Abstract
AIM: To evaluate the efficacy and tolerability of two different preparations of esomeprazole in healing duodenal ulcers.
METHODS: A total of 60 patients with active duodenal ulcers were enrolled and randomized to receive esomeprazole enteric-coated capsules (40 mg) or esomeprazole magnesium (40 mg), once daily, for 4 consecutive wk, with ulcer healing being monitored by endoscopy. Safety and tolerability were also assessed.
RESULTS: Fifty seven patients completed the whole trial. The ulcer healing rates at the end of wk 2 were 86.7% and 85.2% in the esomeprazole enteric-coated capsules and esomeprazole magnesium groups, respectively (P = 0.8410), and reached 100% at the end of wk 4 in both groups. Symptom relief at the end of wk 2 was 90.8% in the esomeprazole enteric-coated capsules group and 86.7% in the esomeprazole magnesium group (P = 0.5406); at the end of wk 4 symptom relief was 95.2% and 93.2%, respectively (P = 0.5786). Adverse events occurred in 16.7% of the esomeprazole enteric-coated capsules group and 14.8% of the esomeprazole magnesium group (P = 1.0000).
CONCLUSION: The efficacies of esomeprazole enteric-coated capsules and esomeprazole magnesium in healing duodenal ulcer lesions and relieving gastrointestinal symptoms are equivalent. The tolerability and safety of both drugs were comparable.
Keywords: Esomeprazole, Duodenal ulcer, Proton pump inhibitor
INTRODUCTION
Esomeprazole, the stereospecific S-isomer of omeprazole, is the first proton pump inhibitor (PPI) to be developed as a single isomer for use in the treatment of acid-related diseases[1,2]. This optical isomer is subject to less first-pass metabolism and lower plasma clearance than omeprazole, thereby offering higher systemic bioavailability[3,4]. Early studies have shown esomeprazole achieves greater and more sustained acid control than omeprazole, with a similar tolerability and safety profile[5,6]. Furthermore, esomeprazole shows a more rapid onset of acid-suppression effect than omeprazole, and less inter-individual variation in acid control[7,8]. Additionally, a recent crossover study demonstrated that esomeprazole at a standard dose of 40 mg once daily provides more effective control of gastric acid at steady state than standard doses of pantoprazole, lansoprazole and rabeprazole in patients with symptomatic gastroesophageal reflux disease (GERD)[9,10]. In addition, esomeprazole treatment yields higher erosive esophagitis healing rates and provides sustained resolution of heartburn in more patients than any other currently available PPI[11].
The current study investigated whether esomeprazole enteric-coated capsules (40 mg; synthesized by Chongqing Lummy Pharmacy, China) provides effective duodenal ulcer healing compared with esomeprazole magnesium (40 mg; Nexium, AstraZeneca Inc), when administered once daily for 4 wk, in a Chinese population.
MATERIALS AND METHODS
Patients
A randomized, double-blind, double-dummy, parallel-controlled study was conducted in accordance with the ethical principles of the Declaration of Helsinki and internationally accepted guidelines for clinical trials in patients with duodenal ulcer disease. Each protocol was approved by an independent ethics committee prior to study commencement. All patients provided written informed consent before entry into the study. The randomization scheme was computer generated. A centralized allocation method was used to assign patients to a treatment group.
Men and women aged 18-65 years, with no more than two active endoscopically confirmed duodenal ulcers (less than 2 cm in diameter), were recruited into the study from April 2006 to July 2006. Major exclusion criteria included: Pregnancy or lactation, any clinically significant abnormal laboratory values at entry, multiple drug allergies, prior gastric surgery, and concurrent treatment with corticosteroids or non-steroidal anti-inflammatory drugs. Discontinuation of any previous PPI therapy was required at least 7 d before randomization. No antisecretory drugs, including H2-receptor antagonists, or any other agents known to alter the pharmacokinetics of PPI, were allowed during the study or within 1 wk before entry. In addition, patients were excluded from the study if they had esophageal erosions or ulceration, esophageal and/or gastric varices, gastric ulcer, pyloric stenosis, endoscopic evidence of active gastrointestinal bleeding or Zollinger-Ellison syndrome. Other exclusion criteria concerned concurrent renal or hepatic insufficiency, treatment for cancer and any history of drug or alcohol dependence.
Of the 60 patients enrolled in the study, 30 in the treatment group received esomeprazole enteric-coated capsules and 30 in the positive control group received esomeprazole magnesium, and 95% of patients completed the study. Three patients (5%) discontinued the study, all in the esomeprazole magnesium group, and were excluded from the evaluable cohort because of consent withdrawal. The baseline demographic and clinical characteristics of the 57 patients in the evaluable cohort, gender, age, height, blood pressure, heart rate, duration of disease, pre-entry score and initial ulcer size and number, were not different between the two groups (Table 1). Overall, the population was predominantly male (70.2%) and most patients were less than 55 years of age (80.7%). Approximately one-third of patients smoked and consumed alcohol, and this proportion was comparable between the two groups. Compliance with study medication was high during 4-wk treatment period, with more than 90% of the patients in each treatment group taking over 75% of the prescribed drugs.
Table 1.
Baseline demographic and clinical characteristics (%, mean ± SD)
| Characteristics | Esomeprazole enteric-coated capsules | Esomeprazole magnesium | Statistics | P value |
| (n = 30) | (n = 27) | |||
| Gender, n (%) | ||||
| Male | 20 (66.7) | 20 (74.1) | ||
| Female | 10 (33.3) | 7 (25.9) | χ2 = 0.3725 | 0.5416 |
| Age (yr) | 44.2 ± 11.9 | 43.6 ± 11.1 | t = -0.1981 | 0.8437 |
| Height (cm) | 163.1 ± 7.2 | 164.7 ± 7.0 | t = 0.8339 | 0.4079 |
| Systolic BP (mmHg) | 114.5 ± 10.5 | 111.6 ± 8.2 | t = -1.1730 | 0.2459 |
| Diastolic BP (mmHg) | 73.4 ± 7.4 | 74.3 ± 9.2 | t = 0.3901 | 0.6980 |
| Heart rate (bpm) | 73.8 ± 8.5 | 75.1 ± 11.3 | t = 0.4713 | 0.6393 |
| Duration of DU (mo) | 58.3 ± 61.2 | 58.6 ± 53.7 | t = 0.0178 | 0.9859 |
| Total score of symptoms | 5.5 ± 2.6 | 5.2 ± 1.6 | t = -0.5580 | 0.5794 |
| Ulcer diameter (mm) | 7.7 ± 2.6 | 7.5 ± 2.4 | t = -0.2764 | 0.7833 |
| Ulcer number | ||||
| 1, n (%) | 23 (76.7) | 23 (85.2) | ||
| 2, n (%) | 7 (23.3) | 4 (14.8) | χ2 = 0.6621 | 0.4158 |
SD: Standard deviation; BPM: Beats per minute; DU: Duodenal ulcer.
Study procedures
Eligible patients were randomly assigned in a double-blind fashion to one of the two groups: The treatment group received esomeprazole enteric-coated capsules (40 mg; Chongqing Lummy Pharmacy, China) and an esomeprazole magnesium-matching placebo, and the other group received esomeprazole magnesium (40 mg; Nexium, AstraZeneca Inc) and an esomeprazole enteric-coated capsule-matching placebo as a positive control group. The study began within 3 d of baseline endoscopy. Patients were administered the medicine once daily in the morning, 30 min before breakfast, for up to 4 wk. All medications were packaged and labeled identically to maintain blinding. Treatment allocation for each patient was provided in individually sealed and blinded randomization envelopes which were collected and checked by the monitor at the end of the study to ensure the integrity of the blinding.
Ulcer healing was determined by sequential endoscopies performed after 2 wk of therapy, and again after 4 wk if the ulcer was not healed. The primary efficacy variable was the rate of ulcer healing, defined as complete regeneration of the mucosa (re-epithelialization) with no visible mucosal breaks at the site of all ulcers identified during the study. An erosion at the original site of any ulceration was considered to be evidence of incomplete healing (Figure 1). Whenever possible, endoscopic examinations in individual patients were performed by the same endoscopist.
Figure 1.
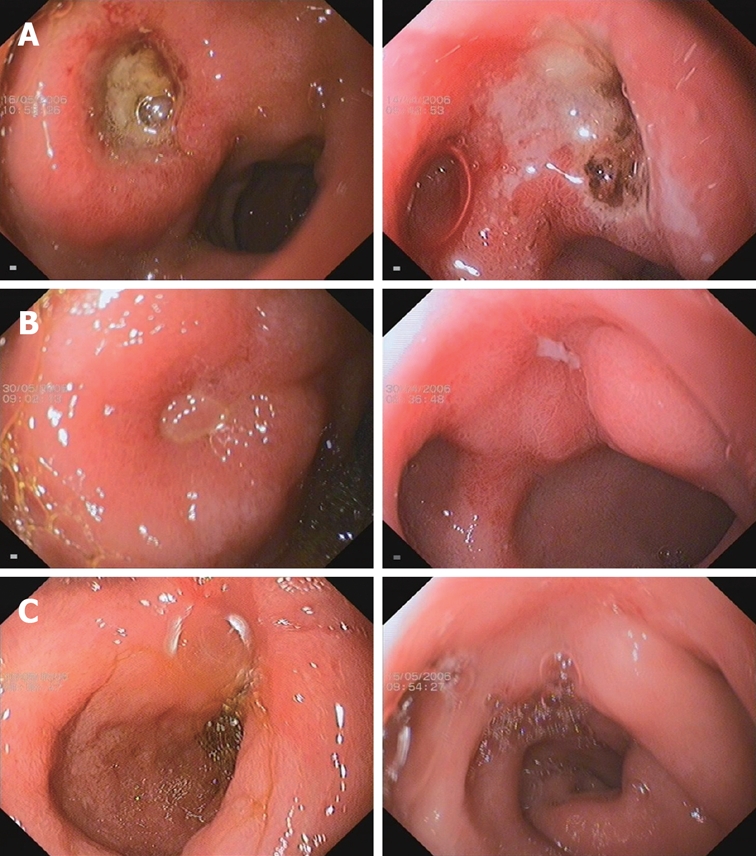
Comparison of Esomeprazole enteric-coated capsules treated group (left column) and Esomeprazole magnesium treated group (right column) in duodenal ulcer under endoscopy. A: Baseline duodenal ulcer under endoscopy; B: Duodenal ulcer under endoscopy at wk 2; C: Duodenal ulcer under endoscopy wk 4.
The secondary end-points of the frequency and intensity of epigastric pain, heartburn, regurgitation, flatulence, belching, nausea and vomiting were assessed at baseline and at the endoscopy visits. Gastrointestinal symptoms were graded on a four-point scale: 0 = none; 1 = mild (aware of symptoms, but easily tolerated); 2 = moderate (discomfort sufficient to cause interference with normal activities); and 3 = severe (incapacitating, with inability to perform normal activities). The patients recorded all of these items in diary cards on a daily basis. The investigator used the diary card information to complete the study case report forms and obtained the total score of all recorded symptoms. The relief of gastrointestinal symptoms was calculated as [(baseline total score-post-treatment total score)/(baseline total score)] × 100%.
Assessment of adverse events
The safety and tolerability of the medication were assessed using physical examination at final visit, review of adverse events as reported by patients at wk 4, and clinical laboratory evaluations at baseline and at the final visit. Clinical laboratory tests included serum chemistry, hematology and urine analysis. The causal relationship of an adverse event to the study drug was classified as being probable, possible or unlikely, and the intensity of the adverse event was rated as mild, moderate or severe. The action taken with study drug in response to the adverse event (none, treatment temporarily stopped, treatment discontinued) was also recorded.
Statistical analysis
Data were analyzed using SAS for Windows, version 6.12; the null hypothesis was rejected if P-values were ≤ 0.05. The primary analysis was carried out on the per-protocol (PP) population, which included all randomized subjects who completed a full course of each treatment, had no appreciable loss of data, and had no major protocol violations. The significance of differences in categorical data was determined using the Pearson χ2 or Monto-Carlo’s exact test. The Student’s t test and Mann-Whitney U test were used when appropriate. Results are reported as means and standard deviations.
RESULTS
Ulcer healing
The duodenal ulcer healing rates at wk 2 and 4 were compared between the two treatment groups (Figure 1). At wk 2, the healing rate was 86.7% in the esomeprazole enteric-coated capsules group compared with 85.2% in the esomeprazole magnesium group (P = 0.8410). At wk 4, 100% ulcer healing was documented in all patients. As shown in Figure 1, case 223 received esomeprazole enteric-coated capsules and case 181 received esomeprazole magnesium. Both patients had much improved duodenal ulcers at wk 2 and had complete ulcer healing at wk 4.
Relief of gastrointestinal symptoms
Assessment of gastrointestinal symptoms showed significant improvements in the frequency and intensity of epigastric pain, heartburn, regurgitation, flatulence, belching, nausea and vomiting at wks 2 and 4, and the two groups demonstrated comparable efficacy. At wk 2, the rate of symptom relief was 90.8% in the esomeprazole enteric-coated capsules treatment group compared with 86.7% in the esomeprazole magnesium positive control group (P = 0.5406). At wk 4, the rates of symptom relief in the two groups were 95.2% and 93.2%, respectively (P = 0.5786).
Safety and tolerability
Of the 60 patients with duodenal ulcers who were randomized with respect to medication, 57 patients received either esomeprazole enteric-coated capsules (n = 30) or esomeprazole magnesium (n = 27) for four wk, and were included in the safety and tolerability analysis. Only a few adverse events were documented with the following distribution: 5/30 patients (16.7%) in the esomeprazole enteric-coated capsules treatment group, and 4/27 patients (14.8%) in the esomeprazole magnesium positive control group (Table 2). There was no difference between the two groups (P = 1.0000). The reported adverse events during the trial were minor and did not require treatment interruption. There were no clinically relevant changes in blood pressure, heart rate or laboratory values during the study.
Table 2.
Adverse events during 4-wk therapy
| Adverse event | Esomeprazole enteric-coated capsules (n = 30) | Esomeprazole magnesium (n = 27) |
| Dizziness | 2 | 2 |
| Diarrhea | 1 | 0 |
| Constipation | 0 | 1 |
| Face puffiness | 1 | 0 |
| Other | 1 (cough) | 1 (palpitation) |
| Total | 5 | 4 |
Of the 3 patients who withdrew from this study, one patient moved to another city because of a change in work place, and the other two patients rejected the gastroscopic operation because of complete symptom relief.
DISCUSSION
There are stereoselective differences in the metabolism of PPIs by the cytochrome P450 (CYP) isoenzymes 2C19 and 3A4, and this is the basis of the observed pharmacodynamic and clinical efficacy differences between esomeprazole and omeprazole[12–15]. A study in which these enzymes were expressed from cDNAs suggested that CYP2C19 is responsible for 40% and 87% of the total intrinsic clearance of S- and R-omeprazole, respectively[16], indicating esomeprazole would be cleared more slowly in vivo[16,17]. Several pharmacological studies using intragastric pH monitoring conducted in either healthy subjects or GERD patients have consistently established the superiority of standard dose esomeprazole over all other currently available standard PPI regimens[18–22]. Recently, another S-isomer of pantoprazole has been used to investigate the efficacy in the treatment of acid-related disease; it has shown better efficacy in the control of GERD symptoms than its racemic mixture of pantoprazole[23].
Miner et al[9,10] demonstrated that, in a five-way crossover study, oral esomeprazole (40 mg) increased intragastric pH more rapidly and maintained intragastric pH above 4.0 longer than lansoprazole (30 mg), omeprazole (20 mg), pantoprazole (40 mg) and rabeprazole (20 mg) did in 34 H pylori-negative patients with symptoms of gastroesophageal reflux disease. In addition, a recent study showed that esomeprazole (20 mg) was more effective at maintaining gastric pH above 4 for longer than lansoprazole (15 mg), pantoprazole (20 mg) and rabeprazole (10 mg)[24].
Two randomized multicenter trials[25,26] which used esomeprazole to treat DUs demonstrated that in H pylori-positive patients with duodenal ulcer, 1 wk of esomeprazole (20 mg twice daily) triple therapy followed by placebo for 3 wk provides the same effective ulcer healing, H pylori eradication and symptom control when compared with 1 wk of omeprazole (20 mg twice daily) triple therapy followed by a 3-wk period of omeprazole monotherapy (20 mg once daily). The authors concluded that 1 wk of esomeprazole-based triple therapy is sufficient to ensure high rates of ulcer healing without the need for follow-on PPI monotherapy in patients with uncomplicated duodenal ulcer disease. Besides, in GERD patients, esomeprazole demonstrated significantly higher healing rates at 4 and 8 wk than other standard dose PPIs, and the magnitude of the benefit that esomeprazole offers increases with the severity of the underlying reflux esophagitis[11].
The present study investigated the efficacy and safety of esomeprazole enteric-coated capsules (synthesized by Chongqing Lummy Pharmacy, China) in the treatment of active duodenal ulcer disease, with esomeprazole magnesium (Nexium, AstraZeneca Inc)-treated patients used as a positive control group. Patients with active duodenal ulcers received esomeprazole (40 mg) once daily for four wk in both groups. At the end of second wk, duodenal ulcer healing was 86.7% in the treatment group and 85.2% in the positive control group (P = 0.8410), and at the end of fourth wk duodenal ulcer healing was 100% in both groups (Figure 1). In the improvement of gastrointestinal symptoms caused by active duodenal ulcer, the esomeprazole enteric-coated capsules treatment group showed 90.8% relief, which was greater than that of the esomeprazole magnesium positive control group (86.7%, P = 0.5406) at the end of the second wk; at the end of the fourth wk, 95.2% and 93.2% symptom relief was seen in the esomeprazole enteric-coated capsules treatment group and the esomeprazole magnesium positive control group, respectively (P = 0.5786).
Since the first PPI, omeprazole, was launched on the market in 1988, it has been widely used to treat acid-related disorders and has demonstrated good efficacy and safety. Treatment with omeprazole over the mean period of 6.5 years causes histologic changes in the stomach which can be detected only by biopsy, but with no specific symptoms[27]. Meanwhile, esomeprazole, the S-isomer of omeprazole, which had increased plasma concentrations and better clinical efficacy than omeprazole, should not be associated with any increase in unwanted effects. In studies involving large numbers of patients, the adverse event rates of esomeprazole have been proven to be similar to those recorded for omeprazole and placebo[28,29]. Our data showed the adverse event rates were 16.7% in the esomeprazole enteric-coated capsules treatment group and 14.8% in the positive control group (P = 1.0000). The results of the current study are consistent with the findings of Richter et al[30], who showed that in the 8-wk treatment of reflux esophagitis, the adverse event rates were 15.3% in the esomeprazole group and 15.1% in the omeprazole group. However, the adverse event rate was much lower in the study by Maton et al[28]. In that study the adverse event rate of esomeprazole was about 3% compared with placebo, in 12-mo treatment for reflux esophagitis. The difference between these studies could be due to variations in therapy duration. Furthermore, in the current study, 3 patients in the esomeprazole magnesium group withdrew from this study, but none of these discontinuations were related to adverse events.
In conclusion, the results of this study indicate treatment with esomeprazole enteric-coated capsules (40 mg) is equivalent to treatment with esomeprazole magnesium (40 mg) in the healing of active duodenal ulcers and improving gastrointestinal symptoms, and that these treatments have similar safety and tolerability profiles.
COMMENTS
Background
Proton pump inhibitors (PPI) were introduced in the late 1980s and have emerged as the drug class of choice for the treatment of most acid-related disorders. Omeprazole was the first PPI on the market, followed by lansoprazole, rabeprazole, pantoprazole and esomeprazole, in that order. Conversely from the other available PPIs, which are all racemic mixtures, esomeprazole, the S-isomer of omeprazole, is the first PPI to be developed as a single isomer and demonstrates pharmacological and clinical benefits beyond those seen with racemic PPIs.
Research frontiers
Studies have shown esomeprazole at a standard dose of 40 mg once daily achieves greater and more sustained acid control than standard doses of any other currently available PPI, with good safety.
Innovations and breakthroughs
This study was designed to evaluate the efficacy and tolerability of two different preparations of esomeprazole, esomeprazole enteric-coated capsules and esomeprazole magnesium, in the healing of active duodenal ulcers.
Applications
This study indicates treatment with esomeprazole enteric-coated capsules is equivalent to treatment with esomeprazole magnesium in healing duodenal ulcer lesions and relieving gastrointestinal symptoms, and could be an alternative in the treatment of acid-related diseases.
Terminology
Isomers are compounds which have the same molecular formula but different chemical structures. Depending on the types of differences there are between the structures, it is possible to classify isomers into various sub-types. Stereo-isomers contain the same functional groups and differ only in the arrangement of atoms in space.
Peer review
The study is performed well and carefully written.
Peer reviewer: Shingo Tsuji, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine (A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
S- Editor Ma L L- Editor McGowan D E- Editor Liu Y
References
- 1.Kendall MJ. Review article: esomeprazole--the first proton pump inhibitor to be developed as an isomer. Aliment Pharmacol Ther. 2003;17 Suppl 1:1–4. doi: 10.1046/j.1365-2036.17.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 2.Niazi M, Ahlbom H, Bondarov P, Karlsson AH, Hassan-Alin M, Rydholm H, Rohss K. Pharmacokinetics of esomeprazole following varying intravenous administration rates. Basic Clin Pharmacol Toxicol. 2005;97:351–354. doi: 10.1111/j.1742-7843.2005.pto_150.x. [DOI] [PubMed] [Google Scholar]
- 3.Dent J. Review article: pharmacology of esomeprazole and comparisons with omeprazole. Aliment Pharmacol Ther. 2003;17 Suppl 1:5–9. doi: 10.1046/j.1365-2036.17.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY, Lu CL, Luo JC, Chang FY, Lee SD, Lai YL. Esomeprazole tablet vs omeprazole capsule in treating erosive esophagitis. World J Gastroenterol. 2005;11:3112–3117. doi: 10.3748/wjg.v11.i20.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fock KM, Ang TL, Bee LC, Lee EJ. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet. 2008;47:1–6. doi: 10.2165/00003088-200847010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Scott LJ, Dunn CJ, Mallarkey G, Sharpe M. Esomeprazole: a review of its use in the management of acid-related disorders. Drugs. 2002;62:1503–1538. doi: 10.2165/00003495-200262100-00006. [DOI] [PubMed] [Google Scholar]
- 7.Andersson T, Hassan-Alin M, Hasselgren G, Rohss K. Drug interaction studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet. 2001;40:523–537. doi: 10.2165/00003088-200140070-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hassan-Alin M, Andersson T, Niazi M, Rohss K. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S-omeprazole (esomeprazole) and R-omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60:779–784. doi: 10.1007/s00228-004-0841-1. [DOI] [PubMed] [Google Scholar]
- 9.Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–2620. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 10.Miner P Jr, Katz PO, Chen Y, Sostek M. Reanalysis of intragastric pH results based on updated correction factors for Slimline and Zinetics 24 single-use pH catheters. Am J Gastroenterol. 2006;101:404–405; author reply 405-406. doi: 10.1111/j.1572-0241.2006.00401_3.x. [DOI] [PubMed] [Google Scholar]
- 11.Edwards SJ, Lind T, Lundell L. Systematic review: proton pump inhibitors (PPIs) for the healing of reflux oesophagitis - a comparison of esomeprazole with other PPIs. Aliment Pharmacol Ther. 2006;24:743–750. doi: 10.1111/j.1365-2036.2006.03074.x. [DOI] [PubMed] [Google Scholar]
- 12.Andersson T, Hassan-Alin M, Hasselgren G, Rohss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet. 2001;40:411–426. doi: 10.2165/00003088-200140060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Isaza C, Henao J, Martinez JH, Arias JC, Beltran L. Phenotype-genotype analysis of CYP2C19 in Colombian mestizo individuals. BMC Clin Pharmacol. 2007;7:6. doi: 10.1186/1472-6904-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006;29:769–784. doi: 10.2165/00002018-200629090-00002. [DOI] [PubMed] [Google Scholar]
- 15.Furuta T, Sugimoto M, Shirai N, Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics. 2007;8:1199–1210. doi: 10.2217/14622416.8.9.1199. [DOI] [PubMed] [Google Scholar]
- 16.Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28:966–972. [PubMed] [Google Scholar]
- 17.Ishizawa Y, Yasui-Furukori N, Takahata T, Sasaki M, Tateishi T. The effect of aging on the relationship between the cytochrome P450 2C19 genotype and omeprazole pharmacokinetics. Clin Pharmacokinet. 2005;44:1179–1189. doi: 10.2165/00003088-200544110-00005. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann D, Eickhoff A, Damian U, Riemann JF, Schilling D. Effect of intravenous application of esomeprazole 40 mg versus pantoprazole 40 mg on 24-hour intragastric pH in healthy adults. Eur J Gastroenterol Hepatol. 2007;19:133–137. doi: 10.1097/01.meg.0000252628.57925.32. [DOI] [PubMed] [Google Scholar]
- 19.Rohss K, Lind T, Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur J Clin Pharmacol. 2004;60:531–539. doi: 10.1007/s00228-004-0804-6. [DOI] [PubMed] [Google Scholar]
- 20.Warrington S, Baisley K, Dunn K, Boyce M, Morocutti A. Effects of single doses of rabeprazole 20 mg and esomeprazole 40 mg on 24-h intragastric pH in healthy subjects. Eur J Clin Pharmacol. 2006;62:685–691. doi: 10.1007/s00228-006-0148-5. [DOI] [PubMed] [Google Scholar]
- 21.Norris V, Baisley K, Dunn K, Warrington S, Morocutti A. Combined analysis of three crossover clinical pharmacology studies of effects of rabeprazole and esomeprazole on 24-h intragastric pH in healthy volunteers. Aliment Pharmacol Ther. 2007;25:501–510. doi: 10.1111/j.1365-2036.2006.03221.x. [DOI] [PubMed] [Google Scholar]
- 22.Hatlebakk JG. Review article: gastric acidity--comparison of esomeprazole with other proton pump inhibitors. Aliment Pharmacol Ther. 2003;17 Suppl 1:10–15; discussion 16-17. doi: 10.1046/j.1365-2036.17.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 23.Pai VG, Pai NV, Thacker HP, Shinde JK, Mandora VP, Erram SS. Comparative clinical trial of S-pantoprazole versus racemic pantoprazole in the treatment of gastro-esophageal reflux disease. World J Gastroenterol. 2006;12:6017–6020. doi: 10.3748/wjg.v12.i37.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röhss K, Wilder-Smith C, Nauclér E, Jansson L. Esomeprazole 20mg provides more effective intragastric Acid control than maintenance-dose rabeprazole, lansoprazole or pantoprazole in healthy volunteers. Clin Drug Investig. 2004;24(1):1–7. doi: 10.2165/00044011-200424010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Tulassay Z, Kryszewski A, Dite P, Kleczkowski D, Rudzinski J, Bartuzi Z, Hasselgren G, Larko A, Wrangstadh M. One week of treatment with esomeprazole-based triple therapy eradicates Helicobacter pylori and heals patients with duodenal ulcer disease. Eur J Gastroenterol Hepatol. 2001;13:1457–1465. doi: 10.1097/00042737-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Subei IM, Cardona HJ, Bachelet E, Useche E, Arigbabu A, Hammour AA, Miller T. One week of esomeprazole triple therapy vs 1 week of omeprazole triple therapy plus 3 weeks of omeprazole for duodenal ulcer healding in Helicobacter pylori-positive patients. Dig Dis Sci. 2007;52:1505–1512. doi: 10.1007/s10620-006-9522-5. [DOI] [PubMed] [Google Scholar]
- 27.Klinkenberg-Knol EC, Nelis F, Dent J, Snel P, Mitchell B, Prichard P, Lloyd D, Havu N, Frame MH, Roman J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661–669. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 28.Maton PN, Vakil NB, Levine JG, Hwang C, Skammer W, Lundborg P. Safety and efficacy of long term esomeprazole therapy in patients with healed erosive oesophagitis. Drug Saf. 2001;24:625–635. doi: 10.2165/00002018-200124080-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hritz I, Herszenyi L, Molnar B, Tulassay Z, Pronai L. Long-term omeprazole and esomeprazole treatment does not significantly increase gastric epithelial cell proliferation and epithelial growth factor receptor expression and has no effect on apoptosis and p53 expression. World J Gastroenterol. 2005;11:4721–4726. doi: 10.3748/wjg.v11.i30.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter JE, Kahrilas PJ, Johanson J, Maton P, Breiter JR, Hwang C, Marino V, Hamelin B, Levine JG. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol. 2001;96:656–665. doi: 10.1111/j.1572-0241.2001.3600_b.x. [DOI] [PubMed] [Google Scholar]


