Abstract
AIM: To investigate the expression of vascular endothelial growth factor (VEGF) and calcium-binding protein S100A4 in pancreatic cancer and their relationship to the clinicopathological parameters and prognosis of pancreatic cancer.
METHODS: Expression status of VEGF and S100A4 was examined in 62 surgical specimens of primary pancreatic cancer by immunohistochemistry. Correlation between the expression of VEGF and S100A4 and clinicopathological parameters was analyzed.
RESULTS: Thirty-eight of 62 (61.3%) specimens of primary pancreatic cancer were positive for S100A4. Thirty-seven (59.7%) specimens showed positive expression of VEGF. The positive correlation between S100A4 and VEGF expression was significant in cancer tissues (P < 0.001). S100A4 expression was significantly correlated with tumor size, TNM stage and poorer prognosis. VEGF expression had a significant correlation with poorer prognosis. The prognosis of 17 S100A4- and VEGF-negative cancer patients was significantly better than that of other patients (P < 0.05). Distant metastasis (P = 0.001), S100A4- (P = 0.008) and VEGF-positive expression (P = 0.016) were significantly independent prognostic predictors (P < 0.05).
CONCLUSION: Over-expression of S100A4 and VEGF plays an important role in the development of pancreatic cancer. Combined examination of the two molecules might be useful in evaluating the outcome of patients with pancreatic cancer.
Keywords: Pancreatic cancer, Prognosis, S100A4, Vascular endothelial growth factor, Immunohistochemistry
INTRODUCTION
Each year there are more than 170 000 new cases of pancreatic cancer in the world. Pancreatic cancer accounts for up to 2.1% of all cancer cases and is the 5th leading cause of cancer death. The survival rate of patients at all stages of the disease is poor. The overall median survival time is 3-5 mo with a 12 mo-survival rate of 10% and a 5-year survival rate less than 5%[1]. Because of lacking of methods for the early diagnosis and limited knowledge on the biological features of pancreatic cancer, the majority of patients are not diagnosed properly until the advanced stage [2].
The S100 protein family is a large family of soluble calcium-binding proteins, first isolated from bovine brain by Moore in 1965[3]. The S100 family members are involved in a variety of physiological functions, such as cell motility, cell proliferation and differentiation, cell cycle control, regulation of enzyme activity, and calcium-dependent transcriptional regulation[4–7]. The S100A4 protein, which was once named as mts1 or p9Ka, belongs to the S100 family and is classified as a ‘metastasis-related gene’[8]. It was reported that over-expression of S100A4 is significantly correlated with tumor invasion and metastasis[9–11]. A number of studies suggested that over-expression of S100A4 is correlated with poor clinical outcomes in a variety of human cancers, such as bladder, colorectal, ovarian, and esophageal carcinoma[12–15]. VEGF plays an important role in tumor angiogenesis and correlates significantly with tumor invasion and metastasis[16]. Elevated levels of VEGF correlate with a poor prognosis of various cancers, including pancreatic cancer[17,18].
This study was to examine the expression status of S100A4 and VEGF in 62 surgical specimens of primary pancreatic carcinoma by immunohistochemistry and study the role of these two molecules in progression and metastasis of pancreatic cancer.
MATERIALS AND METHODS
Specimens
Specimens obtained from 62 patients (36 males, 26 females) with primary pancreatic cancer admitted to Department of Surgery, 6th Affiliated Hospital of Shanghai Jiaotong University in 2002-2005, were formalin-fixed and paraffin-embedded. The age of these patients ranged 30-84 years (mean age of 64.8 years). All cases were diagnosed as primary pancreatic ductal adenocarcinoma by histopathology (well differentiated in 17 cases, moderately differentiated in 15 cases, and poorly differentiated in 30 cases). No patient received any radiotherapy or chemotherapy. The size of tumor was analyzed by maximum diameter. The patients were staged according to the international TNM system by International Union against Cancer (UICC).
Immunohistochemistry and evaluation criteria
Rabbit anti-human S100A4 polyclonal antibody and mouse anti-human VEGF monoclonal antibody were purchased from NeoMarkers. Two consecutive sections of each specimen were incubated. Immunohistochemistry staining was performed according to the manufacture’s instructions. The tissue used as a negative control was incubated with PBS instead of primary antibody. The tissue known to highly express VEGF and S100A4 was used as positive control.
For each slide, cells positive for VEGF or S100A4 were counted and evaluated under 5-10 fields at 200 ×magnification (cells counted: 100-200), and the percentage of positive cells was calculated. Cells were considered positively immune stained when nuclei and cytoplasm were stained. The distribution of stained S100A4 was evaluated with the percentage of stained cells scored as 0: < 5%, 1: 5%-25%, 2: 26%-50%, 3: 51%-75%, 4: > 75% and staining intensity scored as 1: buff, 2: buffy, 3: puce. When the multiplication of the two scores was greater than or equal to 2, S100A4 was considered positively stained. VEGF was considered positively stained when brown-stained granulae were observed in cytoplasm and the percentage of positive cells was greater than 10%.
Statistical analysis
The correlation between S100A4/VEGF expression and clinicopathological parameters was evaluated by chi-square (χ2) test or Fisher’s exact test. Survival curves were plotted using the Kaplan-Meier method. Survival rates for different groups were compared using the log-rank test. Predictors for prognosis of the patients were assessed using Cox multiple hazards regression analysis. Statistical analysis was carried out using the SPSS 13.0. P < 0.05 was considered statistically significant.
RESULTS
Expression of S100A4 and VEGF
S100A4 was immunoreactive in cytoplasm and nuclei (Figure 1A). VEGF was immunoreactive mainly in cytoplasm (Figure 1B). Of the 62 pancreatic cancer patients, 38 (61.3%) had positive S100A4 expression and 24 (38.7%) negative S100A4 expression. Thirty of the 38 (78.9%) patients with positive S100A4 expression had positive VEGF expression. Seventeen of the 24 (70.8%) S100A4-negative patients had negative VEGF expression. The positive correlation between expression of S100A4 and VEGF was statistically significant (P < 0.0001) (Table 1).
Figure 1.
Positive expression of S100A4 (A) and VEGF (B) in pancreatic cancer (× 200).
Table 1.
Correlation analysis of S100A4 and VEGF expression in pancreatic cancer
| S100A4 |
VEGF |
P value | |
| (+) | (-) | ||
| (+) | 30 | 8 | |
| (-) | 7 | 17 | < 0.001 |
Significance was estimated with χ2 test.
The correlation between S100A4/VEGF express-ion and clinicopathological parameters was analyzed (Tables 2-3). Tumors with their maximum diameter greater than 4 cm had a higher S100A4 expression than those with their maximum diameter less than 4 cm. Tumors at III +IV stage had a higher S100A4 expression than those at I + IIstage. The correlation between S100A4 expression and tumor size and TNM stage was statistically significant. VEGF expression was not significantly related with the clinicopathological parameters.
Table 2.
Correlation between S100A4 expression and clinicopathological parameters in pancreatic cancer, n (%)
| Clinicopathological parameters | Cases (n) |
S100A4 expression |
P value | |
| Positive | Negative | |||
| Age (yr) | ||||
| ≥ 70 | 24 | 15 (62.5) | 9 (37.5) | |
| < 70 | 38 | 23 (60.5) | 15 (39.5) | > 0.051 |
| Gender | ||||
| Male | 36 | 22 (61.9) | 14 (38.1) | |
| Female | 26 | 16 (61.5) | 10 (38.5) | > 0.051 |
| Differentiation | ||||
| Well | 17 | 12 (70.6) | 5 (29.4) | |
| Moderately | 15 | 9 (60.0) | 6 (40.0) | |
| Poorly | 30 | 17 (56.7) | 13 (43.3) | > 0.051 |
| Tumor size (cm) | ||||
| < 2.0 | 3 | 0 (0) | 3 (100) | |
| 2.0-4.0 | 38 | 21 (55.3) | 17 (44.7) | |
| > 4.0 | 21 | 17 (81.0) | 4 (19.0) | < 0.052 |
| Lymph node metastasis | ||||
| (-) | 13 | 5 (38.5) | 8 (61.5) | |
| (+) | 49 | 33 (67.3) | 16 (32.7) | > 0.051 |
| Distant metastasis | ||||
| (-) | 39 | 23 (59.0) | 16 (41.0) | |
| (+) | 23 | 15 (65.2) | 8 (34.8) | > 0.051 |
| TNM stage | ||||
| I + II | 30 | 14 (46.7) | 16 (53.3) | |
| III + IV | 32 | 24 (75.0) | 8 (25.0) | < 0.051 |
Significance was estimated with χ2 test;
Significance was estimated with Fisher’s exact test.
Table 3.
Correlation between VEGF expression and clinicopathological parameters in pancreatic cancer, n (%)
| Clinicopathological parameters | Cases (n) |
VEGF expression |
P value | |
| Positive | Negative | |||
| Age (yr) | ||||
| ≥ 70 | 24 | 17 (70.8) | 7 (29.2) | |
| < 70 | 38 | 20 (52.6) | 18 (47.4) | > 0.05 |
| Gender | ||||
| Male | 36 | 24 (66.7) | 12 (33.3) | |
| Female | 26 | 13 (50) | 13 (50) | > 0.05 |
| Differentiation | ||||
| Well | 17 | 10 (58.8) | 7 (41.2) | |
| Moderately | 15 | 8 (53.3) | 7 (46.7) | |
| Poorly | 30 | 19 (63.3) | 11 (36.7) | > 0.05 |
| Tumor size (cm) | ||||
| < 2.0 | 3 | 2 (66.7) | 1 (33.3) | |
| 2.0-4.0 | 38 | 21 (55.3) | 17 (44.7) | |
| > 4.0 | 21 | 14 (66.7) | 7 (33.3) | > 0.05 |
| Lymph node metastasis | ||||
| (-) | 13 | 7 (53.8) | 6 (46.2) | |
| (+) | 49 | 30 (61.2) | 19 (38.8) | > 0.05 |
| Distant metastasis | ||||
| (-) | 39 | 24 (61.5) | 15 (38.5) | |
| (+) | 23 | 13 (56.5) | 10 (43.5) | > 0.05 |
| NM stage | ||||
| I + II | 30 | 17 (56.7) | 13 (43.3) | |
| III + IV | 32 | 20 (62.5) | 12 (37.5) | > 0.05 |
Significance was estimated with χ2 test.
Correlation between expression of S100A4 and VEGF and prognosis of patients
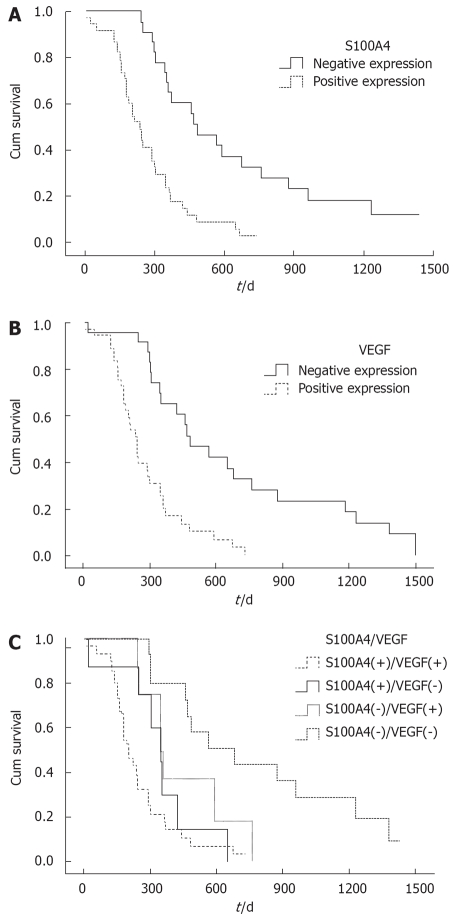
The 62 patients were followed up till December 2006 and their median survival time was 290.6 d. The 1-, 2-, and 3- year survival rate was 37%, 14%, and 7%, respectively. The median survival time of the S100A4 positive and negative patients was 232.8 d and 535.5 d, respectively, while the median survival time of the VFGF positive and negative patients was 229.7 d and 541.6 d, respectively. The survival curve was better for patients with S100A4-negative cancer than for those with S100A4-positive cancer (P < 0.001; log-rank test) (Figure 2A). The survival curve was better for patients with VEGF-negative cancer than for those with VEGF positive cancer (P < 0.001; log-rank test) (Figure 2B).
Figure 2.
Survival curves for group of pancreatic cancer patients according to S100A4 expression (A), group of pancreatic cancer patients according to VEGF expression (B), and four subgroups of pancreatic cancer patients according to the expression of S100A4 and VEGF (C).
According to the expression of S100A4 and VEGF, pancreatic cancer patients were subdivided into four groups: (1) S100A4(+)/VEGF(+), (2) S100A4(+)/VEGF(-), (3) S100A4(-)/VEGF(+), (4) S100A4(-)/VEGF(-). Patients in the S100A4(-)/VEGF(-) group had a significantly better prognosis than those in the other three groups, and their median survival time was 678 d. Patients in the S100A4(+)/VEGF(-) group had a poorer prognosis than those in the S100A4(-)/VEGF(+) and S100A4(-)/VEGF(-) groups (P < 0.05; log-rank test). However, there was no significant difference between the S100A4(+)/VEGF(-) and S100A4(+)/VEGF(-) groups (Figure 2C).
The prognostic value of following parameters was analyzed, including age, differentiation of tumor, size of tumor, lymph node metastasis, distant metastasis, TNM stage and expression of S100A4 and VEGF. The influence of these parameters was evaluated by the Cox proportional hazards model. The results of these analyses showed that distant metastasis, expression of S100A4 and VEGF were significant independent prognostic predictors (Table 4).
Table 4.
Cox multivariate regression analysis of clinicopathological features as a prognostic predictor
| B | SE | Wald | df | P value | OR (95% CI) | |
| Distant metastasis | 1.101 | 0.345 | 10.163 | 1 | 0.001 | 3.006 (1.528, 5.914) |
| S100A4 | 0.893 | 0.338 | 6.989 | 1 | 0.008 | 2.443 (1.260, 4.736) |
| VEGF | 0.821 | 0.340 | 5.815 | 1 | 0.016 | 2.272 (1.166, 4.426) |
DISCUSSION
In this study, the expression of S100A4 and VEGF was evaluated in relation to the clinicopathological parameters of pancreatic cancer. Of the 62 pancreatic cancers patients, 61.3% were positive for S100A4 expression. Pancreatic cancer with a large size and high TNM stage had a higher S100A4 expression. Over-expression of S100A4 was significantly correlated with tumor size, TNM stage and a poor prognosis. These results suggest that over-expression of S100A4 might enhance cell motility and invasiveness.
It was reported that expression of S100A4 is signifi-cantly correlated with lymph node metastasis in several types of cancer, such as breast, colorectal, stomach, lung, and thyroid carcinoma[19–23]. Our results show that positive S100A4 expression was higher in patients with lymph node metastasis than in patients without lymph node metastasis. However, there was no significant difference in S100A4 expression between the two groups, suggesting that expression of S100A4 is not closely related to lymph node metastasis. Further study is needed to confirm our findings.
S100A4 protein may modulate cell cycle, cell motility, invasiveness and adhesion. In cancer cells, S100A4 protein regulates protein kinase C phosphorylation of the heavy chain of nonmuscle myosin in a calcium-dependent manner, resulting in enhanced cell motility and invasiveness[24,25]. Merzak et al[26] reported that expression of S100A4 is closely correlated with in vitro invasiveness of glioma cells. Moreover, increased levels of S100A4 confer metastasis of non-metastatic epithelial cell line in vivo[27,28]. Ambartsumian et al[29] reported that S100A4 induces tumor progression by stimulating angiogenesis. All these findings demonstrate that S100A4 plays an important role in tumor growth, invasion, metastasis and angiogenesis.
VEGF is a mitogen and motor of vascular endothelial cells and also and important factor for angiogenesis. It can induce proliferation and migration of vascular endothelial cells, and formation of blood capillary lumens. VEGF increases vascular permeability and stimulates vascular endothelial cells to secret proteinase and small molecules. In 1993, Brown et al[30] firstly reported the high expression of VEGF in pancreatic cancer specimens, which is closely correlated with the growth and invasion of pancreatic cancer. In our study, S100A4 and VEGF expression in pancreatic cancer was detected, showing that expression of S100A4 and VEGF is closely correlated. The mechanism of S100A4 and VEGF expression and their relationship with the development and progression of pancreatic cancer deserve further study at molecular level.
In conclusion, expression of S100A4(-)/VEGF(-) cancer can be used as a marker to predict the survival of patients. Distant metastasis, positive S100A4 and VEG are highly independent prognostic predictors. Expression of S100A4 and VEGF can be used as an indicator of prognosis in patients with pancreatic cancer. Exploitation and application of S100A4- or VEGF-targeted tumor inhibitors can decrease the recurrence or metastasis rate of pancreatic cancer and improve the prognosis of patients.
COMMENTS
Background
The prognosis of pancreatic cancer is very poor. Many patients are not diagnosed until at its advanced stage. Current treatment for the disease is surgery in combination with radiotherapy or chemotherapy. Growth and metastasis of pancreatic cancer were studied in order to improve its prevention and treatment.
Research frontiers
S100A4 protein expression in different kinds of cancer is closely correlated with the growth, invasion and metastasis of pancreatic cancer. The mechanism of S100A4 protein underlying the growth, invasion and metastasis of tumor is a hot topic in recent researches.
Innovations and breakthroughs
The expression of S100A4 and VEGF in pancreatic cancer and their correlation with prognosis of pancreatic cancer patients were studied. Our results show that expression of S100A4 and VEGF is positively correlated with pancreatic cancer.
Applications
By detecting tumor-associated proteins S100A4 and VEGF, we evaluated the biological characteristics and prognosis of pancreatic cancer, which can improve our understanding of pancreatic cancer and provide scientific basis for the application of S100A4 and VEGF inhibitors in treatment of pancreatic cancer.
Terminology
S100 protein: a member of the large family of soluble acid calcium-binding proteins, first discovered and designated by Moore in 1965. S100 protein can be completely dissolved in saturated ammonium sulfate, and the family consists of 21members, like S100A1-A13, S100B, S100P, Profilaggrin, Trichohyalin and Reperin, etc. S100 protein regulates interaction between Ca++ and target-protein, and is involved in a variety of physiological functions, such as cell proliferation and differentiation, cell cycle control, regulation of enzyme activity, and calcium-dependent transcriptional regulation.
Peer review
In this nice research, the authors investigated the expression of S100A4 and VEGF in pancreatic cancer and discussed the prognostic significance and clinical pathological features of S100A4 and VEGF. The research may offer certain contribution to the treatment of pancreatic cancer. The design of the study is scientific and the results are reliable and have clinical application values.
Peer reviewer: Kazuhiro Hanazaki, MD, Professor and Chairman, Department of Surgery, Kochi Medical School, Kochi University, Kohasu, Okohcho, Nankoku, Kochi 783-8505, Japan
S- Editor Yang RH L- Editor Wang XL E- Editor Liu Y
References
- 1.Spinelli GP, Zullo A, Romiti A, Di Seri M, Tomao F, Miele E, Spalletta B, Eramo A, Hassan C, Tomao S. Long-term survival in metastatic pancreatic cancer. A case report and review of the literature. JOP. 2006;7:486–491. [PubMed] [Google Scholar]
- 2.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 3.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 4.Kriajevska M, Bronstein IB, Scott DJ, Tarabykina S, Fischer-Larsen M, Issinger O, Lukanidin E. Metastasis-associated protein Mts1 (S100A4) inhibits CK2-mediated phosphorylation and self-assembly of the heavy chain of nonmuscle myosin. Biochim Biophys Acta. 2000;1498:252–263. doi: 10.1016/s0167-4889(00)00100-2. [DOI] [PubMed] [Google Scholar]
- 5.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 6.Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–d1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 7.Emberley ED, Murphy LC, Watson PH. S100 proteins and their influence on pro-survival pathways in cancer. Biochem Cell Biol. 2004;82:508–515. doi: 10.1139/o04-052. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Kobayashi R, Ishikawa T, Hidaka H. Isolation and characterization of a calcium-binding protein derived from mRNA termed p9Ka, pEL-98, 18A2, or 42A by the newly synthesized vasorelaxant W-66 affinity chromatography. Arch Biochem Biophys. 1992;292:563–569. doi: 10.1016/0003-9861(92)90031-q. [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Whittaker PA, Usmani BA, Lakshmi MS, Sherbet GV. Induction of 18A2/mts1 gene expression and its effects on metastasis and cell cycle control. DNA Cell Biol. 1994;13:1021–1028. doi: 10.1089/dna.1994.13.1021. [DOI] [PubMed] [Google Scholar]
- 10.Weterman MA, Stoopen GM, van Muijen GN, Kuznicki J, Ruiter DJ, Bloemers HP. Expression of calcyclin in human melanoma cell lines correlates with metastatic behavior in nude mice. Cancer Res. 1992;52:1291–1296. [PubMed] [Google Scholar]
- 11.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K, Irie A, Satoh T, Ishii J, Iwabuchi K, Iwamura M, Egawa S, Baba S. Expression of S100A2 and S100A4 predicts for disease progression and patient survival in bladder cancer. Urology. 2007;70:602–607. doi: 10.1016/j.urology.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Hemandas AK, Salto-Tellez M, Maricar SH, Leong AF, Leow CK. Metastasis-associated protein S100A4--a potential prognostic marker for colorectal cancer. J Surg Oncol. 2006;93:498–503. doi: 10.1002/jso.20460. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi N, Horiuchi A, Osada R, Imai T, Wang C, Chen X, Konishi I. Nuclear expression of S100A4 is associated with aggressive behavior of epithelial ovarian carcinoma: an important autocrine/paracrine factor in tumor progression. Cancer Sci. 2006;97:1061–1069. doi: 10.1111/j.1349-7006.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ninomiya I, Ohta T, Fushida S, Endo Y, Hashimoto T, Yagi M, Fujimura T, Nishimura G, Tani T, Shimizu K, et al. Increased expression of S100A4 and its prognostic significance in esophageal squamous cell carcinoma. Int J Oncol. 2001;18:715–720. doi: 10.3892/ijo.18.4.715. [DOI] [PubMed] [Google Scholar]
- 16.Shinkaruk S, Bayle M, Lain G, Deleris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3:95–117. doi: 10.2174/1568011033353452. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, et al. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122–129. doi: 10.1097/00006676-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gongoll S, Peters G, Mengel M, Piso P, Klempnauer J, Kreipe H, von Wasielewski R. Prognostic significance of calcium-binding protein S100A4 in colorectal cancer. Gastroenterology. 2002;123:1478–1484. doi: 10.1053/gast.2002.36606. [DOI] [PubMed] [Google Scholar]
- 20.Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, Barraclough R. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60:1595–1603. [PubMed] [Google Scholar]
- 21.Zhong XY, Zhang LH, Jia SQ, Shi T, DU H, Hu Y, Zhang GG, Lu AP, Li JY, Ji JF. Nuclear expression of S100A4 is associated with lymph node metastasis in gastric carcinoma. Zhonghua Weichang Waike Zazhi. 2007;10:454–457. [PubMed] [Google Scholar]
- 22.Chen XL, Zhang WG, Chen XY, Sun ZM, Liu SH. Correlations of S100A4 protein expression to invasion and metastasis of non-small cell lung cancer. Ai Zheng. 2006;25:1134–1137. [PubMed] [Google Scholar]
- 23.Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005;93:1277–1284. doi: 10.1038/sj.bjc.6602856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingelhofer J, Ambartsumian NS, Lukanidin EM. Expression of the metastasis-associated mts1 gene during mouse development. Dev Dyn. 1997;210:87–95. doi: 10.1002/(SICI)1097-0177(199710)210:2<87::AID-AJA2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Kriajevska M, Tarabykina S, Bronstein I, Maitland N, Lomonosov M, Hansen K, Georgiev G, Lukanidin E. Metastasis-associated Mts1 (S100A4) protein modulates protein kinase C phosphorylation of the heavy chain of nonmuscle myosin. J Biol Chem. 1998;273:9852–9856. doi: 10.1074/jbc.273.16.9852. [DOI] [PubMed] [Google Scholar]
- 26.Merzak A, Parker C, Koochekpour S, Sherbet GV, Pilkington GJ. Overexpression of the 18A2/mts1 gene and down-regulation of the TIMP-2 gene in invasive human glioma cell lines in vitro. Neuropathol Appl Neurobiol. 1994;20:614–619. doi: 10.1111/j.1365-2990.1994.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 27.Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. Expression of the calcium-binding protein S100A4 (p9Ka) in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene. 1996;13:1631–1637. [PubMed] [Google Scholar]
- 28.Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS. Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene. 1993;8:999–1008. [PubMed] [Google Scholar]
- 29.Ambartsumian N, Klingelhofer J, Grigorian M, Christensen C, Kriajevska M, Tulchinsky E, Georgiev G, Berezin V, Bock E, Rygaard J, et al. The metastasis-associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene. 2001;20:4685–4695. doi: 10.1038/sj.onc.1204636. [DOI] [PubMed] [Google Scholar]
- 30.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]