Abstract
The decision to enter mitosis is mediated by a network of proteins that regulate activation of the cyclin B–Cdk1 complex. Within this network, several positive feedback loops can amplify cyclin B–Cdk1 activation to ensure complete commitment to a mitotic state once the decision to enter mitosis has been made. However, evidence is accumulating that several components of the feedback loops are redundant for cyclin B–Cdk1 activation during normal cell division. Nonetheless, defined feedback loops become essential to promote mitotic entry when normal cell cycle progression is perturbed. Recent data has demonstrated that at least three Plk1-dependent feedback loops exist that enhance cyclin B–Cdk1 activation at different levels. In this review, we discuss the role of various feedback loops that regulate cyclin B–Cdk1 activation under different conditions, the timing of their activation, and the possible identity of the elusive trigger that controls mitotic entry in human cells.
Introduction
Maturation-promoting factor, an activity that can mediate mitotic entry, was discovered in 1971, and during the late 80s was characterized as a complex of cyclin B and Cdk1. Soon after this, the general framework for cyclin B–Cdk1 regulation became clear: cyclin B levels are periodically regulated by transcription and degradation cycles, Cdk1 needs to be phosphorylated on its T loop for full activity, and the activation of cyclin B–Cdk1 is regulated by the opposing activities of Wee1 and Cdc25 (for review see O'Farrell, 2001). Since then, additional layers of complexity have been added to the model.
Cyclin B
In Xenopus egg extracts, cyclin B–Cdk1 is activated when the level of cyclin B reaches a threshold concentration (Solomon et al., 1990). Thus, a critical regulatory step in the activation of cyclin B–Cdk1 is the amount of cyclin B available to form a complex with Cdk1. In human cells, high cyclin B levels are temporally restricted to G2 phase and early mitosis by regulated transcription and protein degradation. Transcription of cyclin B starts in S phase and peaks in late G2 (Fig. 1). Several transcription factors, including NF-Y, FoxM1, and B-Myb, have been shown to activate transcription of the cyclin B1 promoter (for a review on cyclin B transcription and degradation, see Fung and Poon, 2005). Interestingly, all these transcription factors are activated by Cdk activity, ensuring that transcription of cyclin B is efficient only when cyclin A–Cdk2 activity builds up during S and G2 phase (Dynlacht et al., 1994; Ziebold et al., 1997; Saville and Watson, 1998; Chae et al., 2004; Major et al., 2004; Laoukili et al., 2008).
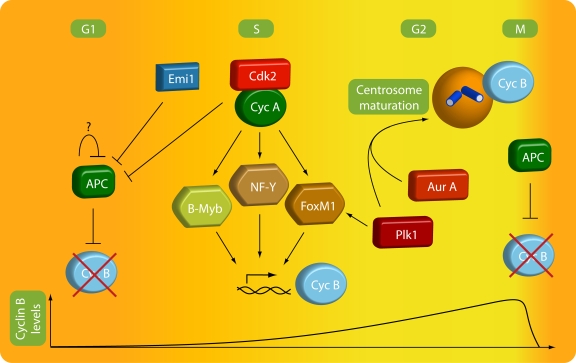
Figure 1.
Regulation of cyclin B accumulation. A combination of temporally regulated degradation and transcription ensures that cyclin B levels peak in late G2 phase and during early mitosis. After silencing of the spindle checkpoint in mitosis, cyclin B is degraded by the APC/C. APC/C activity maintains low cyclin B levels through G1 phase until silencing of APC/C in S phase by a combination of direct inactivation by cyclin A–Cdk2, binding of the inhibitor Emi1, and, possibly, autodestruction of the APC/C E2 ligase UbcH10. Starting in S phase and peaking in late G2 phase, accumulating cyclin A–Cdk2 activity also activates the transcription factors B-MYB, NF-Y, and FoxM1, which directly enhance cyclin B transcription. In addition to transcription and degradation, the local concentration of cyclin B is enhanced during centrosome maturation by Plk1- and Aurora A–dependent targeting of cyclin B to the centrosome.
Degradation of cyclin B is regulated by the anaphase-promoting complex/cyclosome (APC/C), a multisubunit E3 ligase that can poly-ubiquitinylate many mitotic regulators to target them for destruction by the proteasome. Poly-ubiquitinylation of cyclin B starts in metaphase, when the spindle assembly checkpoint is silenced (Acquaviva and Pines, 2006; van Leuken et al., 2008). APC/C continues to promote degradation of cyclin B until early S phase, where its activity is down-regulated due to a combination of phosphorylation by cyclin A–Cdk2 and binding of the APC inhibitor Emi1 (Lukas et al., 1999; Hsu et al., 2002). Autodestruction of the APC E2 ligase UbcH10 has also been suggested to regulate APC/C inactivation, although recent data suggest that UbcH10 destruction may not regulate APC/C inactivation in S phase (Rape and Kirschner, 2004; Walker et al., 2008). Thus, the combined regulation of transcription and protein degradation ensures that high cyclin B expression is limited to G2 and the early stages of mitosis (Fig. 1).
Like many cell cycle regulators, cyclin B shuttles between the nucleus and the cytoplasm. However, in S and the major part of G2 phase, the cyclin B nuclear export outweighs the cyclin B nuclear import, resulting in a predominately cytoplasmic localization of cyclin B (Hagting et al., 1998; Toyoshima et al., 1998; Yang et al., 1998). In mid G2 phase, concomitantly with centrosome maturation, cyclin B starts to accumulate at the centrosomes (see “The outer feedback loops”). Thus, in late G2, the local concentration of cyclin B is highest at the centrosomes (Fig. 1). In agreement with the cyclin B concentration being important for cyclin B–Cdk1 activation in Xenopus extracts (Solomon et al., 1990), in human cells, autophosphorylated cyclin B is first detected on the centrosomes (Jackman et al., 2003).
Collectively, sufficiently high levels of cyclin B to trigger activation of cyclin B–Cdk1 complexes require timely transcriptional activation, inhibition of proteasomal degradation, and, possibly, concentration of cyclin B at specific sites within the cell. However, mere association of cyclin B to Cdk1 is not sufficient to form an active complex, as the Cdk subunit is subject to posttranslational modifications that affect kinase activity. Cdk1 needs to be phosphorylated on T161 in the T loop to produce an active kinase. This is mediated via the Cdk-activating kinase, which appears to function as a constitutively active entity, providing no clues to regulation of Cdk1 activation (Tassan et al., 1994). In addition, when bound to cyclin B in interphase, Cdk1 is phosphorylated on T14 and Y15, resulting in inhibition of kinase activity. The dephosphorylation of these residues forms the core of the feedback loops that control activation of cyclin B–Cdk1 to promote mitotic entry.
The inner feedback loops
Cdk1 T14 and Y15 phosphorylation is controlled by the balance between Wee1/Myt1 kinases and Cdc25 phosphatases. These kinases and phosphatases are in turn directly regulated by Cdk1 activity, which we will refer to as the inner feedback loops. The Wee1 and Myt1 kinases are responsible for T14/Y15 phosphorylation in G2 phase, resulting in the inhibition of cyclin B–Cdk1 activity (O'Farrell, 2001). Wee1 is predominantly nuclear, but has also been found to associate with centrosomes, whereas Myt1 is bound to membrane structures in the cytoplasm (Baldin and Ducommun, 1995; McGowan and Russell, 1995; Liu et al., 1997). Once active, cyclin B–Cdk1 complexes can phosphorylate Wee1 and Myt1 to promote their inactivation, thereby further amplifying Cdk1 activation. Although Cdk1-dependent phosphorylation of Wee1 starts a cascade that promotes Wee1 degradation (Watanabe et al., 2004), Cdk1-dependent phosphorylation of Myt1 starts a cascade that inhibits Myt1 kinase activity (Booher et al., 1997; Nakajima et al., 2003).
In addition, cyclin B–Cdk1 can activate Cdc25 dual-specificity phosphatases, which can dephosphorylate T14 and Y15 in Cdk1 (O'Farrell, 2001). In human cells, there are three Cdc25 isoforms: Cdc25A, Cdc25B, and Cdc25C (for a review on human Cdc25s, see Boutros et al., 2006). All Cdc25s shuttle between the nucleus and the cytoplasm in G2 phase, but Cdc25A appears to be mainly nuclear and Cdc25C appears to be mainly cytoplasmic, whereas there are conflicting reports regarding the localization of Cdc25B (for review see Boutros et al., 2006). Both Cdc25B and Cdc25C are also targeted to the centrosome in G2 phase (Dutertre et al., 2004; Boutros et al., 2006; Busch et al., 2007; Bonnet et al., 2008), where Cdc25B is thought to participate in the initiation of cyclin B–Cdk1 activation (Gabrielli et al., 1996; Lammer et al., 1998; Karlsson et al., 1999; Lindqvist et al., 2005). When active, Cdk1 activates Cdc25C, stabilizes Cdc25A, and affects the localization of Cdc25B (Hoffmann et al., 1993; Baldin et al., 2002; Mailand et al., 2002; Boutros et al., 2006). The functional relevance of cyclin B–Cdk1 phosphorylating human Cdc25s is most likely not completely understood, however, as a recent study has identified many additional cyclin B–Cdk1 phosphorylation sites on Cdc25B (Bouche et al., 2008).
Thus, through the inner feedback loops, cyclin B–Cdk1 can stimulate its further activation by directly activating its activators and deactivating its inactivators (Fig. 2 A).
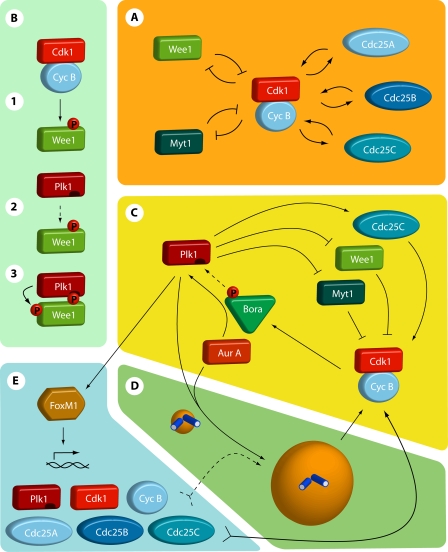
Figure 2.
Feedback loops that regulate cyclin B–Cdk1 activity. (A) The inner feedbacks. Myt1 and Wee1 kinases phosphorylate Cdk1 on T14 and Y15, thereby inhibiting cyclin B–Cdk1 activity. The T14 and Y15 phosphorylations can be antagonized by Cdc25A, -B, and -C. Once activated, cyclin B–Cdk1 activity inhibits Wee1 and Myt1, and activates the Cdc25 phosphatases. Thus, by the inner feedback loops, cyclin B–Cdk1 inhibits its inhibitors and activates its activators. (B) Direct Plk1-dependent feedback. Cyclin B–Cdk1 phosphorylation of many targets, including Wee1, Myt1, and Cdc25C, creates a docking site for Plk1. Binding of Plk1 both guides Plk1 to its substrate and stimulates Plk1 activity by releasing its inhibitory polo-box domains. In this way, cyclin B–Cdk1–mediated phosphorylation can trigger a second round of Plk1-mediated phosphorylation of a target protein. (C) Feedback through Bora-Aurora-Plk1. Cyclin B–Cdk1–mediated phosphorylation of Bora increases Bora binding to Plk1. Bora can also associate with Aurora A, and is required for efficient Aurora A–mediated activation of Plk1. By regulating Bora phosphorylation, cyclin B–Cdk1 can thereby activate Plk1. Plk1 in turn activates cyclin B–Cdk1 at different levels (Fig. 2, B, D, and E). Aurora A can also stimulate cyclin B–Cdk1 activation through activation of Cdc25B and regulation of centrosome maturation (Fig. 2 D). Although Aurora A activity is regulated by Cdk, it is currently unclear if these processes depend on cyclin B–Cdk1–mediated phosphorylation of Bora. (D) Feedback through centrosome maturation. The local concentration of cyclin B–Cdk1, an important regulatory step for cyclin B–Cdk1 activation, is enhanced by targeting to the maturating centrosome in late G2 phase. Moreover, many additional proteins in the mitotic entry network are targeted to centrosomes, thereby creating high local concentrations of cyclin B–Cdk1 activators. Both Aurora A and Plk1 activities are required for centrosome maturation and stimulate cyclin B accumulation on centrosomes. (E) Feedback through transcription. Plk1 directly activates the transcription factor FoxM1. FoxM1 stimulates the expression of multiple proteins in the mitotic entry network, including cyclin B. Thus, cyclin B–Cdk1–mediated activation of Plk1 through Bora/Aurora A increases the production of several cyclin B–Cdk1 activators, thereby further stimulating cyclin B–Cdk1 activation.
The outer feedback loops
Besides direct regulation of Cdk1 T14/Y15 phosphorylation, several feedback mechanisms more indirectly regulate cyclin B–Cdk1 activation. These feedbacks are superimposed on the inner feedback loops, thus we refer to them as the outer feedback loops. Cdk1-dependent phosphorylation not only affects the activity and stability of its targets directly, but can also create a docking site for Polo-like kinase-1 (Plk1; Elia et al., 2003a,b). In this way, Plk1 is targeted to several hundred different Cdk1 substrates (Lowery et al., 2007). Relevant for the inner feedback, Cdk1-dependent phosphorylation of Wee1 mediates Plk1 recruitment and subsequent Plk1-dependent phosphorylation of Wee1 (Watanabe et al., 2005). Dual phosphorylation of Wee1 by Cdk1 and Plk1 creates a phosphodegron on Wee1 that is recognized by the F-box protein β-TrCP, a component of the SCF–β-TrCP ubiquitin ligase, resulting in rapid poly-ubiquitinylation and proteasomal degradation of Wee1 (Watanabe et al., 2004). In parallel, Plk1-dependent phosphorylation of Myt1 results in inhibition of Myt1 kinase activity (Nakajima et al., 2003), and Plk1-dependent phosphorylation of Cdc25C promotes accumulation of Cdc25C in the nucleus (Toyoshima-Morimoto et al., 2002; Elia et al., 2003a). Whether Plk1 can phosphorylate Cdc25A and Cdc25B in a similar Cdk1-dependent manner is currently unclear. In addition, Plk1 can also phosphorylate cyclin B directly, and this coincides roughly with autocatalytic phosphorylation of cyclin B–Cdk1 (Toyoshima-Morimoto et al., 2001; Yuan et al., 2002; Jackman et al., 2003). Thus, Cdk1-dependent phosphorylation of components of the inner feedback can mediate an additional round of phosphorylation by Plk1, thereby strengthening or modulating the feedback (Fig. 2 B). Interestingly, mitotic Plk1 is also phosphorylated on two Cdk consensus sites, and although the relevance of these phosphorylation events is not known, it remains a distinct possibility that Cdk1 can directly modulate Plk1 function (Daub et al., 2008).
In G2 cells, Plk1 is present both at the centrosomes and at the kinetochores, where its localization is strongly influenced by targeting to phosphorylated proteins (Hanisch et al., 2006; Kang et al., 2006; Qi et al., 2006; Soung et al., 2006; Neef et al., 2007). The activation of Plk1 requires phosphorylation of a conserved residue (T210) in the T loop of the kinase. In human cells, the T210 residue of Plk1 is phosphorylated by Aurora A in G2 (Macurek et al., 2008; Seki et al., 2008b). Where exactly this phosphorylation first occurs is not clear, but given that Aurora A is predominately centrosomal and that T210-phosphorylated Plk1 can be detected on the centrosome in G2 phase, it is likely to occur initially on centrosomes (Macurek et al., 2008).
Aurora A can be activated through autophosphorylation of T288 in its T loop (Walter et al., 2000; Littlepage et al., 2002). However, to phosphorylate Plk1 on T210, Aurora A requires the cofactor Bora (Macurek et al., 2008; Seki et al., 2008b). Although Bora has been found to bind to Plk1 in the absence of Cdk1 (Seki et al., 2008a), Cdk1 does phosphorylate Bora (Hutterer et al., 2006; Chan et al., 2008), and this phosphorylation enhances Bora binding to Plk1 (Hutterer et al., 2006; Chan et al., 2008). Thus, by phosphorylating Bora, Cdk1 ensures that Bora can efficiently bind to Plk1, possibly stimulating Bora/Aurora A–mediated activation of Plk1. In this way, an additional feedback loop is formed in which cyclin B–Cdk1 can stimulate its own activation through stimulation of Plk1 activation (Fig. 2, A–C). Moreover, Aurora A was shown to directly phosphorylate Cdc25B, and could thereby stimulate cyclin B–Cdk1 activation even further (Dutertre et al., 2004). However, whether this Aurora A–dependent phosphorylation of Cdc25B requires Bora is currently unclear. In addition, Aurora A can be inactivated by dephosphorylation of T288 in the activatory T loop by the phosphatase PP1. Interestingly, PP1 is also subject to regulation by Cdk1 activity, so cyclin B–Cdk1 can potentially regulate Aurora A through PP1 (Katayama et al., 2001; Marumoto et al., 2002; Satinover et al., 2006). Nonetheless, both Aurora A and Plk1 kinase activities are down-regulated after inhibition of Cdk activity (Abrieu et al., 1998; Marumoto et al., 2002; unpublished data), which is consistent with the notion that these kinases participate in the same feedback loop.
As mentioned in the introduction, cyclin B concentration in late G2 phase is highest at centrosomes. But other players that function in the mitotic entry network, such as Plk1, Aurora A, Cdc25B, and Cdc25C, are also specifically recruited to centrosomes in late G2. Their recruitment coincides with centrosome maturation, when the size of the pericentriolar material (PCM) surrounding the centrioles dramatically increases. Although not completely understood, centrosome maturation and recruitment of cyclin B–Cdk1 to centrosomes both depend on the activities of Plk1 and Aurora A (for review see Barr and Gergely, 2007). Thus, by regulating the size of the PCM and possibly by direct targeting of certain proteins to the PCM, Aurora A and Plk1 can enhance the local concentration of many proteins in the mitotic entry network, thereby facilitating the activation of cyclin B–Cdk1 (Fig. 2 D). Indeed, in Caenorhabditis elegans, centrosomes influence the timing of nuclear envelope breakdown in a manner that is dependent on Aurora A (Hachet et al., 2007; Portier et al., 2007), whereas in human cells, the relevance of centrosomes for timing of mitotic entry remains unclear. Interestingly, both Aurora A and Plk1 centrosomal localization and centrosome maturation requires Cdk11(p58), a kinase specifically produced from an internal ribosomal entry site (IRES) in G2 and M phase (Cornelis et al., 2000; Petretti et al., 2006). The activation of the Cdk11(p58) IRES in G2 phase involves G2- and M-specific binding of hnRNP C1/C2, and it will be interesting to see whether this binding is regulated by any protein in the cyclin B–Cdk1 feedback loops (Schepens et al., 2007).
Recently, Plk1 was also shown to activate FoxM1 directly (Fu et al., 2008). FoxM1 enhances the transcription of multiple genes in the mitotic entry network, including cyclin B, Plk1, and Cdc25 phosphatases (Laoukili et al., 2005; Wang et al., 2005; Wierstra and Alves, 2007). In this way, Cdk1-dependent activation of Plk1 through Bora/Aurora A will enhance the concentration of many proteins in the mitotic entry network, thereby further stimulating cyclin B–Cdk1 activation (Fig. 1 E). In conclusion, multiple feedback loops function in parallel to enhance cyclin B–Cdk1 activation. These feedback loops affect cyclin B–Cdk1 at several levels, ranging from direct activation through the inner feedback to enhancing the concentration of mitotic entry network components through regulation of transcription and specific recruitment to defined subcellular sites. Recent proteomic screens have shown that several thousands of proteins, including at least 30 kinases without known mitotic function, are phosphorylated during mitosis (Daub et al., 2008; Dephoure et al., 2008; Xiang et al., 2008). The selected proteins and feedback loops discussed here are therefore likely to constitute only a minor, albeit central, part of the mitotic entry network.
Redundancy in the mitotic entry network
If many feedback loops function in parallel, what is their relative importance? RNAi-mediated depletion or pharmacological inhibition of Cdc25A, Cdc25B, Cdc25C, Plk1, Bora, Aurora A, cyclin B1, cyclin B2, Wee1, or Myt1 typically elicits a limited effect on mitotic entry (Marumoto et al., 2002; Bulavin et al., 2003; Hirota et al., 2003; van Vugt et al., 2004; Lindqvist et al., 2005; Liu and Ruderman, 2006; Bellanger et al., 2007; Gong et al., 2007; Nakajima et al., 2008; Seki et al., 2008b). Either no effect on the timing of mitotic entry is observed, or at best, entry is delayed by a couple of hours. Although the possibility exists that the limited effects depend on partial inhibition/depletion of the targets, it seems that many of the proteins involved in the molecular network that initiates mitotic entry are redundant for the decision to enter mitosis. Indeed, a more sustained delay is observed when combinations of proteins are inhibited/depleted, like Cdc25A together with Cdc25B or cyclin B1 together with cyclin B2 (Lindqvist et al., 2005; Bellanger et al., 2007; Gong et al., 2007). However, a systematic and exhaustive analysis of the relative contribution of the various components of the mitotic entry network is currently lacking. This is largely caused by technical limitations, as small molecule inhibitors only exist to a limited set of proteins, and RNAi efficiency decreases with simultaneous targeting of multiple genes. Importantly, the centrosomes, which are thought to function as scaffolds to bring together proteins in the mitotic entry network, besides functioning as microtubule organizing centers, are redundant for mitotic entry (Hinchcliffe et al., 2001; Khodjakov and Rieder, 2001; Doxsey et al., 2005). Indeed, Drosophila can develop without centrosomes, showing that their presence is not necessary for cell cycle progression (Basto et al., 2006). Whether the centrosomes play a role for mitotic entry in human cells would require a detailed analysis in the background of inhibition of other feedback loops. Thus, cyclin B–Cdk1 activation is promoted by multiple redundant feedback loops, but the relative contribution of these separate feedback loops to the decision to enter mitosis is currently not well resolved.
Checkpoint recovery
To avoid propagation of harmful mutations, cells arrest their progression through the cell cycle in response to DNA damage. When DNA damage is inflicted on a cell in G2 phase, this cell cycle arrest is achieved through posttranslational modification of many, if not all, proteins in the mitotic entry network (for review see Bartek and Lukas, 2007). Upon repair of the damaged DNA, the cell can in principle resume the cell cycle, a process we refer to as checkpoint recovery (van Vugt et al., 2004). However, due to the modifications caused by DNA-damage signaling, the activity and levels of the proteins in the mitotic entry network are different compared with an unperturbed G2 cell (Smits and Medema, 2001; Karlsson-Rosenthal and Millar, 2006; Bartek and Lukas, 2007; Bassermann et al., 2008). As such, checkpoint recovery constitutes a specialized form of mitotic entry, in which the redundancy in the mitotic entry network might be diminished. Indeed, proteins that are normally redundant for mitotic entry, including Aurora A, Plk1, and Cdc25B, are essential during checkpoint recovery (van Vugt et al., 2004; Macurek et al., 2008). This is a likely consequence of the dramatic changes in the mitotic entry network that have occurred during the checkpoint arrest (e.g., Cdc25A is degraded; Falck et al., 2001). Although the outer feedback (Fig. 2, B–E) is not essential for mitotic entry during an unperturbed cell cycle, at least parts of it become important for a restart of the cell cycle after checkpoint activation. Therefore, depending on the history of a G2 cell, different pathways within the mitotic entry network will be of different importance for the decision to enter mitosis.
The mitotic entry network and mitosis
The feedback loops governing cyclin B–Cdk1 activation discussed earlier will ensure that, concomitantly with cyclin B–Cdk1 activation, Plk1 and Aurora A are activated. Because the latter are both necessary for centrosome maturation and formation of a bipolar spindle in mitosis, this provides efficient coordination of these events with the eventual decision to enter mitosis (Barr and Gergely, 2007; Petronczki et al., 2008). Moreover, Plk1 can enhance the transcription of multiple proteins necessary for mitotic progression via its effect on FoxM1 (Laoukili et al., 2005; Wang et al., 2005; Wierstra and Alves, 2007; Fu et al., 2008). These feedback loops will therefore not only promote an efficient activation of cyclin B–Cdk1, but will also ensure that other regulatory factors needed for successful cell division are coordinately activated. Supporting this notion, short-circuiting the inner feedback by expression of a Wee1-/Myt1–insensitive version of Cdk1 leads to abnormal cell division (Pomerening et al., 2008). Similarly, shortening G2 phase by injection of active cyclin A–Cdk2 leads to mitotic abnormalities (Furuno et al., 1999). Moreover, the amount of cyclin B–Cdk1 activity needed to enter mitosis is relatively low, but cells that do enter mitosis with reduced cyclin B–Cdk1 levels show reduced phosphorylation of at least some of the components of the mitotic entry network, and these cells fail to execute a normal mitosis (Lindqvist et al., 2007). The mitotic defects of cells entering mitosis after deregulation of Cdk1 feedback loops or Cdk1 levels are pleiotropic, probably reflecting the large amount of substrates of Cdk1 and associated components in the feedback loops. Thus, although big parts of the mitotic entry network are redundant for mitotic entry, the activation of the full network, ensured by the multiple feedback loops, appears to be essential for proper coordination of the various mitotic events.
System level considerations
The activation of cyclin B–Cdk1 in Xenopus egg extracts is a bistable, switch-like process (Pomerening et al., 2003; Sha et al., 2003), and because of the feedback loops described in the previous sections, this is probably true in human cells as well (Lindqvist et al., 2007; Pomerening et al., 2008). Bistability here means that the majority of cyclin B–Cdk1 complexes are inactive, active, or approaching one of these states. Therefore, a cell will or will not enter mitosis, but cannot rest in an intermediate state. A consequence of bistability is hysteresis, the memory of a system, which is manifested by a resistance to change between the stable states (for review see Mitrophanov and Groisman, 2008). Because of hysteresis, the threshold level of cyclin B required for cyclin B–Cdk1 activation is higher than the threshold level required for inactivation of cyclin B–Cdk1 (Fig. 3). This means that active cyclin B–Cdk1 can remain active, despite fluctuations in cyclin B levels. These conditions occur during mitotic entry, where a large pool of active cyclin B–Cdk1 translocates to the nucleus. Despite the dip in cyclin B–Cdk1 concentration in the cytoplasm, the cytoplasmic activation of cyclin B–Cdk1 proceeds during the translocation (Lindqvist et al., 2007). Moreover, although not sufficient to execute a normal mitosis, cells that enter mitosis with relatively low levels of cyclin B–Cdk1 retain high cyclin B–Cdk1 activity per complex in mitosis (Lindqvist et al., 2007; Rodriguez-Bravo et al., 2007), and mitotic cells containing unaligned chromosomes can withstand large losses of cyclin B without exiting mitosis (Brito and Rieder, 2006). Thus, because of hysteresis, once cyclin B–Cdk1 is activated, a cell is likely to enter mitosis and remain there until the spindle checkpoint is satisfied, allowing cyclin B degradation. Hysteresis does not only facilitate a faithful mitosis, the resistance to change between stable states also delays the activation of cyclin B–Cdk1 in G2. This may give a cell an extended opportunity to block mitotic entry in case of checkpoint activation, and decreases the chance that a cell will enter mitosis because of local fluctuations in the levels of cyclin B or regulators of cyclin B–Cdk1 activity. Hysteresis regulating cyclin B–Cdk1 activity is most likely complemented by cell cycle–dependent regulation of phosphatases that reverse the phosphorylation of Cdk1 targets (Mochida and Hunt, 2007; Skoufias et al., 2007).
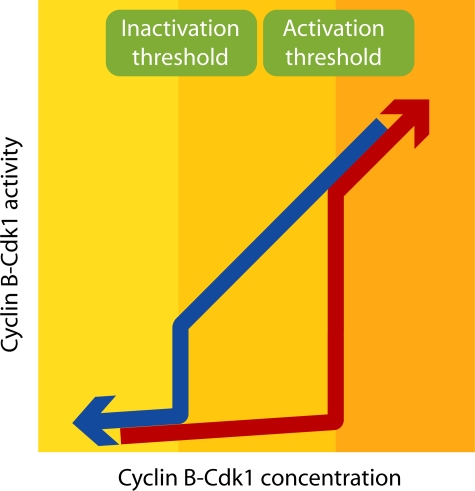
Figure 3.
Bistability and hysteresis governing cyclin B–Cdk1 activity. The graph shows the theoretical relationship between cyclin B–Cdk1 concentration and cyclin B–Cdk1 activity. Note that the graph only shows steady-state end points and therefore does not contain any information on how much time is needed to reach the indicated activity. Because of the cyclin B–Cdk1–dependent feedback loops, the majority of cyclin B–Cdk1 complexes are either active, inactive, or approaching one of these states. A major determinant for whether cyclin B–Cdk1 will be active or inactive is the concentration of cyclin B–Cdk1 complexes; cyclin B–Cdk1 activation is triggered above a threshold concentration of cyclin B–Cdk1 complexes (red arrow). However, once the feedback loops are active, they can sustain cyclin B–Cdk1 activity at cyclin B–Cdk1 concentrations below the activation threshold. Therefore, the inactivation threshold is lower than the activation threshold (blue arrow). In this way, the feedback loops provide a resistance to change between the stable states. This resistance ensures that cyclin B–Cdk1 activity, and thereby the decision to enter or exit mitosis, is relatively insensitive to local fluctuations of mitotic entry network components.
Implications on shuttling for the duration of cyclin B–Cdk1 activation
The bistable, switch-like activation of cyclin B–Cdk1 indicates that at any given time, the majority of cyclin B–Cdk1 complexes are inactive, active, or approaching one of these states. Importantly however, this does not necessarily mean that the initial activation of cyclin B–Cdk1 will occur very rapidly. Indeed, although the activation of the major part of cyclin B–Cdk1 takes place in the last 30 min preceding prometaphase, lower levels of Cdk1 activation can be detected in G2 phase well before centrosome separation (Lindqvist et al., 2007). Similarly, Plk1 activation can be detected ∼5 h before mitosis (Macurek et al., 2008). Both Plk1 and cyclin B–Cdk1 activity rise gradually until mitotic entry, when a rapid increase in activity occurs, arguing that the initial activation of the mitotic entry network is a gradual process that slowly builds up during G2.
So what makes the feedback loops take so long to build up full cyclin B–Cdk1 activation? One clue may come from the subcellular trafficking of cyclin B. Phosphorylation of cyclin B both hides a nuclear export sequence and enhances nuclear import, thereby causing the phosphorylated cyclin B–Cdk1 complex to move to the nucleus (Li et al., 1997; Hagting et al., 1999). The identity of the kinase phosphorylating S147 is unclear, and affirmative proof that S147 is truly phosphorylated in human cells is currently lacking. However, Plk1 and cyclin B–Cdk1 itself can phosphorylate other residues in cyclin B (Toyoshima-Morimoto et al., 2001; Yuan et al., 2002; Jackman et al., 2003). At least in Xenopus, Cdk1 preferentially phosphorylates residues on the cyclin B to which it is bound (Borgne et al., 1999), and phosphorylation of these residues enhances cyclin B–Cdk1 nuclear import (Yang et al., 2001). Thus, it is likely that activation of cyclin B–Cdk1 in human cells will also stimulate autophosphorylation, which in turn increases cyclin B–Cdk1 nuclear import. The regulation of cyclin B–Cdk1 nuclear import is probably also affected by other components of the mitotic entry network. In the nucleus, the Wee1 concentration is high, and cyclin B–Cdk1 can rapidly be inactivated. The now inactivated cyclin B–Cdk1 can no longer sustain its autophosphorylation, but can after export from the nucleus be reactivated and reautophosphorylated. Thus, as the activation is ongoing on the centrosomes, and possibly in the cytoplasm, this causes the activated cyclin B–Cdk1 to relocalize to the nucleus, where it is subsequently inactivated and exported, causing a delay in the activation of the mitotic entry network (Fig. 4).
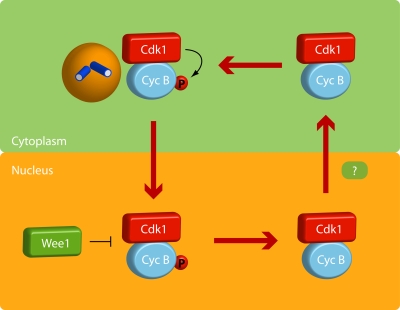
Figure 4.
Model of the effect of cyclin B–Cdk1 activity on cyclin B–Cdk1 nucleocytoplasmic shuttling. Once activated, cyclin B–Cdk1 autophosphorylates, thereby increasing its nuclear import. The nuclear import is most likely also affected by other components of the mitotic entry network. In the nucleus, Wee1 levels are high, leading to inactivation of the cyclin B–Cdk1 complex, which can no longer sustain the phosphorylation of cyclin B. After export to the cytoplasm, cyclin B–Cdk1 can be reactivated. Global activation of cyclin B–Cdk1 will occur when enough active cyclin B–Cdk1 enters the nucleus to promote Wee1 degradation, likely in cooperation with inhibitory phosphorylation of the cyclin B nuclear export sequence by an unknown kinase.
The activation of the majority of cyclin B–Cdk1 complexes will be achieved when enough cyclin B–Cdk1 enters the nucleus to sustain the feedback loops involving stabilization of Cdc25A and degradation of nuclear Wee1. This is likely to coincide with phosphorylation of S147 in cyclin B1, which would reduce cyclin B–Cdk1 nuclear export. Whether the phosphorylation of this residue is regulated by cyclin B–Cdk1–dependent feedback loops is currently not clear. Nonetheless, concomitantly with a rapid increase in cyclin B–Cdk1 activity, cyclin B–Cdk1 import outweighs its export, resulting in an efficient translocation of cyclin B–Cdk1 to the nucleus (Jackman et al., 2003; Lindqvist et al., 2007). This rise in nuclear cyclin B–Cdk1 activity enables the phosphorylation of nuclear cyclin B–Cdk1 targets, including structural lamins, which leads to nuclear envelope breakdown and entry into mitosis shortly after cyclin B–Cdk1 accumulates in the nucleus.
Even though local cyclin B–Cdk1 concentration is above the activation threshold, and activation is ongoing, full activation and entry into mitosis will not occur until a global activation is reached. The centrosomal/cytoplasmic activation, coupled with the nuclear import and subsequent export of cyclin B–Cdk1, can therefore potentially coordinate nuclear and cytoplasmic events; when nuclear envelope breakdown occurs, centrosomes are already mature, and enzymes, including Aurora A and Plk1, are activated to assist in building a bipolar spindle necessary for later mitotic progression. Moreover, the long duration of mitotic entry network activation can potentially provide the transcriptional machinery sufficient time to allow accumulation of mitotic regulators.
Cyclin A
In the nucleus, cyclin A–Cdk activity builds up during G2 phase. RNAi to cyclin A delays mitotic entry, which probably partially depends on reduced transcription and/or enhanced degradation of many players in the mitotic entry network (see Fig. 1). However, in the absence of cyclin A, there is also a delay observed between cytoplasmic Cdk1-dependent events and the rapid nuclear translocation of the bulk of cyclin B–Cdk1 occurring at prophase (Mitra and Enders, 2004; Fung et al., 2007; Gong et al., 2007; De Boer et al., 2008). It is therefore likely that cyclin A also directly contributes to the activation of cyclin B–Cdk1 in G2 phase. Because cyclin–Cdk complexes have many overlapping targets, one possibility is that cyclin A–Cdk can mediate a basal level of Cdk activity that lowers the threshold of cyclin B–Cdk1 necessary to sustain the feedback loops in the nucleus (Mitra and Enders, 2004; Fung et al., 2007; Gong et al., 2007; De Boer et al., 2008). As such, cyclin A–Cdk complexes could create Plk1 docking sites, or contribute to activation of Cdc25s or inactivation of Wee1. Although predominantly nuclear, cyclin A shuttles between the nucleus and the cytoplasm, and can associate with centrosomes, raising the possibility that cyclin A also affects other mitotic entry network components outside the nucleus (Jackman et al., 2002; De Boer et al., 2008). The accumulation of cyclin A is therefore likely to be a major determinant for when the feedback loops regulating cyclin B–Cdk1 activity are initiated.
When is cyclin B–Cdk1 first activated?
Basal Cdk activity is necessary for transcription and inhibition of degradation of many players in the mitotic entry network, which ensures that the initial cyclin B–Cdk1 activation does not come before S phase (Fig. 1; Fung and Poon, 2005). However, checkpoint components such as ataxia telangiectasia and Rad3 related (ATR), microcephalin, and Chk1 have at least a basal level of activity during S phase, and centrosomal Chk1 inhibits cyclin B–Cdk1 activation during S phase in the absence of external DNA damage (Kramer et al., 2004; Alderton et al., 2006; Schmitt et al., 2006; Loffler et al., 2007). Thus, the combined balance of growing Cdk activity during S and G2 phase and the activity of certain checkpoint signaling components during S phase determines when the initial cyclin B–Cdk1 activation can start to build up.
As discussed in this review, the regulation of cyclin B–Cdk1 activation consists of a network of interactions that function in feedback loops. We therefore propose that there is little upstream or downstream in the signal transduction within the mitotic entry network, but rather a large spiral of events that slowly build up to a critical threshold at which full activation is rapidly achieved. The relative importance of individual players in this spiral will depend on the history of the cell, where at least ectopic DNA damage changes the requirement for individual proteins within the mitotic entry network.
Acknowledgments
As mentioned in the article, we only discuss a selected set of feedback loops that regulate cyclin B–Cdk1 activation. We apologize to authors whose work is not cited due to space restrictions. We thank Monica Alvarez, Wytse Bruinsma, Geert Kops, Susanne Lens, and Libor Macurek for critical comments on the manuscript.
Footnotes
Abbreviations used in this paper: APC/C, anaphase-promoting complex/cyclosome; PCM, pericentriolar material; Plk1, Polo-like kinase-1.
References
- Abrieu A., Brassac T., Galas S., Fisher D., Labbe J.C., Doree M. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs.J. Cell Sci. 111:1751–1757 [DOI] [PubMed] [Google Scholar]
- Acquaviva C., Pines J. 2006. The anaphase-promoting complex/cyclosome: APC/C.J. Cell Sci. 119:2401–2404 [DOI] [PubMed] [Google Scholar]
- Alderton G.K., Galbiati L., Griffith E., Surinya K.H., Neitzel H., Jackson A.P., Jeggo P.A., O'Driscoll M. 2006. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling.Nat. Cell Biol. 8:725–733 [DOI] [PubMed] [Google Scholar]
- Baldin V., Ducommun B. 1995. Subcellular localisation of human wee1 kinase is regulated during the cell cycle.J. Cell Sci. 108:2425–2432 [DOI] [PubMed] [Google Scholar]
- Baldin V., Pelpel K., Cazales M., Cans C., Ducommun B. 2002. Nuclear localization of CDC25B1 and serine 146 integrity are required for induction of mitosis.J. Biol. Chem. 277:35176–35182 [DOI] [PubMed] [Google Scholar]
- Barr A.R., Gergely F. 2007. Aurora-A: the maker and breaker of spindle poles.J. Cell Sci. 120:2987–2996 [DOI] [PubMed] [Google Scholar]
- Bartek J., Lukas J. 2007. DNA damage checkpoints: from initiation to recovery or adaptation.Curr. Opin. Cell Biol. 19:238–245 [DOI] [PubMed] [Google Scholar]
- Bassermann F., Frescas D., Guardavaccaro D., Busino L., Peschiaroli A., Pagano M. 2008. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint.Cell. 134:256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. 2006. Flies without centrioles.Cell. 125:1375–1386 [DOI] [PubMed] [Google Scholar]
- Bellanger S., de Gramont A., Sobczak-Thepot J. 2007. Cyclin B2 suppresses mitotic failure and DNA re-replication in human somatic cells knocked down for both cyclins B1 and B2.Oncogene. 26:7175–7184 [DOI] [PubMed] [Google Scholar]
- Bonnet J., Coopman P., Morris M.C. 2008. Characterization of centrosomal localization and dynamics of Cdc25C phosphatase in mitosis.Cell Cycle. 7:1991–1998 [DOI] [PubMed] [Google Scholar]
- Booher R.N., Holman P.S., Fattaey A. 1997. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity.J. Biol. Chem. 272:22300–22306 [DOI] [PubMed] [Google Scholar]
- Borgne A., Ostvold A.C., Flament S., Meijer L. 1999. Intra-M phase-promoting factor phosphorylation of cyclin B at the prophase/metaphase transition.J. Biol. Chem. 274:11977–11986 [DOI] [PubMed] [Google Scholar]
- Bouche J.P., Froment C., Dozier C., Esmenjaud-Mailhat C., Lemaire M., Monsarrat B., Burlet-Schiltz O., Ducommun B. 2008. NanoLC-MS/MS analysis provides new insights into the phosphorylation pattern of Cdc25B in vivo: full overlap with sites of phosphorylation by Chk1 and Cdk1/cycB kinases in vitro.J. Proteome Res. 7:1264–1273 [DOI] [PubMed] [Google Scholar]
- Boutros R., Dozier C., Ducommun B. 2006. The when and wheres of CDC25 phosphatases.Curr. Opin. Cell Biol. 18:185–191 [DOI] [PubMed] [Google Scholar]
- Brito D.A., Rieder C.L. 2006. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint.Curr. Biol. 16:1194–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin D.V., Demidenko Z.N., Phillips C., Moody S.A., Fornace A.J., Jr. 2003. Phosphorylation of Xenopus Cdc25C at Ser285 interferes with ability to activate a DNA damage replication checkpoint in pre-midblastula embryos.Cell Cycle. 2:263–266 [PubMed] [Google Scholar]
- Busch C., Barton O., Morgenstern E., Gotz C., Gunther J., Noll A., Montenarh M. 2007. The G2/M checkpoint phosphatase cdc25C is located within centrosomes.Int. J. Biochem. Cell Biol. 39:1707–1713 [DOI] [PubMed] [Google Scholar]
- Chae H.D., Yun J., Bang Y.J., Shin D.Y. 2004. Cdk2-dependent phosphorylation of the NF-Y transcription factor is essential for the expression of the cell cycle-regulatory genes and cell cycle G1/S and G2/M transitions.Oncogene. 23:4084–4088 [DOI] [PubMed] [Google Scholar]
- Chan E.H., Santamaria A., Silljé H.H., Nigg E.A. 2008. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora.Chromosoma. 117:457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis S., Bruynooghe Y., Denecker G., Van Huffel S., Tinton S., Beyaert R. 2000. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site.Mol. Cell. 5:597–605 [DOI] [PubMed] [Google Scholar]
- Daub H., Olsen J.V., Bairlein M., Gnad F., Oppermann F.S., Korner R., Greff Z., Keri G., Stemmann O., Mann M. 2008. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle.Mol. Cell. 31:438–448 [DOI] [PubMed] [Google Scholar]
- De Boer L., Oakes V., Beamish H., Giles N., Stevens F., Somodevilla-Torres M., Desouza C., Gabrielli B. 2008. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events.Oncogene. 27:4261–4268 [DOI] [PubMed] [Google Scholar]
- Dephoure N., Zhou C., Villen J., Beausoleil S.A., Bakalarski C.E., Elledge S.J., Gygi S.P. 2008. A quantitative atlas of mitotic phosphorylation.Proc. Natl. Acad. Sci. USA. 105:10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S., Zimmerman W., Mikule K. 2005. Centrosome control of the cell cycle.Trends Cell Biol. 15:303–311 [DOI] [PubMed] [Google Scholar]
- Dutertre S., Cazales M., Quaranta M., Froment C., Trabut V., Dozier C., Mirey G., Bouche J.P., Theis-Febvre N., Schmitt E., et al. 2004. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition.J. Cell Sci. 117:2523–2531 [DOI] [PubMed] [Google Scholar]
- Dynlacht B.D., Flores O., Lees J.A., Harlow E. 1994. Differential regulation of E2F transactivation by cyclin/cdk2 complexes.Genes Dev. 8:1772–1786 [DOI] [PubMed] [Google Scholar]
- Elia A.E., Cantley L.C., Yaffe M.B. 2003a. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates.Science. 299:1228–1231 [DOI] [PubMed] [Google Scholar]
- Elia A.E., Rellos P., Haire L.F., Chao J.W., Ivins F.J., Hoepker K., Mohammad D., Cantley L.C., Smerdon S.J., Yaffe M.B. 2003b. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain.Cell. 115:83–95 [DOI] [PubMed] [Google Scholar]
- Falck J., Mailand N., Syljuasen R.G., Bartek J., Lukas J. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis.Nature. 410:842–847 [DOI] [PubMed] [Google Scholar]
- Fu Z., Malureanu L., Huang J., Wang W., Li H., van Deursen J.M., Tindall D.J., Chen J. 2008. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression.Nat. Cell Biol. 10:1076–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.K., Poon R.Y. 2005. A roller coaster ride with the mitotic cyclins.Semin. Cell Dev. Biol. 16:335–342 [DOI] [PubMed] [Google Scholar]
- Fung T.K., Ma H.T., Poon R.Y. 2007. Specialized roles of the two mitotic cyclins in somatic cells: cyclin A as an activator of M phase-promoting factor.Mol. Biol. Cell. 18:1861–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N., den Elzen N., Pines J. 1999. Human cyclin A is required for mitosis until mid prophase.J. Cell Biol. 147:295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli B.G., De Souza C.P., Tonks I.D., Clark J.M., Hayward N.K., Ellem K.A. 1996. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells.J. Cell Sci. 109:1081–1093 [DOI] [PubMed] [Google Scholar]
- Gong D., Pomerening J.R., Myers J.W., Gustavsson C., Jones J.T., Hahn A.T., Meyer T., Ferrell J.E., Jr. 2007. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1.Curr. Biol. 17:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet V., Canard C., Gonczy P. 2007. Centrosomes promote timely mitotic entry in C. elegans embryos.Dev. Cell. 12:531–541 [DOI] [PubMed] [Google Scholar]
- Hagting A., Karlsson C., Clute P., Jackman M., Pines J. 1998. MPF localization is controlled by nuclear export.EMBO J. 17:4127–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., Jackman M., Simpson K., Pines J. 1999. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal.Curr. Biol. 9:680–689 [DOI] [PubMed] [Google Scholar]
- Hanisch A., Wehner A., Nigg E.A., Sillje H.H. 2006. Different Plk1 functions show distinct dependencies on Polo-Box domain-mediated targeting.Mol. Biol. Cell. 17:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe E.H., Miller F.J., Cham M., Khodjakov A., Sluder G. 2001. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase.Science. 291:1547–1550 [DOI] [PubMed] [Google Scholar]
- Hirota T., Kunitoku N., Sasayama T., Marumoto T., Zhang D., Nitta M., Hatakeyama K., Saya H. 2003. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells.Cell. 114:585–598 [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Clarke P.R., Marcote M.J., Karsenti E., Draetta G. 1993. Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis.EMBO J. 12:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.Y., Reimann J.D., Sorensen C.S., Lukas J., Jackson P.K. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1).Nat. Cell Biol. 4:358–366 [DOI] [PubMed] [Google Scholar]
- Hutterer A., Berdnik D., Wirtz-Peitz F., Zigman M., Schleiffer A., Knoblich J.A. 2006. Mitotic activation of the kinase Aurora-A requires its binding partner Bora.Dev. Cell. 11:147–157 [DOI] [PubMed] [Google Scholar]
- Jackman M., Kubota Y., den Elzen N., Hagting A., Pines J. 2002. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm.Mol. Biol. Cell. 13:1030–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M., Lindon C., Nigg E.A., Pines J. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase.Nat. Cell Biol. 5:143–148 [DOI] [PubMed] [Google Scholar]
- Kang Y.H., Park J.E., Yu L.R., Soung N.K., Yun S.M., Bang J.K., Seong Y.S., Yu H., Garfield S., Veenstra T.D., Lee K.S. 2006. Self-regulated Plk1 recruitment to kinetochores by the Plk1-PBIP1 interaction is critical for proper chromosome segregation.Mol. Cell. 24:409–422 [DOI] [PubMed] [Google Scholar]
- Karlsson C., Katich S., Hagting A., Hoffmann I., Pines J. 1999. Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis.J. Cell Biol. 146:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson-Rosenthal C., Millar J.B. 2006. Cdc25: mechanisms of checkpoint inhibition and recovery.Trends Cell Biol. 16:285–292 [DOI] [PubMed] [Google Scholar]
- Katayama H., Zhou H., Li Q., Tatsuka M., Sen S. 2001. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle.J. Biol. Chem. 276:46219–46224 [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C.L. 2001. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression.J. Cell Biol. 153:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Mailand N., Lukas C., Syljuasen R.G., Wilkinson C.J., Nigg E.A., Bartek J., Lukas J. 2004. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase.Nat. Cell Biol. 6:884–891 [DOI] [PubMed] [Google Scholar]
- Lammer C., Wagerer S., Saffrich R., Mertens D., Ansorge W., Hoffmann I. 1998. The cdc25B phosphatase is essential for the G2/M phase transition in human cells.J. Cell Sci. 111:2445–2453 [DOI] [PubMed] [Google Scholar]
- Laoukili J., Kooistra M.R., Bras A., Kauw J., Kerkhoven R.M., Morrison A., Clevers H., Medema R.H. 2005. FoxM1 is required for execution of the mitotic programme and chromosome stability.Nat. Cell Biol. 7:126–136 [DOI] [PubMed] [Google Scholar]
- Laoukili J., Alvarez M., Meijer L.A., Stahl M., Mohammed S., Kleij L., Heck A.J., Medema R.H. 2008. Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain.Mol. Cell. Biol. 28:3076–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Meyer A.N., Donoghue D.J. 1997. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation.Proc. Natl. Acad. Sci. USA. 94:502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A., Kallstrom H., Lundgren A., Barsoum E., Rosenthal C.K. 2005. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome.J. Cell Biol. 171:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A., van Zon W., Karlsson Rosenthal C., Wolthuis R.M. 2007. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression.PLoS Biol. 5:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlepage L.E., Wu H., Andresson T., Deanehan J.K., Amundadottir L.T., Ruderman J.V. 2002. Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A.Proc. Natl. Acad. Sci. USA. 99:15440–15445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Stanton J.J., Wu Z., Piwnica-Worms H. 1997. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex.Mol. Cell. Biol. 17:571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Ruderman J.V. 2006. Aurora A, mitotic entry, and spindle bipolarity.Proc. Natl. Acad. Sci. USA. 103:5811–5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler H., Bochtler T., Fritz B., Tews B., Ho A.D., Lukas J., Bartek J., Kramer A. 2007. DNA damage-induced accumulation of centrosomal Chk1 contributes to its checkpoint function.Cell Cycle. 6:2541–2548 [DOI] [PubMed] [Google Scholar]
- Lowery D.M., Clauser K.R., Hjerrild M., Lim D., Alexander J., Kishi K., Ong S.E., Gammeltoft S., Carr S.A., Yaffe M.B. 2007. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate.EMBO J. 26:2262–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C., Sorensen C.S., Kramer E., Santoni-Rugiu E., Lindeneg C., Peters J.M., Bartek J., Lukas J. 1999. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex.Nature. 401:815–818 [DOI] [PubMed] [Google Scholar]
- Macurek L., Lindqvist A., Lim D., Lampson M.A., Klompmaker R., Freire R., Clouin C., Taylor S.S., Yaffe M.B., Medema R.H. 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery.Nature. 455:119–123 [DOI] [PubMed] [Google Scholar]
- Mailand N., Podtelejnikov A.V., Groth A., Mann M., Bartek J., Lukas J. 2002. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability.EMBO J. 21:5911–5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major M.L., Lepe R., Costa R.H. 2004. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators.Mol. Cell. Biol. 24:2649–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T., Hirota T., Morisaki T., Kunitoku N., Zhang D., Ichikawa Y., Sasayama T., Kuninaka S., Mimori T., Tamaki N., et al. 2002. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells.Genes Cells. 7:1173–1182 [DOI] [PubMed] [Google Scholar]
- McGowan C.H., Russell P. 1995. Cell cycle regulation of human WEE1.EMBO J. 14:2166–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra J., Enders G.H. 2004. Cyclin A/Cdk2 complexes regulate activation of Cdk1 and Cdc25 phosphatases in human cells.Oncogene. 23:3361–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrophanov A.Y., Groisman E.A. 2008. Positive feedback in cellular control systems.Bioessays. 30:542–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Hunt T. 2007. Calcineurin is required to release Xenopus egg extracts from meiotic M phase.Nature. 449:336–340 [DOI] [PubMed] [Google Scholar]
- Nakajima H., Toyoshima-Morimoto F., Taniguchi E., Nishida E. 2003. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate.J. Biol. Chem. 278:25277–25280 [DOI] [PubMed] [Google Scholar]
- Nakajima H., Yonemura S., Murata M., Nakamura N., Piwnica-Worms H., Nishida E. 2008. Myt1 protein kinase is essential for Golgi and ER assembly during mitotic exit.J. Cell Biol. 181:89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef R., Gruneberg U., Kopajtich R., Li X., Nigg E.A., Sillje H., Barr F.A. 2007. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1.Nat. Cell Biol. 9:436–444 [DOI] [PubMed] [Google Scholar]
- O'Farrell P.H. 2001. Triggering the all-or-nothing switch into mitosis.Trends Cell Biol. 11:512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petretti C., Savoian M., Montembault E., Glover D.M., Prigent C., Giet R. 2006. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation.EMBO Rep. 7:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Lenart P., Peters J.M. 2008. Polo on the rise-from mitotic entry to cytokinesis with Plk1.Dev. Cell. 14:646–659 [DOI] [PubMed] [Google Scholar]
- Pomerening J.R., Sontag E.D., Ferrell J.E., Jr. 2003. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2.Nat. Cell Biol. 5:346–351 [DOI] [PubMed] [Google Scholar]
- Pomerening J.R., Ubersax J.A., Ferrell J.E., Jr. 2008. Rapid cycling and precocious termination of G1 phase in cells expressing CDK1AF.Mol. Biol. Cell. 19:3426–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier N., Audhya A., Maddox P.S., Green R.A., Dammermann A., Desai A., Oegema K. 2007. A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown.Dev. Cell. 12:515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Tang Z., Yu H. 2006. Phosphorylation- and polo-box-dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1.Mol. Biol. Cell. 17:3705–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape M., Kirschner M.W. 2004. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry.Nature. 432:588–595 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bravo V., Guaita-Esteruelas S., Salvador N., Bachs O., Agell N. 2007. Different S/M checkpoint responses of tumor and non tumor cell lines to DNA replication inhibition.Cancer Res. 67:11648–11656 [DOI] [PubMed] [Google Scholar]
- Satinover D.L., Brautigan D.L., Stukenberg P.T. 2006. Aurora-A kinase and inhibitor-2 regulate the cyclin threshold for mitotic entry in Xenopus early embryonic cell cycles.Cell Cycle. 5:2268–2274 [DOI] [PubMed] [Google Scholar]
- Saville M.K., Watson R.J. 1998. The cell-cycle regulated transcription factor B-Myb is phosphorylated by cyclin A/Cdk2 at sites that enhance its transactivation properties.Oncogene. 17:2679–2689 [DOI] [PubMed] [Google Scholar]
- Schepens B., Tinton S.A., Bruynooghe Y., Parthoens E., Haegman M., Beyaert R., Cornelis S. 2007. A role for hnRNP C1/C2 and Unr in internal initiation of translation during mitosis.EMBO J. 26:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E., Boutros R., Froment C., Monsarrat B., Ducommun B., Dozier C. 2006. CHK1 phosphorylates CDC25B during the cell cycle in the absence of DNA damage.J. Cell Sci. 119:4269–4275 [DOI] [PubMed] [Google Scholar]
- Seki A., Coppinger J.A., Du H., Jang C.Y., Yates J.R., III, Fang G. 2008a. Plk1- and β-TrCP–dependent degradation of Bora controls mitotic progression.J. Cell Biol. 181:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A., Coppinger J.A., Jang C.Y., Yates J.R., Fang G. 2008b. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry.Science. 320:1655–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W., Moore J., Chen K., Lassaletta A.D., Yi C.S., Tyson J.J., Sible J.C. 2003. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts.Proc. Natl. Acad. Sci. USA. 100:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias D.A., Indorato R.L., Lacroix F., Panopoulos A., Margolis R.L. 2007. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed.J. Cell Biol. 179:671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits V.A., Medema R.H. 2001. Checking out the G(2)/M transition.Biochim. Biophys. Acta. 1519:1–12 [DOI] [PubMed] [Google Scholar]
- Solomon M.J., Glotzer M., Lee T.H., Philippe M., Kirschner M.W. 1990. Cyclin activation of p34cdc2.Cell. 63:1013–1024 [DOI] [PubMed] [Google Scholar]
- Soung N.K., Kang Y.H., Kim K., Kamijo K., Yoon H., Seong Y.S., Kuo Y.L., Miki T., Kim S.R., Kuriyama R., et al. 2006. Requirement of hCenexin for proper mitotic functions of polo-like kinase 1 at the centrosomes.Mol. Cell. Biol. 26:8316–8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J.P., Schultz S.J., Bartek J., Nigg E.A. 1994. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase).J. Cell Biol. 127:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F., Moriguchi T., Wada A., Fukuda M., Nishida E. 1998. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint.EMBO J. 17:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E., Shinya N., Iwamatsu A., Nishida E. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase.Nature. 410:215–220 [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E., Nishida E. 2002. Plk1 promotes nuclear translocation of human Cdc25C during prophase.EMBO Rep. 3:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leuken R., Clijsters L., Wolthuis R. 2008. To cell cycle, swing the APC/C.Biochim. Biophys. Acta. 1786:49–59 [DOI] [PubMed] [Google Scholar]
- van Vugt M.A., Bras A., Medema R.H. 2004. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells.Mol. Cell. 15:799–811 [DOI] [PubMed] [Google Scholar]
- Walker A., Acquaviva C., Matsusaka T., Koop L., Pines J. 2008. UbcH10 has a rate-limiting role in G1 phase but might not act in the spindle checkpoint or as part of an autonomous oscillator.J. Cell Sci. 121:2319–2326 [DOI] [PubMed] [Google Scholar]
- Walter A.O., Seghezzi W., Korver W., Sheung J., Lees E. 2000. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation.Oncogene. 19:4906–4916 [DOI] [PubMed] [Google Scholar]
- Wang I.C., Chen Y.J., Hughes D., Petrovic V., Major M.L., Park H.J., Tan Y., Ackerson T., Costa R.H. 2005. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase.Mol. Cell. Biol. 25:10875–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Nishihara Y., Taniguchi M., Watanabe N., Hunter T., Osada H. 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP.Proc. Natl. Acad. Sci. USA. 101:4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Iwasaki J., Shiina M., Ogata K., Hunter T., Osada H. 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways.Proc. Natl. Acad. Sci. USA. 102:11663–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I., Alves J. 2007. FOXM1, a typical proliferation-associated transcription factor.Biol. Chem. 388:1257–1274 [DOI] [PubMed] [Google Scholar]
- Xiang M., Xue C., Huicai L., Jin L., Hong L., Dacheng H. 2008. Large-scale identification of novel mitosis-specific phosphoproteins.Biochim. Biophys. Acta. 1784:882–890 [DOI] [PubMed] [Google Scholar]
- Yang J., Bardes E.S., Moore J.D., Brennan J., Powers M.A., Kornbluth S. 1998. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1.Genes Dev. 12:2131–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Song H., Walsh S., Bardes E.S., Kornbluth S. 2001. Combinatorial control of cyclin B1 nuclear trafficking through phosphorylation at multiple sites.J. Biol. Chem. 276:3604–3609 [DOI] [PubMed] [Google Scholar]
- Yuan J., Eckerdt F., Bereiter-Hahn J., Kurunci-Csacsko E., Kaufmann M., Strebhardt K. 2002. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1.Oncogene. 21:8282–8292 [DOI] [PubMed] [Google Scholar]
- Ziebold U., Bartsch O., Marais R., Ferrari S., Klempnauer K.H. 1997. Phosphorylation and activation of B-Myb by cyclin A-Cdk2.Curr. Biol. 7:253–260 [DOI] [PubMed] [Google Scholar]