Abstract
The commitment to programmed cell death via apoptosis is largely made upon activation of the proapoptotic mitochondrial proteins Bax or Bak. In this issue, Gallenne et al. (Gallenne, C., F. Gautier, L. Oliver, E. Hervouet, B. Noël, J.A. Hickman, O. Geneste, P.-F. Cartron, F.M. Vallette, S. Manon, and P. Juin. 2009. J. Cell Biol. 185:279–290) provide evidence that the p53 up-regulated modulator of apoptosis (Puma) protein can directly activate Bax.
The Bcl-2 family of proteins participates in the control of the cell's commitment to programmed cell death via the mitochondrial or intrinsic apoptotic pathway. Certain proteins in this family, including Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and Bfl-1/A1, inhibit apoptosis, whereas others in this family promote apoptosis. Proapoptotic Bax and Bak appear to be indispensible for apoptosis (Lindsten et al., 2000; Wei et al., 2001). How does the cell determine fate in the face of competing pro- and antiapoptotic proteins? The rheostat model proposed that when there were more antiapoptotic proteins than proapoptotic proteins, the cell survived and vice versa. However, in many cases, the conversion of a living cell to one committed to death occurs without significant change in the levels of pro- and antiapoptotic proteins. The participation of a third class of proapoptotic proteins largely explained this riddle. These proteins, so-called BH3-only as they share homology only in the proapoptotic Bcl-2 homology 3 domain, appear to act as sentinels of cell damage, which convert initial perturbations into death signals, that act in the mitochondrial pathway. Now, Gallenne et al. (see p. 279 of this issue) provide mechanistic insight into how the BH3-only protein Puma promotes apoptosis. The authors find that Puma, like the BH3-only proteins Bim and Bid, directly activates Bax.
A key event in the commitment to apoptosis is Bax- and Bak-mediated permeabilization of the outer mitochondrial membrane. For this to occur, Bax and Bak alter their conformation from an inactive to an active form, form homo-oligomers in the membrane, and contribute to the formation of pores, which allows the egress of proapoptotic proteins to the cytosol (Fig. 1). Although there is consensus that Bax and Bak must shift from an inactive to an active state for this to occur, there is less consensus about what specific factors cause this crucial switch (Willis et al., 2007). Bid and Bim have been shown to cause activation (conformational change and oligomerization) of Bax and Bak in cellular, mitochondrial, and liposomal systems (Wei et al., 2000; Kuwana et al., 2002; Cartron et al., 2004; Certo et al., 2006). Direct interaction between these activators and Bax has been established experimentally (Gavathiotis et al., 2008; Lovell et al., 2008). Additional studies have suggested that p53 itself may translocate to the mitochondria and activate Bax after select stimuli (Mihara et al. 2003; Chipuk et al., 2004). Even heat has been indicted as a potential activating factor (Pagliari et al., 2005). It is quite possible that many activating factors remain to be discovered.
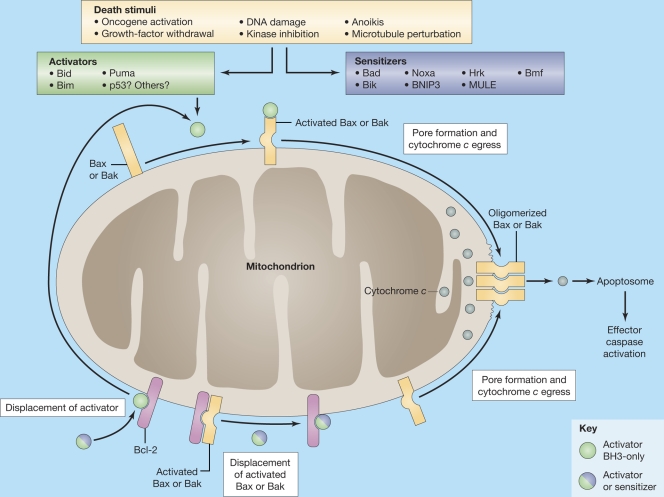
Figure 1.
Control of mitochondrial permeabilization by Bcl-2 family proteins. Activated Bax or Bak are available to oligomerize either when they are directly activated by activating factors, including activator BH3-only proteins (top), or when preactivated Bax or Bak are displaced from antiapoptotic proteins by either activator or sensitizer BH3-only proteins (bottom). Gallenne et al. (2009) provide evidence that Puma is an activator rather than a sensitizer. Oligomerized Bax or Bak participate in forming a pore that allows egress of proapoptotic factors like cytochrome c. Cytochrome c promotes formation of the apoptosome complex, which causes activation of effector caspases. These proteases cleave many key cellular proteins to bring about the apoptotic phenotype. Figure adapted with permission from the Journal of Cell Science (Brunelle, J.K., and A. Letai. 2009. J. Cell Sci. 122:437–441).
Antiapoptotic proteins inhibit apoptosis by binding proapoptotic factors. In many cases, the proapoptotic factors are activator BH3-only proteins like Bid and Bim. However, in some cases, the proapoptotic factors may also include activated monomeric Bax and Bak, which are intercepted before they can oligomerize and form pores. Cells have been described in which antiapoptotic proteins are loaded with abundant prodeath proteins as being “primed for death.” Such cells are particularly sensitive to treatment with chemotherapy and antagonists of antiapoptotic proteins like ABT-737 (Certo et al., 2006; Deng et al., 2007). In most cells, the vast majority of Bax and Bak are in the inactive form, and activated Bax and Bak can be difficult to detect in the absence of toxic perturbation. Nonetheless, BH3-only molecules, which lack the ability to directly activate Bax or Bak, can cause apoptosis by competing for binding to antiapoptotic proteins (Fig. 1). If this competition frees sufficient activator proteins (or activated Bax and Bak), oligomerization of Bax and Bak ensues, committing the cell to death. Based on performance in assays on mitochondria and artificial liposomes spiked with Bax, the BH3-only family has thus been segregated into two subfamilies: the sensitizers and the activators.
Where does Puma fit in? Puma was initially identified as a p53-regulated gene that was induced after DNA damage (Nakano and Vousden, 2001). It has subsequently been found that Puma is responsible for much of the proapoptotic effect of p53 induction but that Puma can also cause apoptosis in a p53-independent fashion (Jeffers et al., 2003; Villunger et al., 2003). The assignment of Puma as either a sensitizer or an activator has been somewhat contentious. The BH3 domains of BH3-only proteins are both necessary and sufficient to interact with Bcl-2 family members and seem to largely recapitulate function of the entire protein. For instance, the BH3 domains of Bid and Bim can activate Bax and Bak in liposomal or mitochondrial settings. The Puma BH3 domain lacked this function in several studies, leading many to classify Puma as a sensitizer (Kuwana et al., 2005; Certo et al., 2006). However, experiments with the full-length protein translated in vitro show an ability to activate Bax comparable with that of Bim and Bid (Kim et al., 2006).
Cartron et al. (2004) has previously found that the BH3 domains of Bim and Puma but not the sensitizer Bad interact with Bax and cause its activation. In Gallenne et al. (2009), the role of Puma as an activator is further supported by three main pieces of evidence. First, Bax preincubated with the Puma BH3 peptide is more toxic to microinjected cells than is Bax alone. This enhancement is blocked by coincubation with a peptide mimicking the putative interaction site on Bax, the Hα1 C-terminal peptide. This suggests that the interaction of the Puma BH3 domain with a site on the first α helix of Bax is necessary for Puma's enhancement of Bax killing. It is worth noting that this interaction site on Bax, first identified by this group 4 yr ago, overlaps with an interaction site of the activator Bim BH3 peptide with Bax recently demonstrated by nuclear magnetic resonance in solution (Gavathiotis et al., 2008). The fact that two groups independently identified a similar and unexpected site for interaction of activating BH3 domains with Bax lends some confidence to this finding.
Additionally, because the Bcl-2 family is absent from the yeast genome, the authors exploit yeast to study Puma and Bax in a setting uncontaminated by the contribution of unmeasured Bcl-2 family proteins. Again, they find that coexpression of Puma is necessary for efficient killing by Bax. Finally, the authors investigate the participation of Puma in killing human colorectal cancer cells with ABT-737. ABT-737 is a BH3 mimetic that promotes apoptosis by binding antiapoptotic proteins and displacing select prebound prodeath proteins. Thus, ABT-737 can only kill cells that are primed with either activators or preactivated Bax or Bak. They find that ABT-737 treatment results in the freeing of Puma, which then interacts with Bax, correlating with the death of the cell. This finding suggests that Puma can play the priming function that is likely critical to sensitivity to many chemotherapeutic agents as well as ABT-737 (Deng et al., 2007). This role may be particularly important in cells in which Bim and Bid are not expressed at high levels.
Some questions remain. It is not clear why several laboratories have consistently failed to observe an activating function for the BH3 domain of Puma in either mitochondrial or liposomal systems. It is possible that even if Puma can play an activating role, the efficiency of this function may vary considerably according to context and perhaps be much less in many contexts than that of Bid or Bim. In a full-length Puma protein, perhaps interactions of the Puma BH3 domain with Bax are enhanced. It is also possible that unknown posttranslational modifications of Puma or Bax, varying according to cellular context, significantly influence the ability of Puma to activate Bax. In any case, Gallenne et al. (2009) have strengthened the case for Puma as an activator so that its potential contribution to this function cannot be ignored. One must now wonder: what other activators might still be out there waiting to be discovered?
References
- Cartron P.F., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA.Mol. Cell. 16:807–818 [DOI] [PubMed] [Google Scholar]
- Certo M., Moore Vdel G., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A. 2006. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members.Cancer Cell. 9:351–365 [DOI] [PubMed] [Google Scholar]
- Chipuk J.E., Kuwana T., Bouchier-Hayes L., Droin N.M., Newmeyer D.D., Schuler M., Green D.R. 2004. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis.Science. 303:1010–1014 [DOI] [PubMed] [Google Scholar]
- Deng J., Carlson N., Takeyama K., Dal Cin P., Shipp M., Letai A. 2007. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents.Cancer Cell. 12:171–185 [DOI] [PubMed] [Google Scholar]
- Gallenne C., Gautier F., Oliver L., Hervouet E., Noël B., Hickman J.A., Geneste O., Cartron P.-F., Vallette F.M., Manon S., Juin P. 2009. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members.J. Cell Biol. 185:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E., Suzuki M., Davis M.L., Pitter K., Bird G.H., Katz S.G., Tu H.C., Kim H., Cheng E.H., Tjandra N., Walensky L.D. 2008. BAX activation is initiated at a novel interaction site.Nature. 455:1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers J.R., Parganas E., Lee Y., Yang C., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., et al. 2003. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways.Cancer Cell. 4:321–328 [DOI] [PubMed] [Google Scholar]
- Kim H., Rafiuddin-Shah M., Tu H.C., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H. 2006. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies.Nat. Cell Biol. 8:1348–1358 [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey M.R., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane.Cell. 111:331–342 [DOI] [PubMed] [Google Scholar]
- Kuwana T., Bouchier-Hayes L., Chipuk J.E., Bonzon C., Sullivan B.A., Green D.R., Newmeyer D.D. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly.Mol. Cell. 17:525–535 [DOI] [PubMed] [Google Scholar]
- Lindsten T., Ross A.J., King A., Zong W.-X., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., et al. 2000. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues.Mol. Cell. 6:1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell J.F., Billen L.P., Bindner S., Shamas-Din A., Fradin C., Leber B., Andrews D.W. 2008. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax.Cell. 135:1074–1084 [DOI] [PubMed] [Google Scholar]
- Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U.M. 2003. p53 has a direct apoptogenic role at the mitochondria.Mol. Cell. 11:577–590 [DOI] [PubMed] [Google Scholar]
- Nakano K., Vousden K.H. 2001. PUMA, a novel proapoptotic gene, is induced by p53.Mol. Cell. 7:683–694 [DOI] [PubMed] [Google Scholar]
- Pagliari L.J., Kuwana T., Bonzon C., Newmeyer D.D., Tu S., Beere H.M., Green D.R. 2005. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat.Proc. Natl. Acad. Sci. USA. 102:17975–17980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A., Michalak E.M., Coultas L., Mullauer F., Bock G., Ausserlechner M.J., Adams J.M., Strasser A. 2003. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa.Science. 302:1036–1038 [DOI] [PubMed] [Google Scholar]
- Wei M.C., Lindsten T., Mootha V.K., Weiler S., Gross A., Ashiya M., Thompson C.B., Korsmeyer S.J. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c.Genes Dev. 14:2060–2071 [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Zong W.X., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death.Science. 292:727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S.N., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak.Science. 315:856–859 [DOI] [PubMed] [Google Scholar]