Abstract
Based on the observation that removal of tumors from metastatic organs reversed their chemoresistance, we hypothesized that chemoresistance is induced by extracellular factors in tumor-bearing organs. By comparing chemosensitivity and proteins in different tumors (primary vs. metastases) and different culture systems (tumor fragment histocultures vs. monolayer cultures derived from the same tumor), we found elevated levels of acidic (aFGF) and basic (bFGF) fibroblast growth factors in the conditioned medium (CM) of solid and metastatic tumors. These CM induced broad spectrum resistance to drugs with diverse structures and action mechanisms (paclitaxel, doxorubicin, 5-fluorouracil). Inhibition of bFGF by mAb and its removal by immunoprecipitation resulted in complete reversal of the CM-induced chemoresistance, whereas inhibition/removal of aFGF resulted in partial reversal. Using CM that had been depleted of aFGF and/or bFGF and subsequently reconstituted with respective human recombinant proteins, we found that bFGF but not aFGF induced chemoresistance whereas aFGF amplified the bFGF effect. aFGF and bFGF fully accounted for the CM effect, indicating these proteins as the underlying mechanism of the chemoresistance. The FGF-induced resistance was not due to reduced intracellular drug accumulation or altered cell proliferation. We further showed that an inhibitor of aFGF/bFGF (suramin) enhanced the in vitro and in vivo activity of chemotherapy, resulting in shrinkage and eradication of well established human lung metastases in mice without enhancing toxicity. These results indicate elevated levels of extracellular aFGF/bFGF as an epigenetic mechanism by which cancer cells elude cytotoxic insult by chemotherapy, and provide a basis for designing new treatment strategies.
Resistance of tumor cells to chemotherapy and the limited efficacy of chemotherapy in metastatic disease are two major challenges in patient management. A common resistance mechanism observed in preclinical studies is the overexpression of drug efflux proteins (1–3). However, clinical studies show that inhibition of the drug efflux proteins does not significantly improve the effectiveness of chemotherapy in patients (4, 5), suggesting the existence of other chemoresistance mechanisms.
Using the transplantable, metastatic rat prostate MAT-LyLu tumor, we have shown that the antitumor activity of paclitaxel in lymph node metastases was 20-fold lower than in s.c. implanted primary tumors. When the metastatic tumor was reimplanted at the s.c. site, the resistance was lost in the second generation primary tumor but regained in the second generation metastases. We further found that the chemoresistance in metastatic tumors is not due to reduced intracellular drug accumulation or retention (6). These results led to the hypothesis of an epigenetic chemoresistance mechanism that is mediated by extracellular factors present in tumor-bearing organs. The present study tested the hypothesis, identified the factors that induce resistance, and determined the restoration of chemosensitivity by using inhibitors of these factors. To determine whether the resistance is broad spectrum and applies to drugs with diverse structures and different mechanisms of action and/or efflux, the study was performed by using several drugs: paclitaxel, which is an antimicrotubule agent and a substrate for drug efflux proteins; doxorubicin, which is a topoisomerase II inhibitor and a substrate for efflux proteins; and 5-fluorouracil, which is an antimetabolite and is not a substrate for efflux proteins (7, 8).
Materials and Methods
Chemicals and Reagents.
Mouse anti-human acidic fibroblast growth factor (aFGF) and basic fibroblast growth factor (bFGF) mAbs were obtained from Sigma, and lactate dehydrogenase (LDH) assay, BrdUrd ELISA, and human recombinant aFGF (r-aFGF) and r-bFGF from Boehringer Mannheim. The anti-aFGF mAb reacts with naturally occurring aFGF and human r-aFGF and does not crossreact with bFGF (9). The anti-bFGF mAb was generated by using the 18-kDa human r-bFGF, is specific for bFGF, and does not crossreact with aFGF (10). r-aFGF and r-bFGF are monomeric peptides and are identical to the 14-kDa human aFGF or the 18-kDa human bFGF, except for the extra methionine at the amino terminus (11, 12).
Tumors and Cultures.
Human prostate PC3 tumor cells were obtained from the American Type Culture Collection. The rat MAT-LyLu tumor cells and PC3-LN cells were gifts from John Isaacs (Johns Hopkins University) and Joy Ware (Virginia Commonwealth University), respectively.
The original clone of the rat MAT-LyLu tumor cells, on implantation in the hind limbs of male Copenhagen rats, yielded primary tumors at the implantation site and, as the primary tumor reached a size of ≥0.5 g, metastasized initially in inguinal lymph nodes and subsequently in the lungs in 50% of animals (13). Using serial reimplantation of the lymph node and lung metastases, we obtained subclones that yielded more rapidly growing metastases in 100% of animals.
Paired primary and metastatic tumors were surgically removed from the same host. Fragments of the nonnecrotic portions were cultured as histocultures. Single-cell suspensions (>95% viability) were obtained by incubating tumor fragments (≈1 g) with collagenase, EDTA, and trypsin. Histocultures (tumor fragments of ≈1 mm3) and monolayers were cultured in medium supplement with 9% heat-inactivated FBS (6).
Collection and Analysis of Proteins in Conditioned Medium (CM).
CM was collected from tumor histocultures (50 ml per 50 to 100 mg tumor fragments) and monolayer cultures (40 ml per 8 × 107 cells) after incubation in serum-free medium for 24 h (14). An aliquot of CM was concentrated about 10,000-fold by using lyophilization followed by reconstitution in 0.1 mM PMSF in water, and then analyzed on 15% SDS/PAGE (85 V for 2.5 h). Protein bands were visualized with silver stain reagents. For Western blotting, proteins were transferred from polyacrylamide gel to a nitrocellulose filter by electrophoresis, followed by sequential incubation with 5% nonfat dry milk in 100 mM Tris/150 mM NaCl/0.1% Tween 20 (pH 7.6), and 5 μg/ml aFGF or bFGF mAb. The mAb-immunoreactive band was visualized by using chemiluminescence blotting.
The levels of aFGF and bFGF in the concentrated CM were quantified by comparing the intensity of their bands on Western blots to the intensity of the bands derived from standard curve samples of r-aFGF and r-bFGF. The standard curves were linear between 3 and 100 ng r-aFGF and between 1 and 160 ng r-bFGF.
Pretreatment with CM and Recombinant r-bFGF.
Before drug treatment, cells were incubated for 4 days with tumor CM or r-aFGF/r-bFGF-containing medium, supplemented with 1% FBS. The medium was renewed every other day.
In Vitro Antitumor Activity Evaluation.
The antiproliferative drug effect in histocultures was measured as inhibition of [3H]thymidine incorporation quantified by autoradiography (6) and, for monolayers, as inhibition of BrdUrd incorporation or as reduction of total proteins by the sulforhodamine B assay (15). For monolayers, the two assays yielded qualitatively similar results, although the sulforhodamine B assay yielded higher inhibitory drug concentration. Cell kill induced by 96-h drug treatment was monitored by the release of LDH into the culture medium. LDH activity was monitored by the conversion of tetrazolium to formazan (detected at 490 nm).
Removal of aFGF and bFGF from CM by Immunoprecipitation.
aFGF or bFGF was immunoprecipitated with its respective mAb (1 μg/ml), in the presence of protein G PLUS/protein A agarose (Oncogene). This procedure reduced the bFGF level in the original CM to below the detection limit of 5 pg/ml by ELISA (Oncogene Science), and the aFGF level in the concentrated CM to below the detection limit by Western blotting.
Intracellular Drug Accumulation.
Tumor cells were treated with [3H]paclitaxel (1 nM for human PC3 cells and 10 nM for rat tumor cells), [14C]doxorubicin (50 and 100 nM, respectively), and [3H]5-fluorouracil (500 nM for both cells) (16). The extracellular drug concentrations were selected based on their IC50 in human and rat cells (data not shown). We measured the plateau intracellular drug concentrations that were attained at 4 h for all three drugs in PC3 cells, and at 1 (doxorubicin and 5-fluorouracil) and 4 h (paclitaxel) in rat cells.
In Vivo Antitumor Activity Evaluation.
Male BALB/c nu/nu mice (6–8 wk old) were used. Animal care was in accordance with institutional guidelines. Human PC3-lymph node (PC3-LN) cells (106 in 0.1 ml physiological saline), which metastasize to lungs in 100% of the animals (17), were injected i.v. via a tail vein. After 4 wk, tumor establishment was determined by visual examination of the lungs of two randomly selected animals, and drug treatment in the remaining animals was initiated when these two animals showed at least five tumor nodules of ≈1 mm diameter. Mice received i.v. injection over 1 min via a tail vein of 200 μl of either physiologic saline or a saline solution delivering 5 mg/kg doxorubicin, 10 mg/kg suramin, or a combination of both drugs, twice weekly for 3 wk. Preliminary pharmacokinetic data in rodents indicate that these doses would result in plasma concentrations of approximately 10 nM for doxorubicin and 10 μM for suramin at 72 h. This doxorubicin concentration was near its IC50 in the monolayer cultures of PC3-LN cells, and the suramin concentration was sufficient to reverse the FGF-induced chemoresistance in cultured cells (see Results). The selected suramin dose has no in vivo antitumor activity against other mouse tumors (18, 19). Three days after completion of drug treatments, animals were euthanized, and their lungs were removed, fixed in Bouin's solution to visualize tumor nodules, and then processed for histologic evaluation. Histologic sections (5 μm) at a depth of between 200–300 μm from the ventral surface and containing all five lobes of the lungs were obtained. The lung surface area (counted as number of pixels) occupied by the tumor was calculated as a fraction of the total lung area, by using Adobe photoshop (Adobe Systems, Mountain View, CA). We also determined microscopically the number of tumor cells in residual tumors and the fraction of apoptotic cells in each tumor. Because apoptotic cells disappear over time, a second measure of the extent of apoptosis was the density of nonapoptotic cells in the residual tumors, which was determined by counting the number of nonapoptotic tumor cells in randomly selected microscopic fields at ×400 magnification. On average, we counted ten fields per animal, or >1,500 cells in the control and suramin groups and >600 cells in the doxorubicin group. In the case of combination therapy where fewer than five tumor nodules remained per animal, we counted all residual cells (between 20 to 600 cells per animal).
Results
Contribution of Tumor Environment and Tumor Location to Chemoresistance.
The contribution of tumor environment and location to chemoresistance was evaluated in three studies (Table 1). Similar results were found for paclitaxel, doxorubicin, and 5-fluorouracil.
Table 1.
Loss of drug resistance, aFGF, and bFGF upon disaggregation of metastatic tumors and upon passaging in monolayer cultures
| Culture conditions | Concentration producing 50% inhibition
|
Amount in CM, pg/ml
|
|||
|---|---|---|---|---|---|
| Pac, nM | Dox, nM | 5-FU, μM | aFGF | bFGF | |
| Histocultures* | |||||
| Primary | >14 | 18 | 33 | 62 | 130 |
| Lymph node | >227† | 165† | NM | 195† | 660† |
| Lung | >1,804† | NM | 122† | 269† | 845† |
| Monolayers | |||||
| (passage 0) | |||||
| Primary | 10.7 | 8.0 | 0.79 | 62 | 93 |
| Lymph node | 32.9† | 31.0† | 1.90† | 100† | 243† |
| Lung | 92.4† | 77.0† | 2.84† | 118† | 302† |
| Monolayers | |||||
| (passage 1) | |||||
| Primary | 10.5 | 8.6 | 0.85 | NM | NM |
| Lymph node | 21.8† | 17.9† | 1.29† | NM | NM |
| Lung | 28.1† | 31.7† | 1.52† | NM | NM |
| Monolayers | |||||
| (passage 2) | |||||
| Primary | 10.3 | 8.0 | 0.79 | NM | NM |
| Lymph node | 15.0† | 11.0† | 1.09† | NM | NM |
| Lung | 21.5† | 16.6† | 1.20† | NM | NM |
| Monolayers | |||||
| (passage 3) | |||||
| Primary | 10.0 | 8.0 | 0.78 | 54 | 95 |
| Lymph node | 11.7 | 7.8 | 0.83 | 51 | 89 |
| Lung | 11.4 | 8.0 | 0.81 | 58 | 103 |
Paired rat primary and metastatic tumors were cultured as histocultures or monolayers. For histocultures, tumors were treated with paclitaxel (Pac) for 24 h (12 pairs of primary and lymph node tumors and 2 pairs of primary and lung tumors), and with doxorubicin (Dox) (3 pairs of primary and lymph node tumors) and 5-fluorouracil (5-FU) (2 pairs of primary and lung tumors) for 96 h. For monolayers, cells were treated with drugs for 96 h, and drug effect was measured by the BrdUrd incorporation method. Mean values. NM, not measured.
In histocultures, Pac at 10,000 nM produced <50% inhibition; data shown are the concentrations that produced 30% inhibition.
P < 0.05 for differences between primary and metastatic tumors (unpaired Student's t test).
The first study compared the chemosensitivity in histocultures of rat primary and metastatic tumors, where the heterogeneous cell types and the three-dimensional structure of solid tumors are maintained, to the chemosensitivity in the corresponding monolayer cultures of cells obtained by trypsin disaggregation of the same tumors. The 2- to 40-fold lower chemosensitivity in histocultures indicates that the unique environment in solid tumors played a role in chemoresistance. The second study shows that histocultures and early monolayer cultures of lung metastases were more resistant than lymph node metastases, which in turn were more resistant than s.c. primary tumors, indicating that tumor location determined the extent of chemoresistance. The third study shows that monolayers derived from lung and lymph node metastases lost their chemoresistance after three passages and became equally sensitive to drugs as monolayers of primary tumor cells where chemosensitivity remained constant for all passages. The latter observation indicates a reversal of chemoresistance in metastatic tumors when cells were removed from the metastatic milieu.
Collectively, these results indicate (i) an epigenetic mechanism of broad spectrum chemoresistance that is mediated by extracellular factors present in solid and metastatic tumors, and (ii) a loss of these factors on removing tumors from metastatic sites and/or disrupting tumor microenvironment.
Induction of Chemoresistance by Extracellular Factors in Solid and Metastatic Tumors.
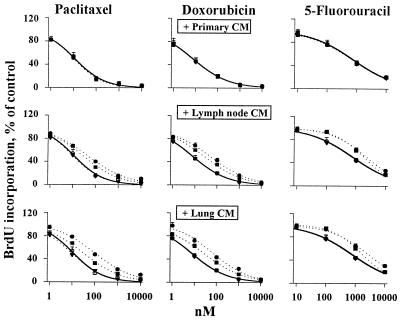
We collected CM from rat tumor cultures and evaluated its effect on chemosensitivity. This and subsequent studies were performed using rat primary tumor cells and human prostate tumor cells. Qualitatively similar results were obtained for both cell types, indicating the general nature of the observations (Fig. 1). CM derived from histocultures and early monolayer cultures of metastases (referred to as active metastatic tumor CM) induced a 3- to 10-fold resistance to drugs (P < 0.05), whereas CM from late monolayer cultures of metastatic tumors and CM from primary tumors (referred to as inactive CM) had no effect. These data confirm the induction of chemoresistance by extracellular factors in metastatic tumors and indicate a progressive loss of these factors on passaging metastatic tumor cells in monolayers.
Figure 1.
Induction of drug resistance in monolayer cultures by CM. CM of primary (Top), lymph node (Middle) and lung (Bottom) tumor cultures. Control with no CM, ○; histoculture CM, ●; CM of early monolayer culture (passage 0); ■; CM of late monolayer culture (passage 3), ▾. Similar results were obtained for rat (shown here) and human PC3 tumor cells. The curves for CM of primary tumor cultures and late monolayer metastatic tumor cultures overlap with the control curves.
Identification of Extracellular Factors That Induce Chemoresistance.
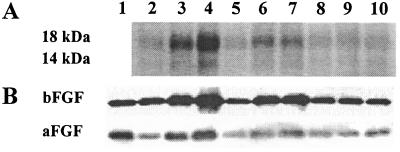
The identity of these factors was established by comparing the proteins in the active and inactive CM (Fig. 2, Table 1). The active CM showed 2- to 7-fold higher concentrations of two proteins, which were identified by immunoblotting as aFGF (14 kDa) and bFGF (18 kDa). Several observations suggest a cause-and-effect relationship between these proteins and resistance. First, the rank order of aFGF/bFGF concentrations in CM of different tumor culture systems was identical to the rank order of the chemoresistance in these cultures. Second, the progressive loss of these proteins on passaging the metastatic tumors in monolayers coincided with the diminishing ability of these monolayer CM to induce chemoresistance. Third, as the protein levels in monolayers of metastatic tumors (passage 3) were reduced to the same levels as in monolayers of primary tumors, equal drug sensitivity was attained in both cultures. Fourth, in monolayer cultures, the aFGF/bFGF concentrations in CM significantly correlated with the relative chemoresistance (P < 0.0001, Pearson test).
Figure 2.
Analysis of proteins in tumor CM. (A) Higher concentrations of 14-kDa and 18-kDa proteins in the active CM compared with the inactive CM. Lanes 2–4, histocultures of primary, LN, and lung tumors, respectively; lanes 5–7, early monolayer cultures (passage 0) of the same tumors; lanes 8–10, late monolayer cultures (passage 3) of the same tumors. (B) Immunoblotting with mouse anti-human aFGF and bFGF mAbs. Lane 1, r-aFGF (20 ng) or r-bFGF (5 ng). Lanes 2–10, same as in A.
Role of Extracellular aFGF and bFGF in Chemoresistance.
The role of extracellular FGF in chemoresistance was evaluated in five studies. Results of the first four studies are summarized in Table 2. The first study used specific inhibitors of extracellular aFGF and bFGF, i.e., mAbs. In the absence of the active CM, treatment with mAbs to aFGF or bFGF did not alter drug activity, indicating no effect of the mAbs on the baseline chemosensitivity. In the presence of the active CM, the bFGF Ab produced a concentration-dependent reversal of the CM-induced chemoresistance, with complete reversal by 5 μg/ml mAb. For aFGF, the mAb treatment partially reversed the CM-induced resistance; the maximum reversal was 60%, which was obtained with 1 μg/ml Ab with no additional reversal at a 5-fold higher concentration. In contrast, a nonspecific Ab (i.e., non-immune rabbit IgG) had no effect. The second study evaluated the effect of removing aFGF and/or bFGF from the active CM by immunoprecipitation. Removal of bFGF abolished the resistance whereas removal of aFGF only partially reversed the CM effect. These two studies indicate that both aFGF and bFGF were involved in the resistance, but had different roles.
Table 2.
Reversal of CM-induced resistance by inhibition and removal of FGF, and induction of resistance by recombinant FGF
| Concentration producing 50% inhibition
|
|||
|---|---|---|---|
| Paclitaxel, nM | Doxorubicin, nM | 5-Fluorouracil, μM | |
| Neutralizing mAb to reverse resistance | |||
| aFGF mAb | |||
| No CM, no mAb (control) | NM | 7.95 ± 0.45 | NM |
| No CM + 5 μg/ml bFGF mAb | NM | 7.64 ± 0.48 | NM |
| + CM, no mAb | NM | 75.4 ± 3.56* | NM |
| + CM + 5 μg/ml IgG | NM | 75.2 ± 3.78* | NM |
| + CM + aFGF mAb, 0.05 μg/ml | NM | 73.4 ± 5.85* | NM |
| + CM + aFGF mAb, 0.1 μg/ml | NM | 45.5 ± 3.16* | NM |
| + CM + aFGF mAb, 1 μg/ml | NM | 30.1 ± 4.05* | NM |
| + CM + aFGF mAb, 5 μg/ml | NM | 29.2 ± 2.98* | |
| bFGF mAb | |||
| No CM, no mAb (control) | 10.1 ± 0.39 | 8.04 ± 0.31 | 0.74 ± 0.05 |
| No CM + 5 μg/ml bFGF mAb | 9.84 ± 3.98 | 8.48 ± 1.15 | 0.75 ± 0.02 |
| + CM, no mAb | 35.7 ± 1.02* | 32.1 ± 0.82* | 1.89 ± 0.15* |
| + CM + bFGF mAb, 0.05 μg/ml | 28.5 ± 1.47* | 27.7 ± 3.61* | 1.62 ± 0.11* |
| + CM + bFGF mAb, 0.1 μg/ml | 21.1 ± 1.00* | 15.0 ± 0.80* | 1.16 ± 0.06* |
| + CM + bFGF mAb, 0.5 μg/ml | 18.0 ± 0.67* | 11.7 ± 1.08* | 1.11 ± 0.12* |
| + CM + bFGF mAb, 5 μg/ml | 14.5 ± 1.90 | 8.24 ± 1.81 | 0.97 ± 0.09 |
| Removal of FGF by immunoprecipitation plus reconstitution with 0.16 ng/ml r-aFGF and/or 0.9 ng/ml r-bFGF | |||
| No CM (control) | NM | 8.99 ± 0.63 | NM |
| + CM | NM | 78.1 ± 5.32* | NM |
| + CM, remove aFGF | NM | 30.6 ± 1.77* | NM |
| + CM, remove aFGF, add r-aFGF | NM | 77.3 ± 3.68* | NM |
| + CM, remove bFGF | NM | 8.22 ± 0.59 | NM |
| + CM, remove bFGF, add r-bFGF | NM | 74.6 ± 2.85* | NM |
| + CM, remove aFGF & bFGF | NM | 8.47 ± 0.91 | NM |
| + CM, remove aFGF & bFGF, add r-aFGF | NM | 8.52 ± 0.74 | NM |
| + CM, remove aFGF & bFGF, add r-bFGF | NM | 28.3 ± 1.93* | NM |
| + CM, remove aFGF & bFGF, add r-aFGF & r-bFGF | NM | 76.8 ± 3.95* | NM |
| Resistance induced by r-aFGF and r-bFGF, independent of CM | |||
| No CM (control) | 1.18 ± 0.04 | 8.05 ± 1.80 | 0.81 ± 0.04 |
| + CM | 3.82 ± 0.07* | 44.6 ± 1.92* | 3.22 ± 0.14* |
| + r-aFGF, 1 ng/ml | 1.15 ± 0.06 | 8.10 ± 0.27 | 0.82 ± 0.08 |
| + r-aFGF, 10 ng/ml | 1.14 ± 0.03 | 8.43 ± 0.24 | 0.70 ± 0.05 |
| + r-aFGF, 50 ng/ml | 1.18 ± 0.05 | 8.05 ± 0.40 | 0.73 ± 0.05 |
| + r-bFGF, 1 ng/ml | 1.21 ± 0.03 | 8.54 ± 2.69 | 0.85 ± 0.12 |
| + r-bFGF, 10 ng/ml | 2.06 ± 0.35 | 23.8 ± 2.21* | 2.41 ± 0.08* |
| + r-bFGF, 50 ng/ml | 3.27 ± 0.85* | 50.8 ± 2.46* | 4.30 ± 0.74* |
| + r-aFGF, 0.04 ng/ml + r-bFGF, 0.9 ng/ml | 1.15 ± 0.05 | 8.53 ± 0.51 | 0.76 ± 0.09 |
| + r-aFGF, 0.08 ng/ml + r-bFGF, 0.9 ng/ml | 2.13 ± 0.06* | 15.2 ± 0.52* | 1.15 ± 0.11* |
| + r-aFGF, 0.16 ng/ml + r-bFGF, 0.9 ng/ml | 3.14 ± 0.14* | 35.3 ± 1.11* | 1.64 ± 0.07* |
| + r-aFGF, 0.32 ng/ml + r-bFGF, 0.9 ng/ml | 4.68 ± 0.09* | 62.2 ± 3.49* | 2.54 ± 0.15* |
| + r-aFGF, 0.64 ng/ml + r-bFGF, 0.9 ng/ml | 5.79 ± 0.16* | 84.9 ± 6.31* | 3.77 ± 0.13* |
| + r-aFGF, 0.9 ng/ml + r-bFGF, 0.9 ng/ml | 6.86 ± 0.22* | 107 ± 2.94* | 5.74 ± 0.28* |
For the neutralizing mAb experiment, the source of CM was lung histocultures for the aFGF mAb experiment, and early monolayer lung cultures for the bFGF mAb experiment. For the immunoprecipitation plus reconstitution experiment, the source of CM was lung histoculture. Mean ± SD. Drug effect was measured by the BrdUrd incorporation method. NM, not measured. Similar results were obtained for rat MAT-LyLu cells and human PC3 cells. Results in the top and middle parts are for rat tumor cells, and results in the bottom part are for PC3 cells. *, P < 0.05 compared with control (one way ANOVA).
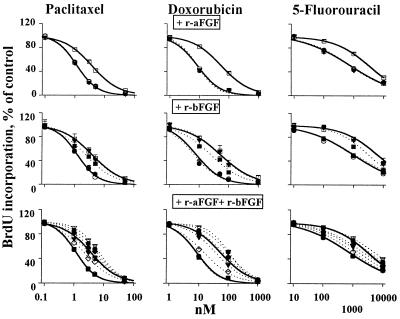
The third study determined whether aFGF and/or bFGF are required for the resistance. We first removed the endogenous aFGF and/or bFGF from the active CM by immunoprecipitation, and then reconstituted the CM by using recombinant proteins. When either the endogenous aFGF or bFGF were removed from the CM, addition of their respective recombinant counterparts, at concentrations comparable to the endogenous levels, fully restored the resistance. When both proteins were removed from the CM, we found (i) that addition of r-aFGF did not induce resistance whereas addition of r-bFGF produced a concentration-dependent resistance, indicating that bFGF but not aFGF was required for the resistance, and (ii) that the concentration of exogenous r-bFGF needed to restore the resistance was 55-fold higher than the endogenous bFGF concentration in the native CM or when endogenous aFGF was present (i.e., 50 ng/ml vs. about 0.9 ng/ml, see Fig. 3 and Table 2). The latter suggests an amplification of bFGF effect by aFGF. The aFGF effect was confirmed in the fourth study, which shows a complete restoration of chemoresistance when the aFGF/bFGF-depleted CM was reconstituted with both r-aFGF and r-bFGF at concentrations of the endogenous proteins. The fifth study confirmed that aFGF and bFGF were the main cause of chemoresistance, by using only r-aFGF and r-bFGF (i.e., without the immunoprecipitated CM) to induce resistance (Fig. 3). When given alone, r-aFGF had no effect whereas r-bFGF induced a concentration-dependent resistance. Combinations of r-aFGF/r-bFGF at concentrations as the endogenous proteins in the CM (i.e., between 0.16 and 0.32 ng/ml r-aFGF plus 1 ng/ml r-bFGF) produced the same extent of resistance as the CM, with an even greater resistance at higher protein concentrations.
Figure 3.
r-aFGF and r-bFGF, independent of CM, induced drug resistance. Two controls: no CM (left solid curves, ○); CM of lung histocultures (right solid curves, □). (Top) r-aFGF. Amounts of 1 (●), 10 (■), and 50 (▾) ng/ml. All three dotted curves overlap with the left control curve. (Middle) r-bFGF. Dotted curves from left to right: 1 (●, overlaps with the left control curve), 10 (■), and 50 (▾, overlaps with the right CM-control curve) ng/ml. (Bottom) r-aFGF/r-bFGF combinations. Dotted curves from left to right: 0.04 and 1 ng/ml (■, overlaps with the left control curve); 0.08 and 1 ng/ml (◊); 0.16 and 1 ng/ml (▾); 0.32 and 1 ng/ml (⧫); 0.64 and 1 ng/ml (●); and 1 ng/ml each (▿). Similar results were obtained in human PC3 (shown here) and rat tumor cells.
Collectively, these results indicate extracellular aFGF and bFGF as the underlying mechanism of the broad spectrum chemoresistance; bFGF but not aFGF was required to induce resistance whereas aFGF amplified the bFGF effect.
FGF-Induced Chemoresistance Applies to Both Antiproliferative and Cell Kill Effects.
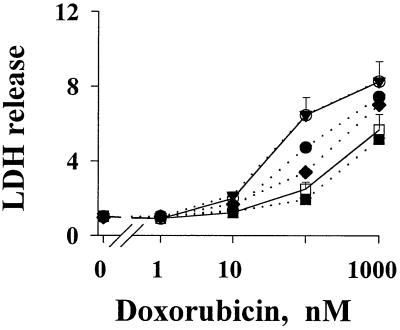
The chemoresistance observed above was measured primarily as antiproliferative drug effect. We observed similar findings for the cell kill effect of drugs, i.e., the active metastatic tumor CM and r-bFGF induced resistance to cell kill by drugs, whereas r-aFGF did not induce resistance but enhanced the effect of r-bFGF (Fig. 4).
Figure 4.
FGF-induced resistance to cell kill by drugs. Drug-induced cell death in PC3 cells was monitored by the activity of LDH released to the culture medium. Results are expressed as a ratio of treated samples to untreated controls. Values for control samples varied by less than 10%. (Top to Bottom) Without CM or FGF (○); 0.9 ng/ml r-aFGF (▾, overlaps with the top control curve); 0.9 ng/ml r-bFGF (●); 0.16 ng/ml r-aFGF plus 0.9 ng/ml r-bFGF (⧫); CM of lung tumor histocultures (□); 0.9 ng/ml r-aFGF plus 0.9 ng/ml r-bFGF (■). Similar results were found for doxorubicin (shown here), paclitaxel, and 5-fluorouracil.
Enhancement of Chemosensitivity by an Inhibitor of aFGF and bFGF.
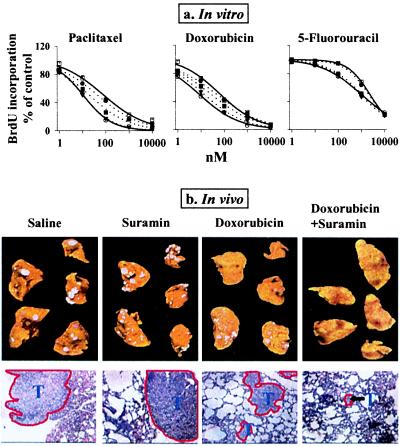
We next determined whether other aFGF/bFGF inhibitors, in addition to the FGF Abs, enhance chemosensitivity. Suramin, a negatively charged molecule that inhibits the action of aFGF and bFGF (20), was used as the inhibitor. Results of in vitro experiments show that addition of suramin produced a concentration-dependent reversal of the CM-induced resistance to paclitaxel, doxorubicin, and 5-fluorouracil in rat tumor cells, PC3 cells, and a metastatic subline of PC3, i.e., PC3-LN cells (Fig. 5a). Complete reversal was attained at between 10 to 15 μM suramin, which had no antitumor effect when used alone (data not shown). Addition of 5 μg/ml bFGF mAb to the drug/suramin combination did not enhance the drug effect (data not shown), indicating that suramin and the mAb shared the same action mechanism and that suramin alone completely inhibited the bFGF-induced resistance. Suramin, at 15 μM, also completely reversed the resistance induced by recombinant aFGF and bFGF (Table 3). Taken together, these data indicate that under in vitro conditions, suramin enhances drug activity by reversing the FGF-induced resistance.
Figure 5.
Enhancement of drug effect by suramin. (a) In vitro. Two controls: no CM, no suramin (left solid curves, ○); CM of lung histocultures, no suramin (right solid curves, □). Dotted curves from right to left, suramin 5 μM (●); 10 μM (■); and 15 μM (▾). Similar results were obtained for rat (shown here), human PC3, and PC3-LN tumor cells. The IC50 of doxorubicin in PC3-LN cells was 17 nM. (b) In vivo. (Top) Visible tumors on the ventral and dorsal surfaces of the lungs in all animals in the control and single agent groups, no visible tumors in most animals in the combination group. (Bottom) Histologic sections (×100), with tumors outlined in red.
Table 3.
Reversal of FGF-induced resistance by suramin
| Drug | Drug concentration required to produce 50% inhibition
|
|||
|---|---|---|---|---|
| Control, no FGF
|
+ 0.3 ng/ml r-aFGF & 1 ng/ml r-bFGF
|
|||
| No suramin | Suramin, 15 μM | No suramin | Suramin, 15 μM | |
| Paclitaxel, nM | 2.15 ± 0.25 | 2.14 ± 0.30 | 7.05 ± 2.61* | 1.89 ± 0.30 |
| Doxorubicin, nM | 27.3 ± 2.8 | 35.7 ± 4.2 | 145 ± 33* | 29.7 ± 3.7 |
| 5-Fluorouracil, μM | 2.20 ± 0.39 | 2.43 ± 0.43 | 5.05 ± 1.17* | 2.19 ± 0.40 |
Human PC3 cells were treated with drugs, with or without suramin and/or a combination of aFGF and bFGF. Drug activity was measured by the sulforhodamine B assay, which measures the cellular protein content. This assay gives higher IC50 values compared with the BrdUrd incorporation assay. Mean ± SD. *, P < 0.05 compared with controls without FGF treatments (one way ANOVA).
The ability of suramin to enhance drug activity under in vivo conditions was evaluated in immunodeficient mice bearing well established human PC3-LN lung metastases, with doxorubicin as the model drug (Fig. 5b and Table 4). Suramin alone had no antitumor effect or toxicity. Doxorubicin alone did not eradicate tumors but reduced the tumor size by ≈80%, tripled the fraction of apoptotic cells and halved the density of nonapoptotic cells in residual tumors, and caused a ≈20% loss in body weight. Addition of suramin to doxorubicin therapy did not enhance weight loss but significantly enhanced the antitumor effect, resulting in (i) tumor eradication in 42% of animals, and, (ii) in the remaining 58% of animals that showed residual tumors, further reduction of the tumor size (additional 10-fold), reduction of density of nonapoptotic tumor cells (additional 4-fold), and enhancement of the apoptotic cell fraction (additional 3-fold).
Table 4.
Suramin enhances the in vivo antitumor effect of doxorubicin in mice bearing well established human lung metastases
| Treatment (n) | % Tumor-free animals | % Lung surface area occupied by tumor | % Apoptotic cells per tumor | Density of nonapoptotic cells in residual tumors, cells/field | End-of-experiment body weight, % of pretreatment value |
|---|---|---|---|---|---|
| Saline control (10) | 0 | 9 ± 4 | 9 ± 6 | 157 ± 37 | 104 ± 5 |
| Suramin, 10 mg/kg (10) | 0 | 7 ± 3 | 10 ± 7 | 139 ± 30 | 101 ± 10 |
| Doxorubicin, 5 mg/kg (10) | 0 | 2 ± 1* | 29 ± 16* | 77 ± 17* | 82 ± 4* |
| Doxorubicin + suramin (12) | 42† | 0.2 ± 0.3† | 77 ± 12† | 22 ± 14† | 84 ± 8* |
The average pretreatment weights for the four groups ranged from 21 g to 22 g. Animals that did not show visible tumors on the lung surface nor microscopic lesions in five random histologic sections are considered tumor-free. Mean ± SD. *, P < 0.05 compared with the control and suramin groups (one way ANOVA).
, P < 0.05 compared with all other groups (one way ANOVA).
FGF-Induced Chemoresistance Is Not Due to Reduced Drug Accumulation nor to Altered Cell Proliferation.
Treatment with the active metastatic tumor CM or r-bFGF did not alter the drug accumulation in tumor cells; the respective intracellular concentrations of paclitaxel, doxorubicin, and 5-fluorouracil remained at ≈0.5, 40, and ≈0.1 pmol/106 cells in PC3 cells; and ≈1, 80, and 24 pmol/106 cells in rat tumor cells (n = 6 each). Treatment with the active CM or r-bFGF also did not alter the doubling time of exponentially growing cells, which remained unchanged at 17 and 24 h for rat and PC3 tumor cells, respectively.
Discussion
Our results establish a mechanism of broad spectrum anticancer drug resistance that is mediated by extracellular aFGF/bFGF. Several growth factors, including bFGF, insulin-like growth factor, and epidermal growth factor, have been shown to induce drug resistance (21–28). To the best of our knowledge, no other study has implicated aFGF in chemoresistance. The aFGF/bFGF resistance mechanism differs, in several ways, from the previously reported induction of resistance to drugs and radiation by exposure to extracellular bFGF or transfection of bFGF gene. First, the aFGF/bFGF resistance was attained at aFGF/bFGF concentrations that are found in patient plasma and urine (29). In contrast, the earlier studies show that resistance to cisplatin and fludarabine is induced at bFGF concentrations that greatly exceed the level found in patient plasma or urine (i.e., 20 and 100 ng/ml vs. ≤1 ng/ml). Second, the resistance to antimetabolites resulting from transfection of bFGF gene occurs via an intracellular mechanism that does not require the presence of extracellular bFGF and cannot be reversed by suramin, and is a result of amplification of several genes in the purine and pyrimidine biosynthesis pathways. In contrast, the aFGF/bFGF-induced resistance reported here was exerted via an extracellular mechanism, as indicated by its reversal by treatment with neutralizing mAb and suramin. The earlier studies show that chemoresistance induced by high extracellular bFGF concentration involves enhancement of mdm2 protein, bcl-2 expression and bcl-2 protein, and reduction in apoptosis. Whereas the mechanism by which aFGF and bFGF induce resistance is unknown, our results show that it is not due to changes in intracellular drug accumulation or cell proliferation. The reversal of the resistance by inhibition of extracellular aFGF/bFGF suggests a mechanism that involves binding of these proteins to their receptors.
The present study shows that, although aFGF does not induce resistance, it drastically enhances the bFGF effect such that the resistance was attained at clinically relevant concentrations of these proteins. This finding provides evidence that interaction between growth factors determines tumor sensitivity to drugs.
The importance and clinical relevance of the aFGF/bFGF-induced resistance is indicated by the 3- to 10-fold resistance induced by aFGF/bFGF at concentrations found in the active metastatic tumor CM and in patients (29, 30). The extent of in vivo chemoresistance may be higher than the CM-induced resistance, because the aFGF/bFGF concentrations in the local environment within solid tumors are likely to exceed their concentrations in the CM, which was diluted 500- to 1,000-fold during collection.
Our results indicate that, under in vitro conditions, suramin reverses the FGF-induced resistance and thereby enhances drug activity. However, because suramin inhibits the action of multiple growth factors, in addition to aFGF and bFGF (20), the possibility that suramin acts via other mechanisms under in vivo conditions cannot be excluded at present. Further studies to elucidate the molecular mechanisms of FGF-induced resistance are needed.
In summary, our findings demonstrate an epigenetic mechanism by which cancer cells use the unique microenvironment of solid tumors and metastases to elude cytotoxic insult, establish an important role of extracellular growth factors in tumor sensitivity to chemotherapy, and indicate a treatment paradigm using combinations of chemotherapy with aFGF/bFGF inhibitors.
Acknowledgments
We thank Dr. Joy L. Ware for providing the PC3-LN cells, Dr. John Isaacs for providing the MAT-LyLu tumor, and Lauren Baylor for her help with image analysis. This work was supported in part by research grants R01CA78577, R37CA49816, and R01CA63363 from the National Cancer Institute, National Institutes of Health.
Abbreviations
- FGF
fibroblast growth factor
- aFGF
acidic FGF
- r-aFGF
recombinant aFGF
- bFGF
basic FGF
- r-bFGF
recombinant bFGF
- CM
conditioned medium
- LDH
lactate dehydrogenase
- LN
lymph node
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140210697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140210697
References
- 1.Lum B L, Fisher G A, Brophy N A, Yahanda A M, Adler K M, Kaubisch S, Halsey J, Sikic B I. Cancer. 1993;72:3502–3514. doi: 10.1002/1097-0142(19931201)72:11+<3502::aid-cncr2820721618>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Barrand M A, Bagrij T, Neo S-Y. Gen Pharmacol. 1997;28:639–645. doi: 10.1016/s0306-3623(96)00284-4. [DOI] [PubMed] [Google Scholar]
- 3.Fidler I J. Cancer Chemother Pharmacol. 1999;43:S3–S10. doi: 10.1007/s002800051091. [DOI] [PubMed] [Google Scholar]
- 4.Ferry D R, Traunecker H, Kerr D J. Eur J Cancer. 1996;32:1070–1081. doi: 10.1016/0959-8049(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 5.Broxterman H J, Lankelma J, Pinedo H M. Eur J Cancer. 1996;32:1024–1033. doi: 10.1016/0959-8049(96)00045-7. [DOI] [PubMed] [Google Scholar]
- 6.Yen W C, Wientjes M G, Au J L-S. Pharm Res. 1996;13:1305–1312. doi: 10.1023/a:1016053412582. [DOI] [PubMed] [Google Scholar]
- 7.Dorr R T, Von Hoff D D. In: Cancer Chemotherapy Handbook. Dorr R T, Von Hoff D D, editors. Norwalk, CT: Appleton and Lange; 1994. [Google Scholar]
- 8.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, Parker R J, Fruehauf J P. Clin Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 9.Ichimori Y, Kinoshita Y, Watanabe T, Seno M, Kondo K. Biochem Biophys Res Commun. 1991;175:291–297. doi: 10.1016/s0006-291x(05)81233-1. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe H, Hori A, Seno M, Kozai Y, Igarashi K, Ichimori Y, Kondo K. Biochem Biophys Res Commun. 1991;175:229–235. doi: 10.1016/s0006-291x(05)81224-0. [DOI] [PubMed] [Google Scholar]
- 11.Jaye M, Howk R, Burgess W, Ricca G A, Chiu I M, Ravera M W, O'Brien S J, Modi W S, Maciag T, Drohan W N. Science. 1986;233:541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- 12.Bohlen P, Esch F, Baird A, Jones K L, Gospodarowicz D. FEBS Lett. 1985;18:177–181. doi: 10.1016/0014-5793(85)80765-1. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs J T, Yu G, Coffey D S. Invest Urol. 1981;19:20–23. [PubMed] [Google Scholar]
- 14.Cavanaugh P G, Nicolson G L. Cancer Res. 1989;49:3928–3933. [PubMed] [Google Scholar]
- 15.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren J T, Bokesch H, Kenney S, Boyd M R. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 16.Au J L-S, Li D, Gan Y, Gao X, Johnson A L, Johnston J, Millenbaugh N J, Jang S H, Kuh H J, Chen C T, Wientjes M G. Cancer Res. 1998;58:2141–2148. [PubMed] [Google Scholar]
- 17.Ware J L, Lieberman A P, Webb K S, Vollmer R T. Exp Cell Biol. 1985;53:163–169. doi: 10.1159/000163308. [DOI] [PubMed] [Google Scholar]
- 18.Chahinian A P, Mandeli J P, Gluck H, Naim H, Teirstein A S, Holland J F. J Surg Oncol. 1998;67:104–111. doi: 10.1002/(sici)1096-9098(199802)67:2<104::aid-jso6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Shin R, Naomoto Y, Kamikawa Y, Tanaka N, Orita K. Scand J Gastroenterol. 1997;32:824–828. doi: 10.3109/00365529708996541. [DOI] [PubMed] [Google Scholar]
- 20.Middaugh C R, Mach H, Burke C J, Volkin D B, Dabora J M, Tsai P K, Bruner M W, Ryan J A, Marfia K E. Biochemistry. 1992;31:9016–9024. doi: 10.1021/bi00152a044. [DOI] [PubMed] [Google Scholar]
- 21.Shaulian E, Resnitzky D, Shifman O, Blandino G, Amsterdam A, Yayon A, Oren M. Oncogene. 1997;15:2717–2725. doi: 10.1038/sj.onc.1201453. [DOI] [PubMed] [Google Scholar]
- 22.Konig A, Menzel T, Lynen S, Wrazel L, Rosen A, Al-Katib A, Raveche E, Gabrilove J L. Leukemia. 1997;11:258–265. doi: 10.1038/sj.leu.2400556. [DOI] [PubMed] [Google Scholar]
- 23.Cohen-Jonathan E, Toulas C, Nonteil S, Couderc B, Maret A, Bard J J, Prats H, Daly-Schveitzer N, Favre G. Cancer Res. 1997;57:1364–1370. [PubMed] [Google Scholar]
- 24.Miyake H, Hara I, Gohji K, Yoshimura K, Arakawa S, Kamidono S. Cancer Lett. 1998;123:121–126. doi: 10.1016/s0304-3835(97)00365-0. [DOI] [PubMed] [Google Scholar]
- 25.Huang A, Wright J A. Oncogene. 1994;9:491–499. [PubMed] [Google Scholar]
- 26.Huang A, Jin H, Wright J A. Exp Cell Res. 1994;213:335–339. doi: 10.1006/excr.1994.1207. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y-S, Jin G-F, Houston C W, Thompson J C, Townsend C M. J Cell Physiol. 1998;175:141–148. doi: 10.1002/(SICI)1097-4652(199805)175:2<141::AID-JCP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Geier A, Hemi R, Haimsoh M, Berry R, Malik Z, Karasik A. In Vitro Cell Dev Biol. 1994;300A:336–343. doi: 10.1007/BF02631455. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen M, Watanabe H, Budson A E, Richie J P, Hayes D F, Folkman H. J Natl Cancer Inst. 1994;86:356–361. doi: 10.1093/jnci/86.5.356. [DOI] [PubMed] [Google Scholar]
- 30.Ikemoto M, Hasegawa K, Kihara Y, Iwakura A, Komeda M, Yamazato A, Fujita M. Clinica Chimica Acta. 1999;283:171–182. doi: 10.1016/s0009-8981(99)00045-5. [DOI] [PubMed] [Google Scholar]