Abstract
Objective
We have previously shown that heparin-binding EGF-like growth factor (HB-EGF) protects the intestines from multiple forms of injury via direct cytoprotective effects on the intestinal mucosa. In this study, we examined the effects of HB-EGF on the hemodynamics of intestinal arterioles, the major resistance vessels that regulate blood flow to the intestines, as an additional mechanism of HB-EGF-mediated intestinal protection.
Methods
The hemodynamic effects of HB-EGF in rodent terminal mesenteric arterioles and human submucosal arterioles were examined ex vivo using a video dimension analyzer. Cultured human intestinal microvascular endothelial cells (HIMEC) were used to elucidate the mechanisms of HB-EGF-induced vasodilation.
Results
HB-EGF significantly increased vessel diameter under conditions of increasing intraluminal pressure and increased flow rate. These HB-EGF-mediated vasodilatory effects were observed in terminal mesenteric arterioles from adult rats and 3 day old rat pups. These effects were confirmed in submucosal arterioles from human intestine. Furthermore, HB-EGF significantly reduced endothelin-1-induced mesenteric arteriolar vasoconstriction. The vasodilatory effects of HB-EGF were blocked by ETB receptor antagonism in adult rat arterioles, and also by nitric oxide synthase inhibition in rat pup and human infant arterioles. In HIMEC, HB-EGF significantly increased endothelin B (ETB) receptor protein expression and provoked intracellular calcium mobilization.
Conclusions
HB-EGF is a potent vasodilator of the intestinal microvasculature, further supporting its use in diseases manifested by decreased intestinal blood flow, including necrotizing enterocolitis.
Keywords: heparin-binding EGF-like growth factor, intestine, arterioles, endothelin, vasodilator, necrotizing enterocolitis
INTRODUCTION
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in newborns, afflicting 7% of all very low birth weight (<1500 gram) prematures (Kliegman and Fanaroff, 1984). The incidence of NEC continues to increase, and has overridden neonatal respiratory distress syndrome as the leading cause of death in premature infants (Rowe et al., 1994). Numerous risk factors contribute to the pathogenesis of NEC, and recent studies demonstrate that compromised intestinal blood flow in the perinatal period is involved (Nowicki, 1990, Hsueh et al., 1994). Severe hypoxia and sustained intestinal ischemia cause a significant reduction of mesenteric blood flow leading to decreased tissue oxygen delivery and intestinal damage. Although it is not clear that ischemia occurs prior to tissue destruction in the pathogenesis of NEC, there is abundant evidence that ischemia is highly associated with the histopathologic findings in NEC (Nowicki, 1990).
Endothelin-1 (ET-1) is a potent vasoconstrictor that is known to play a critical role in regulating the microcirculation of the intestine. We have demonstrated increased tissue concentrations of ET-1 in human intestine acutely afflicted with NEC (Nowicki et al., 2005). These findings were supported by animal studies showing increased expression of ET-1 mRNA in experimental NEC (Ito et al., 2007). These important observations support the argument that ET-1 compromises mesenteric blood flow during the pathologic course of NEC.
The functions of ET-1 are controlled by its two key receptors, ETA and ETB receptors (Pollock et al., 1995). ETA receptors are mainly found in the smooth muscle cells of blood vessels, and binding of endothelin to ETA receptors increases vasoconstriction. The location of ETB receptors is variable. The binding of endothelin to ETB receptors located on endothelial cells leads to the release of nitric oxide or increased prostacyclin formation leading to vasodilation. The activation of ETB receptors also increases natriuresis and diuresis, leading to lower blood pressure (Nakano et al., 2008, Pollock and Pollock, 2008). Furthermore, the ETB receptor acts as an inducible receptor that may cause vasoconstriction when present on vascular smooth muscle cells (Masaki et al., 1991, Adner et al., 1998). The distribution of these two endothelin receptor subtypes in vessels may vary from tissue to tissue, and in physiological or pathological conditions. The tissue distribution or activation state of these two receptor subtypes determines whether a vessel will constrict or dilate. In intestine afflicted with NEC, it appears that the normal balance between endothelin dilator (ETB) and constrictor (ETA) receptors may be lost, favoring net vasoconstriction (Nankervis and Nowicki, 2000, Nankervis et al., 2000, Marasciulo et al., 2006).
HB-EGF is a member of the epidermal growth factor (EGF) family (Higashiyama et al., 1991) that was initially identified in the conditioned medium of cultured human macrophages (Besner et al., 1990). We have shown that HB-EGF protects the intestine from ischemia/reperfusion injury (Pillai et al., 1999, El-Assal and Besner, 2004, Rocourt et al., 2007), hemorrhagic shock and resuscitation (HS/R) (El-Assal et al., 2007), and experimental NEC (Feng et al., 2006). HB-EGF protects the intestine from injury, in part, by promoting restitution (El-Assal and Besner, 2005) and angiogenesis (El-Assal et al., 2007, Mehta and Besner, 2007), by decreasing reactive nitrogen (Lara-Marquez et al., 2002) and oxygen species production (Kuhn et al., 2002), and by decreasing inflammatory cell infiltration (Xia et al., 2003) and pro-inflammatory cytokine production (Rocourt et al., 2007). We have recently demonstrated that HB-EGF increases intestinal villous microvascular blood flow during HS/R (El-Assal et al., 2007) and during experimental NEC (unpublished observations). The goal of the current study was to further examine the effect of HB-EGF on the hemodynamics of terminal mesenteric arterioles (TMA) and submucosal arterioles (SMA), the major resistance vessels controlling blood flow to the intestines, and to investigate the mechanism(s) of HB-EGF-mediated vasodilatory effects.
MATERIALS AND METHODS
Preparation and mounting of terminal mesenteric arterioles (TMA) and submucosal arterioles (SMA)
All procedures were approved by the Institutional Animal Care and Use Committee (protocol #AR07-00058) and the Institutional Review Board (Protocol #IRB06-00267) of the Research Institute at Nationwide Children’s Hospital. Four week old adult male Sprague-Dawley rats (180–200g) or 3 d old rat pups (8–10g) were sacrificed by cervical dislocation following CO2 narcosis and the mesentary removed. The TMA represent the distal-most branches of the mesenteric arterial arcade in rats. A significant pressure drop occurs from the origin to the endpoint of the TMA, a feature that characterizes them as resistance vessels in the mesenteric arterial arcade. SMA are resistance vessels that regulate blood flow to individual villi (Vanner et al., 1990, Nowicki, 2005). SMA are located between the intestinal mucosal layer and the muscular layer. SMA were dissected from human intestinal specimens resected during operative procedures for intestinal atresias or ostomy closures in five patients (age range 2–11 months, average age 5.8 months, mean age 5.8 ± 3.9 months).
Examination of arterioles by video dimension analysis
Rat TMA (adult rat, outer diameter = 229 ± 6 μm, n=54; rat pup, outer diameter = 67 ± 7 μm, n=17), and human SMA (outer diameter = 262 ± 7 μm, n=9) were mounted in the proper proximal to distal orientation on two glass micropipettes seated within a plastic vessel chamber (CH2/AS, Living Systems, Burlington, VT). Arterial pressure and flow were adjusted by means of a pressure-servo system (PS/200/Q), allowing discrete manipulation of pressure and flow within arterioles. Arteriolar perfusion was obtained using standard Krebs buffer of the following composition (in mM): 118.1 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11.1 glucose, 0.26 EDTA. The buffer was continuously aerated with 95% O2–5% CO2 giving it a pH of 7.4 at 38°C. The vessel chamber, and thus the exterior surface of the arterioles, was continuously suffused with warm (38°C) aerated Krebs buffer at a rate of 50 ml/min. All vasoactive agents used in these experiments were added to the suffusion buffer reservoir. All experiments used mature recombinant HB-EGF corresponding to amino acids 74-148 of the HB-EGF precursor, that was produced in our laboratory using recombinant DNA technology and purified as previously described (Davis et al., 1996).
All vessels were kept at a pressure of 20 mmHg for 30 min after mounting to develop spontaneous tone, and then the suffusion buffer was replaced with Krebs buffer containing 40 mM KCl. Vessels which failed to contract to 50% of their baseline diameter were discarded. The suffusion buffer was then replaced with standard Krebs buffer. The relationship between vessel diameter and pressure in the absence of flow or with increasing flow rate was determined independently in adult rat TMA. The microvascular perfusion/suffusion chamber was mounted on the stage of an inverted microscope set in line with a video camera. Vascular dimensions were measured and continuously monitored with a precalibrated video analyzer (V94, Living Systems, Burlington, VT) that displayed vessel luminal diameter. Flow rate across the vessels was measured with a flowmeter set in-line with the perfusion system (Omega, Putnam, CT). Vessel luminal diameters were recorded while intraluminal pressure was increased from 0–100 mmHg in 20 mmHg increments or while flow rate was increased from 0 to 100 μl/min. HB-EGF (10 ng/ml) was then added to the suffusion buffer, and arteriolar diameters were re-measured under conditions of either increasing intraluminal pressure or increasing flow rate three hours later. In preliminary pilot studies, the effect of HB-EGF-induced vasodilation was tested by incubating vessels with HB-EGF for 1, 2, 3, 6 and 12 h, and we found that a 3 h incubation led to satisfactory vasodilatory effects.
Maximal passive diameters were determined by removing Ca2+ from the perfusion and suffusion buffers and adding EGTA (1 mmol) and papaverine (10 μmol) before initiation of the pressure ramp, as previously described (Nankervis et al., 2001a). Additional vasoactive agents added to the suffusion buffer in these experiments included: BQ788 (ETB receptor blocker; 20μmol; Peptides International, Louisville, KY); BQ610 (ETA receptor blocker; 20μmol; Peptides International, Louisville, KY); L-NMMA (non-selective NOS inhibitor; 100μmol; Cayman Chemical, Ann Arbor, MI); indomethacin (COX-1/2 nonselective inhibitor; 10μmol; Sigma, St. Louis, MO), and endothelin-1 (20 pmol; Sigma, St. Louis, MI).
Real time reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from adult rat TMA after incubation with or without HB-EGF (10 ng/ml) for 3 h. Isolated RNA was treated with Rnase free Dnase (Amp Grade; Invitrogen-Gibco, Grand Island, New York) to eliminate DNA contamination, and the quantity and quality of RNA assessed by 1% agarose gel electrophoresis and spectrophotometry. Total RNA was reverse-transcribed with random hexamers using a first-strand cDNA synthesis kit (Invitrogen-Gibco, Grand Island, New York). cDNA (5 μl) was used for PCR amplification. Real time RT-PCR was carried out using a SYBR Green RT-PCR kit (Applied Biosystems Inc., Branchburge, NJ) and an ABI Prism 770 Sequence Detection System (Applied Biosystems, Foster City, California). Similar experiments were performed on harvested HIMEC after a 3, 6 or 24 h incubation with HB-EGF (10ng/ml) in the presence or absence of 2-aminoethoxydiphenylborate (2-APB) (IP3-sensitive calcium channel blocker; 100 μmol; Sigma, St. Louis, MO).
ETA and ETB receptors were amplified using the following primers: ETA sense primer (rat): 5′-TGCCCACAGCAGACTAAACG-3′; anti-sense: 5′-CCAATGGCGGTAATCAAGA-3′; ETB sense primer (rat): 5′-GGCTCTGGGAG ACCTACTA-3′; anti-sense: 5′-TAGCGGCAAGCAGAAGTA-3′; ETB sense primer (human): 5′-GCCAAGGACCCATCGAGAT-3′; anti-sense: 5′-GAAGTGTGGAGTTCCCGATGAT-3′. Amplification of the housekeeper gene (GAPDH) cDNA was used as an internal control for quantification, which was performed using Relative Quantification Software, version 1.01 (Applied Biosystems, Foster City, California).
Immunohistochemistry
Mesentery or intestinal specimens were immersed in cold (4°C) sterile Dulbecco’s modified Eagle’s medium (DMEM, Mediatech, Inc, Manasas, VA) supplemented with 1% fetal bovine serum, penicillin 100 U/ml and streptomycin 100 μg/ml (Sigma, St. Louis, Missouri) immediately upon removal. TMA or SMA were dissected from adherent tissue and cut into 1 mm long segments. Up to a dozen segments were placed in each well of a 12-well plate containing DMEM (1 ml) and incubated at 37°C in humidified 5% CO2 in air. HB-EGF (10 ng/ml) was added to the culture medium in random wells, and segments incubated for 3 h.
Radial cryosections (6 μm) were prepared from adult rat TMA incubated with or without HB-EGF. Primary polyclonal goat anti-ETB receptor antibody (10 μg/ml; Alomone Labs, Jerusalem, Israel) was added with overnight incubation at 4°C. Sections were then incubated with secondary fluorophore-conjugated goat anti rabbit IgG (Green, 4 μg/ml; Alexa 488, Molecular Probes) for 1 h at room temperature, washed in PBS, and mounted using an anti-fade reagent (SlowFade, Molecular Probes, Eugene, Oregon). Fluorescent staining was examined using a Zeiss AxioSkop 2 Plus microscope (Carl Zeiss Inc., Thornwood, New York).
Human intestinal microvascular endothelial cell (HIMEC) culture
HIMEC (ScienceCell Research Laboratories, Carlsbad, CA) were plated onto fibronectin-coated flasks and grown to confluence in Endothelial Cell Medium (ScienceCell Research Laboratories, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS) and endothelial cell growth factor (Upstate Biotechnology, Lake Placid, NY). Cultures of HIMEC were maintained at 37°C in 5% CO2, fed twice a week, and split at confluence. All experiments were carried out on cells between passage 8 to 12, with all experiments repeated at least three times.
Human intestinal microvascular endothelial cell (HIMEC) immunocytochemistry
For immunocytochemistry, HIMEC were seeded in human fibronectin coated 8-well culture slides and starved with serum free ECM for 16 hours. HB-EGF (10 ng/ml) was then added to the cells for 3, 6, 12 or 24 h. ETB receptor immunostaining was performed with rabbit anti-human ETB receptor antibody (USBiological, Swampscott, MA). After incubation, cells were washed in PBS and the incubated in secondary fluorophore-conjugated goat anti-rabbit IgG. Immunostaining was carried out as described above. Image-Probe 6.2 (Bethesda, MD) was used to quantify the fluorescent intensity of ETB receptor staining.
Human intestinal microvascular endothelial cell (HIMEC) endothelin ELISA
The culture medium of HIMEC was collected after a 3 h incubation with or without HB-EGF (10 ng/ml or 100 ng/ml), and endothelin secretion was measured by Enzyme Immunoassay Kit (Cayman, Ann Arbor, MI). Cells treated for 24 h with TGF-β1 (100 ng/ml; Sigma, St. Louis, MO) were used as a positive control for endothelin secretion.
Measurement of HIMEC intracellular calcium levels
HIMEC (106 cells) were loaded with the Ca2+-sensitive fluorescent dye Indo-1 AM (5 μM; Molecular Probes, Eugene, OR) for 30 min at 37°C. To minimize Indo-1 leakage, probenecid (4 mM; Molecular Probes, Eugene, OR) was added. Prior to stimulation, Indo-1 loaded cells were resuspended in 1 ml cell loading media (HBSS plus 1 mM calcium and 1 mM magnesium) maintained at 37°C. Intracellular calcium [Ca2+]i was monitored using a BD LSR II flow cytometer (BD Biosciences, San Jose, CA) with excitation wavelength of 355 nm and emission wavelengths of 405 and 485 nm. [Ca2+]i was expressed as a ratio (R) of the fluorescent intensity at 405 nm divided by the fluorescent intensity at 485 nm, with an increase in the ratio indicating an increase in intracellular calcium. Data were collected for 50 s to establish the baseline 405:485 nm ratio prior to the addition of HB-EGF (10ng/ml). Data were collected for a total of 6 min after HB-EGF addition and analyzed using FlowJo software (Tree Star Software, Ashland, OR). BQ788 (20uM) was added to Indo-1 labeled HIMEC 30 min prior to addition of HB-EGF.
Statistical analyses
ANOVA was used to determine significance within each data set. One- or two-way ANOVA formats were used depending on the number of variables under consideration. If the F-statistic of the ANOVA was significant (p < 0.05), then the Student t test was used to determine the sites of significance at the p < 0.05 level.
RESULTS
HB-EGF amplifies pressure-induced vasodilation in adult rat TMA
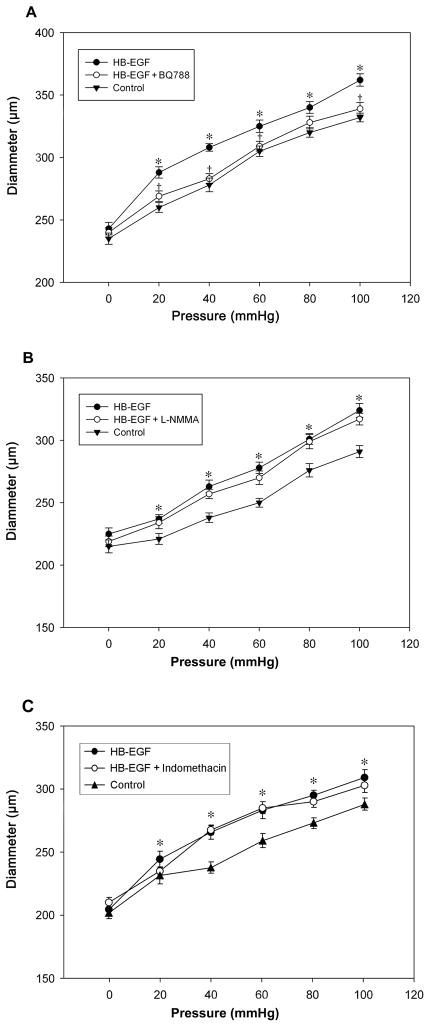
Under conditions of increasing intraluminal pressure (0 to 100 mmHg), adult rat TMA undergo a gradual increase in vessel diameter. After exposure of the vessels to HB-EGF for 3 h, there was significantly increased vasodilation at all measured pressures (Figure 1). To begin to investigate the mechanism(s) of HB-EGF-induced vasodilation, BQ788, a specific ETB receptor inhibitor, was added to the suffusion buffer after HB-EGF treatment. The vasodilatory effect of HB-EGF was specifically blocked by BQ788 (Figure 1A), but was unchanged after the addition of either L-NMMA (a non-selective NOS inhibitor, Figure 1B), or indomethacin (a non-selective COX1/2 inhibitor, Figure 1C). These observations suggest that the vasodilatory effect of HB-EGF was dependent upon the activation of ETB receptors but not nitric oxide or cyclooxygenase activation.
Figure 1.
Effect of HB-EGF on pressure-induced vessel diameter in adult rat TMA. Intraluminal pressures were increased from 0 to 100 mmHg in 20 mmHg increments, initially under control conditions, then after the addition of HB-EGF (10 ng/ml), and then after the application of BQ788 (panel A), L-NMMA (panel B), or indomethacin (panel C) to the suffusion buffer in the presence of HB-EGF. Each point represents the mean vessel diameters of all arterioles tested, with error bars representing SEM. n = 12 vessels in panel A, n = 11 vessels in panel B and n = 6 vessels in panel C. *P< 0.05 for HB-EGF vs. control; †P <0.05 for HB-EGF vs. HB-EGF + BQ788.
HB-EGF amplifies flow-induced vasodilation in adult rat TMA
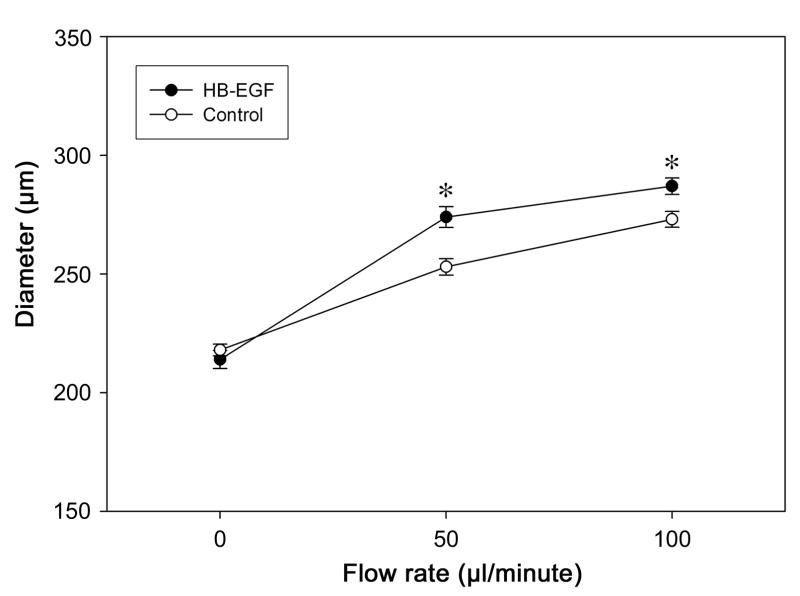
Flow is another characteristic stimulus that induces vessel dilation. We next tested the ability of HB-EGF to induce vasodilation in adult rat TMA under the conditions of increasing flow (0–100 μl/min.) with constant pressure. The addition of HB-EGF significantly increased flow-induced vasodilatation in adult rat TMA (Figure 2).
Figure 2.
Effect of HB-EGF on flow-induced vessel diameter in adult rat TMA. Flow rates were increased from 0 to 100 μl/minute by generating a change in pressure (ΔP) across the vessel. TMA were exposed to HB-EGF in the suffusion buffer for 3h prior to measurement. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. N = 12 vessels; *P < 0.05 for HB-EGF vs. control.
HB-EGF reduces ET-1-induced vasoconstriction in adult rat TMA
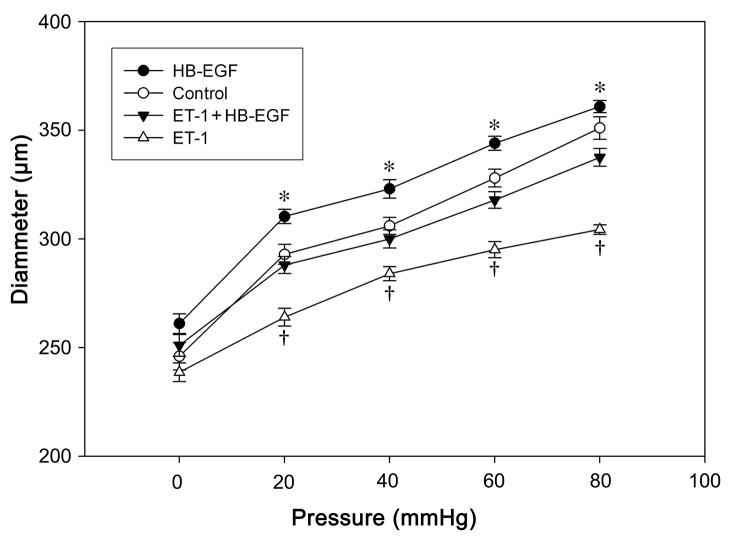
ET-1 is one of the most potent vasoconstrictors known. Similar to the observations of others, we have demonstrated that ET-1-induced vasoconstriction was selectively blocked by the ETA receptor antagonist BQ610 (Supplementary Figure 1). Since ET-1 expression has been shown to be increased in acute necrotizing enterocolitis, we next tested the effect of HB-EGF in adult rat TMA which were pretreated with ET-1. When ET-1 was added to the suffusion buffer it caused a marked vasoconstriction after 10 min that lasted for at least 4 h. When HB-EGF was added to the suffusion buffer in the continued presence of ET-1, HB-EGF-induced arteriolar dilation was still present (Figure 3). This demonstrated that HB-EGF exerts its vasodilitory effects even in the presence of the potent vasoconstrictor ET-1.
Figure 3.
Effect of HB-EGF on pressure-induced vessel diameter in adult rat TMA treated with ET-1. Pressures were increased from 0 to 80 mmHg in 20 mmHg increments initially under control conditions, then after the addition of ET-1 (20 pmol/ml) to the suffusion buffer, and then after the addition of HB-EGF to the suffusion buffer in the presence of ET-1. TMA in the ET-1 + HB-EGF group were initially exposed to ET-1 (20 pmol/ml) and then treated with HB-EGF (10 ng/ml) for 3 h before vessel diameters were measured. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. N = 10 vessels; *P < 0.05 for HB-EGF vs. control; †P < 0.05 for ET-1 + HB-EGF vs. ET-1.
HB-EGF dilates TMA from 3 day old rat pups and submucosal arterioles from human infants
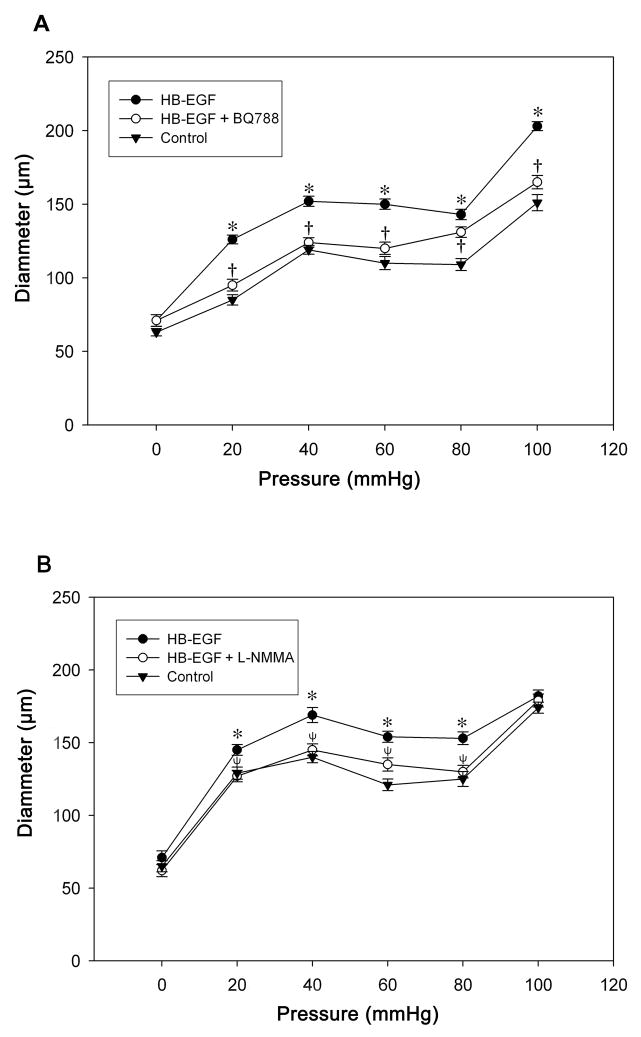
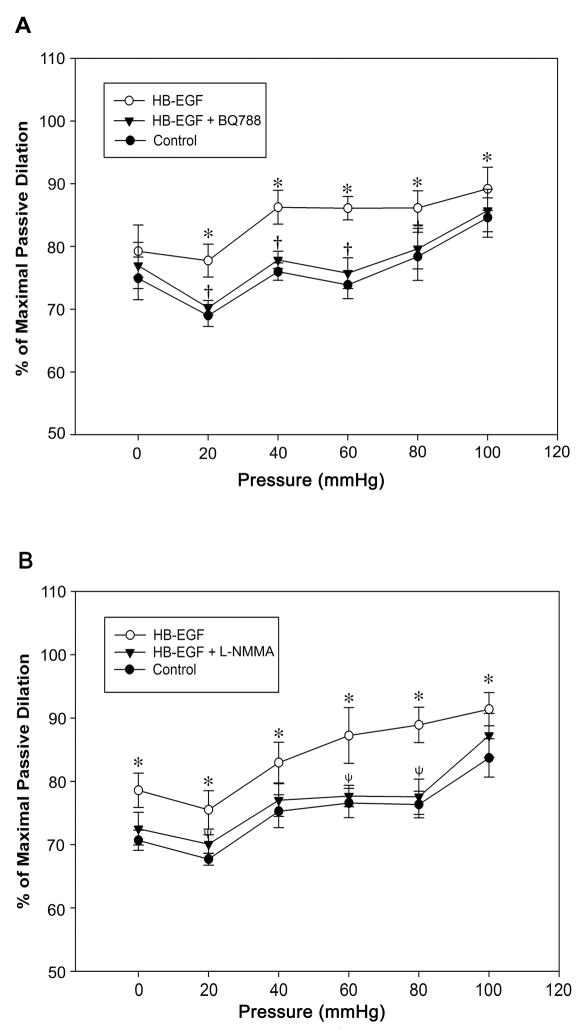
Since several studies have demonstrated important differences in the pressure-flow characteristics of newborn compared to adult TMA (Unthank et al., 1990, Nankervis et al., 2001b, Reber et al., 2002, Nankervis et al., 2008), and since NEC is a disease that only affects neonates, we next tested the effect of HB-EGF on TMA from 3 day old rat pups, the youngest pups from which we could dissect arterioles that could be successfully tested in the video dimension analysis apparatus. We observed a significant myogenic vasoconstrictive response in TMA from 3d-old rats with an increase of intraluminal pressure from 40 to 80 mmHg. Substitution of calcium free buffer containing 1 mmol EGTA and 10 μmol papaverine reversed this response (Supplementary Figure 2). As observed in adult rats, HB-EGF was able to amplify pressure-induced vasodilation in 3 day old rat TMA, and its vasodilatory effect was eliminated in the presence of ETB receptor blockade (Figure 4A). Interestingly, the HB-EGF-induced vasodilatory effects in 3 d old rat TMA were also blocked by the NOS inhibitor L-NMMA (Figure 4B), an effect that was not observed in adult rat TMA (Figure 1B). In addition, similar to our findings in 3 d old rat TMA, HB-EGF-mediated vasodilatory effects were also present in submucosal arterioles harvested from human infants, where the vasodilatory effects of HB-EGF were blocked by either the ETB receptor blocker BQ788 or the NOS inhibitor L-NMMA (Figure 5).
Figure 4.
Effect of HB-EGF on pressure-induced dilation in TMA from 3 d old rat pups. Intraluminal pressures were increased from 0 to 100 mmHg in 20 mmHg increments, initially under control conditions, then after the addition of HB-EGF (10 ng/ml), and then after the addition of BQ788 (panel A) or L-NMMA (panel B) to the suffusion buffer in the presence of HB-EGF. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. N = 7 vessels in panel A and n = 8 vessels in panel B. *P < 0.05 for HB-EGF vs. control; †P <0.05 for HB-EGF vs. HB-EGF + BQ788 in panel A; ψ P <0.05 for HB-EGF vs. HB-EGF + L-NMMA in panel B.
Figure 5.
Effect of HB-EGF on pressure-induced dilation in human submucosal arterioles. Luminal pressures were increased from 0 to 100 mmHg in 20 mmHg increments under control conditions and after the addition of HB-EGF (10 ng/ml) to the suffusion buffer. Vessel diameters are represented as % of maximal passive diameter. Each point represents the mean ratio (vessel diameter/maximal passive diameter x %) of all arterioles tested, with error bars representing SEM. SMA were harvested from 5 patients (average age 5.8 ± 3.9 months). n= 4 vessels in panel A and n= 5 vessels in panel B. *P < 0.05 for HB-EGF vs. control; †P <0.05 for HB-EGF vs. HB-EGF + BQ788 in panel A; ψ P <0.05 for HB-EGF vs. HB-EGF + L-NMMA in panel B.
HB-EGF upregulates ETB receptor expression
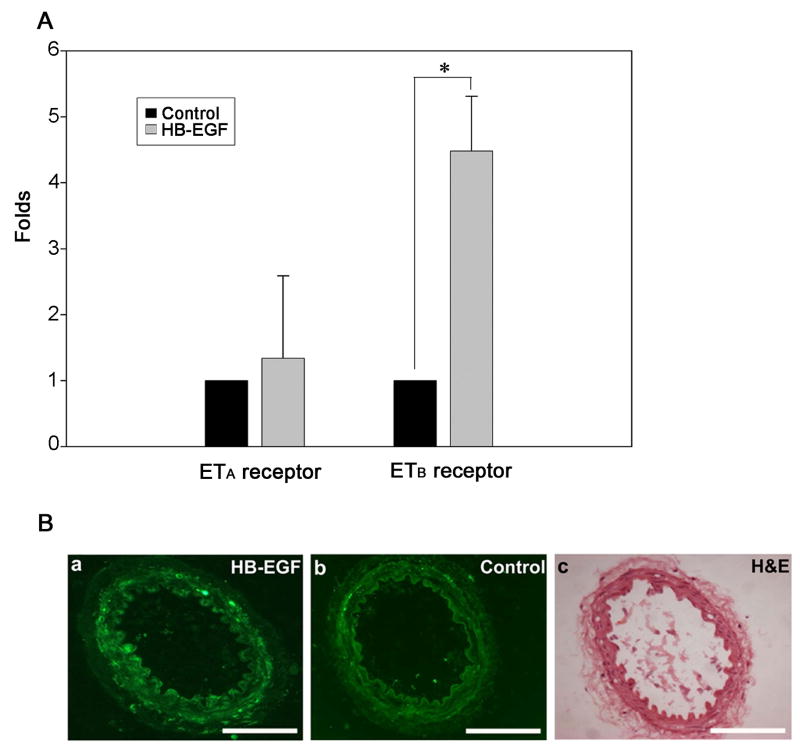
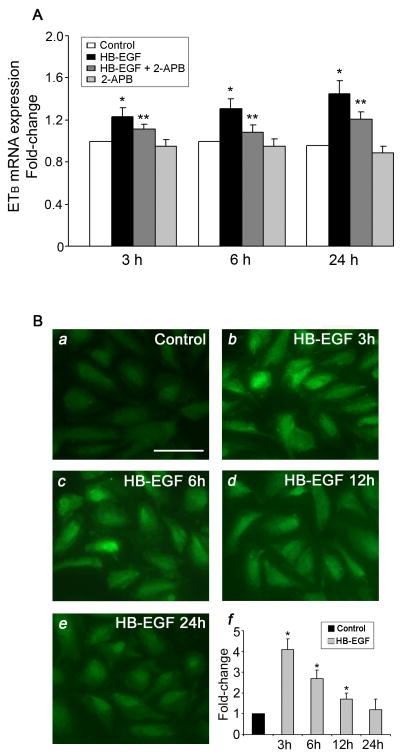
Real time RT-PCR demonstrated a significant increase in ETB receptor mRNA expression in adult rat TMA cultured for 3 h in the presence of HB-EGF (Figure 6). HB-EGF incubation led to a 4-fold increase in ETB receptor mRNA levels, whereas there was no significant effect on ETA receptor mRNA expression (Figure 6A). To further examine ETB receptor protein localization in TMA after treatment with HB-EGF, immunohistochemistry using ETB receptor antibodies was performed. ETB receptor staining was significantly increased after HB-EGF incubation, and was predominantly located in endothelial cells (Figure 6B). To determine whether calcium mobilization was involved in HB-EGF-mediated augmentation of ETB receptor expression, we used real time RT-PCR to quantify ETB mRNA expression in HIMEC after HB-EGF treatment in the presence or absence of the intracellular calcium entry blocker 2-Aminoethyl diphenylborinate (2-APB) (Figure 7A). We found that ETB-receptor mRNA expression was significantly upregulated after HB-EGF treatment for 3, 6 or 24 h. Furthermore, HB-EGF-ETB receptor mRNA upregulation was blocked by the addition of 2-APB. These data suggest that early calcium transients are related to HB-EGF-induced ETB receptor mRNA expression in HIMEC. Immunocytochemistry in HIMEC also demonstrated a significant increase in ETB receptor immunostaining in HIMEC after incubation with HB-EGF (Figure 7B). Maximal ETB immunoreactivity occurred 3 h after addition of HB-EGF. We next examined whether HB-EGF-induced ETB receptor activation could be related to the secretion of the ETB receptor agonist ET-1. We found that HB-EGF did not cause significant ET-1 protein secretion in HIMEC after a 3 h incubation (Supplementary Figure 3).
Figure 6.
Effect of HB-EGF on ETA and ETB mRNA expression and protein distribution in adult rat TMA. A) Real time RT-PCR was performed on total RNA from TMA treated with or without HB-EGF for 3 h using primers for ETA or ETB. ETA or ETB mRNA expression ± SEM is shown, with data shown as fold change compared to control which was set at 1. The data are representative of three independent experiments. *P < 0.05 compared to control ETB receptor expression. B) Panels a and b represent cross-sections of adult rat TMA subjected to immunohistochemical staining with ETB receptor antibodies after incubation for 3 h in the presence (panel a) or absence (panel b) of HB-EGF. C) Hematoxylin & eosin staining of the vessel in panel b. Scale bar=100μm.
Figure 7.
ETB receptor mRNA expression and protein detection in HIMEC. A. Effect of HB-EGF and 2-APB on ETB mRNA expression in HIMEC. ETB mRNA was quantified using real-time RT-PCR after treatment of the cells as indicated. ETB mRNA expression ± SEM is shown, with data shown as fold change compared to control which was set at 1. *P < 0.05 for HB-EGF vs control; **P < 0.05 for HB-EGF + 2-APB vs. HB-EGF. B. Effect of HB-EGF on ETB protein detection in HIMEC. a–e) Fluorescent microscopic images of anti-ETB receptor immunoreactivity in HIMEC in the absence or presence of HB-EGF for 3, 6, 12 or 24 h. f) Quantification of panels a–e using Image-Probe software. Fluorescence intensity was averaged from ten images under each experimental condition. ETB protein intensity ± SEM is shown, with data expressed as fold change compared to control which was set at 1. *P < 0.05 compared to control.
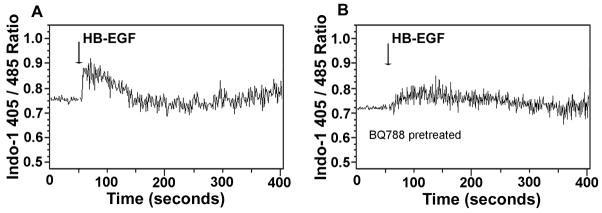
HB-EGF triggers intracellular calcium elevation in HIMEC
Since endothelial cell intracellular calcium spikes are key events in the initiation of vasodilation (Dawson et al., 2006), we next measured the cytostolic calcium concentration of HIMEC after addition of HB-EGF (10 ng/ml) to the Indo-1 labeled cells. Addition of HB-EGF led to an intracellular calcium spike (Figure 8A). However, when HIMEC were pretreated with the ETB blocker BQ788, the HB-EGF-induced intracellular calcium elevation was inhibited (Figure 8B).
Figure 8.
Effect of HB-EGF on intracellular calcium levels in HIMEC. The fluorescent ratio (405 nm/485 nm) was used as an indicator of the relative concentration of intracellular calcium. Data are expressed as the ratio of 405/485 nm over time. The time of addition of HB-EGF is marked by vertical arrows. A) Treatment of HIMEC with HB-EGF alone. B) Pretreatment of HIMEC with the ETB receptor blocker BQ788 prior to addition of HB-EGF.
DISCUSSION
We have previously shown that HB-EGF increases intestinal villous microvascular blood flow in a rat model of hemorrhagic shock and resuscitation (El-Assal et al., 2007). The HB-EGF-induced increase in microcirculatory flow resulted in significant preservation of villous architecture after injury. Recently, we have reproduced these findings in a newborn rat model of experimental NEC (unpublished observations). To further investigate the mechanism(s) of these HB-EGF vasodilatory effects, we have directly tested the effect of HB-EGF on TMA from adult and 3 d old rats, and on SMA from human infants, using video dimension analysis. We found that HB-EGF significantly increases both pressure- and flow-induced vasodilation. This study provides the first direct evidence that HB-EGF acts as a vasodilator, representing an additional potential mechanism underlying its potent intestinal cytoprotective effects.
Necrotizing enterocolitis is a disease that affects neonates, usually those born prematurely. Enhanced vascular tone and elevated intestinal resistance due to an increased myogenic response in neonatal mesenteric arteries have been reported (Nankervis et al., 2001b, Su et al., 2003). The myogenic response is defined as vessel constriction in response to stretch stimuli such as intraluminal pressure, transmural pressure or blood flow. The intensity of the myogenic response in arterioles is higher in 1 day old swine compared to 10 day old swine, suggesting that the myogenic response is age-dependent and developmentally regulated (Su et al., 2003). Consistent with these observations, we have observed the myogenic response in TMA from 3 day old rat pups (Figure 4), but not in adult rat TMA (Figure 1). Since the myogenic response leads to vasoconstriction with flow or pressure stimulation, this response in newborns may make the intestine more vulnerable to ischemia/reperfusion injury. This effect may be abrogated by the vasodilatory effect of HB-EGF.
Ischemia has been implicated in the pathogenesis of NEC (Nowicki, 2005). Even though ischemia may not be the sole basis for NEC-related tissue damage, an initial blood flow perturbation and the consequent cascade of ischemic events contribute to the development of NEC (Markel et al., 2006). Furthermore, the expression of the potent vasoconstrictor ET-1 is increased in the diseased tissue of acute NEC removed in the operating room (Nowicki et al., 2005) and in experimental NEC in rodents (Ito et al., 2007). ET-1 dramatically decreases capillary perfusion and compromises oxygen consumption in vitro (Nowicki and Minnich, 1999, Nankervis et al., 2000). Here we show that HB-EGF retains its vasodilatory capabilities even in the presence of ET-1. These findings suggest that HB-EGF can be used in the treatment of ET-1-mediated ischemic diseases including NEC. Since preservation of blood flow through terminal mesenteric arterioles after ET-1-mediated intestinal ischemic injury determines subsequent tissue viability, we believe that administration of HB-EGF may improve oxygen delivery and restore tissue metabolism by reversing the vasoconstriction caused by ET-1.
Intestinal blood flow is regulated by a delicate balance between arteriolar vasodilators and vasoconstrictors. Nitric oxide is thought to play a pivotal role in vasodilation. Previous studies reported that sustained vasoconstriction occurs in neonatal swine due to reduced production of nitric oxide by newborn gut endothelium (Nankervis and Nowicki, 1995). Lack of nitric oxide production may leave the vessels more vulnerable to the vasoconstrictive effects of ET-1. This type of imbalance appears to contribute to the pathogenesis of NEC (Nankervis and Nowicki, 1995, Nowicki, 1996). The ability of L-NMMA to specifically block HB-EGF-induced vasodilation in rat pup TMA suggests that HB-EGF-induced vasodilation in rat pups may occur via increased endogenous nitric oxide production in endothelial cells. Similar observations were found in SMA resected from the intestine of human infants. However, L-NMMA did not block the effect of HB-EGF in adult rat TMA. In adult rats, the ETB receptor antagonist BQ788 significantly blocked HB-EGF-mediated vasodilation, indicating that the dilatory effects of HB-EGF in adult animals are dependent upon activation of ETB receptors. This was also true in arterioles from rat pups and human infants. Previous studies have documented that activation of ETB receptors on vascular endothelial cells leads to vessel dilation by transient nitric oxide release (Hirata et al., 1993, Tirapelli et al., 2005, Pollock and Pollock, 2008). However, this may not fully explain HB-EGF-induced vessel dilation which lasts for at least 90 minutes. To further study the effect of ETB receptor activation, we used HIMEC and found that HB-EGF increased ETB receptor protein detection in HIMEC cytoplasm after HB-EGF treatment, especially after 3h HB-EGF treatment. Naturally, ETB receptor is constitutively internalized and delivered to lysosomes where it is rapidly degraded (Ozaki et al., 1995, Wu-Wong et al., 1995, Abe et al., 2000). Based on our observation, HB-EGF may promote ETB receptor protein stabilization or slow its degradation. Detail mechanism need to be further illustrated. The ETB receptor has also been reported to couple with calcium pumps and the activation of ETB receptors leads to elevation of intracellular calcium levels (Jouneaux et al., 1993, Jacques et al., 2006). Vascular endothelial cell initial calcium spikes with agonist stimulation commonly occur via release of intracellular calcium stores (Nilius and Droogmans, 2001), which is well known to promote angiogenesis (Mehta et al., 2008), increase vascular permeability (Bates and Curry, 1997, Tiruppathi et al., 2006) and promote secretion of vasoactive substances including nitric oxide and endothelium derived growth factor (Adams et al., 1989, Yamamoto et al., 2000). We found that HB-EGF triggered an instant calcium spike in HIMEC, and that this HB-EGF-induced calcium spike was blocked by ETB receptor antagonism. We believe that HB-EGF-induced vasodilation occurs via the activation of ETB receptors, with associated intracellular calcium mobilization leading to the release of vasodilatory substances.
In summary, we have shown that HB-EGF is a potent vasodilator of the intestinal microvasculature which can counteract the vasoconstrictive effects of ET-1. HB-EGF appears to work by increasing ETB receptor expression in adult rat and rat pup arterioles, and also by increasing NO production in rat pup and human infant arterioles. HB-EGF exerts its vasodilatory effect via triggering intracellular calcium mobilization in vascular endothelial cells. The vasodilatory effects of HB-EGF support its use in the treatment of diseases manifested by intestinal ischemic injury, including necrotizing enterocolitis.
Supplementary Material
Supplementary Figure 1. Effect of BQ610 on pressure-induced vessel diameter in adult rat TMA treated with ET-1. Pressures were increased from 0 to 100 mmHg in 20 mmHg increments initially under control conditions, then after the addition of ET-1 (20 pmol/ml) to the suffusion buffer, and then after the addition of BQ610 (20 μmol/ml) to the suffusion buffer in the presence of ET-1. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. n = 5 vessels; *P < 0.05 for ET-1 vs. control.
Supplementary Figure 2. Relationship between intraluminal pressure and vessel diameter in 3-day-old TMA. Passive vessel dilation was achieved by exposing vessels to Ca2+-free buffer containing EGTA (1 mmol) and papaverine (10 μmol) for at least 30 min. Control vessels were exposed to standard Krebs buffer. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. n = 5 vessels in each group, *P < 0.01 for passive vs. control.
Supplementary Figure 3. Effect of HB-EGF on ET secretion in HIMEC treated with HB-EGF. HIMEC were treated with HB-EGF (10 or 100 ng/ml) for 3 h. HIMEC treated with TGF-β1 (100ng/ml) for 24 h were used as a positive control. Values represent the mean ± SEM. *P<0.01.
Acknowledgments
The authors thank Dave Dunaway for his help with the video dimension analysis system, Dr. Craig Nankervis for his help with human tissue harvesting, and Dr. Philip Nowicki for his helpful advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Nakayama K, Yamanaka A, Sakurai T, Goto K. Subtype-specific trafficking of endothelin receptors. J Biol Chem. 2000;275:8664–8671. doi: 10.1074/jbc.275.12.8664. [DOI] [PubMed] [Google Scholar]
- Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. Faseb J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Adner M, Geary GG, Edvinsson L. Appearance of contractile endothelin-B receptors in rat mesenteric arterial segments following organ culture. Acta Physiol Scand. 1998;163:121–129. doi: 10.1046/j.1365-201X.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am J Physiol. 1997;273:H687–694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KM, Brigstock DR, Johnson PR, Crissman-Combs MA, McCarthy DW, Downing MT, Besner GE. Production of glycosylated heparin-binding EGF-like growth factor in HeLa cells using vaccinia virus. Protein Expr Purif. 1996;8:57–67. doi: 10.1006/prep.1996.0074. [DOI] [PubMed] [Google Scholar]
- Dawson NS, Zawieja DC, Wu MH, Granger HJ. Signaling pathways mediating VEGF165-induced calcium transients and membrane depolarization in human endothelial cells. Faseb J. 2006;20:991–993. doi: 10.1096/fj.05-3923fje. [DOI] [PubMed] [Google Scholar]
- El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor and intestinal ischemia-reperfusion injury. Semin Pediatr Surg. 2004;13:2–10. doi: 10.1053/j.sempedsurg.2003.09.002. [DOI] [PubMed] [Google Scholar]
- El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. discussion 144–149. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W, Caplan MS, Sun X, Tan X, MacKendrick W, Gonzalez-Crussi F. Platelet-activating factor, tumor necrosis factor, hypoxia and necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:11–17. doi: 10.1111/j.1651-2227.1994.tb13234.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Doelle SM, Clark JA, Halpern MD, McCuskey RS, Dvorak B. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res. 2007;61:180–184. doi: 10.1203/pdr.0b013e31802d77db. [DOI] [PubMed] [Google Scholar]
- Jacques D, Sader S, Perreault C, Abdel-Samad D, Jules F, Provost C. NPY, ET-1, and Ang II nuclear receptors in human endocardial endothelial cells. Can J Physiol Pharmacol. 2006;84:299–307. doi: 10.1139/y05-158. [DOI] [PubMed] [Google Scholar]
- Jouneaux C, Goldsmith P, Hanoune J, Lotersztajn S. Endothelin inhibits the calcium pump and stimulates phosphoinositide phospholipase C in liver plasma membranes via two different G proteins, Gs and Gq. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S158–160. doi: 10.1097/00005344-199322008-00042. [DOI] [PubMed] [Google Scholar]
- Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–1103. doi: 10.1056/NEJM198404263101707. [DOI] [PubMed] [Google Scholar]
- Kuhn MA, Xia G, Mehta VB, Glenn S, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal. 2002;4:639–646. doi: 10.1089/15230860260220148. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez ML, Mehta V, Michalsky MP, Fleming JB, Besner GE. Heparin-binding EGF-like growth factor down regulates proinflammatory cytokine-induced nitric oxide and inducible nitric oxide synthase production in intestinal epithelial cells. Nitric Oxide. 2002;6:142–152. doi: 10.1006/niox.2001.0393. [DOI] [PubMed] [Google Scholar]
- Marasciulo FL, Montagnani M, Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem. 2006;13:1655–1665. doi: 10.2174/092986706777441968. [DOI] [PubMed] [Google Scholar]
- Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25:329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991;84:1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Zhou Y, Radulescu A, Besner GE. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors. 2008;26:301–15. doi: 10.1080/08977190802393596. [DOI] [PubMed] [Google Scholar]
- Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol. 2008;294:F1205–11. doi: 10.1152/ajprenal.00578.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankervis CA, Dunaway DJ, Nowicki PT. Determinants of terminal mesenteric artery resistance during the first postnatal month. Am J Physiol Gastrointest Liver Physiol. 2001a;280:G678–686. doi: 10.1152/ajpgi.2001.280.4.G678. [DOI] [PubMed] [Google Scholar]
- Nankervis CA, Giannone PJ, Reber KM. The Neonatal Intestinal Vasculature: Contributing Factors to Necrotizing Enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol. 1995;268:G949–958. doi: 10.1152/ajpgi.1995.268.6.G949. [DOI] [PubMed] [Google Scholar]
- Nankervis CA, Nowicki PT. Role of endothelin-1 in regulation of the postnatal intestinal circulation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G367–375. doi: 10.1152/ajpgi.2000.278.3.G367. [DOI] [PubMed] [Google Scholar]
- Nankervis CA, Reber KM, Nowicki PT. Age-dependent changes in the postnatal intestinal microcirculation. Microcirculation. 2001b;8:377–387. doi: 10.1038/sj/mn/7800110. [DOI] [PubMed] [Google Scholar]
- Nankervis CA, Schauer GM, Miller CE. Endothelin-mediated vasoconstriction in postischemic newborn intestine. Am J Physiol Gastrointest Liver Physiol. 2000;279:G683–691. doi: 10.1152/ajpgi.2000.279.4.G683. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Nowicki P. Intestinal ischemia and necrotizing enterocolitis. J Pediatr. 1990;117:S14–19. doi: 10.1016/s0022-3476(05)81125-4. [DOI] [PubMed] [Google Scholar]
- Nowicki PT. The effects of ischemia-reperfusion on endothelial cell function in postnatal intestine. Pediatr Res. 1996;39:267–274. doi: 10.1203/00006450-199602000-00014. [DOI] [PubMed] [Google Scholar]
- Nowicki PT. Ischemia and necrotizing enterocolitis: where, when, and how. Semin Pediatr Surg. 2005;14:152–158. doi: 10.1053/j.sempedsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Nowicki PT, Dunaway DJ, Nankervis CA, Giannnone PJ, Reber KM, Hammond SB, Besner GE, Caniano DA. Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr. 2005;146:805–810. doi: 10.1016/j.jpeds.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Nowicki PT, Minnich LA. Effects of systemic hypotension on postnatal intestinal circulation: role of angiotensin. Am J Physiol. 1999;276:G341–352. doi: 10.1152/ajpgi.1999.276.2.G341. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Ohwaki K, Ihara M, Fukuroda T, Ishikawa K, Yano M. ETB-mediated regulation of extracellular levels of endothelin-1 in cultured human endothelial cells. Biochem Biophys Res Commun. 1995;209:483–489. doi: 10.1006/bbrc.1995.1527. [DOI] [PubMed] [Google Scholar]
- Pillai SB, Hinman CE, Luquette MH, Nowicki PT, Besner GE. Heparin-binding epidermal growth factor-like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res. 1999;87:225–231. doi: 10.1006/jsre.1999.5764. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. Faseb J. 1995;9:1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Pollock DM. Endothelin and NOS1/nitric oxide signaling and regulation of sodium homeostasis. Curr Opin Nephrol Hypertens. 2008;17:70–75. doi: 10.1097/MNH.0b013e3282f34b02. [DOI] [PubMed] [Google Scholar]
- Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology Clin Perinatol. 2002;29:23–39. doi: 10.1016/s0095-5108(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Rocourt DV, Mehta VB, Besner GE. Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J Surg Res. 2007;139:269–273. doi: 10.1016/j.jss.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MI, Reblock KK, Kurkchubasche AG, Healey PJ. Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg. 1994;29:987–990. doi: 10.1016/0022-3468(94)90264-x. discussion 990–981. [DOI] [PubMed] [Google Scholar]
- Su BY, Reber KM, Nankervis CA, Nowicki PT. Development of the myogenic response in postnatal intestine: role of PKC. Am J Physiol Gastrointest Liver Physiol. 2003;284:G445–452. doi: 10.1152/ajpgi.00259.2002. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Casolari DA, Yogi A, Montezano AC, Tostes RC, Legros E, D’Orleans-Juste P, de Oliveira AM. Functional characterization and expression of endothelin receptors in rat carotid artery: involvement of nitric oxide, a vasodilator prostanoid and the opening of K+ channels in ETB-induced relaxation. Br J Pharmacol. 2005;146:903–912. doi: 10.1038/sj.bjp.0706388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- Unthank JL, Lash JM, Bohlen HG. Maturation of the rat intestinal microvasculature from juvenile to early adult life. Am J Physiol. 1990;259:G282–289. doi: 10.1152/ajpgi.1990.259.2.G282. [DOI] [PubMed] [Google Scholar]
- Vanner S, Jiang MM, Brooks VL, Surprenant A. Characterization of vasopressin actions in isolated submucosal arterioles of the intestinal microcirculation. Circ Res. 1990;67:1017–1026. doi: 10.1161/01.res.67.4.1017. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Chiou WJ, Magnuson SR, Opgenorth TJ. Endothelin receptor in human astrocytoma U373MG cells: binding, dissociation, receptor internalization. J Pharmacol Exp Ther. 1995;274:499–507. [PubMed] [Google Scholar]
- Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434–439. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Effect of BQ610 on pressure-induced vessel diameter in adult rat TMA treated with ET-1. Pressures were increased from 0 to 100 mmHg in 20 mmHg increments initially under control conditions, then after the addition of ET-1 (20 pmol/ml) to the suffusion buffer, and then after the addition of BQ610 (20 μmol/ml) to the suffusion buffer in the presence of ET-1. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. n = 5 vessels; *P < 0.05 for ET-1 vs. control.
Supplementary Figure 2. Relationship between intraluminal pressure and vessel diameter in 3-day-old TMA. Passive vessel dilation was achieved by exposing vessels to Ca2+-free buffer containing EGTA (1 mmol) and papaverine (10 μmol) for at least 30 min. Control vessels were exposed to standard Krebs buffer. Each point represents the mean vessel diameter of all arterioles tested, with error bars representing SEM. n = 5 vessels in each group, *P < 0.01 for passive vs. control.
Supplementary Figure 3. Effect of HB-EGF on ET secretion in HIMEC treated with HB-EGF. HIMEC were treated with HB-EGF (10 or 100 ng/ml) for 3 h. HIMEC treated with TGF-β1 (100ng/ml) for 24 h were used as a positive control. Values represent the mean ± SEM. *P<0.01.