Abstract
In recent years, the authors have been developing novel fluoride-releasing dental composites containing ternary zirconium fluoride chelates. The aim of this study was to improve the physical and mechanical properties of these composites by improving the formulation of the monomers and photoinitiators. The hypothesis was that reduction of hydrophilic monomers and improvement of the photoinitiators could reduce water sorption and significantly increase the mechanical properties of the composite. The degree of conversion of the composites containing different compositions of photoinitiators was studied by Fourier transform near-infrared spectroscopy (FT-NIR). Ten experimental composites containing different compositions of ethoxylated bisphenol-A dimethacrylate (EBPADMA), 1,6-hexanediol dimethacrylate (HDDMA), triethylene glycol dimethacrylate (TEGDMA), and 2,2-bis[4-(2-hydroxy-3-methacryloyloxypropoxy)phenyl]-propane (BisGMA) were tested for flexural strength, viscosity, and water sorption. The experimental composite containing 20% synthesized fluoride-releasing monomer, 30% BisGMA, 30% EBPADMA, and 20% HDDMA showed significantly higher fluoride release and recharge, but physical and mechanical properties similar to those of the control composite containing 40% BisGMA, 40% EBPADMA, and 20% HDDMA.
Keywords: fluoride release, fluoride recharge, dental composite, mechanical properties, water sorption
Introduction
Secondary (recurrent) caries is the most frequent cause for the failure of dental restorations (Mjör, 1997; Wilson et al., 1997; Mjör and Moorhead, 1998; Mjör et al., 2002). Fluoride-releasing dental materials may reduce secondary caries at the restoration margins (Zimmerman et al., 1984; Jensen et al., 1991; Donly and Gomez, 1994; Wiegand et al., 2007). Currently, the fluoride-releasing dental materials can be divided into 4 categories (Burgess et al., 1994; Hicks et al., 2003; Xu and Burgess, 2003; Burke et al., 2006; Wiegand et al., 2007): glass ionomers, resin-modified glass ionomers, compomers, and composites. Our previous study (Xu and Burgess, 2003) showed that current dental materials with higher fluoride release generally have lower mechanical properties. Therefore, high-fluoride-releasing materials, such as glass ionomers and resin-modified glass ionomers, are mainly used clinically to restore decayed non-biting areas in high-caries-risk patients. Composites have been widely used in restorative dentistry, because they have high strength, good wear resistance, and excellent esthetics, but they release only a small amount of fluoride and have low fluoride-recharge capability. Their anti-caries efficacy is questionable. Therefore, the dental composites that have high fluoride release and recharge capabilities, while maintaining their strength and wear resistance, are highly desirable.
In the past two decades, several methods have been used to provide the fluoride sources for resin-based fluoride-releasing dental materials: (1) soluble free salts, such as NaF and SrF2 (Arends and Ruben, 1988; Cook and Youngson, 1989); (2) fluoride-releasing glass fillers (Temin and Csuros, 1988; Sonis and Snell, 1989; Xu and Burgess, 2003); (3) organic fluoride salts, such as tetrabutylammonium tetrafluoroborate (Aasen et al., 1989; Glasspoole et al., 2001); and (4) amine-hydrofluoride-based monomers, such as t-butylaminoethyl methacrylate hydrogen fluoride (Rawls and Zimmerman, 1986; Rawls, 1987).
In recent years, we and others have been developing new fluoride-releasing dental monomers containing ternary heavy-metal-fluoride chelates (Mitra and Wang, 2002; Xu et al., 2004a,b, 2005, 2006a). The results from the experimental composites made of these novel fluoride-releasing monomers have shown significantly higher fluoride-release and recharge capabilities than commercial fluoride-releasing composites, but their physical and mechanical properties are still lower than those of most commercial dental composites.
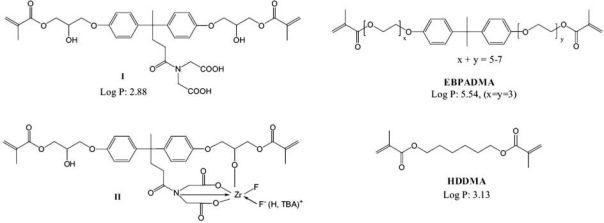
One method of improving the mechanical properties of dental composites is to increase their filler content. However, maximum filler content is limited by monomer viscosity. Most current dental composites use highly viscous BisGMA as the main monomer; therefore, a diluent monomer such as TEGDMA is needed to decrease viscosity. TEGDMA is a hydrophilic monomer (partition coefficient LogP: 2.42 [calculated by means of ChemDraw Ultra 9.0, CambridgeSoft®, Cambridge, MA, USA]; a lower LogP indicates higher hydrophilicity). It can increase water sorption and may have biological side-effects when leaching out (Geurtsen et al., 2001). The challenge is decreasing monomer viscosity while minimizing the content of TEGDMA. Ethoxylated bisphenol-A dimethacrylate (EBPADMA) (LogP: 5.54) and hexanediol dimethacrylate (HDDMA) (LogP: 3.13) are less hydrophilic and less viscous than BisGMA (LogP: 5.09) and TEGDMA, respectively (Cowperthwaite et al., unpublished observations). Their structures are shown in Fig. 1.
Figure 1.
Structures of synthesized monomers I, II, EBPADMA, and HDDMA. The values of LogP (partition coefficient) were calculated by means of ChemDraw Ultra 9.0 (CambridgeSoft).
The objective of this study was to improve the physical and mechanical properties of the fluoride-releasing dental composites by the following approaches: (1) reducing the amount of tetrabutylammonium fluoride used in the synthesis of the fluoride-releasing monomer, (2) optimizing the photoinitiators, and (3) improving the monomer formulation by replacing hydrophilic TEGDMA and part of BisGMA with less-hydrophilic monomers. The hypothesis was that reduction of the hydrophilic components in the monomers and improvement of the photoinitiators could reduce water sorption and significantly increase the mechanical properties of the composites.
Materials & Methods
Reagents and Materials
The chelating dimethacrylate monomer (I), {4,4-bis-(4-[2-hydroxy-3-(2-methacryloyloxy)-propoxy]-phenyl)-pentanoyl-amine}-N, N, -diacetic acid, and its ternary zirconium fluoride chelate (fluoride-releasing monomer II) (Fig. 1) were synthesized by a procedure reported previously (Xu et al., 2004b, 2006a). BisGMA was purchased from Polysciences (Warrington, PA, USA). EBPADMA and HDDMA were provided by Esstech Inc. (Essington, PA, USA). TEGDMA, tetrabutylammonium fluoride (TBAF), camphorquinone (CQ) ethyl 4-dimethylaminobenzoate (4E), bis(2,4,6-trimethylbenzoyl)-phenyl-phosphine oxide (PO), diphenyliodonium hexafluorophosphate (IFP), and zirconium tetrafluoride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Silanized quartz filler (mean, 0.8 µm) was provided by Coltène/Whaledent (Altstätten, Switzerland). The fluoride-releasing filler (fluoroaluminosilicate, mean, 1.3 µm) was provided by Caulk/Dentsply (York, PA, USA).
Formulation and Photopolymerization of Experimental Composites
We formulated and fabricated the experimental and control composites by blending monomers (BisGMA/TEGDMA/ EBPADMA/HDDMA/Synthesized F-releasing monomer in various ratios), photoinitiators, and the fillers, in a 50-mL vacuum blender (National Institute of Standards and Technology, Gaithersburg, MD, USA). All composite samples were light-cured for 40 sec with an Optilux 501 curing light (Kerr, Orange, CA, USA; output > 500 mW/cm ) on each surface.
For optimization of the photoinitiators, all composites contained 65 wt% of the silanized quartz filler and the monomer mixture of BisGMA: TEGDMA (60:40). Degree of conversion (DC) was measured by FT-NIR as described previously (Stansbury and Dickens, 2001; Trujillo et al., 2004; Xu et al., 2006b). Disk composite specimens (5 mm diameter, 2 mm thick, n = 5) were prepared with a rubber ring pressed between a pair of glass slides (22 x 22 x 0.17 mm). The specimen was placed on top of a Smart NIR UpDRIFT (Thermo-Nicolet Instrument Corp., Madison, WI, USA), a top-loading diffuse reflection accessory, and the NIR spectrum of the uncured resin was acquired by means of a Thermo-Nicolet Nexus 670 FT-IR spectrometer. The specimen was then light-cured in situ (without moving the specimen) through the upper glass slides for 40 sec. The NIR spectrum of the cured composite was acquired again. All spectra were recorded at a wavelength range of 4500-7000 cm, resolution of 8 cm, and scan number of 110. The DC was calculated with the area of the first overtone of the vinyl absorption peak, around 6164 cm, and the first overtone of the aromatic absorption peak, around 4621 cm, as the internal standard:
where Ap is the peak area of the cured composite (polymer) and Am is the peak area of uncured resin (monomer) at 6163 cm; Am0 and Ap0 are the peak area at 4621 cm before and after cure, respectively. For optimization of the monomer composition to minimize water sorption, all composites (Control-1 and Exp1 through Exp9) have 70 wt% silanized quartz filler (mean, 0.8 µm) and photoinitiators consisting of 0.5% CQ, 2% 4E, and 1% PO, which was determined by the above-mentioned optimization experiment and was also used in the formulation of Control-2 and Exp10. The monomer of Control-2 contained BisGMA:EBPADMA:HDDMA (40:40:20). The monomer of experimental fluoride-releasing composite Exp10 contained Monomer II BisGMA:EBPADMA:HDDMA (20:30:30:20). Both Control-2 and Exp10 contained 70 wt% silanized fluoride-releasing filler (mean, 1.3 µm).
Fluoride Release and Recharge
Cylindrical specimens (4 mm in diameter and 9 mm in length, n = 5) were prepared and light-cured for 40 sec on each surface. For fluoride release, the specimens were immediately placed in 3 mL de-ionized water. Fluoride release was measured daily for 30 days and then weekly for a total of 100 days, with a fluoride-ion-selective electrode (model 96-09, Thermo-Orion, Beverly, MA, USA) and an Orion 920A PH/ISE meter (Thermo-Orion). For fluoride recharge, the specimens were stored in 500 mL distilled water for 2 wks; the water was replenished daily. We then “recharged” the specimens by applying “60 Seconds Taste Gel” (Pascal Co., Inc., Bellevue, WA, USA), an acidulated phosphate fluoride (containing 1.23 w/w% fluoride ion), for 1 min and rinsing them with running de-ionized water for 1 min. Fluoride release from these recharged specimens was measured daily for 5 days, and the recharge cycles were repeated 3 times.
Measurement of Physical and Mechanical Properties
The complex viscosity (h*) of different monomer mixtures (n = 6) was measured at 25°C on an ARES rheometer (TA Instruments, New Castle, DE, USA), in a 25-mm parallel plate and a Peltier with a 0.5-mm gap. Rectangular specimens for flexural strength (2 x 2 x 25 mm, n = 20) and cylindrical specimens for compressive strength (4 x 9 mm, n = 20) were prepared. One group (n = 10) of each material was tested after storage in de-ionized water at 37°C for 24 hrs. Another group (n = 10) was tested after storage in de-ionized water at 37°C for 30 days. The three-point-bending flexural strength and compressive strengths were tested on an Instron 5566 testing machine (Instron Corp., Canton, MA, USA) with a cross-head speed of 1 mm/min. The water sorption and solubility tests were conducted with disk samples (15.0 mm diameter, 1.0 mm thick, n = 5) according to ISO Specification 4049.
Data Analysis
The data were analyzed by ANOVA and the post hoc Tukey test, with a significance level of 95% (a = 0.05).
Results
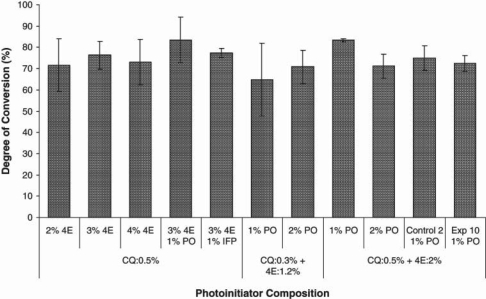
The structures of synthesized chelating monomer I, fluoride-releasing monomer II, and commercial monomers EBPADMA and HDDMA are displayed in Fig. 1. The effects of different photoinitiator compositions on the degree of conversion (DC) at the polymerization time of 40 sec were examined (Fig. 2). The photoinitiator containing 1% PO, 0.5% CQ, and 2% or 3% 4E seemed to produce the highest DC, which is significantly higher than those without PO (p < 0.05). Under this optimal photoinitiator composition, the DCs of fluoride-releasing experimental (Exp10) and control (Control-2) composites containing fluoroaluminosilicate fillers were also measured (Fig. 2). The DC value of Exp10 was similar to that of the optimal composition.
Figure 2.
Effects of photoinitiator systems on the degree of conversion measured by FT-NIR (n = 5, light-cured for 40 sec). All composites (except Exp10 and Control-2) have monomers BisGMA:TEGDMA (60:40) and filler content of 65 wt% silanized quartz (mean, 0.8 µm). Exp10 and Control-2 contain 70 wt% silanized fluoride-releasing filler (mean, 1.3 µm). The error bars are standard deviations.
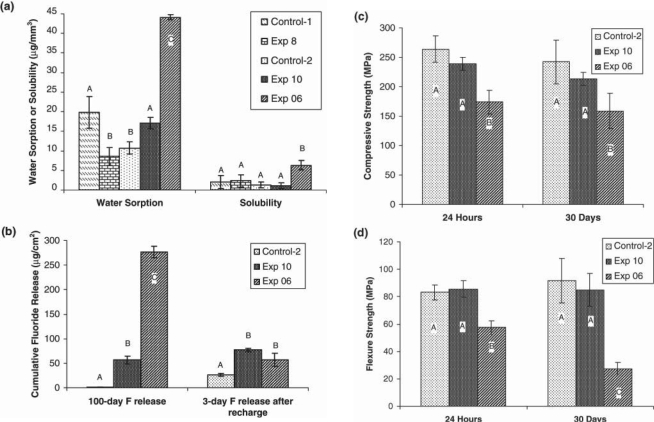
The influence of different monomer compositions on the complex monomer viscosity (h*) and the flexural strength of the corresponding composites was evaluated (Table). The water sorption, solubility, fluoride release and recharge, and compressive and flexural strengths (after 24 hrs and 30 days) of Control-1, Control-2, Exp8, and Exp10 were tested (Fig. 3). The results of the experimental composite from our previous study (Exp6) (Xu et al., 2006a) are also included for comparison (Fig. 3). Exp8 had the highest flexural strength among composites Exp1 through Exp9, and it was statistically the same as Control-1 (Table). However, Exp8 has significantly lower water sorption than Control-1 (Fig. 3a) (p < 0.05). The water sorption and solubility of Exp10 were significantly lower than those of Exp6 (p < 0.01). Exp10 had significantly higher fluoride release than Control-2 (Fig. 3b) (p < 0.01), but significantly lower than Exp6 (Fig. 3b) (p < 0.01). The fluoride recharge capability of Exp10 was also significantly higher than that of Control-2 (p < 0.05) and similar to that of Exp6 (p > 0.05) (Fig. 3b). The compressive and flexural strengths of Exp10 at 24 hrs and 30 days were statistically the same as those of Control-2 (p > 0.05) (Figs. 3c, 3d), but significantly higher than those of Exp6 (p < 0.05).
Table.
Effects of Monomer Compositions on the Viscosity and Flexural Strength of Composites
| Monomer Composition (wt%) |
Mono mer Viscosity (mean ± SD) (n = 6) |
Flexural Strength* (mean ± SD) (n = 10) |
||||
|---|---|---|---|---|---|---|
| Material | BisGMA | TEGDMA | EBPADMA | HDDMA | (Pa•s) | (MPa) |
| Control-1 | 60 | 40 | 0.55 ± 0.01 | 101.6 ± 5.9A | ||
| Exp. 1 | 60 | 10 | 30 | 0.72 ± 0.01 | 80.1 ± 6.8B | |
| Exp. 2 | 60 | 20 | 20 | 2.25 ± 0.02 | 91.3 ± 10.2A,B | |
| Exp. 3 | 50 | 30 | 20 | 0.60 ± 0.01 | 89.8 ± 10.0A,B | |
| Exp. 4 | 50 | 30 | 20 | 1.14 ± 0.02 | 80.9 ± 6.9B | |
| Exp. 5 | 50 | 20 | 30 | 0.42 ± 0.01 | 90.9 ± 10.2A,B | |
| Exp. 6 | 40 | 20 | 40 | 0.84 ± 0.02 | 89.4 ± 5.7A,B | |
| Exp. 7 | 40 | 30 | 30 | 0.33 ± 0.00 | 88.0 ± 7.7A,B | |
| Exp. 8 | 40 | 40 | 20 | 0.70 ± 0.01 | 101.9 ± 5.5A | |
| Exp. 9 | 40 | 50 | 10 | 2.01 ± 0.01 | 82.7 ± 5.7B | |
| M10 | 100 | 515.95 ± 1.68 | ||||
| M11 | 100 | 0.013 ± 0.001 | ||||
| M12 | 100 | 0.98 ± 0.02 | ||||
| M13 | 100 | 0.0046 ± 0.0004 | ||||
The same superscript letter indicates no significant difference between different groups (p > 0.05).
BisGMA: 2,2-bis[4-(2-hydroxy-3-methacroyloxypropoxy)phenyl]-propane. EBPADMA: ethoxylated bisphenol-A dimethacrylate.
HDDMA: 1,6-hexanediol dimethacrylate.
TEGDMA: triethylene glycol dimethacrylate.
Figure 3.
Properties of experimental and control composites (the data for Exp6 are from Xu et al., 2006a): (a) water sorption and solubility (n = 5); (b) cumulative fluoride release in 100 days and in 3 days after recharge (n = 5) in de-ionized water; (c) compressive strength after 24 hrs (n = 10) and 30 days (n = 10); (d) flexural strength after 24 hrs (n = 10) and 30 days (n = 10). The error bars are standard deviations. The letters represent grouping. The same letter indicates no significant difference between groups in the same property (p > 0.05).
Discussion
We synthesized the fluoride-releasing monomer (II) by first reacting zirconium fluoride with tetrabutylammonium fluoride (TBAF) to form the soluble fluorozirconate complex salts, which then reacted with the chelating monomer (I). In our previous work (Xu et al., 2006a), the 1:4 ratio of ZrF4 to TBAF was used. The extra amount of TBAF allowed for easy dissolution of ZrF4, but the extra free salts could not be removed, thus leading to higher water sorption, higher solubility, and poor mechanical properties. In this study, the amount of TBAF was reduced from ZrF4/TBAF 1:4 to 1:2. To facilitate the dissolution of ZrF4, we used a large amount (100 times that of ZrF4) of solvent (methanol) during synthesis. We investigated the effects of different combinations of photoinitiators, particularly the effect of bis(2,4,6-trimethylbenzoyl)-phenyl-phosphine oxide (PO), which can form free radicals without tertiary amine as an accelerator. This is important for the acidic monomers, such as the chelating monomer and fluoride-releasing monomer in this study, and self-etching dental bonding agents. In general, the addition of 1% of PO to a two-component (CQ + 4EDMAB) photoinitiator system can enhance the degree of conversion (DC). However, when CQ and amine concentrations are already high (e.g., 0.5% CQ and 2% or higher 4E), further addition of PO (e.g., 2%) will reduce DC. One percent of diphenyliodonium hexafluorophosphate (DPIHFP or IFP) was also added to 0.5% CQ + 3% 4E, because it might increase DC by converting the reversible free-radical-forming reaction between CQ and 4E to an irreversible reaction. However, IFP has shown little effect on DC, probably because the CQ and 4E contents are already high.
In composites Expl-Exp9, various portions of BisGMA and TEGDMA were replaced by EBPADMA and HDDMA, which have higher LogP values and are more hydrophobic than BisGMA and TEGDMA, respectively. Therefore, Exp8 composite has significantly lower water sorption than Control-1 (p < 0.05) and solubility similar to that of Control-l. The monomer composition of Exp8 was chosen to formulate fluoride-releasing composites because it generates the highest flexural strength (the same as Control-1) and contains no TEGDMA.
Based on these results, 2 fluoride-releasing composites were formulated: Control-2, containing the same monomer compositions as Exp8, and Exp10, containing 20% synthesized fluoride-releasing monomer (II) replacing 10% BisGMA and 10% EBPADMA. Both composites contained the same amount of F-releasing filler and photoinitiators. The compressive and flexural strengths of Exp10 were statistically the same as those of Control-2 at 24 hrs and 30 days (p > 0.05), but they were significantly higher than those of Exp6. More importantly, the flexural strength of Exp10 after 30-day storage in water improved dramatically (223%) compared with Exp6.
Exp10 also maintained its flexural strength at the same value as at 24 hrs, which indicates better hydrolytic stability. Water sorption and solubility of Exp10 were significantly lower than those of Exp6 and similar to those of Control-2. These results are consistent with our previous finding that a negative correlation exists between water sorption and mechanical properties (Xu et al., 2006a). Higher filler content (70 wt%) in Exp10 than in Exp6 (65 wt%) also contributed to the improved mechanical properties. In contrast, the cumulative fluoride release of Exp10 was significantly lower than that of Exp6. This reduction was probably due to the decreased amount of free tetrabutylammonium fluoride (TBAF) salt and the decreased hydrophilicity (and water sorption). Nevertheless, the fluoride release of Exp10 was still significantly higher than that of Control-2 (p < 0.01). The fluoride recharge capability of Exp10 was also significantly higher than that of Control-2 (p < 0.05) and similar to that of Exp6. Since Control-2 contained only the fluoride-releasing filler particles, it had very low initial fluoride release, which diminished to a negligible level after about 1 wk. Exp10 contained both fluoride-releasing filler particles and the fluoride-exchanging monomer, which can promote the transport of fluoride through the polymer matrix by an ion-exchange mechanism and led to sustained higher fluoride release and recharge capability.
In summary, the careful formulation of the monomers and improvement of the photoinitiators can significantly reduce water sorption and increase mechanical properties of the composite. The improved fluoride-releasing composite can achieve significantly higher fluoride release and recharging capability and physical and mechanical properties similar to those of conventional non-fluoride-releasing dental composites. Therefore, the original hypothesis was proved. Further improvement in the physical and mechanical properties of dental composites releasing anti-caries agents can be achieved by the incorporation of ceramic whiskers or nanofibers (Xu et al., 2002, 2007). This work is currently ongoing in our group.
Acknowledgments
The authors thank Ms. Xiaohua Du, LSU School of Dentistry, for statistical analysis, and Esstech, Coltène/Whaledent, and Caulk/Dentsply for their generous donations of monomers and filler particles.
Footnotes
This study is supported by the Joe W. and Dorothy Brown Foundation and by a US NIH/NCRR COBRE grant (No. 1 P20RR020160).
References
- Aasen SM, Oxman JD, Ubel A. inventors (1989). Organic fluoride sources. U.S. Patent 4,871,786, October
- Arends J, Ruben J. (1988). Fluoride-releasing from a composite resin. Quintessence Int 19:513-514 [Google Scholar]
- Burgess J, Norling B, Summitt J. (1994). Resin ionomer restoration materials: the new generation. J Esthetic Dent 6:207-215 [DOI] [PubMed] [Google Scholar]
- Burke FM, Ray NJ, McConnell RI. (2006). Fluoride-containing restorative materials. Int Dent J 56:33-44 [DOI] [PubMed] [Google Scholar]
- Cook PA, Youngson CC. (1989). A fluoride-containing composite resin—an in vitro study of a new material for orthodontic bonding. Br J Orthod 16:207-212 [DOI] [PubMed] [Google Scholar]
- Donly K, Gomez C. (1994). In vitro demineralizatio-remineralization of enamel caries at restoration margins utilizing fluoride-releasing composite resin. Quintessence Int 25:355-358 [PubMed] [Google Scholar]
- Geurtsen W, Leyhausen G. (2001). Chemical-biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J Dent Res 80:2046-2050 [DOI] [PubMed] [Google Scholar]
- Glasspoole EA, Erickson RL, Davidson CL. (2001). A fluoride-releasing composite for dental applications. Dent Mater 17:127-133 [DOI] [PubMed] [Google Scholar]
- Hicks J, Garcia-Godoy F, Donly K, Flaitz C. (2003). Fluoride-release restorative materials and secondary caries. J CA Dent Assoc 31:229-245 [PubMed] [Google Scholar]
- Jensen ME, Wefel JS, Hammesfahr PD. (1991). Fluoride releasing liners: in vitro recurrent caries. Gen Dent 39:12-17 [PubMed] [Google Scholar]
- Mitra SB, Wang B. inventors (2002). Use of metallofluorocomplexes for dental compositions. U.S. Patent 6,391,286, May 21
- Mjör IA. (1997). The reasons for replacement and the age of failed restorations in general dental practice. Acta Odontol Scand 55:58-63 [DOI] [PubMed] [Google Scholar]
- Mjör IA, Moorhead JE. (1998). Selection of restorative materials, reasons for replacement, and longevity of restorations in Florida. J Am Coll Dent 65:27-33 [PubMed] [Google Scholar]
- Mjör IA, Shen C, Eliasson ST, Richter S. (2002). Placement and replacement of restorations in general dental practice in Iceland. Oper Dent 27:117-123 [PubMed] [Google Scholar]
- Rawls HR. (1987). Fluoride-releasing acrylics. J Biomater Appl 1:382-405 [DOI] [PubMed] [Google Scholar]
- Rawls HR, Zimmerman BF. inventors (1986). Fluoride interpolymeric resin. U.S. Patent 4,572,920, February 25
- Sonis AL, Snell W. (1989). An evaluation of a fluoride-releasing, visible light-activated bonding system for orthodontic bracket placement. Am J Orthod Dentofacial Orthop 95:306-311 [DOI] [PubMed] [Google Scholar]
- Stansbury JW, Dickens SH. (2001). Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater 17:71-79 [DOI] [PubMed] [Google Scholar]
- Temin SC, Csuros Z. (1988). Long-term fluoride release from a composite resin. Dent Mater 4:184-186 [DOI] [PubMed] [Google Scholar]
- Trujillo M, Newman SM, Stansbury JW. (2004), Use of near-IR to monitor the influence of external heating on dental composite photopolymerization. Dent Mater 20:766-777 [DOI] [PubMed] [Google Scholar]
- Wiegand A, Buchalla W, Attin T. (2007). Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 23:343-362 [DOI] [PubMed] [Google Scholar]
- Wilson NH, Burke FJ, Mjör IA. (1997). Reasons for placement and replacement of restorations of direct restorative materials by a selected group of practitioners in the United Kingdom. Quintessence Int 28:245-248 [PubMed] [Google Scholar]
- Xu HH, Quinn JB, Smith DT, Antonucci JM, Schumacher GE, Eichmiller FC. (2002). Dental resin composites containing silica-fused whiskers— effects of whisker-to-silica ratio on fracture toughness and indentation properties. Biomaterials 23:735-742 [DOI] [PubMed] [Google Scholar]
- Xu HH, Weir MD, Sun L, Takagi S, Chow LC. (2007). Effects of calcium phosphate nanoparticles on Ca-PO4 composite. J Dent Res 86:378-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Burgess JO. (2003). Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 24:2451-2461 [DOI] [PubMed] [Google Scholar]
- Xu X, Burgess JO, Ding X, Ling L. inventors (2004a). Fluoride-releasing compositions. U.S. Patent 6,703,518, March 9
- Xu X, Ling L, Ding X, Burgess JO. (2004b). Synthesis and characterization of a novel fluoride-releasing dimethacrylate monomer and its dental composite. J Polym Sci: Part A: Polym Chem 42:985-998 [Google Scholar]
- Xu X, Ding X, Ling L, Burgess JO. (2005). Synthesis and characterization of novel fluoride-releasing monomers 2: Dimethacrylates containing bis(aminodiacetic acid) and their ternary zirconium-fluoride complexes. J Polym Sci A Polym Chem 43:3153-3166 [Google Scholar]
- Xu X, Ling L, Wang R, Burgess JO. (2006a). Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater 22:1014-1023 [DOI] [PubMed] [Google Scholar]
- Xu X, Sandras D, Burgess JO. (2006b). Shear bond strength with increasing light guide distance from dentin. J Esthet Restor Dent 18:19-28 [DOI] [PubMed] [Google Scholar]
- Zimmerman BF, Rawls HR, Querens AE. (1984). Prevention of in vitro secondary caries with an experimental fluoride-exchanging resin. J Dent Res 63:689-692 [DOI] [PubMed] [Google Scholar]