Abstract
Background
C. elegans has been established as a powerful genetic system. Use of a chemically defined medium (C. elegans Maintenance Medium (CeMM)) now allows standardization and systematic manipulation of the nutrients that animals receive. Liquid cultivation allows automated culturing and experimentation and should be of use in large-scale growth and screening of animals.
Results
We find that CeMM is versatile and culturing is simple. CeMM can be used in a solid or liquid state, it can be stored unused for at least a year, unattended actively growing cultures may be maintained longer than with standard techniques, and standard C. elegans protocols work well with animals grown in defined medium. We also find that there are caveats to using defined medium. Animals in defined medium grow more slowly than on standard medium, appear to display adaptation to the defined medium, and display altered growth rates as they change the composition of the defined medium.
Conclusions
As was suggested with the introduction of C. elegans as a potential genetic system, use of defined medium with C. elegans should prove a powerful tool.
Background
Cultivation in chemically defined media was promoted at the initial suggestion of the utility of C. elegans as a genetic model system [1]. However, the development of a defined medium (C. elegans Maintenance Medium (CeMM), [2]) lagged far behind the development of C. elegans as a genetic system [3]. As a result monoxenic cultivation of C. elegans on Nematode Growth Medium agar plates with E. coli (NGM) is standard [4]. The ability to alter systematically the composition of CeMM should make it a staple in metabolism and aging studies. Additionally, cultivation using CeMM now allows removal of the variables introduced by monoxenic cultivation. Some of these variables impact nematode biology [5], with reduction of lifespan being the impact most recently demonstrated [6]. Monoxenic cultivation also introduces variables into pharmaceutical studies on C. elegans [4,7], an example being lack of drug effect being due to E. coli metabolizing the drug. Large scale screening of pharmaceutical and nematicidal compounds on C. elegans can now be achieved when liquid CeMM is used with equipment for automated culturing [8] and experimentation ([9], updated version available from Union Biometrica). CeMM may also be useful in growing large numbers of animals for genomic or proteomic work and in development of C. elegans biosensors. We made arrangements with Fisher to have a 2× stock of CeMM produced by MediaTech. We provided Fisher a protocol (see Additional file 1) adapted from Lu and Goetsch [2]. All studies presented here utilized the same batch, though growth rates did not vary between two batches.
Results and Discussion
Growing C. elegans in liquid CeMM
Actively growing cultures may be maintained longer using CeMM than NGM. Growth arrested L1s and dauers are observed after approximately one week on NGM plates, whereas healthy, reproducing populations were observed for at least one week, at room temperature, in unchanged liquid CeMM, in all tested materials (Table 1). Healthy, reproducing populations were observed for at least one month in the flasks, Opticells™, and FEP bags. With small initial populations healthy, reproducing animals were observed after two or three months on 1.7% agar CeMM plates or in unchanged liquid CeMM.
Table 1.
Equipment compatible with C. elegans in liquid CeMM
| Material: | Manufacturer: |
| Tubes: | |
| 1 ml | Eppendorf |
| 15 ml conical | Falcon |
| 50 ml conical | Falcon |
| Dishes: | |
| 60 mm petri | Falcon |
| 100 mm petri | Falcon |
| 60 mm tissue culture | Falcon |
| 100 mm tissue culture | Falcon |
| 24 well tissue culture | Corning |
| 96 well tissue culture | Corning |
| Flasks: | |
| 25 cm2 cell culture | Corning |
| 75 cm2 cell culture | Corning |
| Membranous devices: | |
| 10 mm membrane tubing | Spectrapor |
| Opticell™ | BioCrystal |
| FEP bag | American Fluoroseal |
Significant evaporation of liquid CeMM was noted in 75 cm2 flasks and 10 mm tubing. In 10 mm tubing, a starting volume of 1 ml of worms in liquid CeMM completely evaporated in seven days when sealed in a 100 mm petri dish or in six days when sealed in a Kennedy Space Center fixation tube (KFT [10]). Inclusion of a moist paper towel inside a 100 mm petri dish or a KFT allowed growth for 22 days with 300 μ l of liquid CeMM remaining, a loss of two thirds the starting volume of CeMM. In contrast, evaporation of two-thirds of the starting volume of liquid CeMM in 75 cm2 flasks is observed to take four to five weeks. While not extensively quantitated, evaporation does occur in other culture devices. The Opticell™ or FEP bag allowed adequate oxygenation of stationary cultures and appeared to minimize evaporation. After nine months a FEP bag contained 8 ml of the 11 ml starting culture volume of liquid CeMM. We presume animal movement was sufficient to mix liquid CeMM, if indeed mixing CeMM is necessary to dilute wastes and evenly distribute nutrients. Use of either device also facilitated transport and shipment of animals. More animals were observed to settle to the bottom of the culture in FEP bags than in Opticells™, but sedimentation did not have obvious effects on population growth or health. Sedimentation appears to be the result of increased depth of the culture surface and lack of FEP bag rigidity versus the Opticell™. These results demonstrate CeMM can easily be used with a variety of laboratory devices and that CeMM grown animals require less maintenance than NGM grown animals.
In all devices, we often noted clumps of eggs in the medium, perhaps due to self-association, ruptured adult animals, or both. As many as 80% of these eggs failed to develop when clumps were placed in fresh liquid CeMM. This is in contrast to individual unclumped eggs which appeared to develop normally when placed in fresh liquid CeMM. Clumping was not observed on NGM plates, on CeMM plates, or when individual animals were cultured in liquid CeMM. These results suggest there are differences between CeMM and NGM grown animals.
Adaptation to growth in liquid CeMM
Animals in liquid CeMM grow more slowly than on NGM plates and appear to adapt to the medium. As shown in Figure 1, wildtype animals (Strain N2) grown on NGM plates reached a length of 1 mm in times consistent with those published [11]. Wildtype N2 animals that had been maintained in liquid CeMM for three years prior to this study took two to three times as long to reach 1 mm in length when grown in liquid CeMM (Fig. 1). Animals grown on 1.7% agar CeMM plates, reached a length of 1 mm in times not significantly different from those in liquid CeMM (Table 2), implying growth rate differences are the effect of CeMM composition but not the physical state. When animals were switched from NGM plates into liquid CeMM or from liquid CeMM to NGM plates, animals grew as predicted for the medium to which they were switched (not shown). Animals developed from eggs transferred into liquid CeMM from NGM plates had no gross phenotypic differences from animals exclusively grown in liquid CeMM. Animals developed from eggs transferred onto NGM plates from liquid CeMM displayed a moderate egg-laying defect (36%) and massive internal hatching (8%). These data suggest adaptation to growth in liquid CeMM occurs. Therefore we have provided the wildtype N2 grown in CeMM for three years with a new name under our registered lab designation, strain CC1. Using a wildtype strain maintained in axenic medium for several years in another laboratory (Strain LSJ1) we observed social feeding behavior when grown on NGM plates. In liquid CeMM LSJ1 took two days longer to reach 1 mm than either of the other wildtype strains (N2, CC1). Surprisingly, LSJ1 started laying eggs at approximately the same time as strains N2 and CC1 despite being shorter. LSJ1 started laying eggs at 0.8 ± 0.04 mm as opposed to the > 1 mm classically associated with reaching the egg laying stage of adulthood [11]. These results suggest different adaptations to growth in axenic media are possible and that the life-cycle of CeMM grown animals is altered compared to that of animals on NGM plates.
Figure 1.
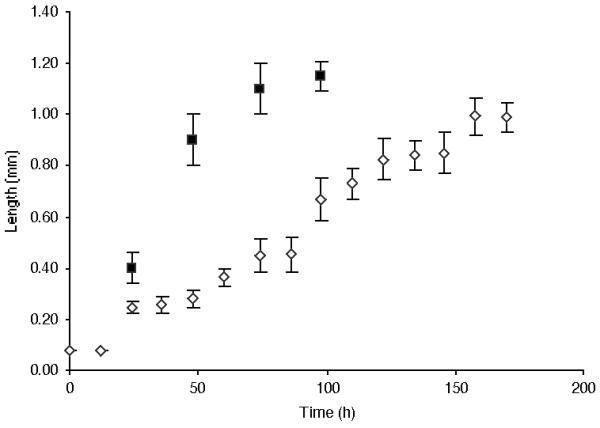
Growth curve at 20°C for animals grown on NGM plates (filled squares) and in liquid CeMM (open diamonds). Eggs were obtained by bleach treatment of animals grown for at least three years on NGM plates or in liquid CeMM. Approximately 50 eggs from NGM plate grown worms were placed on fresh NGM plates at t = 0. Approximately 50 eggs from liquid grown CeMM worms were place in 50 μ l of fresh CeMM in a microfuge tube cap in a humidified chamber at t = 0. Length of ten animals was optically measured every twenty-four (NGM) or twelve (CeMM) hours, until adults were observed in consecutive timepoints.
Table 2.
Time animals take to reach 1 mm in length when CeMM is altered
| Condition1,2 | Days to reach 1 mm at 25°C3 |
| Fresh liquid CeMM | 5 |
| Fresh CeMM plate | 5 |
| Fresh liquid CeMM heated to 50°C for 1 h | 5 |
| Liquid CeMM conditioned 4.5 weeks, heated to 50°C for 1 h | 4 |
| Liquid CeMM conditioned 4.5 weeks, heated to 50°C for 1 h, filtered | 8 |
| Fresh liquid CeMM with added E. coli strain OP50 | 4 |
| Liquid CeMM stored 1 y in the dark @ 4°C | 5 |
| Liquid CeMM stored 1 y in the dark @ 4°C, subject to periodic removal from storage for use | 7–10 |
1 CeMM plates were 60 × 15 mm 2 Liquid CeMM was 200 μ l in a 24 well tissue culture dish 3 All times represent the average length of ten animals from three independent runs
Dynamic nature of CeMM cultures
CeMM composition changes as the result of animal growth in the medium and growth rates are consequently altered as the medium changes. When culturing at 22.5°C, liquid CeMM remains at pH 6 for the first week, increases to pH 7 after one week, increases to pH 8.5 after three weeks, and remains at pH 8.5 after six weeks, suggesting that animals condition CeMM. To evaluate growth in conditioned liquid CeMM, we used 75 cm2 flasks to grow animals at 25°C for 4.5 weeks, at which point liquid CeMM had decreased in volume from 10 to 3.5 ml. Animals were killed by heating to 50°C for one hour. New animals added to this medium reached 1 mm one day sooner than animals grown in fresh CeMM subjected to 50°C for one hour (Table 2). Particulate material, in the form of shed cuticles and dead animals, accumulates in conditioned liquid CeMM. Particulate material has been reported to increase the growth rate of the related species C. briggsae by providing physical stimulation [12]. To test if particulate material accounted for the increased growth rate we grew animals in conditioned, heat killed liquid CeMM subject to filtration through a 0.2 micron filter (Millipore). Animals reached a length of 1 mm three days later than animals grown in fresh liquid CeMM (Table 2), suggesting both that conditioned CeMM is nutritionally depleted or otherwise altered in a toxic fashion, and that the presence of accumulated particulate material improves the growth rate in liquid CeMM. We believe increased growth rate is the result of cannibalism of dead animals in liquid CeMM. Accordingly, we find addition of E. coli strain OP50 to fresh liquid CeMM results in growth rates not significantly different from animals grown in conditioned, unfiltered liquid CeMM (Table 2). Together these results also suggest that animals grown in fresh liquid CeMM are receiving suboptimal nutrients. It is important to note this is defined by growth rate which may or may not be the appropriate end point to measure [13]. These results suggest careful consideration should be given to using animals in fresh versus conditioned media for experimentation and that composition changes must be taken into account when comparing data from separate experiments.
CeMM can be stored, but repeated handling will decrease shelf life, presumably due to degradation of components because of photosensitivity or oxidation. Animals grown at 25°C displayed an unaltered growth rate in liquid CeMM that had been stored unused in the dark at 4°C for one year as compared to fresh liquid CeMM (Table 2). In contrast, animals displayed a variably decreased growth rate reaching 1 mm two to five days later than animals in fresh liquid CeMM when grown at 25°C in liquid CeMM stored in the dark at 4°C but subject to periodic removal for culturing animals over the course of a year (Table 2). We find storing and handling media in small aliquots minimizes concerns with media degradation.
Using standard protocols with CeMM grown animals
Most protocols developed for NGM grown animals work with CeMM grown animals. Standard protocols for settling worms or bleaching eggs to create age synchronous populations were easily performed with CeMM grown animals, as were protocols for preparation of dauers, dauer recovery, and freezing of strains [4,7]. Individual animals were manipulated using a pipettor and microscopic observation and optical measurement of animals were conducted with ease. Matings were not easily carried out but were possible on small CeMM plates in 24 well tissue culture dishes.
Much as with bleaching eggs from NGM grown animals, it is possible to overbleach CeMM grown animals and get no viable eggs, underbleach CeMM grown animals and get eggs that develop within the incompletely dissolved parents, and it is possible to bleach just long enough to get eggs from which 98% or greater develop to adulthood. As with NGM grown animals the age, and thus strength, of the bleach solution and the density of worms being bleached appear to affect efficiency of bleaching CeMM grown animals.
On NGM plates dauer formation is determined by an integrated response to food, pheromone, and temperature [14]. While dauers may be in a developmental stasis, dauers are active living animals with an altered metabolism compared to adults [15,16]. Dauers generated at 27°C are often paler than dauers generated at 25°C [17] suggesting that dauers generated by various means are not the same. It therefore stands to reason that dauers generated by various techniques may not respond identically to changes in environment. Rather than study dauer formation and recovery for CeMM grown animals extensively, we simply assayed the small scale ability to generate and recover dauers. In three separate attempts, all of the viable progeny of four adults placed on CeMM plates at 28°C for one week were dauers and no dauer animals were noted on the plates after an additional week at 20°C.
The number of animals frozen directly impacts the number of animals recovered for both liquid CeMM grown animals and NGM plate grown animals. Since it is easier to generate larger volumes of worms using liquid CeMM than on a NGM plate it is possible to get larger numbers of animals surviving freezing. Mixed stage animals grown in liquid CeMM can be directly mixed 1:1 with freezing medium [4,7] and slowly frozen to -80°C. Compared to typical starved L1 freezes prepared from NGM grown animals, larger numbers of animals are viable after three years. We have not attempted to quantitate viability nor compare viability between culture conditions. We also have not attempted to generate starved L1 freezes from liquid CeMM grown animals even though starved L1s have been reported to freeze better than mixed stage NGM preparations [4,7]. The ability to recover large numbers of viable animals from liquid CeMM cultures may indicate CeMM grown animals are partially starved, are somehow otherwise prone to survive freezing better than NGM grown animals, or may simply reflect the ease with which large numbers of animals can be generated in liquid CeMM.
CeMM has a very broad absorbance spectra ranging from below 200 to just below 600 nm (Fig 2a). CeMM has two major emission peaks. When excited at 350 nm these peaks are centered at 434 and 522 nm (Fig 2b). This is a widely used range for a number of applications therefore these emissions may present a difficulty for some applications. Due to CeMM fluorescence GFP was difficult to observe in transgenic animals containing GFP in nerve and muscle when a dissecting microscope was used. (Strains MU1085 (bwIs2(flp-1::gfp; rol-6(su1006))) and PJ707 (jIs01(myo-3::GFP; rol-6(su1006))). When viewed with a compound microscope we experienced no difficulty in observing GFP and little problem with background fluorescence from CeMM. We believe the difference in GFP visualization on our microscopes reflects the relative depth of liquid CeMM in which worms were observed. However, width of field and slight differences in the filter sets used are other potential explanations.
Figure 2.
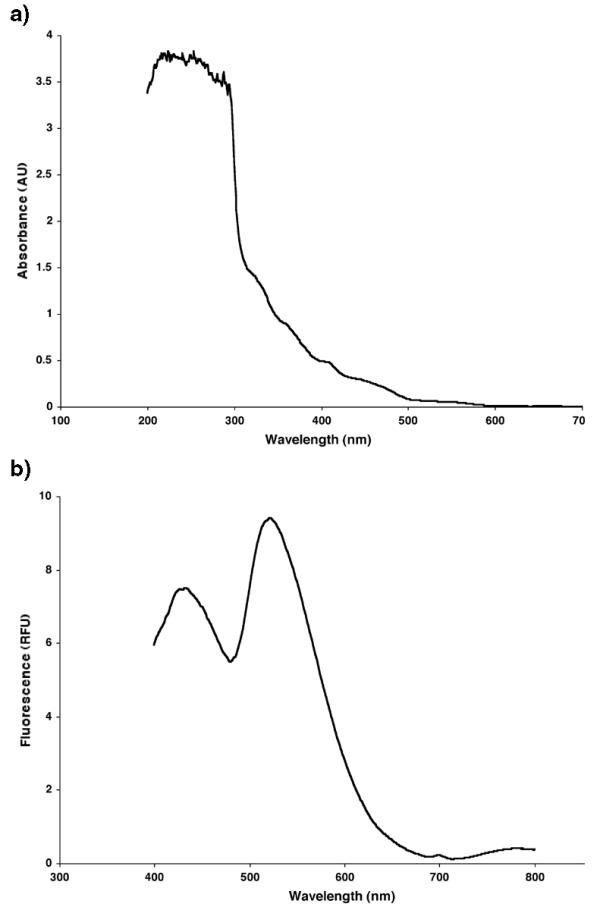
Absorbance and emission spectra for CeMM. a) Absorbance of CeMM is shown as a function of wavelength. Absorbance was measured for each nm increment between 200 and 1000 nm. No appreciable signal was noted between 700 and 1000 nm. b) Emissions of CeMM as excited at 350 nm. Emissions were measured for each nm increment between 400 and 800 nm.
The only procedure we had difficulty carrying out with CeMM grown animals was mating. Consistent with the a priori prediction that males would not mate well in liquid, we observed successful matings on CeMM plates but not in liquid CeMM, both in 24 well tissue culture dishes (Strains CB1489 (him-8(e1489) IV) and PJ1046 (cha-1(p1182ts) IV; ccIs55(unc-54::lacZ) V)). Successful mating was noted on one of three attempts on a CeMM plate made in a 24 well tissue culture dish and not at all on three attempts on a CeMM plate made in a 60 × 15 mm or 35 × 15 mm petri dish. We presume matings were unsuccessful most of the time because the animals move throughout the plate rather than remaining on the surface. As such it was possible to observe cross progeny but not determine mating efficiency. It is possible that male mating ability is reduced when animals are grown using CeMM, however we have no data on this. These results demonstrate that although there may be differences between NGM and CeMM grown animals, knowledge of NGM grown animals can be applied to CeMM grown animals.
Conclusions
We have presented technical aspects of our initial work with animals grown in liquid CeMM. These animals are clearly different from those grown on NGM plates. Those wishing to use CeMM should be aware that, as with all media, there are caveats associated with use of CeMM and potential pitfalls of applying what is known of animals on NGM plates to animals grown using CeMM.
Authors' contributions
All authors participated in study conception, manuscript preparation, the testing of laboratory hardware, and the testing of procedures with animals grown in CeMM. NJS performed the growth rate, media switching, and effects of conditioned medium experiments. EK performed the experiments to assess pH changes of the medium.
Supplementary Material
Chemically Defined C. elegans Medium Preparation. This file is a protocol adapted from Lu & Goetsch (1993) for the preparation of CeMM.
Acknowledgments
Acknowledgements
Strain N2 was provided by Stuart Kim; MU1085 by Christine Li; CB1489, PJ717, and PJ1046 by Lewis Jacobson; and LSJ1 was provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). David Hoffman prepared the protocol for CeMM preparation. Beth Little, Gerri Stephenson, and Tim Pohle provided library support. Thanks to Chad Paavola for assistance with the spectra. Thanks to Lewis Jacobson, Ingrid Udranszky, Charles Wade, and Seth Winfree for helpful suggestions and critical reading of drafts this manuscript. This research was funded by NASA Fundamental Biology and the NASA Astrobiology Institute.
Contributor Information
Nathaniel J Szewczyk, Email: nate@alumni.cmu.edu.
Elena Kozak, Email: ekozak@mail.arc.nasa.gov.
Catharine A Conley, Email: Cassie.Conley@nasa.gov.
References
- Dougherty EC, Calhoun HG. Possible Significance of Free-living Nematodes in Genetic Research. Nature. 1948;161:29. doi: 10.1038/161029a0. [DOI] [PubMed] [Google Scholar]
- Lu NC, Goetsch KM. Carbohydrate requirement of Caenorhabditis elegans and the final development of a chemically defined medium. Nematologica. 1993;39:303–331. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C. Elegans II. Cold Spring Harbor, Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Nicholas WL, Dougherty EC, Hansen EL. Axenic cultivation of Caenorhabditis briggsae (Nematoda: Rhabditidae) with chemically undefined supplements, comparative studies with related nematodes. Ann NY Acad Sci. 1959;77:218–236. [Google Scholar]
- Larsen PL, Clarke CF. Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science. 2002;295:120–123. doi: 10.1126/science.1064653. [DOI] [PubMed] [Google Scholar]
- Epstein H, Shakes D. Methods in Cell Biology. Vol. 48. San Diego, Academic Press; 1995. Caenorhabditis elegans: Modern Biological Analysis of an Organism. [Google Scholar]
- Janssen D. Simplifying Cell Culture. Genomics and Proteomics. 2002;2:23. [Google Scholar]
- Byerly L, Cassada RC, Russell RL. Machine for rapidly counting and measuring the size of small nematodes. Review of Scientific Instruments. 1975;46:517–522. doi: 10.1063/1.1134262. [DOI] [PubMed] [Google Scholar]
- Wells HW, Best M. Portable Device for Chemical Fixation of a Biological Sample. NASA Tech Briefs. 1999;23:55 (also KSC–11993). [Google Scholar]
- Byerly L, Cassada RC, Russell RL. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev Biol. 1976;51:23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Lu NC, Briggs GM, Stokstad ELR. Effect of Particulate Materials on Population Growth of the Free-Living Nematode Caenorhabditis briggsae. Proceedings of the Society for Experimental Biology and Medicine. 1979;160:203–207. doi: 10.3181/00379727-160-40420. [DOI] [PubMed] [Google Scholar]
- Dougherty EC. Introduction to axenic culture of invertebrate metazoa: A goal. Annals of the New York Academy of Sciences. 1959;77:27–54. [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- O'Riordan VB, Burnell AM. Intermediary metabolism in the dauer larva of the nematode Caenorhabditis elegans: I. Glycolysis, gluconeogenesis, oxidative phosphorylation, and the tricarboxylic acid cycle. Comparative Biochemistry and Physiology B Comparative Biochemistry. 1989;92:233–238. doi: 10.1016/0305-0491(89)90271-X. [DOI] [Google Scholar]
- O'Riordan VB, Burnell AM. Intermediary metabolism in the dauer larva of the nematode Caenorhabditis elegans: II. The glyoxylate cycle and fatty acid oxidation. Comparative Biochemistry and Physiology B Comparative Biochemistry. 1990;95:125–130. doi: 10.1016/0305-0491(90)90258-U. [DOI] [Google Scholar]
- Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemically Defined C. elegans Medium Preparation. This file is a protocol adapted from Lu & Goetsch (1993) for the preparation of CeMM.


