Abstract
Objective:
To determine the association between changes in serum levels of cartilage oligomeric matrix protein (COMP) and serum N-telopeptide crosslinks (NTX) over a 6 year interval with the development and progression of radiographically apparent hip osteoarthritis (RHOA) in a community sample of elderly women over 8.3 years of follow-up.
Methods:
Pelvic radiographs were obtained a mean of 8.3 years apart in Caucasian women ≥ 65 years of age enrolled in the Study of Osteoporotic Fractures. From a cohort of 5,928 subjects, we randomly sampled study subjects (∼ 170 per group) to perform two nested case-control studies, one of RHOA incidence and the other of RHOA progression. Baseline and year six serum COMP and serum NTX levels were measured by ELISA in duplicate and percent change in serum levels were calculated. Odds ratios (ORs) and 95% confidence intervals (CIs) for 1 standard deviation (SD) change in the serum COMP and NTX level differences were calculated using logistic regression analysis and used to predict the development or progression of RHOA, adjusting for potential covariates,
Results:
The percent change in the level of serum COMP from baseline to year six was found to be a risk factor for the development of incident RHOA [adjusted OR of 1.58 per 1 SD increase (95% CI: 1.19- 2.09)], and reduction of progression of RHOA [adjusted OR of 0.74 per 1 SD increase (95% CI: 0.58 - 0.96)]. Quartile analysis of serum COMP changes revealed that the three highest quartiles of change in serum COMP were associated with 1) a 5-fold greater risk of developing incident RHOA [adjusted OR= 5.42 (95% CI: 2.80 - 10.60)], and 2) a 50% decreased risk of developing progression of RHOA [adjusted OR= 0.48 (95% CI: 0.30 - 0.80)]. No significant association was found between changes in serum NTX levels from baseline to year six with either incident RHOA or the progression of existing RHOA.
Conclusion:
Measurement of serum COMP at two distinct timepoints may be a method of identifying patients at risk for developing incident RHOA and those with baseline RHOA that will not rapidly progress.
Introduction
Osteoarthritis (OA) is a common disease and a common cause of disability in the elderly. Currently, over 230,000 people in the U.S. will undergo either hip or knee joint replacement annually for advanced osteoarthritis (1).
There are no widely-used and reliable biomarkers for predicting who is at risk of developing hip or knee osteoarthritis, and no validated non-radiologic methods for monitoring progression of OA. While several validated methods exist for grading severity of existing OA on standard radiographs, these methods can be insensitive to early changes of cartilage and bone metabolism.
Recently, several groups have evaluated the use of serial measurements of serum and urine biomarkers in OA to gauge severity and progression of disease with promising results (2-4). Serial time measurements of biomarkers have also been used in attempts to evaluate response to potential disease modifying osteoarthritis drugs (DMOADs) (5). Thus, by evaluating potential markers of bone and cartilage turnover over two or more points in time, it may be possible to recognize active attempts at cartilage repair while they are occurring; this measurement, in turn, may provide a window of opportunity for preventing worsening of joint degeneration and a means of measuring tissue response to potential DMOADs.
We recently reported that baseline measurements of the serum markers cartilage oligomeric matrix protein (COMP) and N-telopeptide cross links of type I collagen (NTX) were modest risk factors associated with the development of incident radiographic hip osteoarthritis (RHOA) (6). Since there have been reports that serial measurements may be more informative regarding disease progression (2-4), the purpose of this study was to investigate whether serial measurements of a potential biomarker are better able to predict patients at risk of incident or progressive OA than baseline measurements alone.
Patients and Methods
Study Population
Patients were white women ≥ 65 years of age at baseline who were enrolled in the Study of Osteoporotic Fractures (SOF), a cohort whose characteristics have been described in our previous paper (6). Participants were recruited between September 1986 and October 1988 from population-based listings in 4 areas of the United States (7). Nonwhite women were excluded from the original cohort because of their low incidence of hip fracture, as were women who were nonambulatory or who had undergone bilateral hip replacement (7). In addition, women with radiographically confirmed rheumatoid arthritis, Paget's disease, or hip fracture at baseline were excluded from this analysis.
Two nested case-control studies were performed, similar to the methods reported previously (6). From the SOF cohort, 5,928 subjects with supine pelvic x-rays at baseline and 8.3 years apart were identified. Of these subjects, 677 subjects had COMP and NTX measured on serum collected at baseline and at year 6. These subjects were assigned to one of two nested case-control studies, one focusing on RHOA incidence (n= 336) and the other on RHOA progression (n= 341) (see Figure 1).
Figure 1.
Study Group Selection. COMP= Cartilage Oligomeric Matrix Protein; NTX= N-telopeptide Crosslinks; RHOA= Radiographic Hip Osteoarthritis.
Radiography and Interpretation
At the baseline and follow-up visits (average 8.3 years follow-up time), supine anteroposterior radiographs of the pelvis were obtained using a standard protocol (7). Radiographs were assessed for five individual radiographic features (IRFs) of hip OA (joint space narrowing, osteophyte formation, subchondral sclerosis, cysts, and deformity) using atlas photographs to improve the reliability of the readings (8, 9). Minimum joint space (MJS) was measured using published methods (10). The methods for radiographic interpretation have been previously published (7, 11, 12).
Radiograph pairs were initially read and measured by one primary reader (NEL) side-by-side with the reader blinded to the order by masking identifying information and randomly assigning the order of films. Radiograph pairs with either definite osteophytes or definite narrowing (severity score ≥ 2) in any location on the initial reading were jointly evaluated by 2 readers to reach consensus scoring. A total of 21% of the film pairs underwent a consensus reading. Inter-rater reliability for the radiographic readings evaluated from a random sample of 178 pairs was good to excellent: for MJS <1.5, kappa = 0.95; for definite joint space narrowing (JSN), kappa= 0.91; and for definite osteophytes, kappa = 0.71 (7, 11, 12).
Definitions of radiographic hip OA and radiographic progression
The definitions of radiographic hip OA and radiographic progression that were used have been previously described (6, 11, 12). A summary grade of 0-4, modified from Croft, was assigned to each hip based on individual radiographic features. Grade 2 hips required the presence of either definite (severity grade ≥ 2) JSN or osteophytes plus at least one other feature (cysts or subchondral sclerosis). Grade 3 hips required the presence of three of the following features: 1) definite osteophytes, and 2) definite JSN, plus 3) either cysts or sclerosis. Grade 4 hips met the criteria for grade 3, and had femoral head deformity present.
Hips were considered to have baseline radiographic findings of hip OA if any of the following 3 findings were present: 1) a summary grade ≥ 2, 2) JSN severity grade ≥ 2 superolaterally or severity grade ≥ 3 superomedially, or 3) definite osteophytes in any location and definite JSN in any location. For this analysis, hips were divided into those with and without baseline findings of RHOA. Those without baseline RHOA were eligible to develop RHOA at the follow-up visit. Hips with baseline RHOA were eligible for progression at the follow-up visit. A hip was defined as having developed OA (incident disease) if any of the above 3 findings were present on the 8.3 year follow-up radiograph in a hip without any radiographic features of OA at the baseline. A hip was defined as having progressed radiographically if any of the following occurred between baseline and follow-up: 1) a decrease in MJS of ≥ 0.5mm, 2) an increase of one or more in the summary grade, 3) an increase of 2 or more in total osteophyte score, or 4) total hip replacement for OA between baseline and follow-up which was assessed by radiography and review of the medical records.
Biochemical Measurements
Baseline and year 6 serum levels of COMP and NTX were measured in duplicate on fasting serum samples. Serum COMP was measured with a commercial ELISA kit (Anamar Medical, Lund, Sweden) which utilized 2 mouse monoclonal antibodies (12-11 and 6-8) directed against separate antigenic determinants on the human COMP molecule. Methods of calibration and correlation with other reported results have also been previously published (6). Serum NTX was also measured, using a commercially available ELISA kit (Osteomark, Princeton, NJ) that used a horseradish-peroxidase labeled monoclonal antibody to NTX. The mean intra-assay coefficient of variation for both assays was 5.02%. We previously published baseline results of COMP and NTX and the development and progression of RHOA (6). Approximately 20% of the subjects from the previous study did not have serum samples at Visit 4 (Year 6) and a small number of subjects no longer had a serum sample at the baseline visit. Therefore, the study subject samples analyzed in this study were somewhat different from the initial report. However, all available serum samples were re-analyzed and there was a correlation of nearly 90% between the initial and follow-up COMP samples assayed.
Statistical Analyses
Comparison of baseline characteristics between cases and controls in the incidence and progression studies was done using the chi-squared (dichotomous variables) or t-test (continuous variables) analyses. We calculated the percent change between the two timepoints (baseline and after 6 years) for serum COMP and NTX, and performed logistic regression to assess the odds ratio and 95% confidence interval for incidence and progression of RHOA per standard deviation (SD) of percent and absolute change. Analyses adjusted for the following covariates: age, weight, height, hip pain, estrogen use, vitamin D use and femoral neck BMD.
Results
Study subject characteristics
Table 1 shows the baseline characteristics for the subjects in the two nested case-control studies. In general the case and control subjects in both the incidence and progression studies were not different. For the RHOA incidence study, subjects in both groups were similar except that subjects with incident RHOA were older. In the progression study, the baseline subject characteristics were also similar except that subjects were taller in the RHOA progression group (see Table 1).
Table 1.
Baseline Characteristics of Study Subjects for the Incidence Radiographic hip OA (RHOA) and the Progression RHOA study.
| Incidence Study |
Progression Study |
|||
|---|---|---|---|---|
| No RHOA (n=169)* |
Incident RHOA (n=167)* |
Stable RHOA (n=173)* |
Progression of RHOA (n=168)* |
|
| Age (years) (Mean ± SD) |
69.6 ± 3.7 | 70.8 ± 4.6 † | 71.8 ± 5.5 | 72.2 ± 5.5 |
| Weight (kg) (Mean ± SD) |
67.5 ± 11.2 | 67.4 ± 12.3 | 67.2 ± 11.4 | 68.3 ± 12.5 |
| Height (cm) (Mean ± SD) |
159.4 ± 5.6 | 159.9 ± 6.1 | 157.7 ± 6.1 | 160.0 ± 6.6 ‡ |
| Hip pain (% Positive) |
58 (35%) | 57 (35%) | 64 (37%) | 79 (47%) |
| Estrogen use (% Positive) |
24 (14%) | 24 (14%) | 16 (9%) | 26 (16%) |
| Vitamin D use (% Positive) |
83 (51%) | 75 (46%) | 87 (51%) | 82 (50%) |
| Femoral Neck BMD (g/cm2) (Mean ± SD) |
0.66 ± 0.10 | 0.67 ± 0.11 | 0.65 ± 0.11 | 0.70 ± 0.13 |
| Baseline serum COMP (u/L) (Mean ± SD) |
11.06 ± 3.32 | 10.55 ± 2.93 | 10.66 ± 3.21 | 11.17 ± 3.60 |
| Visit 4 Serum COMP (u/L) (Mean ± SD) |
10.78 ± 2.98 | 12.27 ± 3.73 | 11.60 ± 3.54 | 11.08 ± 3.58 |
| Baseline serum NTX (nM BCE) (Mean ± SD) |
20.29 ± 8.97 | 20.52 ± 7.98 | 20.68 ± 7.29 | 20.89 ± 7.24 |
| Visit 4 Serum COMP (nM BCE) (Mean ± SD) |
19.54 ± 5.66 | 20.95 ± 7.86 | 20.43 ± 7.62 | 20.34 ± 6.47 |
Number of subjects ( ) represents all subjects with baseline characteristics and covariates collected.
RHOA=radiographic hip osteoarthritis, BMD=bone mineral density
p<0.05 versus subjects without RHOA
p<0.005 versus subjects with stable RHOA
Change in serum levels of COMP from baseline to visit 4 and the association of incident and progressive RHOA
Incidence study
The mean percent change in serum COMP levels were nearly four times greater in the incident RHOA group compared to the control group that did not develop RHOA, though this difference was not statistically significant (Table 2). There was a 58% increase in risk of incident RHOA for every 1 SD increase in change of COMP [age-adjusted OR= 1.60 (95% CI: 1.22 – 2.09); adjusted OR = 1.58 (95% CI: 1.19 – 2.09)] (Table 2). Results remained similar after adjusting for baseline serum COMP level [adjusted OR= 1.66 (95%CI: 1.20- 2.30)].
Table 2.
Association of Percentage Change in Serum Levels of Cartilage Oligomeric Matrix Protein (COMP) and N-telopeptide (NTX) with Incidence and Progression of RHOA per 1 SD Increase
| % Change in Serum COMP |
Adjusted Odds Ratio* (95% CI) |
% Change Serum NTX |
Adjusted Odds Ratio* (95% CI) |
||
|---|---|---|---|---|---|
| Incidence Study |
No RHOA | 4.0 ± 37.6 | Referent Group |
9.0 ± 45.1 | Referent Group |
| Incident RHOA |
19.2 ± 31.1 | 1.58 (1.19 – 2.09) |
11.9 ± 44.2 | 1.05 (0.81 – 1.36) |
|
| Progression Study |
Stable RHOA |
13.5 ± 34.1 | Referent Group |
8.9 ± 48.6 | Referent Group |
| Progression of RHOA |
5.3 ± 36.7 | 0.74 (0.58 – 0.96) |
6.9 ± 44.6 | 0.93 (0.73 – 1.19) |
Adjusted for age, weight, height, estrogen use, hip pain, vitamin D use, and femoral neck BMD.
Progression Study
The mean percent change in serum COMP was 61% more in those that had stable RHOA compared to those who had RHOA progression, though this change did not reach a level of statistical significance. The odds of progression were reduced 26% per every 1 SD increase in COMP [age-adjusted OR= 0.79 (95%CI: 0.62 – 1.00); adjusted OR= 0.74 (95%CI: 0.58 - 0.96)] (Table 2). Results were not different after additional adjustment for baseline serum COMP levels [adjusted OR= 0.69 (95%CI: 0.51-0.94)] and baseline RHOA severity [adjusted OR= 0.73 (95%CI: 0.56 – 0.96)].
Quartile Analysis in Incidence and Progression Studies
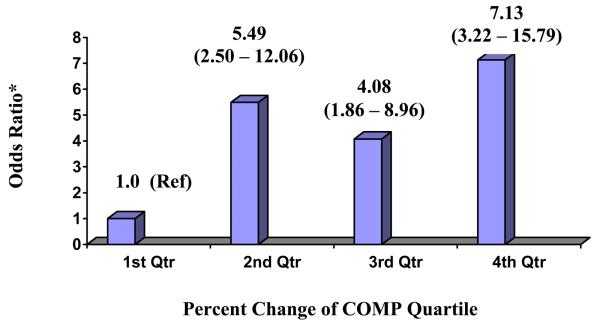
The risk of incident RHOA increased with an increasing change in COMP with a greater than seven-fold risk of developing RHOA in the highest quartile of change compared to the first quartile (P-value for trend < 0.0001) (Figure 2). When the change in serum COMP was combined for quartiles 2-4 and compared to the first quartile, the risk of incident RHOA was five-fold greater [adjusted OR= 5.42 (95% CI: 2.80 - 10.60)]. The risk of progression of RHOA decreased with an increasing change in COMP from baseline to visit 4, and this risk was 52% lower in the highest three quartiles of change in COMP compared to the first quartile [adjusted OR= 0.48, (95%CI: 0.30 - 0.90)]. No differences were found after adjustment including baseline serum COMP level.
Figure 2.
Percent Change in serum COMP by Quartiles and Association with Incident RHOA
* Adjusted for the following covariates: age, weight, hip pain, estrogen use, Vitamin D use, and femoral neck BMD (P-value for trend <0.0001).
Change in serum levels of NTX from baseline to visit 4 and the association of incident and progressive RHOA
There was no significant association between percent change in serum NTX and the development of RHOA or worsening of baseline RHOA. The odds of incident and progressive RHOA per 1 SD increase in serum NTX change over 6 years was 1.05 (95% CI: 0.81 - 1.36), and 0.93 (95%CI= 0.73 – 1.19), respectively. Results were not different in the progression group, after adjusting for baseline RHOA severity [OR= 0.94 (95%CI: 0.73 – 1.21)]. Similar results were observed in the quartile analysis for incident RHOA [adjusted OR for 4th v. 1st quartile= 1.06 (95% CI: 0.52 – 2.20)], and progressive RHOA [adjusted OR for 4th v. 1st quartile= 0.87, (95% CI: 0.40 – 1.70)].
Discussion
We found a higher percentage increase in serum COMP from baseline to year six is a risk factor for the development of incident RHOA but not for progression of RHOA in a community sample of elderly Caucasian women. A stratified analysis found that the three highest quartiles of change in serum COMP were associated with 1) a 5-fold greater risk of developing incident RHOA per 1 SD of increase, and 2) a 50% decreased risk of progression of RHOA per 1 SD increase in COMP.
COMP itself is a pentameric glycoprotein of the thrombospondin family that is found predominantly in articular cartilage, but also in other tissues such as tendons and synovium.(13, 14). Changes in serum COMP are believed to reflect changes in cartilage breakdown. Animal studies of OA support this hypothesis: in studies of transgenic mice with impaired collagen type II production, a transient upregulation of serum COMP mRNA correlated temporally with the onset of histological cartilage degeneration (15). In pediatric studies of patients with active juvenile idiopathic arthritis, periods of pronounced growth plate impairment and decreased cartilage turnover were associated with decreased serum COMP levels, compared to healthy controls (16).
Attempts to correlate serum COMP levels with the development and progression of RHOA must take into account the complex pathogenesis of OA and the non-linear timeline in which tissue destruction occurs. Altered joint biomechanics and/or focal injury can lead to repeated articular cartilage insult and attempts at regeneration, which may dominate in the preradiologic stage (17, 18). Metabolically active cartilage in early OA can undergo multiple attempts at repair, which in turn are associated with increased levels of serum COMP. This phase of OA can also be accompanied by synovial inflammation, another tissue source of elevated serum COMP (19, 20). As repeated insult to the joint occurs, OA can become more established, and continued loss of articular cartilage may outpace attempts at repair, leading the remaining cartilage to be less metabolically active overall. This stage may be reflected serologically by a relatively lower increase in COMP levels, and radiographically by subchondral sclerosis, osteophyte formation and joint space narrowing (17, 18).
Early changes in cartilage metabolism in OA may explain our finding that changes in serum COMP levels have different associations with incident versus progressive RHOA. Patients with incident RHOA in this study may have had more active cartilage turnover, resulting in the association of a greater percentage increase in serum COMP levels with incident disease. The progressive RHOA group, on the other hand, likely had more advanced, less metabolically active cartilage, which may explain the finding that a smaller percentage increase in serum COMP was associated with less RHOA progression. Patients with progressive RHOA were also more likely to have a significant association between the percentage increase in COMP and the onset of hip pain by Visit 4, possibly as a result of more advanced hip disease [age-adjusted OR= 2.431 (95%CI: 1.28, 4.60)].
The strong association of higher quartile increases in COMP with the development of incident RHOA further supports the hypothesis that COMP levels increase at a more rapid rate during periods associated with the development of incident RHOA. Sharif et al. measured serial levels of serum COMP over 60 months, correlated levels of this biomarker with radiographic findings of knee OA (2), and reported serum COMP levels in a patient to be significantly increased over any 12-24 month period in which the greatest radiographic change occurred. These authors also note that serum COMP levels may differ among subtypes of knee OA (21), but to date, no such distinctions in subtypes of hip OA have been reported.
Our results differ from previously published reports of the relationship of serum COMP and RHOA progression. Conrozier et al.(3) followed 48 patients with painful hip OA over one year, and found no change in serum COMP levels measured 12 months apart, and subsequently, no correlation between radiographic changes of joint space narrowing and changes in serum COMP levels. In contrast, our study examined the effect of two measurements of serum COMP over six years in a larger cohort of patients, potentially increasing the ability to link changes in a potential biomarker to disease progression over time; direct clinical application of our results is limited, for reasons we outline further below.
We found no statistically significant association between changes in serum NTX levels and the development or progression of RHOA. Our group had reported previously that baseline serum NTX was associated with a modest risk of incident and progressive RHOA (6). Urine NTX has been evaluated previously by Bettica et al. as a risk marker in progressive knee OA, and was found to be elevated in patients who later had radiographic progression of knee OA (4). This finding suggests that progression of established OA reflects changes of bone metabolism, rather than cartilage turnover. Since urine samples were not available in the SOF cohort, we could not confirm these findings. The serum NTX assay has been used to evaluate certain patient populations with osteoporosis with some success (22, 23), but there are few published data to support its reliability as a measure of OA progression. It may lack the sensitivity to bone changes occurring in different phases of OA compared to urinary markers of bone turnover.
This study has several strengths. First, the radiographic methods used to evaluate the incident and progressive nested groups have been validated extensively. In addition, the strong associations found between percentage increase in serum COMP over two time points, while limited in immediate clinical applicability, point towards a potential goal of following the course of this disease. Serial measurements of a potential biomarker of disease have already been used in other subspecialties to gauge treatment response and predict further disease progression; recently, serial measurements of high sensitivity C-reactive protein (hsCRP) over several months following an acute ischemic event have been shown to be helpful in assessing statin therapy response and in predicting long-term survival in patients with coronary disease (24). Similarly, a strong association of serum COMP changes with incident or progressive RHOA could serve as a potential tool for assessing and predicting the clinical course of OA in a patient.
Some of the shortcomings of this study have been outlined previously (6). An additional potential weakness is that we chose an 8.3 year interval between radiographs, while serum assays were measured six years apart. The discrepancy in measurement times makes it more difficult to determine with certainty whether the assay level changes or radiographic changes occurred first. This uncertainty, in turn, may limit our ability to correlate serum biomarker changes with the true onset of RHOA. Additionally, while these results are statistically significant and interesting in generating hypotheses about the role of COMP and NTX in the development of OA, clinical decision-making based on these results is limited. Significant overlap in the absolute values and percentage increase between the different groups was observed (See Table 1, 2); further investigation would be required prior to instituting a clinical intervention based on these results.
In addition, while we controlled for the effect of osteoporosis of the femoral neck on levels of COMP and NTX, we were not able to control for possible OA at sites other than the hip, and cannot exclude the possibility that OA in other sites contributed to the changes in COMP and NTX values. Also, since this was a racially homogenous cohort of elderly women, these results cannot be generalized to other patient groups.
Lastly, since several studies have shown that serum COMP levels increase with exercise (25, 26, 27), changes in a study subject's activity level may have influenced the association we observed between serum COMP levels and incident and progressive RHOA. Therefore,, we reanalyzed our data and adjusted for two activity-associated variables: 1) the number of kcal/week expended by the patient at baseline and 2) the number of hours spent sedentary at baseline and the associations we found were not changed.
In summary, the change in serum levels of COMP over two distinct timepoints was found to be a risk factor for predicting the development of incident or progressive RHOA. While a percent increase in COMP over six years was found to be associated with an increased risk of developing RHOA, a smaller percent increase in serum COMP was found to be potentially protective against progression of RHOA. We found no significant relationship between serum NTX changes and the development of incident or progressive RHOA in our study.
Acknowledgements
This work was supported by NIH grants (grants K24-AR-048841-01 to Dr. Lane, 1R01-Ag-054007, and 1R01-AR-40431SOF) the Arthritis Foundation of Northern California, and the American College of Rheumatology Research and Education Foundation Physician Scientist Award 2006-2007 to RKC.
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding, under the following grant numbers: AG05407, AR35582, AG05394, AR35584, and AR35583, R01 AG005407, 2 R01 AG005394-22A1, R01 AG027576-22, 2 R01 AG027574-22A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Katz JN. Total joint replacement in osteoarthritis. Best practice & Research Clinical Rheumatology. 2006;30:145–153. doi: 10.1016/j.berh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of Nonlinear or Phasic progression of Knee Osteoarthritis Based on Measurements of Serum Cartilage Oligomeric Matrix Protein Levels Over Five Years. Arthritis Rheum. 2004;50:2479–2488. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 3.Conrozier T, Saxne T, Fan CS, Mathieu P, Tron AM, Heinegard D, et al. Serum Concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: a one-year prospective study. Ann Rheum Dis. 1998;57:527–32. doi: 10.1136/ard.57.9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettica P, Cline G, Hart D, Meyer J, Spector T. Evidence for Increased Bone Resorption in Patients with Progressive Knee Osteoarthritis. Arthritis Rheum. 2002;46(12):3178–3184. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- 5.Bingham CO, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, et al. Risedronate Decreases Biochemical Markers of Cartilage Degradation but Does Not Decrease Symptoms or Slow Radiographic Progression in Patients with Medial Compartment Osteoarthritis of the Knee. Arth and Rheum. 2006;54:3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 6.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of Higher Levels of Serum Cartilage Oligomeric Matrix Protein and N-Telopeptide Crosslinks with the Development of Radiographic Hip Osteoarthritis in Elderly Women. Arthritis & Rheumatism. 2006;54(1):236–243. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 7.Lane NE, Nevitt MC, Hochberg MC, Hung YY, Palermo L. Progression of radiographic hip osteoarthritis over eight years in a community sample of elderly white women. Arthritis Rheum. 2004;50:1477–86. doi: 10.1002/art.20213. [DOI] [PubMed] [Google Scholar]
- 8.Altman RD, Hochberg M, Murphy WA, Jr., Wolfe F, Lesquene M. Atlas of radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 9.Lane NE, Nevitt MC, Genant HK, Hochberg MC, Scott JC, Pressman AR, et al. Reliability of new indices of radiographic osteoarthritis of the hand and hip and lumbar disc degeneration. J Rheumatol. 1993;20:1911–8. [PubMed] [Google Scholar]
- 10.Lesquene M. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol. 1997;24:779–81. [PubMed] [Google Scholar]
- 11.Lane NE, Gore LR, Cummings SR, Hochberg MC, Scott JC, Pressman AR, et al. for the Study of Osteoporotic Fractures Research Group. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Arthritis Rheum. 1999;42:854–60. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK, et al. Radiographic osteoarthritis of the hip and bone mineral density. Arthritis Rheum. 1995;38:907–16. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 13.DiCesare PE, Hauser N, Lehman D, Pasumarti D, Paulsson M. Cartilage Oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354:237–40. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 14.DiCesare PA, Carlson CS, Stollerman ES, Chen PA, Leslie M, Perris R. Expression of cartilage oligomeric protein by human synovium. FEBS Lett. 1997;412:249–52. doi: 10.1016/s0014-5793(97)00789-8. [DOI] [PubMed] [Google Scholar]
- 15.Salminen H, Perala M, Lorenzo P, Saxne T, Heinegard D, et al. Up-Regulation of Cartilage Oligomeric Matrix Protein at the Onset of Articular Cartilage Degeneration in a Transgenic Mouse Model of Osteoarthritis. Arthritis and Rheum. 2000;43:1742–1748. doi: 10.1002/1529-0131(200008)43:8<1742::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Urukami T, Manki A, Inoue T, Oda M, Tanaka H, et al. Clinical Significance of Decreased Serum Concentration of Cartilage Oligomeric Matrix Protein in Systemic Juvenile Idiopathic Arthritis. J Rheum. 2006;33:996–1000. [PubMed] [Google Scholar]
- 17.Bullough PG. Pathology of Osteoarthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. Third ed. Mosby; Philadelphia: 2003. pp. 1835–1845. [Google Scholar]
- 18.Burr DB. Increased Biological Activity of Subchondral Mineralized Tissues Underlies the Progressive Deterioration of Articular Cartilage in Osteoarthrosis. J Rheum. 2005;32(6):1156–1158. [PubMed] [Google Scholar]
- 19.Jung YO, Do JH, Kang HJ, Yoo SA, Yoon CH, Kim HA, et al. Correlation of Sonographic severity with biochemical markers of synovium and cartilage in knee osteoarthritis patients. Lin Exp Rheum. 2006;24:253–259. [PubMed] [Google Scholar]
- 20.Bruyere O, Collette J, Kothari M, Zaim S, White D, Genant H, et al. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65:1050–1054. doi: 10.1136/ard.2005.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharif M, Granell R, Johansen J, Clarke S, Elson C, Kirwan J. Serum Cartilage matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology. 2006;45:522–526. doi: 10.1093/rheumatology/kei216. [DOI] [PubMed] [Google Scholar]
- 22.Maeno Y, Inaba M, Okuno S, Yamakawa T, Ishimura E, Nishizawa Y. Serum Concentrations of Cross-Linked N-Telopeptides of Type I Collageb: New Marker for Bone Resorption in Hemodialysis Patients. Clin Chemistry. 2005;51(12):2312–2317. doi: 10.1373/clinchem.2005.051524. [DOI] [PubMed] [Google Scholar]
- 23.Fall PM, Kennedy D, Smith JA, Seibel MJ, Raisz LG. Comparison of Serum and Urine Assays for Biochemical Markers of Bone Resorption in Postmenopausal Women with and without Hormone Replacement Therapy and in Men. Osteoporosis Intl. 2000;11:481–485. doi: 10.1007/s001980070089. [DOI] [PubMed] [Google Scholar]
- 24.Morrow D, Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, et al. Clinical Relevance of C-Reactive Protein During Follow-Up of Patients with Acute Coronary Syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 25.Mundermann A, Dyrby CO, Andriacchi TP, King KB. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthritis and Cartilage. 2005;13:34–38. doi: 10.1016/j.joca.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Neidhart M, Muller-Ladner U, Frey W, Bosserhoff AK, Colombani PC, et al. Increased serum levels of non-collageneous matrix proteins (cartilage oligomeric matrix protein and melanoma inhibitory activity) in marathon runners. Osteoarthritis and Cartilage. 2000;8:222–229. doi: 10.1053/joca.1999.0293. [DOI] [PubMed] [Google Scholar]
- 27.Andersson MLE, Thorstensson CA, Roos EM, Petersson IF, Heinegard D, et al. Serum levels of Cartilage Oligomeric Protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskeletal Disorders. 2006;7:98–106. doi: 10.1186/1471-2474-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]