Abstract
Focal adhesion kinase (FAK) is important for breast cancer progression and invasion and is necessary for the dynamic turnover of focal adhesions. However, it has not been determined whether FAK also regulates the dynamics of invasive adhesions formed in cancer cells known as invadopodia. In this study, we report that endogenous FAK functions upstream of cellular Src (c-Src) as a negative regulator of invadopodia formation and dynamics in breast cancer cells. We show that depletion of FAK induces the formation of active invadopodia but impairs invasive cell migration. FAK-deficient MTLn3 breast cancer cells display enhanced assembly and dynamics of invadopodia that are rescued by expression of wild-type FAK but not by FAK that cannot be phosphorylated at tyrosine 397. Moreover, our findings demonstrate that FAK depletion switches phosphotyrosine-containing proteins from focal adhesions to invadopodia through the temporal and spatial regulation of c-Src activity. Collectively, our findings provide novel insight into the interplay between FAK and Src to promote invasion.
Introduction
Efficient cancer cell migration through tissue barriers requires dynamic interactions between the ECM and the actin cytoskeleton as well as the capacity for matrix degradation (Wolf and Friedl, 2006). A prominent feature of the cell migration machinery is the generation of organized adhesive structures. The most extensively characterized of these structures, focal adhesions, contain clusters of integrin receptors tethered to ends of actin stress fibers (Schoenwaelder and Burridge, 1999; Carragher and Frame, 2004). Highly invasive cancer cells also form specialized actin-rich ECM-degrading membrane protrusions known as invadopodia, which, in contrast to focal adhesions, are primary sites of rapid actin polymerization (Buccione et al., 2004, 2009; Yamaguchi et al., 2006; Linder, 2007). Significant progress has been made in characterizing the form and function of invadopodia, which are comprised of actin regulators such as Arp2/3, neural Wiscott-Aldrich syndrome protein, gelsolin, and cortactin (Yamaguchi et al., 2005; Clark et al., 2007) and are dependent on Src kinase activity (Spinardi et al., 2004). Invadopodia also contain concentrated foci of matrix-degrading proteases such as matrix metalloproteinases (MMPs; Linder and Aepfelbacher, 2003). A stepwise model for invadopodia formation has been proposed (Artym et al., 2006); however, the precise mechanisms that regulate invadopodia formation and dynamics are not well understood.
FAK is a nonreceptor tyrosine kinase that plays a central role in cell migration (Mitra et al., 2005). FAK has been shown to coordinate lamellipodial formation and focal adhesion turnover in fibroblasts (Webb et al., 2004; Tilghman et al., 2005). Increased expression of FAK has been detected in metastatic tumors of the breast, prostate, colon, and brain (Owens et al., 1995; Cance et al., 2000; Gabarra-Niecko et al., 2003; Lark et al., 2005). Recent evidence suggests that FAK may be involved in cancer progression, as FAK is required for ErbB2-mediated oncogenic transformation and invasion (Benlimame et al., 2005). Furthermore, mammary epithelial-specific disruption of FAK has been shown to block the transition to carcinoma formation in a mouse transgenic cancer model (Lahlou et al., 2007). In addition, stable expression of the FAK-related nonkinase, an alternative transcript of FAK, has been shown to block mammary carcinoma lung metastases (van Nimwegen et al., 2005).
FAK promotes cell migration by its ability to bridge signals between integrin and growth factor receptors (Sieg et al., 2000). Downstream of integrin or growth factor stimulation, FAK is cis- or trans-phosphorylated at Tyr-397, which provides a critical binding site for Src family kinases (Schaller et al., 1994), the p85 regulatory subunit of phosphatidylinositol 3-kinase (Chen and Guan, 1994), and phospholipase Cγ (Zhang et al., 1999). In particular, Src binding to Tyr-397 is important for phosphorylation of Tyr-576/577, which is important for full FAK activation. Subsequently, an activated FAK–Src complex mediates the phosphorylation of multiple adhesion components involved in the dynamic regulation of cell motility, including p130Cas (Tachibana et al., 1997) and paxillin (Bellis et al., 1995).
In this study, we sought to determine whether FAK is involved in cell invasion through the regulation of invadopodia, which are thought to be responsible for the ability of cancer cells to degrade and invade through the matrix. Intriguingly, we find that depletion of FAK enhances the formation and dynamics of matrix-degrading invadopodia, which is rescued upon expression of wild-type FAK but not by expression of FAK that cannot be phosphorylated at Tyr-397. We demonstrate that enhanced invadopodia formation in FAK-depleted cells is not sufficient for cancer cell invasion. We also provide evidence that depletion of FAK expression regulates a switch from phosphotyrosine-containing proteins at focal adhesions to invadopodia via the spatial and temporal regulation of active Src. Together, our findings provide novel insight into the mechanism of FAK–Src signaling in cancer cell invasion.
Results
FAK negatively regulates invadopodia formation and dynamics
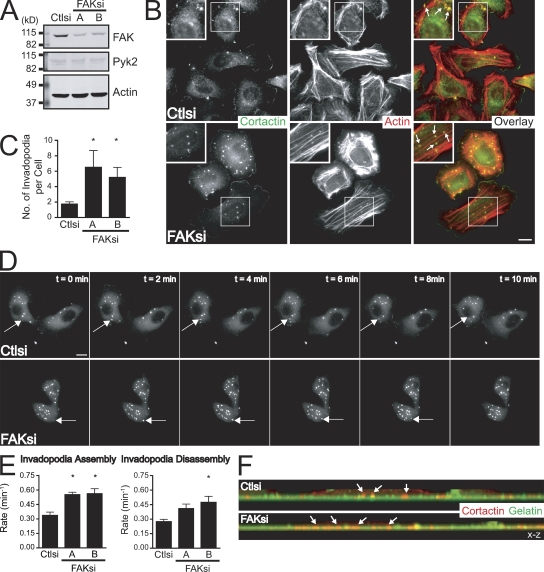
Previous studies have demonstrated a critical role for FAK in regulating the turnover of focal adhesions (Webb et al., 2004; Schober et al., 2007); however, a role for FAK in regulating the dynamics of invadopodia in cancer cells has not been characterized. MTLn3 mammary adenocarcinoma cells form invadopodia when plated on fibronectin or fibronectin-gelatin substrates, and the formation of these structures is thought to correlate with invasive capacity (Yamaguchi et al., 2005). To generate FAK-deficient MTLn3 breast cancer cells, we used two independent siRNAs that depleted endogenous FAK by 80% (Fig. 1 A) without affecting the expression of FAK-related proline-rich tyrosine kinase (Pyk2; Lim et al., 2008). Invadopodia structures were identified in cells cultured on fibronectin-coated coverslips stained for the invadopodia markers cortactin and actin (Fig. 1 B). We unexpectedly found that FAK-deficient MTLn3 cells formed increased numbers of invadopodia on fibronectin, fibronectin-gelatin, and Matrigel surfaces (Fig. 1 C and unpublished data). We also observed increased invadopodia formation in FAK-deficient MDA-MB-231 human mammary adenocarcinoma cells (Fig. S1) and SNB19 human glioblastoma cells (unpublished data), indicating that the ability of endogenous FAK to suppress invadopodia formation may be a general property of invasive cancer cells.
Figure 1.
FAK negatively regulates invadopodia formation and dynamics. (A) Cell lysates from MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi A and FAKsi B) were analyzed by Western blotting and probed for FAK or Pyk2. Actin was probed as a loading control. (B) MTLn3 cells were plated on fibronectin-coated coverslips and stained with anticortactin antibody (green) and rhodamine-phalloidin (red). Boxed regions depict regions of invadopodia shown magnified in insets. (C) Quantification of cortactin- and actin-containing invadopodia is expressed as the mean number of invadopodia per cell. (D) GFP-cortactin was transiently cotransfected with control siRNA or FAK siRNA (FAK siRNA B is shown) into MTLn3 cells. Cells were plated on fibronectin-coated glass-bottomed dishes and analyzed by time-lapse fluorescence microscopy. Time-lapse montages demonstrate representative images of the dynamics of the invadopodia marker GFP-cortactin over a period of 10 min. (E) Rate constants for assembly and disassembly were calculated from plots of fluorescence intensities of GFP-cortactin as described in Materials and methods (Videos 1–3). (F) Representative x-z confocal images of invadopodia in MTLn3 cells show cortactin-containing invadopodia (red) projecting into the gelatin matrix (green). Arrows indicate representative invadopodia. Data shown are means ± SEM of three independent experiments. *, P < 0.05 by t test compared with control siRNA. Bars, 10 µm.
To determine how FAK regulates invadopodia formation, we visualized the dynamics of the invadopodia marker GFP-cortactin (Perrin et al., 2006; Cortesio et al., 2008) in control or FAK-deficient MTLn3 cells by time-lapse microscopy (Fig. 1 D and Videos 1–3). FAK-deficient cells formed significantly greater numbers of GFP-cortactin–containing invadopodia than control cells. In this study, we analyzed the dynamics of short-lived invadopodia precursor structures (Yamaguchi et al., 2005; Cortesio et al., 2008). Live fluorescence imaging demonstrated that FAK-depleted cells formed invadopodia that were present for a shorter duration than those formed in control cells by ∼2 min (unpublished data). From plots of GFP-cortactin fluorescence intensities over time, we generated rate constants for net invadopodia assembly and disassembly (Fig. 1 E). The rate of invadopodia assembly was significantly enhanced by twofold in FAK-deficient cells as compared with control cells. We also found modest increases in the rate of disassembly of invadopodia, indicating that invadopodia dynamics are enhanced in FAK-deficient breast cancer cells. Additionally, confocal microscopy revealed that invadopodia formed by FAK-deficient MTLn3 cells were still able to project vertically from the ventral membrane surface into a gelatin matrix (Fig. 1 F). Collectively, our results identify a novel role for endogenous FAK as a negative regulator of invadopodia formation and dynamics in breast cancer cells. These findings are especially intriguing in light of data demonstrating that depletion of FAK results in impaired focal adhesion dynamics (Webb et al., 2004).
Enhanced invadopodia formation in FAK-deficient MTLn3 cells is not sufficient for invasion
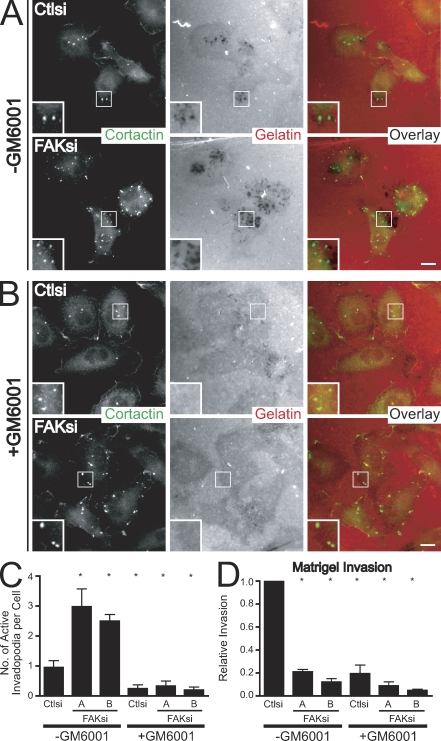
Because the matrix-degrading function of invadopodia has been attributed to the focalized activity of MMPs (Chen and Wang, 1999; Artym et al., 2006), we examined whether the invadopodia formed in FAK-deficient MTLn3 cells retained their capacity to degrade the ECM. We cultured cells on fibronectin–Alexa Fluor 568 gelatin substrates in the absence or presence of the pan-MMP inhibitor GM6001 (Fig. 2, A and B). FAK-deficient MTLn3 cells displayed a threefold increase in the formation of active invadopodia associated with focal areas of matrix degradation as compared with control cells (Fig. 2 C). The regions of matrix degradation formed in both control and FAK-deficient cells required the activity of MMPs because GM6001 impaired matrix degradation without affecting the formation of cortactin-containing puncta. To determine whether formation of invadopodia correlates with the invasive capacity of MTLn3 cancer cells, we assayed the ability of serum-starved MTLn3 cells to invade toward serum through Matrigel-coated membranes (Fig. 2 D). Invasion of MTLn3 control and FAK-deficient cells across Matrigel was impaired in the presence of GM6001, suggesting that the formation of functional invadopodia is necessary for Matrigel invasion. However, FAK-deficient cells, despite forming more active invadopodia, displayed substantially impaired Matrigel invasion as compared with control cells, demonstrating that enhanced formation of invadopodia in FAK-deficient cells is not sufficient to mediate invasive cell migration.
Figure 2.
Enhanced invadopodia formation in FAK-deficient MTLn3 cells requires MMP activity but is not sufficient for invasion. (A) MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi) were cultured on fibronectin–Alexa Fluor 568 gelatin coverslips in the presence of DMSO vehicle control (−GM6001) and stained with anticortactin antibody (green). (B) MTLn3 cells transiently transfected with control siRNA or FAK siRNA were cultured on fibronectin–Alexa Fluor 568 gelatin-coated coverslips in the presence of 50 µM GM6001 (+GM6001) and stained with anticortactin antibody (green). Boxed regions in A and B depict regions of invadopodia shown magnified in insets. (C) Quantification of active invadopodia, defined as colocalizing areas of cortactin staining and matrix degradation, is expressed as the mean number of active invadopodia per cell. (D) MTLn3 cells transiently transfected with control siRNA or FAK siRNA were cultured in the presence of vehicle control (−GM6001) or 50 µM GM6001 (+GM6001) and were assayed for their ability to invade through Matrigel-coated membranes. Relative invasion was determined by normalizing the number of cells invaded to those transfected with control siRNA (−GM6001). Data shown are means ± SEM of three independent experiments. *, P < 0.05 by one-way ANOVA compared with control siRNA (−GM6001). Bars, 10 µm.
Expression of wild-type FAK but not Y397F-FAK rescues enhanced invadopodia formation and impaired invasion upon FAK depletion in MTLn3 cells
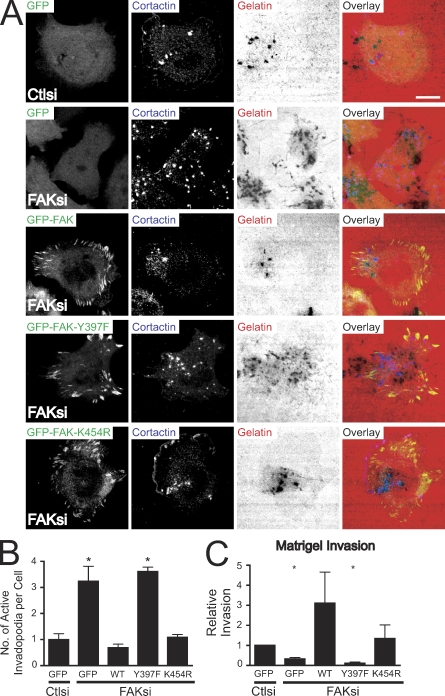
To begin to investigate how FAK regulates invadopodia formation, we generated MTLn3 cell lines stably expressing GFP, wild-type GFP-FAK, the SH2 domain-binding mutant GFP-FAK-Y397F, and the kinase-inactive mutant GFP-FAK-K454R. Treatment with FAK siRNA (which targets a sequence in the 5′ untranslated region of FAK) effectively reduced endogenous FAK expression without affecting the expression of GFP-FAK, GFP-FAK-Y397F, or GFP-FAK-K454R (Fig. S2 A). We tested the capacity of the cell lines cultured on fibronectin–Alexa Fluor 568 gelatin substrates to form invadopodia and degrade the ECM (Fig. 3 A). Depletion of endogenous FAK in GFP-expressing cells resulted in significantly enhanced numbers of active invadopodia (Fig. 3 B). Expression of wild-type FAK restored numbers of active invadopodia to those observed in control cells. However, GFP-FAK-Y397F expression failed to rescue invadopodia numbers. Expression of kinase-dead GFP-FAK also rescued invadopodia but not to the same extent as wild-type FAK. We next assayed the ability of the cell lines to invade through Matrigel-coated membranes (Fig. 3 C). Depletion of FAK in MTLn3 cells expressing GFP resulted in significantly impaired invasion. Invasion was restored in cells expressing wild-type and kinase-dead GFP-FAK but not in cells expressing GFP-FAK-Y397F, suggesting that phosphorylation of FAK at Tyr-397 plays a critical role in limiting invadopodia formation and is also required for efficient invasion.
Figure 3.
Expression of wild-type but not Y397F-FAK restores enhanced matrix-degrading invadopodia and impaired invasion in FAK-deficient MTLn3 cells. (A) MTLn3 cells stably expressing GFP, GFP-FAK (wild type), GFP-FAK-Y397F, and GFP-FAK-K454R were transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi), cultured on fibronectin–Alexa Fluor 568 gelatin coverslips, and stained with anticortactin antibody (blue). Bar, 10 µm. (B) Quantification of active invadopodia, defined as colocalizing areas of cortactin staining and matrix degradation, is expressed as the mean number of active invadopodia per cell. (C) MTLn3 cells treated as in A were assayed for their ability to invade through Matrigel-coated membranes. Relative invasion was determined by normalizing the number of cells invaded to those transfected with control siRNA. WT, wild type. Data shown are means ± SEM of three independent experiments. *, P < 0.05 by t test compared with GFP-expressing cells transfected with control siRNA.
FAK has been shown to be important for the regulation of focal adhesion morphology and turnover in fibroblasts, which contribute to efficient migration (Sieg et al., 1999; Webb et al., 2004). In accordance with these studies, FAK-deficient MTLn3 cells displayed enlarged vinculin-containing focal adhesions at the cell periphery, which is consistent with impaired focal adhesion disassembly in fibroblasts depleted of FAK (Fig. S2 B). This corresponded to a threefold reduction in transwell migration as compared with control cells expressing GFP (Fig. S2 C). Focal adhesion morphology and migration were restored in cells expressing GFP-FAK or GFP-FAK-K454R but not GFP-FAK-Y397F. Collectively, these data are consistent with the hypothesis that FAK and its phosphorylation at Tyr-397 are critical in the differential regulation of focal adhesion and invadopodia dynamics to modulate migration and invasion.
Previous studies have suggested that FAK localizes to invadopodia structures (Hauck et al., 2002; Hsia et al., 2003). To determine whether FAK localizes to invadopodia in MTLn3 cells, we performed immunofluorescent cell staining in cells stably expressing GFP-FAK and visualized them by confocal microscopy (Fig. S2 D). We plated cells on fibronectin-gelatin coverslips and stained for vinculin, a known marker of focal adhesions, and Arp2/3, which is known to localize to invadopodia (Yamaguchi et al., 2005). Z sectioning revealed that GFP-FAK and vinculin were localized to focal adhesions, and we did not detect the presence of vinculin or FAK in Arp2/3-containing invadopodia. These data indicate that the invadopodia formed by MTLn3 cells are morphologically distinct from podosomes, which contain vinculin-rich adhesive rings (Buccione et al., 2004; Oikawa et al., 2008). We were unable to stain for endogenous FAK, but we found that endogenous phosphorylated FAK at Tyr-397 localized with GFP-vinculin at focal adhesions but not cortactin-containing invadopodia (Fig. S2 E). Collectively, these data suggest that FAK does not localize to invadopodia in MTLn3 cells, which is consistent with experiments performed in MDA-MB-231 breast cancer cells (Bowden et al., 2006), but nevertheless, is able to modulate invadopodia formation.
FAK functions upstream of Src kinase activity to negatively regulate invadopodia formation
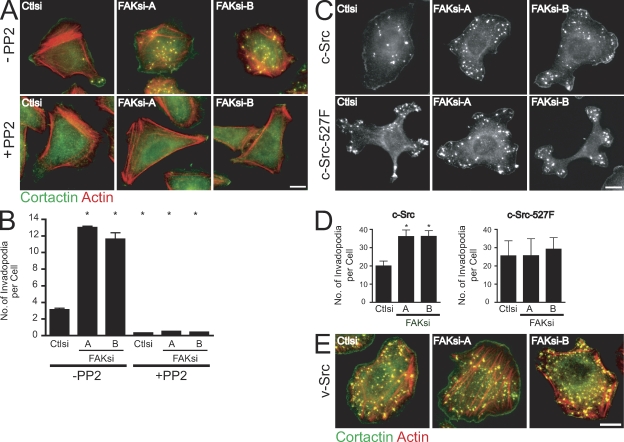
Because phosphorylation of FAK at Tyr-397 is known to mediate Src binding (Schaller et al., 1999; Sieg et al., 1999), we next wanted to determine whether FAK modulates invadopodia formation through a Src-dependent pathway. Substantial evidence supports a critical role for Src tyrosine kinases in podosome and invadopodia formation (Linder and Aepfelbacher, 2003; Spinardi et al., 2004). Thus, we assayed the ability of control or FAK-deficient MTLn3 cells to form invadopodia on fibronectin-gelatin coverslips in the absence or presence of the Src kinase inhibitor PP2 (Fig. 4 A). Accordingly, we found that the enhanced invadopodia formation observed in FAK-deficient cells required Src activity (Fig. 4 B). To determine whether FAK functions upstream or downstream of Src, we examined invadopodia formation in MTLn3 cells stably expressing cellular Src (c-Src) or activated c-Src–527F (Fig. 4 C). Cells expressing c-Src or c-Src–527F formed dramatically enhanced numbers of cortactin-containing invadopodia as compared with control cells. Depletion of FAK in cells that overexpress c-Src resulted in a significant increase in invadopodia (Fig. 4 D). However, in cells expressing c-Src–527F or constitutively active viral Src (v-Src; Fig. 4 E), we found that depletion of FAK did not enhance invadopodia formation, suggesting that c-Src–527F or v-Src functions independently of endogenous FAK to modulate invadopodia formation. This is the first study to show that endogenous FAK functions as a negative regulator of invadopodia formation in cancer cells upstream of c-Src.
Figure 4.
FAK functions upstream of Src kinase to regulate invadopodia formation. (A) MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi-A and FAKsi-B) were cultured on fibronectin-gelatin coverslips in the presence of vehicle control (−PP2) or 2 µM PP2 (+PP2). Cells were stained with anticortactin antibody (green) and rhodamine-phalloidin (red). (B) Quantification of cortactin- and actin-containing invadopodia is expressed as the mean number of invadopodia per cell. (C) MTLn3 cells expressing c-Src or c-Src–527F were transiently transfected with control siRNA or FAK siRNA, cultured on fibronectin-gelatin coverslips, and stained with anticortactin antibody. (D) Quantification of cortactin-containing invadopodia in c-Src– or c-Src–527F-expressing cell lines is expressed as the mean number of invadopodia per cell. (E) MTLn3 cells expressing v-Src were transiently transfected with control siRNA or FAK siRNA and cultured on fibronectin-gelatin coverslips. Cells were stained with anticortactin antibody (green) and rhodamine-phalloidin (red). Data shown are means ± SEM of three independent experiments. *, P < 0.05 by one-way ANOVA compared with control siRNA (−PP2). Bars, 10 µm.
FAK depletion regulates a switch between phosphotyrosine-containing proteins at focal adhesions to invadopodia
The FAK–Src complex can bind to and phosphorylate diverse substrates to regulate adhesion dynamics and cell migration (Mitra et al., 2005). Also, tyrosine phosphorylation is a critical mechanism by which focal adhesion dynamics are regulated (Crowley and Horwitz, 1995; Zamir et al., 1999). Therefore, we reasoned that FAK may differentially regulate invadopodia and focal adhesions through its effects on localized tyrosine phosphorylation. We examined the distribution of tyrosine-phosphorylated proteins in control and FAK-deficient MTLn3 cells cultured on fibronectin-gelatin substrates (Fig. 5 A). Control cells displayed robust tyrosine phosphorylation at focal adhesions that contained GFP-paxillin and GFP-vinculin (unpublished data) and at invadopodia positive for Arp2/3 staining. In contrast, FAK-deficient MTLn3 cells exhibited dramatically reduced tyrosine phosphorylation at focal adhesions but displayed strong tyrosine phosphorylation at invadopodia. Additionally, we tested the localization of a phosphotyrosine reporter construct consisting of two tandem Src SH2 phosphotyrosine-binding domains (YFP-dSH2), which specifically detects tyrosine phosphorylation at focal adhesions (Fig. 5 B; Kirchner et al., 2003). YFP-dSH2 was strongly localized with phosphotyrosine at focal adhesions, but not invadopodia, in control cells. However, upon FAK depletion, phosphotyrosine staining at focal adhesions and YFP-dSH2 localization were severely impaired. Accordingly, live fluorescence microscopy of YFP-dSH2 in control cells revealed its localization at dynamic focal adhesion structures (Videos 4 and 5). YFP-dSH2 localization at focal adhesions in FAK-deficient cells was decreased, and the YFP-dSH2 that was present at focal adhesions displayed impaired dynamics. Interestingly, we did not detect any clear localization at invadopodia puncta, indicating the predominant specificity of the YFP-dSH2 probe for tyrosine-phosphorylated substrates at focal adhesions. Together, these data support the hypothesis that FAK regulates a balance between phosphotyrosine-containing proteins at focal adhesions and invadopodia.
Figure 5.
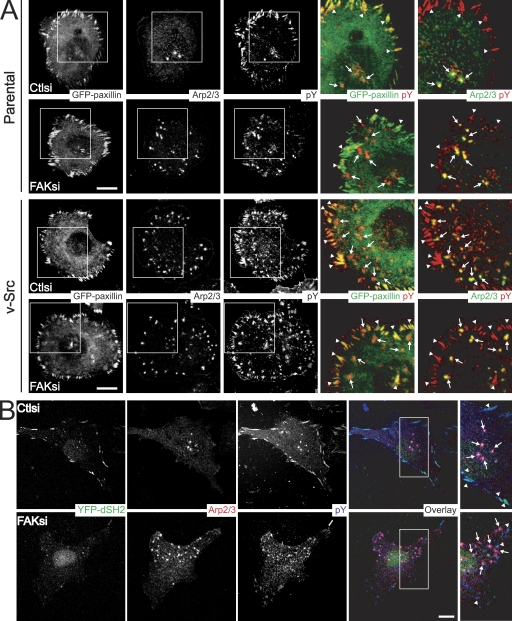
FAK differentially regulates tyrosine phosphorylation at focal adhesions and invadopodia. (A) MTLn3 (parental) cells or v-Src–transformed MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi) and cotransfected with GFP-paxillin were cultured on fibronectin-gelatin coverslips and stained with anti-p34Arc (Arp2/3 subunit) antibody and antiphosphotyrosine antibody. Two sets of overlays are depicted to illustrate localization of GFP-paxillin or Arp2/3 (green) with phosphotyrosine (pY) staining (red). (B) MTLn3 cells transiently transfected with control siRNA or FAK siRNA and cotransfected with YFP-dSH2 were cultured on fibronectin-gelatin coverslips and stained with anti-p34Arc antibody (red) and antiphosphotyrosine antibody (blue). Regions outlined by boxes indicate images of invadopodia and focal adhesions. Arrows indicate representative invadopodia, and arrowheads indicate representative focal adhesions. Bars, 10 µm.
To determine whether FAK modulates tyrosine phosphorylation at focal adhesions through a Src-dependent pathway, we investigated the effects of FAK depletion on phosphotyrosine localization in Src-transformed MTLn3 cells (Fig. 5 A). Similar to untransformed cells, we observed strong phosphotyrosine staining at both focal adhesions that contained GFP-paxillin and at invadopodia positive for Arp2/3 in v-Src–transformed MTLn3 cells. In contrast, phosphotyrosine staining at focal adhesions in FAK-deficient v-Src–transformed cells remained robust, suggesting that FAK is not necessary for tyrosine phosphorylation at focal adhesions in Src-transformed cells. We also examined the invasive capacity of untransformed and v-Src–transformed MTLn3 cells (Fig. S3). We found that FAK-deficient cells were impaired 80% in their ability to invade through Matrigel-coated membranes. However, FAK-deficient MTLn3 cells expressing v-Src were impaired only 40% in their invasive capacity despite similar knockdown efficiency. These results indicate that depletion of FAK likely modulates localized tyrosine phosphorylation at focal adhesions and invadopodia upstream of Src to regulate invasion.
The FAK–Src complex has been demonstrated to mediate the phosphorylation of multiple adhesion components involved in cell migration, the best characterized of which are p130Cas (Tachibana et al., 1997) and paxillin (Bellis et al., 1995). To distinguish the specific phosphoprotein components at focal adhesions and invadopodia, we first examined the localization of tyrosine-phosphorylated paxillin and p130Cas in MTLn3 cells cultured on fibronectin-gelatin coverslips. Phosphorylated paxillin at Tyr-31 was absent in cortactin-positive invadopodia in control cells (Fig. 6 A). FAK-deficient cells displayed an increase in invadopodia that also lacked phosphopaxillin. In contrast, phosphopaxillin localized strongly with vinculin-positive focal adhesions in control cells (Fig. 6 B). Depletion of FAK impaired phosphopaxillin staining at focal adhesions but did not result in its redistribution to other structures.
Figure 6.
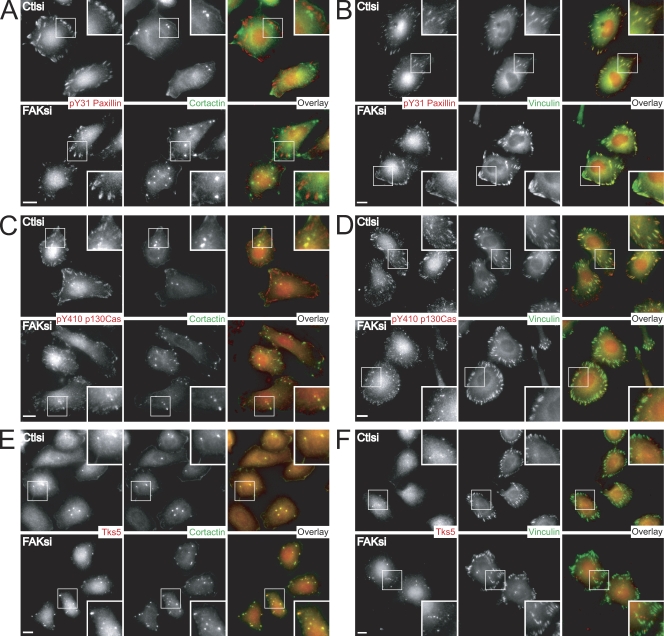
FAK modulates the localization of pY31-paxillin, pY410 p130Cas, and Tks5 in MTLn3 cells. (A–F) MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi) were cultured on fibronectin-gelatin coverslips and stained with anti-pY31 paxillin (A and B), anti-pY410 p130Cas (C and D), or anti-Tks5/FISH antibody (E and F; red). Cells were costained with the invadopodia marker cortactin (A, C, and E; green) or the focal adhesion marker vinculin (B, D, and F; green). Regions outlined by boxes correspond to magnified images of pY31-paxillin, pY410 p130Cas, or Tks5 localization at focal adhesions and/or invadopodia shown in insets. Bars, 10 µm.
Previous studies have shown that p130Cas is critical for podosome organization and Src transformation (Honda et al., 1998; Brabek et al., 2005). We observed some localization of phospho-p130Cas at cortactin-containing invadopodia (Fig. 6 C), although phospho-p130Cas was predominantly localized to focal adhesion–like structures in control cells, which was confirmed by immunostaining with an antivinculin antibody (Fig. 6 D). Similar to phosphorylated paxillin, FAK-deficient cells displayed a dramatic reduction in the localization of phospho-p130Cas at focal adhesions. However, in MTLn3 cells depleted of FAK, we detected an increase in phospho-p130Cas at dotlike structures, some of which contained cortactin. These results indicate that FAK is critical for the localized tyrosine phosphorylation of paxillin and p130Cas at focal adhesions.
Recent evidence has also implicated the Tks5/FISH family of adaptor proteins in podosome formation (Seals et al., 2005; Oikawa et al., 2008). In accordance with these studies, we observed localization of Tks5 with cortactin-containing invadopodia (Fig. 6 E) but not at vinculin-containing focal adhesions in control cells (Fig. 6 F). FAK-deficient cells displayed increased numbers of Tks5/cortactin-positive invadopodia. In addition, we detected an enhancement of invadopodia that contained phosphorylated cortactin in FAK-deficient MTLn3 cells (Fig. S4). Collectively, concomitant with a decrease in phosphorylated paxillin and p130Cas localization at focal adhesions was an increase in the localization of Tks5/FISH and phosphorylated cortactin at invadopodia, supporting a switch in localized tyrosine phosphorylation mediated by FAK depletion.
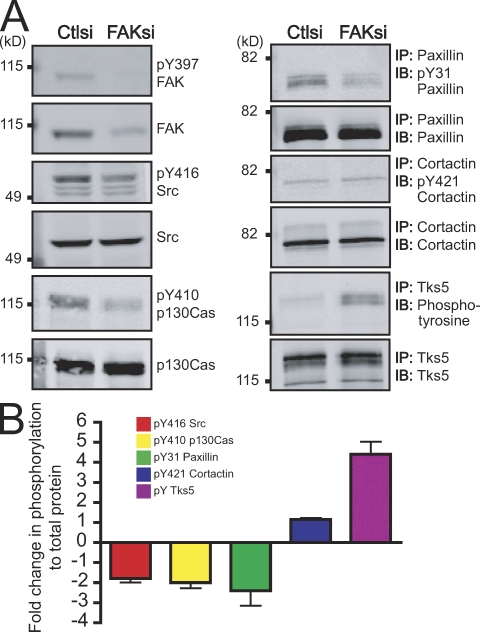
To confirm our hypothesis, we performed a phosphoprotein profile analysis of the focal adhesion and invadopodia components that we examined by immunolocalization (Fig. 7). In FAK-depleted cells, we observed a twofold reduction in global tyrosine phosphorylation of paxillin and p130Cas as compared with control cells. In addition, although we found enhanced numbers of invadopodia containing phosphorylated cortactin, we only detected a modest increase in global cortactin phosphorylation. Intriguingly, we found a substantial increase in tyrosine phosphorylation of Tks5 in FAK-deficient cells. Together, our data indicate that FAK depletion mediates a switch in tyrosine phosphorylation of FAK–Src substrates at focal adhesions to invadopodia.
Figure 7.
FAK regulates a switch in tyrosine phosphorylation at focal adhesions and invadopodia. (A) Cell lysates from MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi) were analyzed by immunoblotting (IB) or immunoprecipitation (IP)/immunoblotting and probed with phospho-specific antibodies anti-pY397 FAK, anti-pY416 Src, anti-pY410 p130Cas, anti-pY31 paxillin, anti-pY421 cortactin, or antiphosphotyrosine and antibodies to total proteins anti-FAK, anti-Src, anti-p130Cas, antipaxillin, anticortactin, or anti-Tks5/FISH. (B) Quantification of immunoblots or immunoprecipitation/immunoblots is expressed as fold change in phosphorylation to total protein. Fold change was determined by the ratio of normalized phosphorylation to total protein in FAK siRNA compared with control siRNA. Data shown are means ± SEM of three independent experiments.
Depletion of FAK modulates the redistribution of active Src from focal adhesions to invadopodia
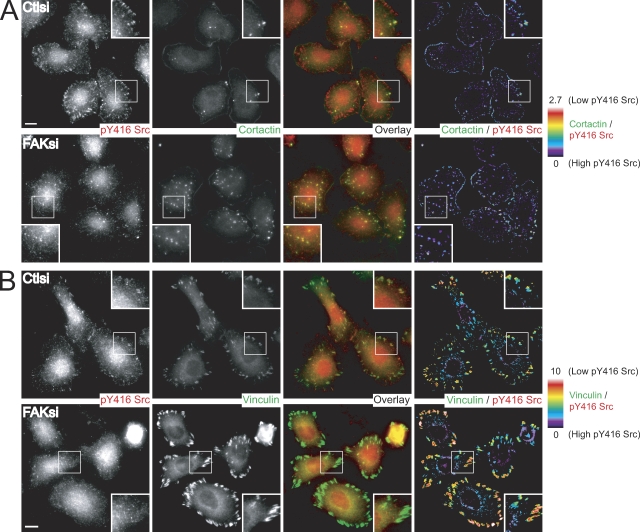
We observed that FAK was strongly present at focal adhesions but not at invadopodia (Fig. S2, D and E). It was intriguing that a focal adhesion protein could modulate the dynamics of distal invadopodia structures. A previous study suggested that the localization and activity of Src could be regulated by association with FAK (Schaller et al., 1999). Thus, we wanted to determine how FAK depletion impaired tyrosine phosphorylation of FAK–Src substrates at focal adhesions but enhanced tyrosine phosphorylation of substrates at invadopodia. To address this, we examined the localization of activated c-Src by staining for Tyr-416 c-Src in control and FAK-deficient MTLn3 cells on fibronectin-gelatin substrates (Fig. 8). In control cells, we detected the robust presence of active Src at both cortactin-containing invadopodia and focal adhesion–like structures as well as within several cytoplasmic dots, which is consistent with possible endosomes (Fig. 8 A). Although we found that global Src activity was decreased in MTLn3 cells depleted of FAK (Fig. 7), we observed a substantial increase in the number of invadopodia containing active Src. Ratio images of cortactin to phospho-Src did not reveal any clear differences in the amount of active Src at invadopodia in control and FAK-deficient cells. In contrast, phospho-Src was almost completely absent at focal adhesion–like structures in FAK-deficient cells. Staining with an antivinculin antibody in control cells verified that active Src was localized to focal adhesions (Fig. 8 B). Depletion of FAK resulted in reduced localization of active Src at enlarged vinculin-positive focal adhesions. Additionally, we generated ratio images of vinculin to phospho-Src. The vinculin/phospho-Src ratio was greater in FAK-deficient cells than control cells, suggesting a decrease in active Src at focal adhesions in FAK-deficient cells. Collectively, these data highlight a novel role for FAK in the differential modulation of focal adhesion and invadopodia composition and dynamics to regulate invasion through the spatial and temporal regulation of Src activity.
Figure 8.
Recruitment of active Src to focal adhesions but not invadopodia requires FAK. (A and B) MTLn3 cells transiently transfected with control siRNA (Ctlsi) or FAK siRNA (FAKsi) were cultured on fibronectin-gelatin coverslips and stained with anti-pY416 Src antibody (red) and anticortactin antibody (A, green) or antivinculin antibody (B, green). (A) Ratiometric images are presented as a ratio of cortactin/pY416 Src. (B) Ratiometric images are presented as a ratio of vinculin/pY416 Src. Regions outlined by boxes correspond to magnified images of pY416 Src localization at focal adhesions and invadopodia shown in insets. The color bars indicate the ratio on a scale of black = 0 (high pY416 Src) to white = 10 (low pY416 Src). Bars, 10 µm.
Discussion
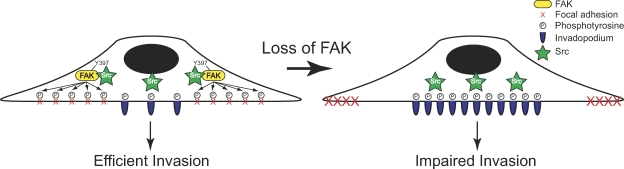
A hallmark of invasive tumor cells is the ability to degrade the ECM through the generation of invadopodia. Previous studies have suggested a critical role for FAK in breast cancer progression and metastatic disease; however, the role of FAK in invadopodia formation has not been characterized (Owens et al., 1995; Cance et al., 2000; Gabarra-Niecko et al., 2003; Lark et al., 2005). In this study, we describe a novel function for endogenous FAK in the regulation of invadopodia formation and dynamics (Fig. 9). FAK-deficient MTLn3 breast cancer cells display enhanced dynamics of invadopodia that are rescued by expression of wild-type FAK but not by expression of FAK that cannot be phosphorylated at Tyr-397. Furthermore, we show that depletion of endogenous FAK by siRNA induces the formation of active invadopodia and mediates a switch from robust phosphotyrosine-containing proteins at focal adhesions to invadopodia through the spatial and temporal regulation of Src activity. Our findings provide novel insight into the mechanism of FAK–Src signaling in cancer cell invasion.
Figure 9.
A model for FAK in invadopodia regulation. FAK and its phosphorylation at Tyr-397 recruit a pool of active Src to mediate localized tyrosine phosphorylation of substrates at focal adhesions. A balance between tyrosine phosphorylation at focal adhesions and invadopodia is required for proper dynamics and cell migration. Depletion of FAK releases active Src to mediate enhanced phosphorylation of substrates at invadopodia, which impairs focal adhesion dynamics but enhances the formation and dynamics of invadopodia; however, this is not sufficient for effective invasion.
Significant progress has been made in understanding the molecular mechanisms by which adhesive structures assemble and disassemble during cell migration. It appears that many of the signaling pathways that govern focal adhesion formation and disassembly also play roles in the regulation of invadopodia dynamics. For example, Src kinases and calpain proteases are important for the disassembly of both focal adhesions and podosome-type adhesions, including podosomes (Calle et al., 2006) and invadopodia (Cortesio et al., 2008). Nevertheless, the mechanisms that regulate the formation of invadopodia and focal adhesions can be distinct. For example, Src kinase activity is necessary for the formation of podosome-type adhesions (Spinardi et al., 2004), but its role in focal adhesion formation is less clear. Indeed, expression of constitutively active Src in cells that normally form focal adhesions promotes a switch to the formation of podosome-type adhesions (Gavazzi et al., 1989) and has been associated with a more invasive phenotype. Many cancer cells form both focal adhesions and invadopodia, but the molecular determinants that mediate the balance between formation of firm adhesive structures through focal adhesions and the generation of invasive matrix-degrading adhesion structures remain poorly understood.
Our findings suggest that FAK is poised to play a key role in regulating a dynamic interplay in signaling between focal adhesions and invadopodia during invasive cell migration. In accordance with a recent publication (Vitale et al., 2008), our data indicate that FAK is not necessary for the formation of invadopodia but instead can function to limit the formation of matrix-degrading invadopodia in cancer cells. However, our study is the first to show that depletion of endogenous FAK provides a critical switch between focal adhesion and invadopodia dynamics. We find that FAK depletion induces the formation of functional invadopodia in several different cancer cell lines, including MTLn3 and MDA-MB-231 breast cancer cells (Fig. S1) and SNB19 glioblastoma cells (unpublished data). Furthermore, our study presents a novel separation between enhanced generation of invadopodia and the invasive capacity of cancer cells. FAK-deficient MTLn3 cells showed impaired invasion across Matrigel-coated filters despite showing enhanced invadopodia formation on many different matrices including fibronectin, fibronectin-gelatin, and Matrigel (unpublished data). Although we found that MTLn3 invasion across Matrigel requires proteinase activity, enhanced matrix degradation alone is not sufficient for efficient tumor cell invasion in FAK-deficient cells. These findings suggest that FAK plays a complicated role in cancer cell invasion. It is intriguing to speculate that mechanisms that limit matrix degradation, through FAK or related pathways, may be necessary to optimize the matrix environment for invasive cell motility. This is an especially attractive hypothesis because FAK expression is associated with the invasive activity of human tumors and has been implicated in breast cancer progression and invasion in mouse models (Owens et al., 1995; Cance et al., 2000; Gabarra-Niecko et al., 2003; Lark et al., 2005; Mitra et al., 2006; Lahlou et al., 2007).
We have substantial evidence indicating that FAK functions upstream of c-Src to suppress invadopodia formation. For example, we found that endogenous FAK suppresses invadopodia formation in wild-type breast cancer cells and cells that overexpress wild-type c-Src. However, FAK siRNA had no effect on invadopodia formation in cells that express either active c-Src–527F or transforming v-Src. In addition, expression of wild type but not Y397F-FAK, which abrogates Src binding, suppressed invadopodia formation in FAK-deficient cells. Interestingly, we observed partial effects in cells expressing kinase-inactive FAK, suggesting that the kinase activity of FAK may also contribute to the ability of FAK to suppress invadopodia formation. Collectively, our data demonstrate that phosphorylation of FAK at Tyr-397 is important for limiting invadopodia formation by FAK upstream of Src.
Previous studies have demonstrated that tyrosine phosphorylation is a critical mechanism by which focal adhesion dynamics are regulated (Crowley and Horwitz, 1995; Zamir et al., 1999). We found that tyrosine phosphorylation was decreased at focal adhesions but not at invadopodia in FAK-deficient MTLn3 cells. Furthermore, depletion of FAK resulted in enlarged peripheral focal adhesions and impaired localization and dynamics of a specific phosphotyrosine reporter at focal adhesions, which is consistent with previous reports of impaired focal adhesion disassembly in fibroblasts (Sieg et al., 1999; Webb et al., 2004). In contrast, FAK-deficient cells displayed enhanced formation and dynamics of matrix-degrading invadopodia. Together, our data indicate that FAK is critical for tyrosine phosphorylation at focal adhesions and mediates a balance between tyrosine phosphorylation and dynamic turnover at invadopodia and focal adhesions.
It is interesting that although we find that FAK does not localize to invadopodia in MTLn3 cells, it is still able to modulate their formation and dynamics, implicating a common signaling mechanism that regulates focal adhesion and invadopodia dynamics. It has been proposed that FAK may be important for localized Src activity and phosphorylation of substrates that regulate cell migration (Schaller et al., 1999). In this context, our study is the first to demonstrate that depletion of FAK induces a redistribution of active Src from focal adhesions to invadopodia. Although we found that global Src activity is decreased, we observed a substantial increase in the number of invadopodia containing active Src in FAK-deficient cells. These findings suggest that FAK depletion induces a switch from focal adhesion initiation to invadopodia initiation through the spatial regulation of Src activity and localized tyrosine phosphorylation. Accordingly, depletion of FAK results in decreased tyrosine phosphorylation of p130Cas and paxillin at focal adhesions and enhanced tyrosine phosphorylation of cortactin and Tks5/FISH at invadopodia. Our findings demonstrate that FAK is critical for localization of active Src to focal adhesions, and it will be important to determine what localizes and activates Src at invadopodia.
Interestingly, with the substrates we examined, we found that Tks5/FISH phosphorylation is substantially increased in FAK-deficient cells. In accordance with previous reports, we find that Tks5 is required for invadopodia/podosome formation in MTLn3 cells using RNAi (Seals et al., 2005; Oikawa et al., 2008; unpublished data). It is intriguing to speculate that phosphorylation of Tks5 by Src may play a role in modulating the enhancement of invadopodia upon FAK depletion. Mapping the Src phosphorylation sites of Tks5 will be critical for addressing this hypothesis. However, it is unlikely that a single substrate will be sufficient to block the enhancement in invadopodia upon FAK depletion because Src targets multiple substrates involved in invadopodia and focal adhesion dynamics (Luo et al., 2008). Collectively, our experiments provide novel insight into how FAK differentially modulates focal adhesion and invadopodia dynamics through the spatial and temporal regulation of localized c-Src activity.
Our findings demonstrate a novel role for FAK as a negative regulator of invadopodia formation and provide mechanistic insight into the dynamic regulation of adhesive structures involved in cell invasion. It is especially intriguing that endogenous FAK and c-Src display opposing functions on invadopodia. Src is required for the generation of invadopodia, whereas FAK suppresses the formation and dynamics of invadopodia. A challenge for future investigation will be to elucidate the downstream mechanisms by which the FAK–Src complex differentially regulates the balance between adhesive and degradative structures and to characterize their precise functions in vivo.
Materials and methods
Reagents and antibodies
Fibronectin was purified as described previously (Ruoslahti et al., 1982). The following reagents were used: α-MEM, Opti-MEM I, Ham's F12 medium, Oregon green 488 gelatin conjugate, rhodamine phalloidin, Alexa Fluor 568 protein labeling kit (Invitrogen), gelatin from porcine skin (Sigma-Aldrich), PP2 (in ethanol used at 2 µM; Axxora; Enxo Biochem, Inc.), and GM6001 (in DMSO used at 50 µM; EMD). The following mouse monoclonal antibodies were used: anticortactin (clone 4F11), anti-Src (clone GD11), antiphosphotyrosine (4G10; Millipore), antivinculin (clone h-VIN1), anti–β-actin (clone AC-15; Sigma-Aldrich), anti-Pyk2 (clone 11), antipaxillin (clone 349), anti-p130Cas (clone 21), and anti-FAK (clone 77; BD). The following rabbit polyclonal antibodies were used: anti-p34Arc, anti-pY421 cortactin (Millipore), anti-FAK (clone C-20), anti-Tks5/FISH (clone M-300; Santa Cruz Biotechnology, Inc.), anti-pY31 paxillin, anti-pY397 FAK (Invitrogen), anti-pY410 p130Cas, and anti-pY416 Src (Cell Signaling Technology). Rabbit monoclonal antibody used for staining was anti-pY397 FAK (Invitrogen). The following secondary antibodies were used: Alexa Fluor 680 goat anti–mouse IgG (Invitrogen), IRDye 800CW goat anti–rabbit IgG (Rockland Immunochemicals, Inc.), FITC sheep anti–mouse IgG (whole molecule; Chroma Pure), FITC donkey anti–rabbit IgG, TRITC donkey anti–rabbit IgG (Jackson ImmunoResearch Laboratories), Rhodamine red-X goat anti–mouse IgG, and Marina blue goat anti–mouse IgG (Invitrogen). Immunoprecipitation control IgGs used were mouse IgG (whole molecule; Chroma Pure) and rabbit IgG (Jackson ImmunoResearch Laboratories).
Cell lines and siRNA transfection
The following cell lines were used: MTLn3 rat mammary adenocarcinoma (provided by J. Condeelis and J. Segall, Albert Einstein College of Medicine, New York, NY), MDA-MB-231 human mammary adenocarcinoma (provided by A. Rapraeger, University of Wisconsin, Madison, WI), and SNB19 human glioblastoma (provided by M. Symons, The Feinstein Institute for Medical Research at North Shore-LIJ, Manhasset, NY). MTLn3 cells were cultured in α-MEM supplemented with 5% FBS (HyClone) and antibiotics (Cellgro; Mediatech) as described previously (Segall et al., 1996). MDA-MB-231 and SNB19 cells were cultured in DME (Cellgro; Mediatech) supplemented with 10% FBS, nonessential amino acids (Sigma-Aldrich), and antibiotics. RNAi transfections were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. In brief, MTLn3 cells were plated at 105 in 6-well plates 24 h before transfection. MTLn3 cells were transfected with 120 pmol siRNA and 4 µl Lipofectamine 2000 in 2 ml Opti-MEM I for 45 min to 1 h. Sense sequences for Stealth siRNA oligonucleotides (Invitrogen) used are as follows: control siRNA, 5′-GAAUCUCAUCUAUUUCGUAACGGAC-3′; FAK siRNA (target A), 5′-UGACAGAUACCUAGCAUCUAGCAAA-3′; and FAK siRNA (target B), 5′-UCUCCAUGCCUGAUAAUACUGGCCC-3′. FAK siRNA (target A) targets a sequence in the 5′ untranslated region of FAK. All cells were cultured for 48 h before experimental analysis.
DNA constructs
pMX-GFP-FAK was generated by subcloning murine GFP-FAK from pEGFP-C1-FAK-HA (provided by D. Schlaepfer, University of California, San Diego, La Jolla, CA) into the pMX-IRES-GFP retroviral vector (provided by C. Svendsen, University of Wisconsin) from which the IRES-GFP sequence was excised. GFP-FAK was amplified using the following primers: forward, 5′- ACAGGATCCGCCACCATGGTGAGCAAGGGCGAG-3′ and reverse, 5′-ATATGTATTCTAGATGATCGTCTGTCGACTCAGTGTGGCCGTGTCTGCCCTAGC-3′.
pMX-GFP-FAK-Y397F and pMX-GFP-FAK-K454R were generated using the Quikchange site-directed mutagenesis kit (Agilent Technologies) with the following primers: Y397F (forward), 5′-CTGTGTCAGAGACAGATGACTTTGCAGAGATCATCGATGAGG-3′ and (reverse) 5′-CCTCATCGATGATCTCTGCAAAGTCATCTGTCTCTGACACAG-3′; and K454R (forward), 5′-GGCTGTTGCAATCAGAACATGTAAAAACTG-3′ and (reverse) 5′-CAGTTTTTACATGTTCTGATTGCAACAGCC-3′. Retroviral transfection was performed as described previously (Franco et al., 2004). Cells expressing GFP were sorted by FACS (University of Wisconsin Comprehensive Cancer Center Flow Cytometry Core Facility). c-Src was amplified from LCNX–c-Src (provided by P. Keely, University of Wisconsin), and c-Src–527F was amplified from pCDNA3.1(+)–c-Src–527F (Cortesio et al., 2008) using the following primers: forward, 5′-ATTGTGGGATTCATGGGGAGCAGCAAGAGC-3′ and reverse, 5′-GCCAGGGAATTCCTATAGGTTCTCTCCAGGCTG-3′. pMX–c-Src–IRES-GFP or pMX–c-Src–527F-IRES-GFP were generated by subcloning into the BamHI and EcoRI sites of the pMX-IRES-GFP vector. Cells positive for c-Src or c-Src–527F were identified by GFP expression. FPGV v-Src was a provided by M. Frame (Edinburgh Cancer Research Centre, Edinburgh, Scotland, UK). v-Src transformation of MTLn3 cells was performed as described previously (Cortesio et al., 2008). EGFP-cortactin has been described previously (Perrin et al., 2006; Cortesio et al., 2008). YFP-dSH2 was provided by T. Gomez (University of Wisconsin) and has been previously described (Kirchner et al., 2003). GFP-vinculin was provided by A. Bershadsky and B. Geiger (The Weizmann Institute of Science, Rehovot, Israel). GFP-paxillin has been previously described (Laukaitis et al., 2001). Transient transfections of YFP-dSH2, GFP-vinculin, or GFP-paxillin were performed using Lipofectamine 2000 with 2 µg DNA 24 h after siRNA transfection.
Immunoblot analysis and immunoprecipitation
Cells were scraped into lysis buffer (50 mM Tris, pH 7.6, 500 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, 0.5 mM MgCl2, 0.2 mM PMSF, 1 µg ml−1 pepstatin, 2 µg ml−1 aprotinin, 1 µg ml−1 leupeptin, and 1 mM sodium orthovanadate) on ice and clarified by centrifugation. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Immunoblotting of cell lysates was performed as previously described (Cortesio et al., 2008), and blots were imaged with an Infrared Imaging System (Odyssey; LI-COR Biosciences). For immunoprecipitations, MTLn3 cells were plated 48 h after siRNA transfection on 15-cm dishes coated with 10 µg/ml fibronectin. Cells were incubated for 3 h at 37°C in 5% CO2, washed once with PBS, and lysed in ice-cold modified RIPA buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 2 mM EDTA, 0.2 mM PMSF, 1 µg ml−1 pepstatin, 2 µg ml−1 aprotinin, 1 µg ml−1 leupeptin, and 1 mM sodium orthovanadate). 500 µg cleared lysates were incubated overnight at 4°C with 5 µg antipaxillin (mouse monoclonal), anticortactin (mouse monoclonal), or anti-Tks5/FISH (rabbit polyclonal) antibodies or with 5 µg mouse or rabbit IgG. Immune complexes were captured on GammaBind G–Sepharose beads (GE Healthcare), washed three times in RIPA, and analyzed by immunoblotting.
Immunofluorescence
Glass coverslips were acid washed, ethanol sterilized, and coated with ECM as previously described (Cortesio et al., 2008). Fibronectin coverslips were prepared by coating with 10 µg/ml fibronectin for 1 h at 37°C. Fibronectin-gelatin (10 µg/ml fibronectin and 0.2% gelatin) coverslips were prepared by coating gelatin coverslips as described previously (Artym et al., 2006) and subsequently coating with 10 µg/ml fibronectin for 1 h at 37°C. Matrigel (BD) coverslips were prepared by coating a thin layer of a 1:10 dilution of Matrigel for 1 h at 37°C. Cells were cultured on coverslips for 16 h, fixed, and stained with antibodies. Coverslips were imaged using a 60×/1.40 oil objective on an inverted microscope (1X-70; Olympus) equipped with a cooled charge-coupled device (CCD) camera (CoolSNAP FX; Photometrics) or an inverted microscope (TE300; Nikon) equipped with a cooled CCD camera (Orca II; Hamamatsu Photonics). Images were captured into MetaVue (version 6.2; MDS Analytical Technologies) or MetaMorph imaging software (version 7.1; MDS Analytical Technologies). At least 40 cells were counted in each of at least three separate experiments performed on separate days. Dotlike structures containing cortactin and/or actin were scored as invadopodia. For ratiometric analysis, images were first subjected to high-pass filtration based on the water algorithm (Zamir et al., 1999) to remove diffuse background fluorescence. The images were thresholded based on the vinculin image because it had the largest signal to noise ratio, providing the clearest distinction between focal adhesions and background. The thresholded vinculin image was used to generate a binary image in which values at focal adhesions equal 1 and all other values equal 0. A ratio image of vinculin/pY416 Src was generated, and high frequency noise was removed with a low-pass filter. Ratio images were displayed with similar nucleocytoplasmic background as a control for possible differences in staining. Ratiometric images presented are representative of at least 50 cells visualized by immunofluorescence.
Fluorescent gelatin degradation assay
Alexa Fluor 568–conjugated gelatin was prepared using the Alexa Fluor 568 protein labeling kit according to the manufacturer's instructions (Invitrogen). Oregon green 488–conjugated and Alexa Fluor 568 gelatin-coated coverslips were prepared as described previously (Artym et al., 2006). Fluorescent gelatin coverslips were subsequently coated with 10 µg/ml fibronectin for 1 h at 37°C. Cells were cultured on coverslips for 16 h, fixed, and stained with anticortactin antibody. Active invadopodia were identified as areas of cortactin-containing dots that localized with areas of matrix degradation. A total of 40–60 cells were counted in at least three independent experiments performed on separate days. All measurements shown are the mean ± SEM.
Time-lapse fluorescence microscopy and quantification of invadopodia dynamics
Fluorescence imaging of YFP-dSH2 and EGFP-cortactin dynamics were performed using a 60×/1.40 oil objective on an inverted microscope (1X-70; Olympus) in a 37°C closed system as previously described (Franco et al., 2004; Webb et al., 2004; Cortesio et al., 2008). Glass-bottomed dishes (35 mm) were coated with 10 µg/ml fibronectin for 1 h at 37°C. Cells were plated in Ham's F12 containing 5% FBS and 20 mM Hepes, pH 7.2, and were allowed to adhere for 3 h. Fluorescent images were collected using a cooled CCD camera (CoolSNAP FX; Hamamatsu Photonics) and captured into MetaVue every 1 min for 1 h. Time-lapse sequences from live fluorescence imaging were first subjected to high-pass filtration based on the water algorithm (Zamir et al., 1999) to remove diffuse background fluorescence. For each rate constant, measurements were made on a total of 40–60 invadopodia in five to six cells from at least three independent experiments performed on separate days.
Migration and invasion assays
Transwell filters (Corning) with 8-µm pores were coated on the bottom surface with 10 µg/ml fibronectin for 1 h at 37°C and were dried after coating. Cells were serum starved for 3 h in α-MEM with 1% FBS. 105 cells were plated in the top chamber and were allowed to migrate toward α-MEM containing 10% FBS for 16 h. Cell invasion experiments were performed using similar conditions with 8-µm porous chambers coated with Matrigel (BD). Cells were allowed to invade through the Matrigel membrane for 48 h. In GM6001 treatment experiments, DMSO vehicle or GM6001 were added to both upper and lower chambers. Cells on the bottom surface were fixed and stained using a Hema-3 stain kit (Thermo Fisher Scientific). Cells were counted from four to six representative 20× or 40× fields per condition in at least three independent experiments performed on different days. All data are shown normalized to control as mean ± SEM.
Confocal microscopy
Confocal images were collected using a laser-scanning confocal microscope (Fluoview FV-1000; Olympus) using a 60× Plan Apo/1.45 oil immersion objective with a 3.0 zoom factor and were captured into Fluoview software (FV10-ASW version 01.07; Olympus). 3D projections (x-z shown) were generated from 3.0–4.0-µm z series to illustrate invadopodial projections into fluorescent gelatin matrix. For FAK localization, single projections were generated from 3.0–4.0-µm z series with a 0.37-µm step size. Images were subsequently processed using MetaMorph software.
Statistical analyses
Prism 4 software (GraphPad Software, Inc.) was used to perform tests for statistical significance. For statistical comparison, a two-tailed paired t test or one-way analysis of variance (ANOVA) was used with P < 0.05 considered significant.
Online supplemental material
Fig. S1 corresponds to Fig. 1 and illustrates invadopodia formation in MDA-MB-231 cells expressing control or FAK siRNA. Fig. S2 corresponds to Fig. 3 and shows expression of GFP-FAK variants in MTLn3 cells, focal adhesion morphology and migration, and FAK localization. Fig. S3 corresponds to Fig. 5 and shows invasion by MTLn3 cells or v-Src–transformed MTLn3 cells. Fig. S4 corresponds to Fig. 6 and demonstrates localization of phosphorylated cortactin at invadopodia. Videos 1–3 correspond to Fig. 1 and show dynamics of GFP-cortactin–containing invadopodia in MTLn3 cells expressing control siRNA, FAK siRNA (target A), or FAK siRNA (target B), respectively. Videos 4 and 5 show dynamics of YFP-dSH2 in MTLn3 cells expressing control siRNA or FAK siRNA (target A). Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200809110/DC1.
Acknowledgments
We thank Dr. S.K. Yoo for helpful discussions and technical assistance.
We would like to acknowledge funding by grants from the National Institutes of Health to A. Huttenlocher (R01 CA085862-08) and by the Department of Defense predoctoral fellowship to C. Cortesio (BC073295).
Footnotes
Abbreviations used in this paper: ANOVA, analysis of variance; c-Src, cellular Src; CCD, charge-coupled device; MMP, matrix metalloproteinase; v-Src, viral Src.
References
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., Mueller S.C. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function.Cancer Res. 66:3034–3043 [DOI] [PubMed] [Google Scholar]
- Bellis S.L., Miller J.T., Turner C.E. 1995. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase.J. Biol. Chem. 270:17437–17441 [DOI] [PubMed] [Google Scholar]
- Benlimame N., He Q., Jie S., Xiao D., Xu Y.J., Loignon M., Schlaepfer D.D., Alaoui-Jamali M.A. 2005. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion.J. Cell Biol. 171:505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden E.T., Onikoyi E., Slack R., Myoui A., Yoneda T., Yamada K.M., Mueller S.C. 2006. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells.Exp. Cell Res. 312:1240–1253 [DOI] [PubMed] [Google Scholar]
- Brabek J., Constancio S.S., Siesser P.F., Shin N.Y., Pozzi A., Hanks S.K. 2005. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells.Mol. Cancer Res. 3:307–315 [DOI] [PubMed] [Google Scholar]
- Buccione R., Orth J.D., McNiven M.A. 2004. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles.Nat. Rev. Mol. Cell Biol. 5:647–657 [DOI] [PubMed] [Google Scholar]
- Buccione R., Caldieri G., Ayala I. 2009. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix.Cancer Metastasis Rev. 28:137–149 [DOI] [PubMed] [Google Scholar]
- Calle Y., Carragher N.O., Thrasher A.J., Jones G.E. 2006. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility.J. Cell Sci. 119:2375–2385 [DOI] [PubMed] [Google Scholar]
- Cance W.G., Harris J.E., Iacocca M.V., Roche E., Yang X., Chang J., Simkins S., Xu L. 2000. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes.Clin. Cancer Res. 6:2417–2423 [PubMed] [Google Scholar]
- Carragher N.O., Frame M.C. 2004. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion.Trends Cell Biol. 14:241–249 [DOI] [PubMed] [Google Scholar]
- Chen H.C., Guan J.L. 1994. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase.Proc. Natl. Acad. Sci. USA. 91:10148–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.T., Wang J.Y. 1999. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization.Ann. N. Y. Acad. Sci. 878:361–371 [DOI] [PubMed] [Google Scholar]
- Clark E.S., Whigham A.S., Yarbrough W.G., Weaver A.M. 2007. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia.Cancer Res. 67:4227–4235 [DOI] [PubMed] [Google Scholar]
- Cortesio C.L., Chan K.T., Perrin B.J., Burton N.O., Zhang S., Zhang Z.Y., Huttenlocher A. 2008. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion.J. Cell Biol. 180:957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley E., Horwitz A.F. 1995. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions.J. Cell Biol. 131:525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco S.J., Rodgers M.A., Perrin B.J., Han J., Bennin D.A., Critchley D.R., Huttenlocher A. 2004. Calpain-mediated proteolysis of talin regulates adhesion dynamics.Nat. Cell Biol. 6:977–983 [DOI] [PubMed] [Google Scholar]
- Gabarra-Niecko V., Schaller M.D., Dunty J.M. 2003. FAK regulates biological processes important for the pathogenesis of cancer.Cancer Metastasis Rev. 22:359–374 [DOI] [PubMed] [Google Scholar]
- Gavazzi I., Nermut M.V., Marchisio P.C. 1989. Ultrastructure and gold-immunolabelling of cell-substratum adhesions (podosomes) in RSV-transformed BHK cells.J. Cell Sci. 94:85–99 [DOI] [PubMed] [Google Scholar]
- Hauck C.R., Hsia D.A., Ilic D., Schlaepfer D.D. 2002. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion.J. Biol. Chem. 277:12487–12490 [DOI] [PubMed] [Google Scholar]
- Honda H., Oda H., Nakamoto T., Honda Z., Sakai R., Suzuki T., Saito T., Nakamura K., Nakao K., Ishikawa T., et al. 1998. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas.Nat. Genet. 19:361–365 [DOI] [PubMed] [Google Scholar]
- Hsia D.A., Mitra S.K., Hauck C.R., Streblow D.N., Nelson J.A., Ilic D., Huang S., Li E., Nemerow G.R., Leng J., et al. 2003. Differential regulation of cell motility and invasion by FAK.J. Cell Biol. 160:753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner J., Kam Z., Tzur G., Bershadsky A.D., Geiger B. 2003. Live-cell monitoring of tyrosine phosphorylation in focal adhesions following microtubule disruption.J. Cell Sci. 116:975–986 [DOI] [PubMed] [Google Scholar]
- Lahlou H., Sanguin-Gendreau V., Zuo D., Cardiff R.D., McLean G.W., Frame M.C., Muller W.J. 2007. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression.Proc. Natl. Acad. Sci. USA. 104:20302–20307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark A.L., Livasy C.A., Dressler L., Moore D.T., Millikan R.C., Geradts J., Iacocca M., Cowan D., Little D., Craven R.J., Cance W. 2005. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype.Mod. Pathol. 18:1289–1294 [DOI] [PubMed] [Google Scholar]
- Laukaitis C.M., Webb D.J., Donais K., Horwitz A.F. 2001. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells.J. Cell Biol. 153:1427–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y., Lim S.T., Tomar A., Gardel M., Bernard-Trifilo J.A., Chen X.L., Uryu S.A., Canete-Soler R., Zhai J., Lin H., et al. 2008. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility.J. Cell Biol. 180:187–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. 2007. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation.Trends Cell Biol. 17:107–117 [DOI] [PubMed] [Google Scholar]
- Linder S., Aepfelbacher M. 2003. Podosomes: adhesion hot-spots of invasive cells.Trends Cell Biol. 13:376–385 [DOI] [PubMed] [Google Scholar]
- Luo W., Slebos R.J., Hill S., Li M., Brabek J., Amanchy R., Chaerkady R., Pandey A., Ham A.J., Hanks S.K. 2008. Global impact of oncogenic Src on a phosphotyrosine proteome.J. Proteome Res. 7:3447–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S.K., Hanson D.A., Schlaepfer D.D. 2005. Focal adhesion kinase: in command and control of cell motility.Nat. Rev. Mol. Cell Biol. 6:56–68 [DOI] [PubMed] [Google Scholar]
- Mitra S.K., Lim S.T., Chi A., Schlaepfer D.D. 2006. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model.Oncogene. 25:4429–4440 [DOI] [PubMed] [Google Scholar]
- Oikawa T., Itoh T., Takenawa T. 2008. Sequential signals toward podosome formation in NIH-src cells.J. Cell Biol. 182:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L.V., Xu L., Craven R.J., Dent G.A., Weiner T.M., Kornberg L., Liu E.T., Cance W.G. 1995. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors.Cancer Res. 55:2752–2755 [PubMed] [Google Scholar]
- Perrin B.J., Amann K.J., Huttenlocher A. 2006. Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration.Mol. Biol. Cell. 17:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E.G., Pierschbacher M., Engvall E. 1982. Fibronectin: purification, immunochemical properties, and biological activities.Methods Enzymol. 82:803–831 [DOI] [PubMed] [Google Scholar]
- Schaller M.D., Hildebrand J.D., Shannon J.D., Fox J.W., Vines R.R., Parsons J.T. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src.Mol. Cell. Biol. 14:1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Hildebrand J.D., Parsons J.T. 1999. Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases.Mol. Biol. Cell. 10:3489–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M., Raghavan S., Nikolova M., Polak L., Pasolli H.A., Beggs H.E., Reichardt L.F., Fuchs E. 2007. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics.J. Cell Biol. 176:667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Burridge K. 1999. Bidirectional signaling between the cytoskeleton and integrins.Curr. Opin. Cell Biol. 11:274–286 [DOI] [PubMed] [Google Scholar]
- Seals D.F., Azucena E.F., Jr., Pass I., Tesfay L., Gordon R., Woodrow M., Resau J.H., Courtneidge S.A. 2005. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells.Cancer Cell. 7:155–165 [DOI] [PubMed] [Google Scholar]
- Segall J.E., Tyerech S., Boselli L., Masseling S., Helft J., Chan A., Jones J., Condeelis J. 1996. EGF stimulates lamellipod extension in metastatic mammary adenocarcinoma cells by an actin-dependent mechanism.Clin. Exp. Metastasis. 14:61–72 [DOI] [PubMed] [Google Scholar]
- Sieg D.J., Hauck C.R., Schlaepfer D.D. 1999. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration.J. Cell Sci. 112:2677–2691 [DOI] [PubMed] [Google Scholar]
- Sieg D.J., Hauck C.R., Ilic D., Klingbeil C.K., Schaefer E., Damsky C.H., Schlaepfer D.D. 2000. FAK integrates growth-factor and integrin signals to promote cell migration.Nat. Cell Biol. 2:249–256 [DOI] [PubMed] [Google Scholar]
- Spinardi L., Rietdorf J., Nitsch L., Bono M., Tacchetti C., Way M., Marchisio P.C. 2004. A dynamic podosome-like structure of epithelial cells.Exp. Cell Res. 295:360–374 [DOI] [PubMed] [Google Scholar]
- Tachibana K., Urano T., Fujita H., Ohashi Y., Kamiguchi K., Iwata S., Hirai H., Morimoto C. 1997. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates.J. Biol. Chem. 272:29083–29090 [DOI] [PubMed] [Google Scholar]
- Tilghman R.W., Slack-Davis J.K., Sergina N., Martin K.H., Iwanicki M., Hershey E.D., Beggs H.E., Reichardt L.F., Parsons J.T. 2005. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells.J. Cell Sci. 118:2613–2623 [DOI] [PubMed] [Google Scholar]
- van Nimwegen M.J., Verkoeijen S., van Buren L., Burg D., van de Water B. 2005. Requirement for focal adhesion kinase in the early phase of mammary adenocarcinoma lung metastasis formation.Cancer Res. 65:4698–4706 [DOI] [PubMed] [Google Scholar]
- Vitale S., Avizienyte E., Brunton V.G., Frame M.C. 2008. Focal adhesion kinase is not required for Src-induced formation of invadopodia in KM12C colon cancer cells and can interfere with their assembly.Eur. J. Cell Biol. 87:569–579 [DOI] [PubMed] [Google Scholar]
- Webb D.J., Donais K., Whitmore L.A., Thomas S.M., Turner C.E., Parsons J.T., Horwitz A.F. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly.Nat. Cell Biol. 6:154–161 [DOI] [PubMed] [Google Scholar]
- Wolf K., Friedl P. 2006. Molecular mechanisms of cancer cell invasion and plasticity.Br. J. Dermatol. 154(Suppl 1):11–15 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T., Condeelis J. 2005. Molecular mechanisms of invadopodium formation: the role of the N-WASP–Arp2/3 complex pathway and cofilin.J. Cell Biol. 168:441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Pixley F., Condeelis J. 2006. Invadopodia and podosomes in tumor invasion.Eur. J. Cell Biol. 85:213–218 [DOI] [PubMed] [Google Scholar]
- Zamir E., Katz B.Z., Aota S., Yamada K.M., Geiger B., Kam Z. 1999. Molecular diversity of cell-matrix adhesions.J. Cell Sci. 112:1655–1669 [DOI] [PubMed] [Google Scholar]
- Zhang X., Chattopadhyay A., Ji Q.S., Owen J.D., Ruest P.J., Carpenter G., Hanks S.K. 1999. Focal adhesion kinase promotes phospholipase C-gamma1 activity.Proc. Natl. Acad. Sci. USA. 96:9021–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]