Abstract
Centromeres are chromosomal structures required for equal DNA segregation to daughter cells, comprising specialized nucleosomes containing centromere protein A (CENP-A) histone, which provide the basis for centromeric chromatin assembly. Discovery of centromere protein components is progressing, but knowledge related to their establishment and maintenance remains limited. Previously, using anti-CENP-A native chromatin immunoprecipitation, we isolated the interphase–centromere complex (ICEN). Among ICEN components, subunits of the remodeling and spacing factor (RSF) complex, Rsf-1 and SNF2h proteins, were found. This paper describes the relationship of the RSF complex to centromere structure and function, demonstrating its requirement for maintenance of CENP-A at the centromeric core chromatin in HeLa cells. The RSF complex interacted with CENP-A chromatin in mid-G1. Rsf-1 depletion induced loss of centromeric CENP-A, and purified RSF complex reconstituted and spaced CENP-A nucleosomes in vitro. From these data, we propose the RSF complex as a new factor actively supporting the assembly of CENP-A chromatin.
Introduction
Centromeres are the key to the faithful partition of sister chromatids. As important as they are for cells, and implicitly the life of whole organisms, they are intricate and mysterious in structure, function, and uniqueness. Abnormalities in their architecture often impair their function in equal distribution and inheritance of genetic material to daughter cells. An abnormal centromere/kinetochore structure is correlated with infertility, birth defects, and cancers. Native human centromeres comprise a highly repetitive alphoid DNA (Willard, 1998), in which specialized nucleosomes containing centromere protein A (CENP-A), an H3 histone variant, are embedded (Sullivan, 2001; Cleveland et al., 2003). CENP-A is a conserved protein found in centromeres from lower eukaryotes to humans (Sullivan et al., 1994; Stoler et al., 1995; Buchwitz et al., 1999; Henikoff et al., 2000; Takahashi et al., 2000). CENP-A nucleosomes can be assembled in vitro from purified CENP-A and histones H2A, H2B, and H4 (Yoda et al., 2000). There is no specific DNA sequence at which CENP-A is considered to be targeted, because as a rare event, CENP-A nucleosomes can be de novo deposited on the chromosomal arms to form neocentromeres (du Sart et al., 1997; Saffery et al., 2000). These results imply that establishment of CENP-A nucleosomes is the defining process of active centromere formation. Although it is certain that centromeric chromatin formation is of epigenetic nature, little is known about the processes and factors governing CENP-A loading and maintenance at the centromeric region. The presence of CENP-A in the parental chromatid (Shelby et al., 1997), chromatin tension at anaphase (Mellone and Allshire, 2003), or a heterochromatic environment in the pericentromeric region (Henikoff et al., 2000; Folco et al., 2008) have been proposed to date. The ability of CENP-A to target centromeres partly lies on its own specific structure called the centromere targeting domain (CATD), which is located in the conserved histone fold domain (Shelby et al., 1997; Black et al., 2004). In Schizosaccharomyces pombe, several proteins are required for centromeric CENP-A loading, such as Mis6 (Takahashi et al., 2000), Sim4 (Pidoux et al., 2003), GATA-like factor Ams2 (Chen et al., 2003), and Mis16 and Mis18 (Hayashi et al., 2004).
Previously, tandem mass spectrometric analysis of the interphase–centromere complex (ICEN), which had been purified using immunoprecipitation with anti-CENP-A monoclonal antibody, revealed 40 proteins (Obuse et al., 2004; Izuta et al., 2006). Along with five canonical centromere proteins—CENP-A/ICEN40, -B/20, -C/7, -H/35, and hMis6/19 (CENP-I)—the ICEN contained seven novel proteins related to kinetochore function (ICEN 22, 24, 32, 33, 36, 37, and 39). Two other laboratories reported 11 novel proteins (CENP-K, -L… to -U) (Foltz et al., 2006; Okada et al., 2006), among which seven proteins were identical to ours: ICEN22 = CENP-T, 24 = U, 32 = N, 33 = L, 36 = O, 37 = K, and 39 = M.
Known as the two subunits of the remodeling and spacing factor (RSF) complex, Rsf-1 (ICEN2) and SNF2h (ICEN8) were found along with the components of the ICEN complex in CENP-A affinity precipitates (Obuse et al., 2004; Izuta et al., 2006). The RSF complex has been identified as an ATP-dependent nucleosome remodeling and spacing factor that favors in vitro chromatin transcription initiation along with the FACT complex comprising FACTp140 (ICEN6) and FACTp80 (ICEN12) (LeRoy et al., 1998; Orphanides et al., 1998). Previous work has described Rsf-1/XAP8 as a PHD-finger protein that interacts with the X protein of HBV (Shamay et al., 2002), and recent reports have found that its gene is amplified in ovarian carcinoma (Shih et al., 2005). SNF2h is an ATPase-containing subunit that belongs to the ISWI family and associates with various proteins to form different chromatin remodeling complexes, such as ACF, CHRAC, NURF, and RSF (Loyola et al., 2003).
In this work, we addressed questions relating to the function of RSF at centromeres considering its putative implication in remodeling centromeric chromatin. We used native chromatin immunoprecipitation (nChIP), siRNA-mediated depletion, and an in vitro nucleosomes remodeling assay to ascertain the biological importance of the Rsf-1–SNF2h complex in CENP-A nucleosome formation. We propose a new role for the RSF complex as an ATP-dependent remodeling and spacing factor actively supporting CENP-A deposition to centromeric chromatin.
Results
The Rsf-1–SNF2h complex associates with CENP-A chromatin at the mononucleosome level
We sought to further elucidate the quality of the RSF and CENP-A interaction, and first performed native immunoprecipitation of bulk chromatin (nChIP) that had undergone mild digestion with micrococcal nuclease (MNase) (Fig. 1 A, lane 1) using anti-CENP-A, anti-CENP-H, and anti-SNF2h antibodies. The precipitates were examined with ACA serum. All antibodies coprecipitated CENP-A, -B, and -C (Fig. 1 B). Notably, anti-SNF2h antibody coprecipitated CENP-A (middle panel, lane 4) in approximately the same amount as anti-CENP-H antibody (middle panel, lane 3). These results suggest that SNF2h directly or indirectly associates with CENP-A chromatin. Additionally, anti-SNF2h antibody also coprecipitated significant amounts of H3 core histones (bottom panel, lane 4) compared with anti-CENP-A and anti-CENP-H antibodies (bottom panel, lanes 2 and 3). The approximate concentration of CENP-A nucleosomes versus total nucleosomes in each sample is shown at the bottom of Fig. 1 B. This suggests that nChIP with anti-SNF2h also coprecipitated H3 nucleosomes in addition to CENP-A. Fig. 1 C shows that Coomassie staining of the anti-SNF2h nChIP membrane revealed the presence of three major bands corresponding to 250, 170, and 135 kD (lane 1), and immunostaining revealed that the bands at 250 and 135 kD were Rsf-1 and SNF2h, respectively (lanes 2 and 3). Proteomic analysis also confirmed these two major bands as Rsf-1 and SNF2h, and revealed the third major band as WSTF, as well as two other SNF2h partners: BPTF and Tip5 (Fig. S1). Combined with our previous data showing that only the Rsf-1–SNF2h complex was identified in the CENP-A affinity eluates (ICEN) (Obuse et al., 2004), these results suggest that Rsf-1, and not the other SNF2h partners (WSTF, BPTF, and Tip5), may associate with CENP-A chromatin. Importantly, as shown in Fig. 1 D (lane 2), antibodies against CENP-A coprecipitated Rsf-1 and SNF2h even after extensive MNase digestion of bulk chromatin (Fig. 1 A, lane 2), whereas CENP-B and CENP-C detached from CENP-A chromatin as previously reported (Ando et al., 2002). Conversely, antibodies against SNF2h and Rsf-1 coprecipitated CENP-A (Fig. 1 E, lanes 2 and 4) under identical conditions. Overall, these results led us to conclude that the RSF complex physically associates with CENP-A chromatin at the mononucleosome level.
Figure 1.
RSF complex interacts with CENP-A chromatin at the mononucleosome level. (A) Ethidium bromide–stained gel showing DNA nucleosomal ladder of bulk chromatin that had undergone mild (200 U/ml × min, lane 1) and extensive (12,000 U/ml × min, lane 2) digestion with micrococcal nuclease in the presence of 0.3 M NaCl. Percentages of mono-, di-, and tri-nucleosomes for lane 2 are shown at the right. (B) The bulk chromatin of asynchronous HeLa cells was mildly digested with MNase as in A, immunoprecipitated using anti-CENP-A (lane 2), anti-CENP-H (lane 3), and anti-SNF2h antibodies (lane 4), and nonimmune IgG (lane 5). Input bulk chromatin, one-tenth of the other samples, was applied in lane 1. Samples were run on a 5–20% gel and immunostained with ACA serum on the same membrane. The apparent molecular weight of CENP-A is calculated as ∼17 kD. The bottom panel shows a Coomassie blue–stained duplicate SDS-PAGE gel with histones as loading controls. The ratio of CENP-A to histone H4 of lanes 2 and 4 relative to the input sample (lane 1) was calculated and is depicted at the bottom. (C) Identification of proteins recovered by nChIP with anti-SNF2h antibodies of bulk chromatin after mild digestion with MNase (A, lane 1). The proteins eluted using the antigen peptide p4c were separated on 7.5% SDS-PAGE, transferred to a PVDF membrane, and identified with Coomassie Brilliant blue staining (lane 1) or with anti-Rsf-1 (lane 2) and anti-SNF2h (lane 3) antibodies. Fig. S1 shows mass spectrometry analysis of an identical sample. The apparent molecular weight of Rsf-1 or SNF2h is calculated from the molecular weight marker as ∼250 kD or ∼135 kD, respectively. (D) The anti-CENP-A nChIP samples of mild (lane 1) and extensive (lane 2) MNase digestion were separated in a 5–20% gel and immunostained using ACA serum, and anti-Rsf-1 and -SNF2h antibodies. (E) Western blot analysis of the nChIP samples using antibodies against SNF2h (lanes 1 and 2), Rsf-1 (lanes 3 and 4), and control IgG (lanes 5 and 6) after mild (lanes 1, 3, and 5) and extensive (2, 4, and 6) MNase digestion of bulk chromatin.
Rsf-1/RSF transiently associates with CENP-A chromatin to localize at the centromere region in middle G1
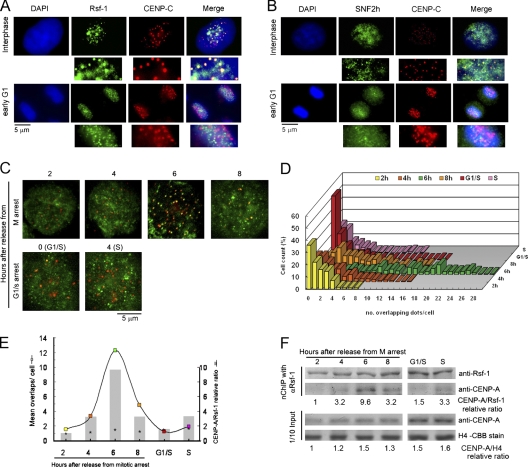
Using fluorescent immunostaining, we next examined subcellular localization of Rsf-1 and SNF2h in regards to centromeres recognized by anti-CENP-C antibody. Images revealed some Rsf-1 and SNF2h punctated foci as more evident, and some of them overlapped CENP-C signals in interphase cells, but not in early G1 (Fig. 2, A and B). From metaphase to telophase, Rsf-1 signals faded from chromosomal DNA, while SNF2h was omnipresent but did not overlap CENP-C, as shown in Fig. S2. Because Rsf-1 exists in cells only in the RSF complex (Loyola et al., 2003), and SNF2h binds a few other partners as shown in Fig. S1, we focused mostly on analyzing the Rsf-1 subunit of the RSF complex in later experiments. To determine the exact interphase stage at which RSF transits the centromere, Rsf-1 and CENP-A colocalized signals were counted with immunofluorescence staining using mouse monoclonal antibody against Rsf-1 and rat monoclonal antibody against CENP-A, respectively, in cells synchronized at six different interphasic time-points as follows: four points into G1 (specifically 2, 4, 6, and 8 h after release from the mitotic arrest), G1/S, and 4 h into S (Fig. 2 C). After merging dots were counted for each cell, the percentages of cells showing equal numbers of overlaps were plotted against each interphasic time-point (Fig. 2 D). Identical data are shown as mean overlaps per cell for each time-point in Fig. 2 E (colored box). Although little colocalization was observed through interphase, cells enriched in overlapping dots increased in mid-G1, showing a peak at 6 h when up to 30 detectable overlaps were counted (Fig. 2 D), corresponding to a mean of 12 overlaps/cell (Fig. 2 E, green box). Next, to investigate the cell cycle dependency of the association of the RSF complex to CENP-A chromatin biochemically on the basis of the results obtained in Fig. 1 E, we performed nChIP with anti-Rsf-1 antibody using bulk chromatin of synchronized cells (Fig. 2 F). Co-precipitated CENP-A levels against the recovered Rsf-1 were examined on the immunostained blot for each of the synchronized cells (Fig. 2 F, nChIP panel). The intensity of each band was measured by densitometer tracing, and the relative ratio of CENP-A to Rsf-1 is shown under the nChIP panel of Fig. 2 F. While the input CENP-A amount relative to histone H4 only slightly changed between 1 and ∼1.6 during the G1- to S-phase (Fig. 2 F, Input panel), the relative amount of coprecipitated CENP-A started to increase at 4 h after release from mitotic arrest (Fig. 2, E and F; CENP-A/Rsf-1 = 3.2), reached peak at 6 h (CENP-A/Rsf-1 = 9.6), and then decreased at 8 h (CENP-A/Rsf-1 = 3.2). The results strikingly coincided with the upper microscopy observation (compare boxes with bars in Fig. 2 E). Thus, the RSF complex started to associate with CENP-A at 4 h after release from M-phase; it increased at 6 h, then decreased from 8 h to G1/S. From these results, we conclude that Rsf-1/RSF transiently associates with CENP-A chromatin and localizes at the centromere region in middle G1.
Figure 2.
Rsf-1/RSF transits centromeres in middle G1. (A and B) Immunolocalization of Rsf-1 (A, green) and SNF2h (B, green) regarding centromeres recognized by anti-CENP-C antibodies (red). DAPI (blue) was used for DNA staining. The enlarged insets show clear overlapping and nonoverlapping. (C) HeLa cells synchronized at G1 at 2, 4, 6, and 8 h after release from TN16-induced mitotic arrest; G1/S border at 0 h and S phase at 4 h after release from thymidine block were coimmunofluorescent stained using mouse monoclonal antibody against anti-Rsf-1 (green) and rat monoclonal antibody against CENP-A (red). (D) Quantification of Rsf-1 and CENP-A overlapping signals in each of the 100 cells evaluated per interphasic time-point. (E) Interaction of Rsf-1 with CENP-A is maximal at 6 h after release from mitotic arrest. The colored boxes show results from D as the mean number of Rsf-1 and CENP-A overlapping signals per cell for each interphasic stage (n = 1). Shaded bars show the relative ratio of CENP-A to Rsf-1 recovered after nChIP with anti-Rsf-1 antibody from F, of the cells synchronized as in C (n = 1). Asterisks show relative ratio of CENP-A to histone H4 of input samples in F (n = 1). (F) Cell cycle dependency of association of the Rsf-1/RSF complex with CENP-A chromatin. Bulk chromatin of approximately 3 × 108 HeLa cells synchronized as in C was extensively digested with MNase (4,000 U/ml x min) and subjected to nChIP using anti-Rsf-1 antibody. The relative amounts of CENP-A to Rsf-1 were determined on immunoblotted membrane by densitometer tracing of the bands and drawn in E as shaded columns. Input bulk chromatin fractions (one-tenth of nChIP sample) were separately run on two duplicate gels, one transferred to a membrane and probed for CENP-A, and one stained with Coomassie Brilliant blue to visualize histone H4. The amounts of CENP-A relative to histone H4 in input samples were measured and depicted as asterisks in E. Relative molecular mass of Rsf-1 or CENP-A is ∼250 kD or ∼17 kD, respectively.
Rsf-1/RSF is required for normal mitotic progression
To investigate the biological importance of transient localization of the Rsf-1–SNF2h complex at the centromeric region, we performed siRNA-mediated depletion of each Rsf-1 and/or SNF2h proteins (Fig. 3). Depletion was confirmed using immunofluorescence microscopy (Fig. 3 A) and Western blot analysis (Fig. 3 B). The siRNA treatment decreased Rsf-1 and SNF2h proteins to less than one-tenth levels at 48 h or 24 h post-transfection, respectively (Fig. 3 B). Despite extensive examinations, apparent differences in cell growth and chromosome segregation were scarcely found between depleted and control cells (unpublished data). Therefore, we quantified the cell cycle distribution of cells from the G2 phase to the telophase for up to 6 d post-transfection for each depletion type (Fig. 3 C). All depletion types caused conspicuous accumulation of prometaphase cells of 40–60% compared with ∼30% in the control cells (Fig. 3 C, green). The ratio of prometaphase to metaphase cells was as high as ∼3 at 4 d post-transfection, but was only ∼1 in control cells (Fig. 3 D). In comparison, we found accumulation of prometaphase cells when CENP-A was depleted with siRNA (Fig. 3 D and Fig. S3). These results suggest that siRNA depletion induced a delay in the chromosomes' congression to the equator plate because of some defects at the centromeres. Fig. 3 E shows normal metaphase and prometaphase cells seen in the control (control panels) and corresponding abnormal ones observed in Rsf-1–depleted cells (siRsf-1 panels), with misaligned kinetochores seen to the left near the spindle poles, whereas most kinetochores congressed to the spindle equator in metaphasic cells (siRsf-1, top). In prometaphasic cells, disparate chromosomes with scattered kinetochores partly bound to the spindle were observed (siRsf-1, bottom). At 4 d post-siRNA transfection, a marked increase in misaligned metaphase cells was observed in Rsf-1– (24%) and SNF2h- (22%) depleted cells compared with control cells (5%), and the abnormalities of prometaphase in Rsf-1 knockdown cells increased slightly to 11% (Fig. 3 F). These inhibition effects of Rsf-1 depletion became more evident by blocking anaphase onset using MG132 (Fig. S4). These results show that Rsf-1/RSF is necessary for normal mitotic progression; its depletion affects the chromosomal congression to the metaphase plate.
Figure 3.
siRNA-mediated depletion of Rsf-1 and SNF2h of RSF delays cell cycle progression and causes kinetochore misalignment. (A) Immunostaining of HeLa cells with anti-Rsf-1 (top panels) and anti-SNF2h antibodies (bottom panels) at 48 h after mock and specific siRNA transfection. (B) Western blot analysis of Rsf-1 and SNF2h levels in cells siRNA-depleted of Rsf-1 (right) or SNF2h (left) at 0, 24, 48, and 72 h post-transfection. WB, Western blot; CBB, Coomassie Brilliant blue staining of a gel-duplicate. Rsf-1 = ∼250 kD; SNF2h = ∼135 kD. (C) Results of microscopic observation of cell stage distribution in G2 (blue), prometaphase (green), metaphase (red), and anaphase/telophase (yellow) at 2–6 d after siRNA-transfection of Rsf-1, SNF2h, and co-depletion (RSF) (n = 200–400 cells/depletion type/day post-transfection). Discrimination of each cell stage was based on the shape and condensation degree of chromosomes, distribution pattern of centromeres, and/or presence or absence of mitotic microtubules. Differences between Pm and M are shown in E as an example. (D) Prometaphase to metaphase cell ratio at 4 d post-transfection for each depletion type in C, and siCENP-A–transfected cells (see also Fig. S3). (E) Co-immunofluorescent staining of HeLa prometaphase and metaphase cells in control and siRNA-depleted samples using anti-β-tubulin (green) and anti-CENP-C (red) antibodies. DNA is shown in blue. (F) Percentage of abnormal prometaphase and metaphase cells at 4 d post-transfection, from C (n = 1).
RSF is required for CENP-A assembly at centromeric core chromatin
Because RSF is a nucleosome remodeling and spacing factor (Loyola et al., 2003), the above data, showing cell cycle delay and misaligned chromosomes as a result of Rsf1 depletion, led us to investigate whether these defects result from impairment of the loading process of CENP-A at centromeric chromatin. Therefore, we quantified the amount of CENP-A after siRsf-1 depletion using Western blot analysis. After two consecutive rounds of siRsf-1 treatments, the whole cell lysate, nuclei fraction, and chore chromatin fraction were prepared (see Materials and methods and Fig. 4 A, e), and CENP-A levels were examined and quantified as 82%, 78%, and 31 ± 10% of each mock sample, respectively (Fig. 4 A). Although siRsf-1 depletion reduced cellular (Fig. 4 A, b) and nuclear (see Fig. 5 A, c) CENP-A to a limited extent, a significant CENP-A reduction was observed in the core chromatin fraction, which is the 0.6 M NaCl-washed nuclear fraction (Fig. 4 A, d), and the reduction was comparable to that of CENP-A–depleted whole cell lysate (30%; Fig. 4 A, a). These findings suggest that Rsf-1 knockdown inhibited CENP-A from being loaded onto the stable centromeric nucleosomes, because CENP-A as well as H3 nucleosomes were stable enough to resist 0.6 M NaCl treatment (Ando et al., 2002), but the CENP-A chromatin complex (ICEN) was disrupted and many of the ICEN components were eluted to the soluble fraction (Fig. S5). Complementary to this result, we also detected CENP-A in the nuclear extract fraction as shown in Fig. 4 A, e, and its amount relative to the input control, histone H4, in the siRsf-1–depleted sample was 2.3 times higher than that in the control sample. Next, the reduction of centromeric CENP-A fluorescent signal intensity after siCENP-A or siRsf-1 treatment was examined (Fig. 4, B and C). Using low salt (PBS) washing, the intensity of the centromeric CENP-A signal was not affected by siRsf-1 treatment (106 ± 9% of mock sample), whereas it was greatly reduced by siCENP-A depletion (32 ± 5%) (Fig. 4 B, top panels; Fig. 4 C, −NaCl). Interestingly, siRsf-1 depletion greatly diminished the CENP-A signals (20 ± 5%) in high salt (0.5 M NaCl) conditions (Fig. 4 B, bottom panels; Fig. 4 C, +NaCl). These observations were statistically confirmed by quantifying the CENP-A signal intensity of each depletion type in up to several hundred cells as summarized in Fig. 4 C. These results suggest that CENP-A exists in two states at centromere regions: an unstable, centromere weak-associated fraction, and a stable, centromere strong-associated fraction. As RSF depletion reduced the stable form of CENP-A and enhanced the unstable form of CENP-A, this complex is implicated in the establishment of the centromere strong-associated form of CENP-A, the centromeric core chromatin.
Figure 4.
Rsf-1 depletion impairs deposition of CENP-A to the centromeric core chromatin. (A) Immunoblot of Rsf-1 and CENP-A in CENP-A– (a) and Rsf-1–depleted (b–d) cells. Analysis was performed in whole-cell lysate (a and b), nuclei after low salt (c), and high salt (0.6 M NaCl) wash (d). “Core chromatin” represents the high salt insoluble nuclear fraction and “nuclear extract” represents the high salt soluble nuclear fraction, prepared as schematically depicted in e. Depletion was induced by two rounds of specific siRNA transfection at d 0 and d 2 before sample preparation at d 5 for Rsf-1, and d 4 for CENP-A. The intensity of each CENP-A band of mock and siRNA-treated sample was quantified, and the intensity relative to mock sample is shown at the bottom of each lane. Histone H4 was visualized on a duplicate gel stained with Coomassie Brilliant blue. Rsf-1 = ∼250 kD; SNF2h = ∼135 kD; CENP-A = ∼17 kD. (B) The effect of high salt wash on centromere stability of CENP-A immunofluorescent signals, in siCENP-A– and siRsf-1–depleted cells. Mock-, CENP-A–, and Rsf-1–depleted cells were fixed and washed in low salt (0.15 M NaCl; top) or high salt (0.5 M NaCl; bottom). Depletion of each protein was induced by two rounds of siRNA transfection as in A. Nuclei were visualized by DAPI staining. Images showing CENP-A (green) and CENP-C (red) were merged. (C) Quantification of the CENP-A fluorescent signals on photographed cells prepared in B. The intensity of the fluorescent signal was measured for each of 300–1,000 cells per depletion type, and the mean intensity value per cell was normalized with mock sample in each washing condition. Error bars represent ± SEM.
Figure 5.
RSF complex can reconstitute and regularly space CENP-A nucleosomes using CENP-A core histones and naked DNA template. (A) Partially purified Flag-tagged (2 µl, left) and native (35 µl, right) RSF fractions were resolved on 7.5% SDS-PAGE and visualized with Coomassie Brilliant blue. Concentration of Rsf-1 was measured by densitometer tracing as 50 ng/µl for recombinant and 1 ng/µl for native fraction using BSA as control protein. (B) 15% SDS-PAGE stained with Coomassie Brilliant blue showing an equimolar ratio of reconstituted H3 (left) and CENP-A (right) core histones. CENP-A = ∼17 kD. (C) RSF-mediated H3 and CENP-A nucleosomes assembly assay. The ability of the RSF complex to reconstitute and space H3 and CENP-A nucleosomes was evaluated by the presence of periodic nucleosomal ladders following partial micrococcal nuclease digestion at two different dilutions (lanes 1–14) or a single dilution (15–18). Reactions were performed without (lanes 1–2), or with 10 µl each of recombinant (lanes 3–8) or native RSF fraction (9–18). The native fraction's activity was inhibited by immunodepletion with anti-Rsf-1 antibody (lane 17) and restored by the readdition of the same RSF sample (lane 18). Immunodepletion reduced Rsf-1 to ∼15% as detected from the analysis of the immunoblot shown under lanes 16 and 17. The nucleosomal ladder was resolved on a 1% agarose gel and stained with ethidium bromide.
RSF reconstitutes CENP-A nucleosomes in vitro
Finally, we addressed the question of whether RSF can assemble CENP-A nucleosomes in vitro. For this purpose, recombinant RSF from insect cells coinfected with baculoviruses encoding Flag-Rsf-1-SNF2h (Fig. 5 A, left) and native complex from HeLa nuclei (Fig. 5 A, right) were immunopurified. We reconstituted native H3 core histones (Fig. 5 B, lane 1) and native CENP-A core histones (Fig. 5 B, lane 2) (Yoda et al., 2000), and using the two partially purified RSF fractions, the nucleosome assembly reaction was performed (Fig. 5 C). Both recombinant and native RSF fractions supported regular assembly of H3 nucleosomes (Fig. 5 C, lanes 5–6 and 11–12) in an ATP-dependent manner (Fig. 5 C, lanes 3–4 and 9–10). Remarkably, both recombinant (lanes 7–8) and native (lanes 13–14) RSF fractions were able to reconstitute CENP-A nucleosomes. Although the native RSF fraction apparently contains WSTF (Fig. 5 A, right), the CENP-A nucleosome assembly activity of the native fraction relies on RSF activity because immunodepletion of the Rsf-1 subunit by anti-Rsf-1 antibody (Fig. 5 C, bottom inset) inhibited the nucleosomal ladder release (Fig. 5 C, lanes 16 vs. 17), whereas the readdition of native RSF fraction rescued it (Fig. 5 C, lane 18). These results suggest that RSF could reconstitute and space the CENP-A nucleosomes in vitro.
Discussion
Purification of the CENP-A chromatin (ICEN)
Continuing our previous work with ICEN components (Obuse et al., 2004; Izuta et al., 2006), this paper relates the RSF complex to centromere formation and function. Another CENP-A chromatin complex was isolated recently and named CENP-A nucleosome-associated complex (NAC) (Foltz et al., 2006). Stable HeLa cell lines expressing CENP-A–TAP were established; then, based on our nChIP preparation method (Ando et al., 2002; Yoda and Ando, 2004), the authors performed two successive rounds of affinity purification. The purified CENP-A–TAP complex contained FACT but not other chromatin remodeling factors. A single complex with histone chaperone activities would be insufficient to explain the CENP-A nucleosome remodeling, because FACT is reported to be responsible for H2A/H2B heterodimer exchange (Belotserkovskaya et al., 2003). It has been speculated that CENP-A assembly would be mediated by a remodeling complex other than CAF-1 or HIRA (Shelby et al., 2000; Foltz et al., 2006; Mellone et al., 2006; Black and Bassett, 2008). Unlike the CENP-A–TAP complex, the ICEN contains RSF as well as FACT. It has recently been reported that Drosophila RSF governs silent chromatin formation through replacement of histone H2A variants (Hanai et al., 2008). Human histone H2AZ, a homologue of Drosophila H2Av, is concentrated more in the CENP-A–TAP complex than that of H3.1 (Foltz et al., 2006). An interesting possibility is that CENP-A nucleosome remodeling at centromeres might be a concerted effort of both the RSF complex and the FACT complex for CENP-A/H4 tetramer as well as H2A/H2B dimer deposition and/or exchange.
Specificity of RSF for CENP-A chromatin
Our results showing that the RSF complex is able to associate with CENP-A nucleosomes, but not the other three SNF2h partners (Fig. 1 and Fig. S1), suggest at the centromeric region the RSF complex may associate selectively with CENP-A nucleosomes at the mononucleosome level (Fig. 1). What, then, is the signal driving RSF to centromeric CENP-A nucleosomes? Our data suggest that the affinity for the CENP-A nucleosome may be a function of Rsf-1, the specificity subunit of the complex, and not of the SNF2h subunit, an ISWI type of ATPase energy subunit. It may be that the CATD region in CENP-A (Black et al., 2004) is related to the association between CENP-A nucleosome and the RSF complex. It remains to be seen whether specificity for CENP-A nucleosomes results from a direct interaction of Rsf-1 with CENP-A, or whether it is mediated by one or more ICEN components or other factors. The driving signal might be posttranslational modification of CENP-A or other histones at deposition sites, for example through the work of the hMis18 complex (Hayashi et al., 2004; Fujita et al., 2007).
Timing of RSF association to CENP-A chromatin
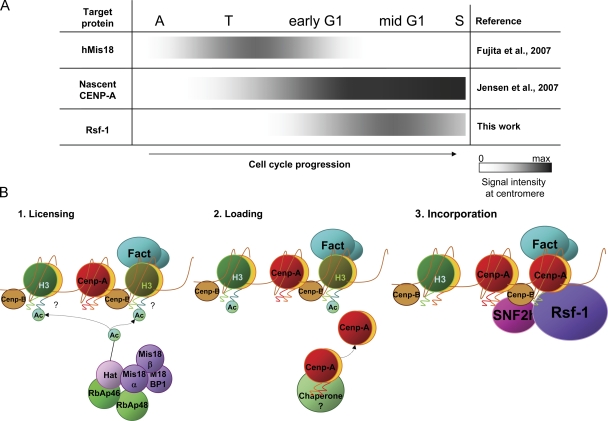
Microscopic detection of the RSF complex at the centromere was laborious because its interaction with CENP-A chromatin is ephemeral and qualitative, occurring in a short window in the interphase. Extensive analysis revealed that RSF localization at centromeres, through its association with CENP-A chromatin, starts in early to mid-G1, 4 h after release from the mitotic block, and reaches a peak 6 h post-release in middle G1 (Fig. 2 and Fig. 6 A). Reportedly, centromere replication takes place during the mid-S to late S phase, but CENP-A loading to centromeres is uncoupled with DNA synthesis and its cellular levels increase in G2 (Shelby et al., 2000). The complex of hMis18α, hMis18β, and M18BP/KNL2 is necessary for CENP-A recruitment to centromeres, and localizes transiently to centromeres from the late anaphase/telophase to early G1 (Fujita et al., 2007; Maddox et al., 2007), performing a centromere licensing process (presumably consisting of H4 acetylation), together with pRbAp46/48 (Hayashi et al., 2004; Fujita et al., 2007; Maddox et al., 2007). Using SNAP-tagged CENP-A, Jansen et al. (2007) elegantly showed that newly synthesized CENP-A starts to localize at the centromeres as early as reconstitution of the nuclear envelopes, with ∼50% accumulation by early G1 and over 90% by mid-G1. For comparison, Fig. 6 A summarizes the centromere localization timing of these factors, including RSF. It is reasonable to consider that licensing work by the hMis18 complex precedes CENP-A targeting in the early G1 phase. Timing of RSF centromeric localization coincides with that of CENP-A at the early to mid-G1 phase, but RSF loading is slightly more delayed than the start of nascent CENP-A loading.
Figure 6.
Sequential establishment in two steps of centromeric CENP-A chromatin. (A) Timing cascade of centromere localization of hMis18, nascent CENP-A, and Rsf-1. Dark shade gradients represent gradual accumulation or loss of the indicated factor at centromeres throughout the cell cycle progression from anaphase to the synthetic phase. (B) Putative two-step model for CENP-A nucleosomes establishment at centromeres. Establishment of CENP-A nucleosomes at the centromere region occurs after a licensing process engaged by the Mis18 complex (1) in two sequences: first, loading of CENP-A occurs at or around centromere regions (2), followed by incorporation/assembly into the centromeric nucleosomes and spacing by RSF remodeling function (3).
In vitro reconstitution of CENP-A nucleosomes by the purified RSF fraction
We confirmed that the purified recombinant and native RSF fractions were both able to reconstitute regularly spaced CENP-A nucleosomes as well as H3 nucleosomes (Fig. 5).
Although the purity and protein concentration of recombinant RSF were much higher than those of native RSF (Fig. 5 A), the enzyme activity was lower (Fig. 5 C, lane 8 vs. lane 14). The estimated specific enzyme activity was lower by two orders of magnitude compared with that of native RSF. We confirmed that the remodeling activity of the native fraction was attributable to the RSF complex, because Rsf-1 immunodepletion inhibited its enzyme activity (lanes 16–17).
Other factor(s) might be required for optimizing RSF remodeling and/or spacing activity. Considering its very weak chaperon activity, in vivo activity of RSF might mainly be remodeling of the preexisting CENP-A or histone H3 at the centromeric regions.
Rsf-1/RSF facilitates CENP-A incorporation into centromeric chromatin
Our results suggest that Rsf-1 function is required in interphasic centromeres (Fig. 2) to establish active kinetochores later in the mitotic phase (Fig. 3), and further that Rsf-1 facilitates CENP-A incorporation into centromeric core chromatin (Fig. 4). From another perspective, a prominent feature of the inhibition of chromosome segregation by Rsf-1 knockdown was its “mildness.” Although we have extensively examined the effect of seven kinds of siRsf-1 and three kinds of siSNF2h in single or co-double (siRSF) transfections, including tandem transfections for reboosting the same siRNA, no apparent depletion effects were detected in mitotic chromosome segregation, and the major effect was only prometaphasic cell accumulation (Fig. 3 C). These characteristics were common to CENP-A depletion by siRNA, as we scarcely observed any reproducible abnormality in chromosome segregation except for prometaphasic cell accumulation (Fig. 3 D and Fig. S3). In CENP-A knockout chicken cells, cell growth is normal up to 4 d after CENP-AOFF, and prometaphasic cells start to accumulate from this time-point (prometa/meta = ∼4) (Regnier et al., 2005). The mild and gradual abnormality in chromosome segregation is a distinctive phenotype that is specific to CENP-A nucleosome reduction from centromeric chromatin for the following reasons: (1) centromeric CENP-A nucleosomes are stable and transmitted to subsequent generations, diluted by half per generation; and (2) CENP-A nucleosome is a basic component of centromeric core chromatin and the kinetochore structure might be somewhat conserved despite CENP-A reduction to some extent. CENP-H and CENP-I amounts were reportedly invariable even when the CENP-A amount was reduced to one-tenth (Liu et al., 2006). The fact that the Rsf-1 depletion phenotype mimics that of CENP-A depletion also supports our argument that Rsf-1/RSF facilitates CENP-A incorporation into centromeric chromatin.
Two-step model for centromeric CENP-A nucleosomes formation
We observed a reduction in core chromatin incorporated CENP-A only after washout of the noncore chromatin materials by 0.5–0.6 M NaCl of cells or nuclei (Fig. 4), implying that nascent CENP-A is incorporated into centromeres in two steps: first, CENP-A is recruited to the preexisted centromere chromatin complex with relatively weak association, and next, is assembled into the stable centromeric core chromatin. RSF is required for the second step. Based on the results obtained in this paper and other works, we argue for a two-step deposition model for centromeric CENP-A nucleosome formation as follows (Fig. 6 B). First, CENP-A is recruited to the centromeric chromatin through relatively weak association in early G1 (Jansen et al., 2007). Meanwhile, from arrival to actual assembly into nucleosomes, ICEN components might trap CENP-A at deposition sites, potentially through the CENP-H/I (ICEN35/19) complex (Okada et al., 2006) (Fig. 6 B, loading step) or other factors. Then, the predeposited CENP-A is assembled into the centromeric nucleosomes by RSF remodeling function in mid-G1 (Fig. 6 B, incorporation step). A predeposition model was previously proposed to explain the delay between DNA replication and CENP-ACID loading (Schuh et al., 2007). The lag between the start of CENP-A loading and RSF colocalization (Fig. 6 A) might be necessary for accumulation of a certain amount of CENP-A, or modification of CENP-A itself, other histones, or ICEN components at deposition sites. Our findings may help decipher other cellular processes with established epigenetic mechanisms.
Materials and methods
Cell culture
HeLa S3 cells were grown at 37°C in DMEM (Sigma-Aldrich) for monolayer culture or RPMI 1640 (Nissui) for suspension culture, supplemented with 5 or 10% calf serum and antibiotics.
Cell synchronization
Synchronization at the G1/S border was achieved by two cycles of 2 mM thymidine block and at mitosis by a 12-h incubation with 100 ng/ml TN16. About 3 × 108 cells per interphasic time-point were subjected to nChIP with anti-Rsf-1. Cells in the S phase were obtained 4 h after release from the second thymidine block. Cells in G1 were obtained by release after 2, 4, 6, and 8 h from the TN16 mitotic block. Cells were exposed to 10 µM MG132 after one thymidine block in order to block the anaphase onset.
Microscopy
Cells grown on chamber slides (Thermo Fisher Scientific) were fixed in ice-cold 95% acetone or 4% paraformaldehyde, and blocked in 0.5% skim milk or signal enhancer (Invitrogen). For CENP-A signal quantification, cells were incubated in PBS with 0.1% digitonin or Triton X-100 and with or without a final concentration of 0.5 M NaCl for 30 min at room temperature, before acetone fixation. Cells were incubated at 37°C for 1 h with each of the primary and secondary antibodies. Cells were observed using a microscope (model BX51; Olympus). Images were acquired with a 20, 40, or 60x objective for FITC or TRITC using a CoolSNAP monochrome camera (Roper Scientific), processed with Lumina Vision software (Mitani Co.). CENP-A immunofluorescence was quantified with the same exposure conditions for all images and depletion types. About 10–15 images for each depletion type were captured arbitrarily. Integrated CENP-A fluorescent intensity and cell number in each image were measured after background subtraction. The average value per cell of the CENP-A signal intensity from 300–1,000 cells was calculated and normalized to the intensity of the CENP-A signal in mock-depleted cells.
Transfection of siRNA and/or DNA
siRNA was transfected using Lipofectamine 2000 (Invitrogen). The following short double-stranded RNAs with 25 nt (Invitrogen) or 21 nt (JBios) were used: ICEN2/Rsf-1: 5′-AAGAGUCUCAGCCAACUGGUUUCGA-3′; ICEN8/SNF2h: 5′-GGUCCGAGGAUUAAACUGGCUCAUU-3′; ICEN7/CENP-C: 5′-GCUGGUGAGUUGAAGGUATT-3′. ICEN40/CENP-A was described in Goshima et al. (2003).
Antibodies
Monoclonal antibodies against SNF2h (m22E7) and Rsf-1 (m9E5 for Western blotting or m38B5 for nChIP), and rat monoclonal antibodies against human CENP-A (r8C5) were newly prepared. Antibodies against CENP-A (mAb3-19, mouse monoclonal), CENP-C (guinea pig serum), and ACA serum (human) were described previously (Ando et al., 2002). Anti-β-tubulin (mouse monoclonal, 4G5) was purchased from Sigma-Aldrich.
Western blots
Methods for Western blot analyses were performed as described previously (Ando et al., 2002). Band intensity was quantified using NIH ImageJ software. The core chromatin sample was prepared as follows: the isolated nuclei were incubated in WB supplemented with 0.6 M NaCl and 0.1% NP-40 for 30 min at 4°C, and then centrifuged at 30,000 rpm for 30 min at 4°C. The 0.6 M NaCl soluble fraction was subjected to immunoprecipitation using anti-CENP-A antibodies.
Bulk chromatin and native-chromatin immunoprecipitation (nChIP)
Bulk chromatin of 5 ∼10 × 109 HeLa cells was prepared as described previously (Yoda et al., 2004) with slight modifications. Antibodies against CENP-A, CENP-H, Rsf-1, and Sn2h were preadsorbed to protein G–Sepharose (GE Healthcare), and incubated with bulk chromatin samples overnight at 4°C. Unless otherwise specified, the immunoprecipitates were eluted by boiling for 5 min in SDS buffer and the eluates were subjected to SDS-PAGE. For native RSF purification, anti-SNF2h affinity immunoprecipitates were eluted using 0.1 mg/ml antigen peptide p4c (CKRKMDGAPDGRGRKKKLKL). For proteomic analysis, CENP-A affinity complex was eluted in 8 M urea or stepwise in 0.6 M and 2 M NaCl using a 1-ml column (Fig. S5).
Purification of the RSF complex and H3 and CENP-A core histones
Purification and reconstitution of H3 and CENP-A core histones were performed as described previously (Yoda et al., 2000). The recombinant RSF complex was purified from insect cells coinfected with baculoviruses each carrying FLAG-Rsf-1- and SNF2h-gene using antibody against FLAG-tag. Native RSF complex purification was performed in two steps. First, the bulk chromatin was subjected to nChIP with anti-SNF2h antibody, and eluted using antigen peptide p4c. After elution, DEAE column purification was performed as described elsewhere (Loyola et al., 2003).
In vitro reconstitution of CENP-A nucleosomes
Nucleosome assembly was performed essentially as described previously (Loyola et al., 2003). The reaction mixture (20 µl) containing 2 µg pUCα11 (pUC119 containing 11-mer type I α-satellite) (Ikeno et al., 1998) as template, 2 µg H3 or CENP-A core histones, and 10 µl RSF fraction was incubated overnight at 30°C. The assembled nucleosomes were digested using micrococcal nuclease, electrophoresed on a 1% agarose gel, and visualized with ethidium bromide.
Proteomic analysis
All procedures were performed fundamentally as described previously (Obuse et al., 2004). The eluate by p4c of SNF2h nChIP fraction was separated by 7.5% SDS-PAGE (Fig. S1), and the gel area from 50–350 kD was analyzed. CENP-A affinity complex was eluted in 8 M urea or stepwise in 0.6 M and 2 M NaCl using a 1-ml column (Fig. S5). The eluted products were concentrated by acetone precipitation and separated by 12.5% SDS-PAGE.
Online supplemental material
Figure S1 shows results of mass spectrometry analysis of the proteins recovered after nChIP with anti- SNF2h antibody. Figure S2 shows subcellular localization of Rsf-1 and SNF2h. Figure S3 shows gradual decrease of CENP-A amount after CENP-A-siRNA transfection. Figure S4 shows that blocking of anaphase onset using MG132 treatment disclosed the impairment by siRsf-1 knockdown of the chromosomal congression to the equatorial plate. Figure S5 shows the protein components of ICEN that were eluted to the soluble fraction with 0.6 M NaCl treatment. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200903088/DC1.
Acknowledgments
The authors thank Danny Reinberg for generously providing baculoviruses carrying the hSNF2h and 3×FLAG tagged Rsf-1 genes; Koichiro Ohtake for assistance in the native RSF purification step; Florin Perpelescu for helpful assistance in the manuscript preparation; and Hiroshi Masumoto (Kazusa DNA Research Institute), Tatsuo Fukagawa (National Institute of Genetics), William C. Earnshaw (University of Edinburgh), and Hisao Masukata (Osaka University) for helpful discussions and critically reading the manuscript.
This work was supported by Grants-in-aid for Scientific Research on Priority Areas (K. Yoda), the Uehara Memorial Foundation (K. Yoda), and the Japan Society for the Promotion of Science (M. Perpelescu).
Footnotes
Abbreviations used in this paper: CENP, centromere protein; ICEN, interphase–centromere complex; nChIP, native chromatin immunoprecipitation; RSF, remodeling and spacing factor; SNF2h, sucrose nonfermenting protein 2 homologue.
References
- Ando S., Yang H., Nozaki N., Okazaki T., Yoda K. 2002. CENP-A, -B, and -C chromatin complex that contains the I-type alpha-satellite array constitutes the prekinetochore in HeLa cells.Mol. Cell. Biol. 22:2229–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotserkovskaya R., Oh S., Bonarenko V.A., Orphanides B., Studitsky V.M., Reinberg D. 2003. FACT facilitates transcription-dependent nucleosome alteration.Science. 301:1090–1093 [DOI] [PubMed] [Google Scholar]
- Black B.E., Bassett E.A. 2008. The histone variant CENP-A and centromere specification.Curr. Opin. Cell Biol. 20:91–100 [DOI] [PubMed] [Google Scholar]
- Black B.E., Foltz D.R., Chakravarthy S., Luger K., Woods V.L., Jr., Cleveland D.W. 2004. Structure determinants for generating centromeric chromatin.Nature. 430:578–582 [DOI] [PubMed] [Google Scholar]
- Buchwitz B.J., Ahmad K., Moore L.L., Roth M.B., Henikoff S. 1999. A histone-H3-like protein in C. elegans.Nature. 401:547–548 [DOI] [PubMed] [Google Scholar]
- Chen E.S., Saitoh S., Yanagida M., Takahashi K. 2003. A cell cycle-regulated GATA factor promotes centromeric localization of CENP-A in fission yeast.Mol. Cell. 11:175–187 [DOI] [PubMed] [Google Scholar]
- Cleveland D.W., Mao Y., Sullivan K.F. 2003. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling.Cell. 112:407–421 [DOI] [PubMed] [Google Scholar]
- du Sart D., Cancilla M.R., Earle E., Mao J., Saffery R., Tainton K.M., Kalitsis P., Martyn J., Barry A.E., Choo A. 1997. A functional neo-centromere formed through activation of a latent human centromere and consisting of non-alpha-satellite DNA.Nat. Genet. 16:144–153 [DOI] [PubMed] [Google Scholar]
- Folco H.D., Pidoux A.L., Urano T., Allshire R.C. 2008. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres.Science. 319:94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz D.R., Jansen L.E.T., Black B.E., Bailey A.O., Jr., Yate I.I.I., Cleveland D.W. 2006. The human CENP-A centromeric nucleosome-associaed complex.Nat. Cell Biol. 8:458–469 [DOI] [PubMed] [Google Scholar]
- Fujita Y., Hayashi T., Kiyomitsu T., Toyoda Y., Kokubu A., Obuse C., Yanagida M. 2007. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1.Dev. Cell. 12:17–30 [DOI] [PubMed] [Google Scholar]
- Goshima G., Kiyomitsu Y., Yoda K., Yanagida M. 2003. Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway.J. Cell Biol. 160:25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai K., Furuhashi H., Yamamoto T., Akasaka K., Hirose S. 2008. RSF governs silent chromatin formation via histone H2Av replacement.PLoS Genet. 4:e1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. 2004. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres.Cell. 118:715–729 [DOI] [PubMed] [Google Scholar]
- Henikoff S., Ahmad K., Platero J.S., Steensel B.V. 2000. Heterochromatic deposition of centromeric histone H3-like proteins.Proc. Natl. Acad. Sci. USA. 97:716–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno M., Grimes B., Okazaki T., Nakano M., Saitoh K., Hoshino H., McGill N., Cooke H., Masumoto H. 1998. Construction of YAC based mammalian artificial chromosomes.Nat. Biotechnol. 16:431–439 [DOI] [PubMed] [Google Scholar]
- Izuta H., Ikeno M., Suzuki N., Tomonaga T., Nozaki N., Obuse C., Kisu Y., Goshima N., Nomura F., Nomura N., Yoda K. 2006. Comprehensive analysis of the ICEN (Interphase Centromere Complex) components enriched in the CENP-A chromatin of humancells.Genes Cells. 11:673–684 [DOI] [PubMed] [Google Scholar]
- Jansen L.E., Black B.E., Foltz D.R., Cleveland D.W. 2007. Propagation of centromeric chromatin requires exit from mitosis.J. Cell Biol. 176:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy G., Orphanides G., Lane W.S., Reinberg D. 1998. Requirement of RSF and FACT for transcription of chromatin templates in vitro.Science. 282:1900–1904 [DOI] [PubMed] [Google Scholar]
- Liu S.T., Rattner J.B., Jablonski S.A., Yen T.J. 2006. Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells.J. Cell Biol. 175:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola A., Huang J.Y., LeRoy G., Hu S., Wang Y.H., Donelly R.J., Lane W.S., Lee S.C., Reinberg D. 2003. Functional analysis of the subunits of the chromatin assembly factor RSF.Mol. Cell. Biol. 23:6759–6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox P.S., Hyndman F., Monen J., Oegema K., Desai A. 2007. Functional genomics identifies a Myb domain-containing protein family required for assembly of CENP-A chromatin.J. Cell Biol. 176:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellone B.G., Allshire R.C. 2003. Stretching it: putting the CEN(P-A) in centromere.Curr. Opin. Genet. Dev. 13:191–198 [DOI] [PubMed] [Google Scholar]
- Mellone B., Erhardt S., Karpen G.H. 2006. The ABCs of centromeres.Nat. Cell Biol. 8:427–429 [DOI] [PubMed] [Google Scholar]
- Obuse C., Yang H., Nozaki N., Goto S., Okazaki T., Yoda K. 2004. Proteomics analysis of the centromere complex from HeLa interphase cells: uv-Damaged DNA Binding Protein-1 (DDB-1) is a component of the CEN-complex, while BMI-1 is transiently colocalized with the centromeric region in interphase.Genes Cells. 9:105–120 [DOI] [PubMed] [Google Scholar]
- Okada M., Cheeseman I.M., Hori T., Okawa K., McLeod I.X., Yates J.R., III, Desai A., Fukagawa T. 2006. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A.Nat. Cell Biol. 8:446–457 [DOI] [PubMed] [Google Scholar]
- Orphanides G., LeRoy G., Chang C.-H., Luse D.S., Reinberg D. 1998. FACT, a factor that facilitates transcript elongation through nucleosomes.Cell. 92:105–116 [DOI] [PubMed] [Google Scholar]
- Pidoux A.L., Richardson W., Allshire R.C. 2003. Sim4: a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation.J. Cell Biol. 161:295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier V., Vagnarelli P., Fukagawa T., Zerjal T., Burns E., Trouche D., Earnshaw W., Brown W. 2005. CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1.Mol. Cell. Biol. 25:3967–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffery R., Irvine D.V., Griffiths B., Kalitsis P., Worderman L., Choo K.H. 2000. Human centromeres and neocentromeres show identical distribution patterns of >20 functionally improtant kinetochore-associated proteins.Hum. Mol. Genet. 9:175–185 [DOI] [PubMed] [Google Scholar]
- Schuh M., Lehner C.F., Heidman S. 2007. Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase.Curr. Biol. 17:237–243 [DOI] [PubMed] [Google Scholar]
- Shamay M., Barak O., Doitsh G., Ben-Dor I., Shaul Y. 2002. Hepatitis B virus pX interacts with HBXAP, a PHD finger protein to coactivate transcription.J. Biol. Chem. 277:9982–9988 [DOI] [PubMed] [Google Scholar]
- Shelby R.D., Vafa O., Sullivan K.F. 1997. Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites.J. Cell Biol. 136:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby R.D., Monier K., Sullivan K.F. 2000. Chromatin assembly at kinetochores is uncoupled from DNA replication.J. Cell Biol. 151:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih I.-M, Sheu J.J., Santillan A., Nakayama K., Yen M.J., Bristow R.E., Vang R., Parmigiani G., Kurman R.J., Trope C.G., et al. 2005. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma.Proc. Natl. Acad. Sci. USA. 102:14004–14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S., Keith K.C., Curnick K.E., Fitzgerald-Hayes M. 1995. A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis.Genes Dev. 9:573–586 [DOI] [PubMed] [Google Scholar]
- Sullivan K.F. 2001. A solid foundation: functional specialization of centromeric chromatin.Curr. Opin. Genet. Dev. 11:182–188 [DOI] [PubMed] [Google Scholar]
- Sullivan K.F., Hechenberger M., Khaled M. 1994. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere.J. Cell Biol. 127:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Chen E.S., Yanagida M. 2000. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast.Science. 288:2215–2219 [DOI] [PubMed] [Google Scholar]
- Willard H.F. 1998. Centromeres: the missing link in the development of human artificial chromosomes.Curr. Opin. Genet. Dev. 8:219–225 [DOI] [PubMed] [Google Scholar]
- Yoda K., Ando S. 2004. Immunological analysis and purification of centromere complex.Methods Enzymol. 375:270–277 [DOI] [PubMed] [Google Scholar]
- Yoda K., Ando S., Morishita S., Houmura K., Hashimoto K., Takeyasu K., Okazaki T. 2000. Human centromere protein A (CENP-A) can replace histone H3 in nucleosome reconstitution in vitro..Proc. Natl. Acad. Sci. USA. 97:7266–7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda K., Morishita S., Hashimoto K. 2004. Histone variant CENP-A purification, nucleosome reconstitution.Methods Enzymol. 375:253–269 [DOI] [PubMed] [Google Scholar]