase the susceptibility to RLRTI.
In summary, our results contribute to the existing evidence supporting the role of maternal smoking and recurrent chest infections in asthma development and the mediator role of breastfeeding for at least 3 months. At the population level, the impact of the risk-triad depends on the prevalence of exposure in the study population. The population-attributable risk percent is the proportion of decrease in asthma cases that would be achieved if the effects of the respective triads were completely reversible and there was complete exposure cessation in the general population. We estimated this quantity using the Levin’s formula (62). For instance, the prevalence of asthma at age 1 to 10 in the population could be reduced by 31% if, in combination with extending breastfeeding duration beyond 3 months, maternal smoking and recurrence of lower respiratory infection is prevented (label 2, 4, and 5 in Table 3). Focusing on age 4 and 10, breastfeeding promotion in combination with prevention of smoking and recurrence of respiratory infections, would reduce the prevalence of asthma by 17%. From a public health standpoint, due to the associations between prenatal smoking and RLRTI and between breastfeeding and RLRTI, RLRTI prevention could be achieved through smoking cessation and breastfeeding promotion.
CONCLUSION
We suggest that future etiologic studies not focus on individual early childhood risk factors, but apply combinations of known risk factors such as the risk-triad approach used in this analysis. We believe that our results provide strong evidence for asthma reduction by promoting breastfeeding, by preventing smoking during pregnancy, and thus reducing the risk of lower respiratory tract infections, an important risk factor for asthma in early and late childhood.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health grant R01 AI061471.
Abbreviations
- BF ≥ 3 m
breastfeeding for at least 3 months
- BF < 3 m
breastfeeding for less than 3 months
- RLRTI
recurrent lower respiratory tract infections
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- 1.National Center for Environmental Health. Asthma’s impact on children and adolescents. Vol. 2003. Centers of Disease and Control; 2003. [Google Scholar]
- 2.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Homa DM, Akinbami LJ, et al. Surveillance for asthma–United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 4.Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–907. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 5.Wright AL, Holberg CJ, Taussig LM, et al. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax. 2001;56:192–197. doi: 10.1136/thorax.56.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess SW, Dakin CJ, O’Callaghan MJ. Breastfeeding does not increase the risk of asthma at 14 years. Pediatrics. 2006;117:e787–e792. doi: 10.1542/peds.2005-1753. [DOI] [PubMed] [Google Scholar]
- 7.Matheson MC, Erbas B, Balasuriya A, et al. Breast-feeding and atopic disease: A cohort study from childhood to middle age. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Alati R, Al Mamun A, O’Callaghan M, et al. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology. 2006;17:138–144. doi: 10.1097/01.ede.0000198148.02347.33. [DOI] [PubMed] [Google Scholar]
- 9.Bosdure E, Dubus JC. The effects of tobacco on children. Rev Mal Respir. 2006;23:694–704. doi: 10.1016/s0761-8425(06)72083-6. [DOI] [PubMed] [Google Scholar]
- 10.Carter S, Percival T, Paterson J, et al. Maternal smoking: risks related to maternal asthma and reduced birth weight in a Pacific Island birth cohort in New Zealand. N Z Med J. 2006;119:U2081. [PubMed] [Google Scholar]
- 11.Jaakkola JJ, Kosheleva AA, Katsnelson BA, et al. Prenatal and postnatal tobacco smoke exposure and respiratory health in Russian children. Respir Res. 2006;7:48. doi: 10.1186/1465-9921-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lannero E, Wickman M, Pershagen G, et al. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE) Respir Res. 2006;7:3. doi: 10.1186/1465-9921-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnusson LL, Olesen AB, Wennborg H, et al. Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin Exp Allergy. 2005;35:1550–1556. doi: 10.1111/j.1365-2222.2005.02374.x. [DOI] [PubMed] [Google Scholar]
- 14.Moshammer H, Hoek G, Luttmann-Gibson H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 15.Pattenden S, Antova T, Neuberger M, et al. Parental smoking and children’s respiratory health: independent effects of prenatal and postnatal exposure. Tob Control. 2006;15:294–301. doi: 10.1136/tc.2005.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raherison C, Penard-Morand C, Moreau D, et al. In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir Med. 2007;101:107–117. doi: 10.1016/j.rmed.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 17.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 18.Vonk JM, Boezen HM. Predicting adult asthma in childhood. Curr Opin Pulm Med. 2006;12:42–47. doi: 10.1097/01.mcp.0000188371.30508.54. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FD. Links between pediatric and adult asthma. J Allergy Clin Immunol. 2001;107:S449–S455. doi: 10.1067/mai.2001.114993. [DOI] [PubMed] [Google Scholar]
- 20.Rehan VK, Wang Y, Sugano S, et al. Mechanism of nicotine-induced pulmonary fibroblast transdifferentiation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L667–L676. doi: 10.1152/ajplung.00358.2004. [DOI] [PubMed] [Google Scholar]
- 21.Le Souef PN. Adverse effects of maternal smoking during pregnancy on innate immunity in infants. Eur Respir J. 2006;28:675–677. doi: 10.1183/09031936.06.00101206. [DOI] [PubMed] [Google Scholar]
- 22.Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 23.Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127:502–508. doi: 10.1378/chest.127.2.502. [DOI] [PubMed] [Google Scholar]
- 24.Nafstad P, Magnus P, Jaakkola JJ. Early respiratory infections and childhood asthma. Pediatrics. 2000;106:E38. doi: 10.1542/peds.106.3.e38. [DOI] [PubMed] [Google Scholar]
- 25.Copenhaver CC, Gern JE, Li Z, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 26.Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet. 1995;346:1065–1069. doi: 10.1016/s0140-6736(95)91742-x. [DOI] [PubMed] [Google Scholar]
- 27.Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139:261–266. doi: 10.1067/mpd.2001.117006. [DOI] [PubMed] [Google Scholar]
- 28.Takemura Y, Sakurai Y, Honjo S, et al. Relation between breastfeeding and the prevalence of asthma: the Tokorozawa Childhood Asthma and Pollinosis Study. Am J Epidemiol. 2001;154:115–119. doi: 10.1093/aje/154.2.115. [DOI] [PubMed] [Google Scholar]
- 29.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Alarcon M, Villalpando S, Fajardo A. Breast-feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr. 1997;127:436–443. doi: 10.1093/jn/127.3.436. [DOI] [PubMed] [Google Scholar]
- 31.Stein RT, Holberg CJ, Sherrill D, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol. 1999;149:1030–1037. doi: 10.1093/oxfordjournals.aje.a009748. [DOI] [PubMed] [Google Scholar]
- 32.Floreani AA, Rennard SI. The role of cigarette smoke in the pathogenesis of asthma and as a trigger for acute symptoms. Curr Opin Pulm Med. 1999;5:38–46. doi: 10.1097/00063198-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94:136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurukulaaratchy RJ, Fenn M, Twiselton R, et al. The prevalence of asthma and wheezing illnesses amongst 10-year-old schoolchildren. Respir Med. 2002;96:163–169. doi: 10.1053/rmed.2001.1236. [DOI] [PubMed] [Google Scholar]
- 35.Ehrlich RI, Du Toit D, Jordaan E, et al. Risk factors for childhood asthma and wheezing. Importance of maternal and household smoking. Am J Respir Crit Care Med. 1996;154:681–688. doi: 10.1164/ajrccm.154.3.8810605. [DOI] [PubMed] [Google Scholar]
- 36.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 37.Chan-Yeung M, Dimich-Ward H. Respiratory health effects of exposure to environmental tobacco smoke. Respirology. 2003;8:131–139. doi: 10.1046/j.1440-1843.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 38.Hide DW, Arshad SH, Twiselton R, et al. Cord serum IgE: an insensitive method for prediction of atopy. Clin Exp Allergy. 1991;21:739–743. doi: 10.1111/j.1365-2222.1991.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 39.Tariq SM, Matthews SM, Hakim EA, et al. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101:587–593. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- 40.Arshad SH, Stevens M, Hide DW. The effect of genetic and environmental factors on the prevalence of allergic disorders at the age of two years. Clin Exp Allergy. 1993;23:504–511. doi: 10.1111/j.1365-2222.1993.tb03238.x. [DOI] [PubMed] [Google Scholar]
- 41.Arshad SH, Tariq SM, Matthews S, et al. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 42.Duncan B, Ey J, Holberg CJ, et al. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics. 1993;91:867–872. [PubMed] [Google Scholar]
- 43.Arshad SH, Bateman B, Sadeghnejad A, et al. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119:307–313. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 44.Arshad SH, Karmaus W, Matthews S, et al. Association of allergy-related symptoms with sensitisation to common allergens in an adult European population. J Investig Allergol Clin Immunol. 2001;11:94–102. [PubMed] [Google Scholar]
- 45.Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Rhinitis in 10-year-old children and early life risk factors for its development. Acta Paediatr. 2002;91:1334–1338. doi: 10.1111/j.1651-2227.2002.tb02830.x. [DOI] [PubMed] [Google Scholar]
- 46.Ramadas RA, Sadeghnejad A, Karmaus W, et al. Interleukin-1R antagonist gene and pre-natal smoke exposure are associated with childhood asthma. Eur Respir J. 2007;29:502–508. doi: 10.1183/09031936.00029506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurukulaaratchy RJ, Matthews S, Arshad SH. Relationship between childhood atopy and wheeze: what mediates wheezing in atopic phenotypes? Ann Allergy Asthma Immunol. 2006;97:84–91. doi: 10.1016/S1081-1206(10)61375-0. [DOI] [PubMed] [Google Scholar]
- 48.Kurukulaaratchy RJ, Waterhouse L, Matthews SM, et al. Are influences during pregnancy associated with wheezing phenotypes during the first decade of life? Acta Paediatr. 2005;94:553–558. doi: 10.1111/j.1651-2227.2005.tb01938.x. [DOI] [PubMed] [Google Scholar]
- 49.Kurukulaaratchy RJ, Fenn M, Matthews S, et al. Characterisation of atopic and non-atopic wheeze in 10 year old children. Thorax. 2004;59:563–568. doi: 10.1136/thx.2003.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 51.SAS Institute. Statistical Analysis System, Version 9.1. Gary, NC: 2007. [Google Scholar]
- 52.Mandhane PJ, Greene JM, Sears MR. Interactions between breast-feeding, specific parental atopy, and sex on development of asthma and atopy. J Allergy Clin Immunol. 2007;119:1359–1366. doi: 10.1016/j.jaci.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 53.Guedes HT, Souza LS. Exposure to maternal smoking in the first year of life interferes in breast-feeding protective effect against the onset of respiratory allergy from birth to 5 yr. Pediatr Allergy Immunol. 2008 doi: 10.1111/j.1399-3038.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 54.Brooks AM, Byrd RS, Weitzman M, et al. Impact of low birth weight on early childhood asthma in the United States. Arch Pediatr Adolesc Med. 2001;155:401–406. doi: 10.1001/archpedi.155.3.401. [DOI] [PubMed] [Google Scholar]
- 55.Nepomnyaschy L, Reichman NE. Low birthweight and asthma among young urban children. Am J Public Health. 2006;96:1604–1610. doi: 10.2105/AJPH.2005.079400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.PausJenssen ES, Cockcroft DW. Sex differences in asthma, atopy, and airway hyperresponsiveness in a university population. Ann Allergy Asthma Immunol. 2003;91:34–37. doi: 10.1016/S1081-1206(10)62055-8. [DOI] [PubMed] [Google Scholar]
- 57.Lemanske RF, Jr., Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Brooks GD, Lemanske RF., Jr Infections and asthma. Semin Respir Crit Care Med. 2002;23:339–345. doi: 10.1055/s-2002-34329. [DOI] [PubMed] [Google Scholar]
- 59.Gern JE, Martin MS, Anklam KA, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002;13:386–393. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 60.Montalbano MM, Lemanske RF., Jr Infections and asthma in children. Curr Opin Pediatr. 2002;14:334–337. doi: 10.1097/00008480-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Kurukulaaratchy RJ, Matthews S, Holgate ST, et al. Predicting persistent disease among children who wheeze during early life. Eur Respir J. 2003;22:767–771. doi: 10.1183/09031936.03.00005903. [DOI] [PubMed] [Google Scholar]
- 62.Skzlo M, Nieto J. Epidemiology: Beyond the Basics. 2nd edition. Sudbury, MA: Jones & Bartlett Pub.; 2007. pp. 99–100. [Google Scholar]