Abstract
Background
Experiencing emotions engages high-order orbitofrontal and medial prefrontal areas, and expressing emotions involves low-level autonomic structures and peripheral organs. How is information from the cortex transmitted to the periphery? We used two parallel approaches to map simultaneously multiple pathways to determine if hypothalamic autonomic centres are a key link for orbitofrontal areas and medial prefrontal areas, which have been associated with emotional processes, as well as low-level spinal and brainstem autonomic structures. The latter innervate peripheral autonomic organs, whose activity is markedly increased during emotional arousal.
Results
We first determined if pathways linking the orbitofrontal cortex with the hypothalamus overlapped with projection neurons directed to the intermediolateral column of the spinal cord, with the aid of neural tracers injected in these disparate structures. We found that axons from orbitofrontal and medial prefrontal cortices converged in the hypothalamus with neurons projecting to brainstem and spinal autonomic centers, linking the highest with the lowest levels of the neuraxis. Using a parallel approach, we injected bidirectional tracers in the lateral hypothalamic area, an autonomic center, to label simultaneously cortical pathways leading to the hypothalamus, as well as hypothalamic axons projecting to low-level brainstem and spinal autonomic centers. We found densely distributed projection neurons in medial prefrontal and orbitofrontal cortices leading to the hypothalamus, as well as hypothalamic axonal terminations in several brainstem structures and the intermediolateral column of the spinal cord, which innervate peripheral autonomic organs. We then provided direct evidence that axons from medial prefrontal cortex synapse with hypothalamic neurons, terminating as large boutons, comparable in size to the highly efficient thalamocortical system. The interlinked orbitofrontal, medial prefrontal areas and hypothalamic autonomic centers were also connected with the amygdala.
Conclusions
Descending pathways from orbitofrontal and medial prefrontal cortices, which are also linked with the amygdala, provide the means for speedy influence of the prefrontal cortex on the autonomic system, in processes underlying appreciation and expression of emotions.
Background
Neural processing of emotions engages diverse structures from the highest to the lowest levels of the neuraxis. On one hand, high-order association areas are necessary to understand the significance of an emotional situation, and on the other hand, low level structures must be activated to express the emotion through changes in the rhythm of peripheral organs. The orbitofrontal cortex participates in both of these processes (reviewed in [1,2]) and when damaged, patients lack emotional propriety, and do not show changes in heart rate and skin responses that normally accompany emotional arousal [3,4]. A related medial prefrontal area, in the anterior cingulate, has a role in emotions as well, specializing in the expression of emotions through pathways to autonomic structures in primates [5,6] as well as in rats (e.g., [7-12]). Both prefrontal regions are connected with the amygdala, a structure with a key role in emotions (reviewed in [13-15]).
The existence of diverse pathways that underlie emotional processing through the prefrontal cortex in primates has been described in piecemeal fashion in separate studies (for reviews see [15-17]). It is not clear if pathways from the prefrontal cortex to autonomic structures are circuitous or relatively direct. Another open question is whether previously described pathways from prefrontal areas synapse in autonomic structures [5,6], or merely pass through. This information is critical, because key autonomic structures in the hypothalamus and brainstem are major thoroughfares for numerous and unrelated pathways (for review see [18]). Here we provide direct evidence that pathways synapsing in the hypothalamus link high-level prefrontal association cortex with low-level autonomic structures. These serial pathways may allow direct cortical control of autonomic functions in response to complex emotional situations.
Results
Serial pathways link prefrontal cortices with hypothalamic, spinal, and brainstem autonomic centers
We first investigated if pathways that can transmit information from prefrontal cortex to autonomic centers overlap in the hypothalamus with pathways that innervate the lowest autonomic level in the intermediolateral column of the spinal cord. We addressed this question by placing a bidirectional tracer in orbitofrontal area 12 to investigate if axons from this area terminate in the hypothalamus (Fig. 1A). In the same animal, we placed a different retrograde tracer in the intermediolateral column of the thoracic spinal cord (Fig. 1B) to determine if neurons in the hypothalamus project to this spinal autonomic region (Table 1, case AV). This approach indicated that the two pathways overlap in the hypothalamus, demonstrated by intermixing of axonal terminations from the prefrontal pathway, and labeled projection neurons giving rise to the lower pathway leading to the spinal cord. Specifically, the pathways overlapped in several hypothalamic centers that are involved in autonomic control, including the dorsal hypothalamic area and tuberomammillary nucleus (Fig. 1C, DA, TM), the perifornical nucleus (Fig. 1D, Pef), and the fields of Forel (Fig. 1E, FF). Axons originating in prefrontal area 12 also reached the lateral hypothalamic area (LA), anterior hypothalamic area, and posterior hypothalamic area. In the same experiment we found that prefrontal area 12 received projections from the basolateral (BL) and lateral (L) nuclei of the amygdala (Fig. 1F, red dots).
Figure 1.

Overlap in hypothalamus of serial pathways from prefrontal area 12 and the intermediolateral spinal column. (A) The bidirectional tracer fluororuby (fr) was injected in prefrontal area 12 (red area). (B) The injection of the retrograde tracer fast blue (fb) was in the spinal cord, covering the intermediolateral cell column, the lowest central autonomic center (blue area). Fast blue labeled neurons projecting to the spinal cord (blue dots) and labeled axons (brown lines) originating in area 12 were intermingled in the following hypothalamic areas: (C) dorsal hypothalamic area (DA) and tuberomammillary nucleus (TM); (D) perifornical nucleus (Pef); (E) fields of Forel (FF). (F) In the same case, labeled neurons in the basolateral (BL) and lateral (L) nuclei of the amygdala projected to area 12 (red dots).
Table 1.
Summary of cases, injection sites and hemisphere, tracers, and analyses
| Case | Injection site(s), (hemisphere) | Tracer | Label type* #, areas analysed |
| AV | O12, IML (left) | fr, fb | hypothalamus* (fr); # (fb), amygdala# (fr); |
| AW | LA (right) | fb | prefrontal cortex#; amygdala# |
| AX | LA (left) | BDA | prefrontal cortex#,*; amygdala#,*; brainstem, IML* |
| AY | 32 (right) | BDA | LA*; synapses from area 32 in LA (EM) |
| BG | 32 (right) | BDA | PA*; synapses from area 32 in PA (EM) |
*Anterograde label; #retrograde label
We then used a different approach to obtain an overview of the origin and relative strength of serial pathways leading from the prefrontal cortex to the hypothalamus, and from the hypothalamus to autonomic regions in the brainstem, in addition to the spinal autonomic center, demonstrated above. We addressed this question by placing a bidirectional tracer in the lateral hypothalamic area (Fig. 2B; Table 1, case AX), which has robust and bidirectional connections with prefrontal cortices [5]. We then mapped projection neurons which originated most densely in posterior orbitofrontal (areas 13, 25, O12) and medial (areas 24, 32, 14) prefrontal areas, leading to the hypothalamus (Fig. 2A). In turn, axons from the same hypothalamic area terminated in the intermediolateral column (IML) of the spinal cord (Fig. 2E), and in several brainstem sites (Fig. 2C,2D), including the reticular formation (RF), the parabrachial nucleus (nPB), the raphe nuclei (nRph), the periaqueductal gray (PAG), all of which participate in autonomic control (for reviews see [17,18]). In addition, the amygdala issued projections to the same hypothalamic site, revealed by labeled projection neurons in its basal complex, and in the medial, central, and cortical nuclei (Fig. 2F). We confirmed these findings in another case by placing a retrograde tracer in the lateral hypothalamic area (Fig. 3C; Table 1, case AW). We mapped projection neurons originating densely from the medial sector of area 25, area 32, the caudal orbitofrontal cortex (areas OPAll, OPro), and in moderate numbers from areas 24, 14, 13, 11 and 12 (Fig. 3A,3B) As in the previous case, we found projection neurons in the amygdala (Fig. 3D). Double-labeling experiments indicated that a subpopulation of these projection neurons in the amygdala were positive for the calcium binding protein calbindin, but not parvalbumin, which label distinct classes of inhibitory interneurons in the amygdala (e.g., [19,20]). We found double-labeled, and presumably inhibitory projection neurons, mostly in the central and medial nuclei of the amygdala (not shown), confirming and extending previous findings [21,22], but not in the basolateral nucleus (BL), which projects to the hypothalamus as well (Figs. 2F; 3D).
Figure 2.

Serial pathways from the prefrontal cortex reach central autonomic structures. Pathways were mapped after injection of the bidirectional tracer BDA in the lateral (LA) hypothalamic area. (A) The first pathway is marked by projection neurons (blue dots) originating most densely from orbitofrontal and medial prefrontal cortices leading to the injection site (brown area in B). (B) The second pathway is marked by labelled axons emanating from the injection site and terminating in several autonomic nuclei (brown lines): (C, D) brainstem nuclei; (E) the thoracic spinal cord; (F) A bidirectional pathway links the amygdala with the same hypothalamic nuclei. The shaded areas in the amygdala show the specific termination zones of axons from orbitofrontal cortex (yellow), and the diffuse termination zone by axons from medial prefrontal cortex (light brown), as described by Ghashghaei and Barbas [24]. The dotted line in A indicates the upper border of cortical layer V, and brown lines show the terminations of hypothalamic axons.
Figure 3.
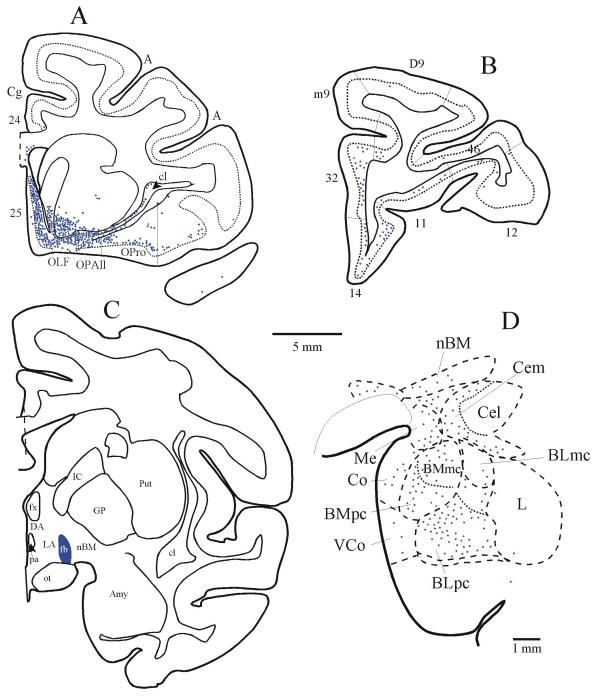
Simultaneous labelling of pathways from orbitofrontal cortex and the amygdala to the lateral hypothalamic area. (A) Densely distributed projection neurons in the posterior orbitofrontal cortex (areas OPAll, OPro), and in medial area 25 projected to the lateral hypothalamic area (LA in C). (B) Projection neurons in prefrontal areas 32 and 14 and in other prefrontal areas directed to LA; (C) The injection site of fast blue (fb) was in LA of the hypothalamus. (D) Projection neurons from the amygdala directed to the same area (LA) of the hypothalamus. The dotted line in A and B indicates the upper border of cortical layer V.
The connections of prefrontal cortices with the lateral hypothalamic area were bidirectional and showed a specific laminar distribution. Projection neurons in prefrontal cortices originated mostly from the deep layers (V and VI; Fig. 2A; 3A,3B). Hypothalamic efferents reached all areas of the prefrontal cortex, terminating most densely in layer I, followed by the deep layers (V-VI), and then layers II and the upper part of layer III (Fig. 2A). Only a small number of hypothalamic fibres terminated in layer IV (7–10%), and this pattern was seen only in orbitofrontal area OPro, area 32, and to a lesser extent in area 24.
Pathways from medial area 32 synapse in hypothalamic autonomic centers
The independent observations from the two approaches suggest that descending pathways from the prefrontal cortex to the hypothalamus, and from the hypothalamus to the brainstem and the spinal cord are serially connected. However, because numerous pathways pass through the hypothalamus, it was necessary to determine if labeled axons from the prefrontal cortex synapse there, or simply pass through. We addressed this question at the electron microscopic level by investigating if axons from prefrontal area 32 synapse in hypothalamic autonomic centers (Table 1, case AY). We focused on area 32 because we previously found that it is heavily and bidirectionally connected with the amygdala [23,24], and issues robust projections to hypothalamic autonomic areas, including the lateral hypothalamic area, the dorsal hypothalamic area, the posterior hypothalamic area, the perifornical nucleus, the paramammillary nucleus, the supramammillary nucleus and the tuberomammillary nucleus [5]. Consistent with previous findings in macaque monkeys [5,6,25], projections from area 32 did not extend to the endocrine-related paraventricular nucleus of the hypothalamus (for review see [26]), a pathway described in rats (e.g., [27]).
For analysis at the ultrastructural level we focused on synapses formed between axonal boutons from area 32 in the lateral hypothalamic area (LA). We found many synaptic interactions between boutons from axons emanating from area 32 and neuronal elements in the lateral hypothalamic area (Fig. 4), and all synapses were asymmetric, suggesting that they are excitatory (n = 143). Boutons from medial area 32 synapsed with spines (55%; Fig. 4A) and dendrites (45%; Fig. 4B) of the lateral hypothalamic area. The distribution of labeled synapses differed significantly from a randomly selected population of unlabeled synapses in the region (n = 112), most of which involved dendrites (79%), while a smaller proportion (21%) involved spines (X2, P << 0.00001). Moreover, there were significant differences in the synaptic features of a sample of labeled (n = 108), in comparison with unlabeled synapses (n = 75). The average area of profiles of labeled boutons (0.701 ± 0.003 μm2) was higher than the unlabeled (0.50 ± 0.03 μm2; P < 0.0001), as was the average length of the synaptic density of labeled terminals (0.592 ± 0.015 μm), in comparison with unlabeled terminals (0.382 ± 0.014 μm; P < 0.0001). These findings indicate that prefrontal axons target a specific population of postsynaptic elements.
Figure 4.
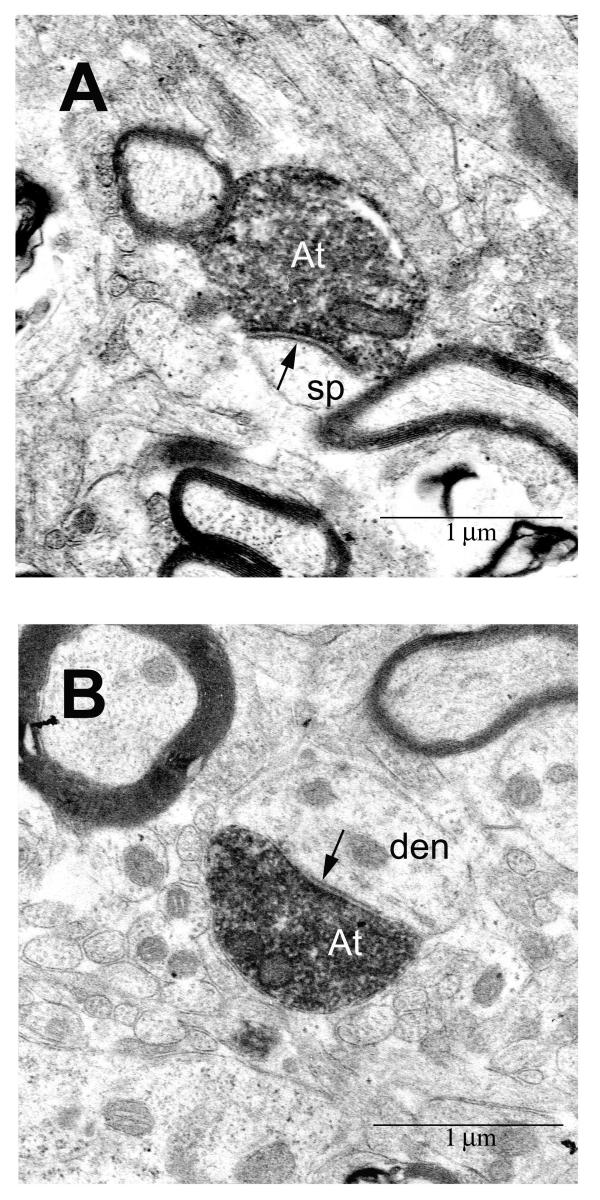
Axons from prefrontal area 32 synapse in the lateral hypothalamic area. Axon terminals (At) were identified by BDA label injected in area 32. (A) synapse (arrow) with dendritic spine (sp); (B) synapse (arrow) with dendrite (den). Scale bar = 1 μm.
We confirmed and extended the above data in another case, by demonstrating synaptic interactions between boutons from prefrontal area 32 and postsynaptic elements of another area engaged in autonomic control, the posterior hypothalamic area (Table 1, case BG). Double-labeling for GABA revealed that while a significant proportion of hypothalamic elements were positive for GABA, none formed synapses with boutons from the prefrontal cortex.
Discussion
Combining two independent approaches to label multiple pathways simultaneously, we have demonstrated that the prefrontal cortex has relatively direct access to central autonomic structures. This evidence suggests that high-level prefrontal association areas in the posterior medial and orbitofrontal cortex can rapidly influence autonomic organs in complex emotional situations. This influence reaches as far as the spinal cord, the final central autonomic site, now demonstrated in a primate species. The present data do not directly address whether prefrontal axons terminate directly on projection neurons that innervate spinal or brainstem autonomic centers. However, in view of the massive projections from medial prefrontal cortices to hypothalamic autonomic centers [5], their strong synaptic interactions with presumptive excitatory neurons demonstrated here, and the robust projections leading from the hypothalamus to brainstem autonomic centers (e.g., [28]), it is highly likely that the serial pathways are relatively direct.
Although presumptive support for some of these pathways was provided previously [5,6,25], the possibility was not ruled out that prefrontal axons merely pass through autonomic structures, as do many other pathways [18]. The present study provided compelling evidence that prefrontal axons synapse with autonomic structures in the hypothalamus, terminating as large boutons, comparable in size to the highly efficient thalamocortical system [29]. The serial pathways mapped simultaneously provide the means for orbitofrontal and medial prefrontal cortices to influence the expression of emotions. Orbitofrontal and medial prefrontal cortices are in a strategic position to influence the expression of emotions through their robust and bidirectional connections with the amygdala (e.g., [23-25],[30-36]).
Orbitofrontal and medial prefrontal cortices show some key differences in their connections, suggesting that they may have complementary roles in emotions, as summarized in Figure 5. Orbitofrontal areas share with the amygdala a panoramic view of the entire sensory periphery, through robust connections with cortical areas representing every sensory modality (for reviews see [37,38]). Moreover, the same sites in the amygdala that receive sensory input from the cortex also innervate the orbitofrontal cortex [24,39]. This remarkably specific network provides the orbitofrontal cortex with dual access to the sensory environment: directly from sensory cortices (Fig. 5, pathway so), and indirectly from the amygdala [37] (pathways s and s'). The massive indirect pathway through the amygdala may allow the orbitofrontal cortex to extract the emotional significance of events [37]. This interpretation is supported by evidence that neurons in the orbitofrontal cortex and the amygdala respond to sensory stimuli only when they are significant for behavior, and cease to respond when the stimuli lose their motivational value [40-42].
Figure 5.

Summary of pathways linking prefrontal cortex with structures associated with perception and expression of emotions. Line thickness represents the density of projections. Direct and indirect sensory input to the orbitofrontal cortex: The orbitofrontal cortex and the amygdala receive projections from every sensory modality through the cortex (pathways so, s), and are robustly linked with each other, providing the structural basis for direct (so), and indirect (s and s') sensory input to the orbitofrontal cortex. The orbitofrontal cortex disinhibits hypothalamic autonomic centers: Orbitofrontal axons terminate heavily in the intercalated masses of the amygdala (IM, pathway a), which project to the central nucleus (a'), which projects to hypothalamic autonomic centers (pathway b). Activation of pathways (a, a') leads to disinhibition of hypothalamic autonomic centers, which innervate brainstem and spinal autonomic centers (pathways c', o'). Direct and indirect pathways from medial prefrontal areas to hypothalamic autonomic centers: The direct pathway courses from medial prefrontal cortex to hypothalamic autonomic centers (c), forming asymmetric (and presumed excitatory) synapses in the lateral and posterior hypothalamic areas. The indirect pathway courses from medial prefrontal cortices to the parvicellular sector of the basolateral nucleus of the amygdala (BLpc, pathway d), which projects to hypothalamic autonomic centers (d'), and is presumed to be excitatory. Activation of the direct or indirect pathways ultimately activates brainstem and spinal autonomic nuclei (pathways c, c', o'), which innervate peripheral organs. Red, inhibitory pathways; green, excitatory pathways.
The pathways from orbitofrontal cortex to the amygdala are equally robust and specific, terminating heavily on presumed inhibitory neurons in the intercalated masses [24] (Fig. 5, pathway a), which innervate the central nucleus of the amygdala [43] (pathway a'). In turn, the central nucleus innervates and inhibits hypothalamic and brainstem autonomic structures [21,22] (pathway b). The specific projection of the orbitofrontal cortex on the intercalated masses can essentially quiet the activity of the central nucleus of the amygdala, and allow the hypothalamus and brainstem to be activated in emotional situations (Fig. 5). This evidence suggests that joint responses of the orbitofrontal cortex and the amygdala could effectively signal emotional value of the environment to the autonomic nervous system.
The medial prefrontal cortex differs from the orbitofrontal by its comparatively sparse connections with sensory areas [44], stronger projections to hypothalamic autonomic centers [5], and widespread and diffuse innervation of several nuclei of the amygdala [24], which project to the hypothalamus (for review see [45]; Fig. 2). Therefore, the medial prefrontal cortex has dual access to the emotional motor system: through a direct pathway to hypothalamic and brainstem autonomic centers [5,6,46] (pathway c), and indirectly through the amygdala [24] (pathways d, d1 and d') and the extended amygdala [47]. The direct pathway from medial area 32 avoids inhibitory interneurons, and forms asymmetric, and presumed excitatory synapses, targeting preferentially spines in the hypothalamus, which are enriched on dendrites of excitatory neurons [48]. A significant component of the second part of the indirect pathway courses from the basolateral nucleus to hypothalamic autonomic centers (pathway d'). This pathway, which also appears to have excitatory bias, has a role in the process of learning the significance of motivationally relevant cues [49]. If proved to be excitatory in functional studies, then the medial prefrontal cortex may amplify the output of the basolateral nucleus to the hypothalamus (pathway d'), while also exerting a direct excitatory influence on the hypothalamus through the direct pathway to the hypothalamus (Fig. 5, pathway c). This evidence is consistent with the designation of medial prefrontal cortex as the emotional motor system [50,51].
Appreciation of emotions is a complex and conscious process that must involve the cortex [52], as the amygdala is activated even when humans are unaware of having 'seen' masked pictures of fearful faces [53]. Instead, the amygdala may be wired for vigilance and fast reaction when danger lurks, through thalamic pathways that bypass the cortex [54]. Repetitive activation of the remarkably specific and bidirectional pathways linking the amygdala with the orbitofrontal cortex [24] may be necessary for conscious appreciation of the emotional significance of events. Orbitofrontal and medial prefrontal cortices are connected with each other, as well as with lateral prefrontal cortices, which are implicated in cognitive functions [55]. Acting in concert, the prefrontal cortex likely can evaluate emotional events and influence rapidly the lowest levels of the autonomic nervous system for emotional expression.
Conclusions
We demonstrated that serial pathways link relatively directly prefrontal cortices with efferent autonomic structures. Thus, medial prefrontal and orbitofrontal cortices, which are associated with appreciation of emotions, project to hypothalamic autonomic centers, which innervate brainstem and spinal autonomic structures. The latter, in turn, innervate peripheral organs whose activity is markedly increased in emotional arousal. The pathways are robust, and at least the medial pathway from area 32 forms excitatory connections in the hypothalamus, which are comparable in size to the highly efficient thalamocortical pathways. Disruption of the prefrontal-autonomic circuits will likely have widespread repercussions in behavior. The hypothalamic sites innervated by prefrontal pathways project onto neurochemically specific structures in the brainstem [56], which broadcast widely to the cortex (e.g., [57]; for review see [58]). In the prefrontal cortex normal function is critically dependent on a delicate balance of transmission by dopaminergic and adrenergic fibers [59]. Damage to orbital and medial prefrontal areas disconnects the prefrontal cortex from the amygdala, from central autonomic structures, and from lateral prefrontal areas associated with executive functions. Such disconnection may help explain why patients with orbitofrontal lesions have inappropriate affect, lack emotional responsiveness, and make poor decisions (for review see [2]). At the opposite extreme, psychiatric conditions characterized by chronic anxiety, such as obsessive-compulsive disorder, activate excessively the orbitofrontal cortex [60], and by extension may influence the function and integrity of autonomic structures and peripheral systems, such as the heart.
List of abbreviations
A, arcuate sulcus; Amy, amygdala; At, axon terminal; BDA, biotinylated dextran amine; BL, basolateral nucleus of the amygdala (mc, magnocellular division; pc, parvicellular division); BM, basomedial nucleus of the amygdala (also known as accessory basal); Ce, central nucleus of the amygdala; Cel, central nucleus of the amygdala, lateral division; Cem, central nucleus of the amygdala, medial division; Cg, cingulate sulcus; cl, claustrum; Co, cortical nucleus of the amygdala; cp, cerebral peduncle; DA, dorsal hypothalamic area; den, dendrite; EM, electron microscopy; fb, fast blue; FF, fields of Forel; fr, fluororuby; fx, fornix; GP, globus pallidus; ic, internal capsule; IM, intercalated masses; IML, intermediolateral column of the spinal cord; L, lateral nucleus of the amygdala; LA, lateral hypothalamic area; Me, medial nucleus of the amygdala; mt, mammillothalamic tract; nBM, nucleus basalis of Meynert; nPB, parabrachial nucleus; nRph, nucleus raphe; nT, nucleus tractus solitarii; ot, optic tract; PA, posterior hypothalamic area; PAG, periaqueductal grey; Pef, perifornical nucleus of the hypothalamus; PLBL, paralamellar basolateral nucleus of the amygdala; Put, putamen; RF, reticular formation; SN, substantia nigra; sp, spine; STh, subthalamic nucleus; T6, spinal thoracic level 6; TM, tuberomammilllary nucleus of the hypothalamus; VCo, ventral cortical nucleus of the amygdala; Vm, ventromedial nucleus of the hypothalamus.
Methods
Surgery
Experiments were conducted on 5 rhesus monkeys (Macaca mulatta) according to the NIH guide for the Care and Use of Laboratory Animals (NIH pub. 86–23, revised 1996). Experimental protocols were approved by the IACUC at Boston Univ. Sch. of Medicine, Harvard Medical School and New England Primate Research Center. Surgery was performed under general gas anesthesia (isoflurane) and sterile conditions for injection of neural tracers, as described previously [39]. We determined the stereotaxic coordinates of cortical area 32 and the lateral hypothalamic area, an autonomic center, from magnetic resonance images (MRI), using as reference hollow ear bars filled with betadine salve, which is visible in MRI.
In one animal we injected fluororuby (dextran tetramethylrhodamine, MW 3000, Molecular Probes, cat. # D3308, Eugene, OR) in prefrontal area 12, and fast blue (Sigma, cat. # F5756, St. Louis, MO) in the intermediolateral column at the upper thoracic region (T5-T6) to label in the hypothalamus axonal terminations emanating from area 12 and projection neurons in the hypothalamus directed to the spinal cord (Table 1, case AV). In two other animals we injected the retrograde tracer fast blue, or the bidirectional tracer BDA (biotinylated dextran amine, Molecular Probes, cat. # D-7135, Eugene, OR) in the lateral hypothalamic area to label neurons in the prefrontal cortex and the amygdala projecting to the hypothalamus, and BDA labeled axons in the brainstem and spinal autonomic centers, and prefrontal cortex (Table 1, cases AW, AX). In two other animals we injected BDA in medial prefrontal area 32 to investigate if axons emanating from the injection site form synapses with neurons in hypothalamic autonomic centers, including the lateral hypothalamic area and the posterior hypothalamic area (Table 1, cases AY, BG).
Tissue processing and histological procedures
Tissue sectioning into matched series
After a survival period of 18 days, animals were anesthetized and perfused through the heart with saline followed by 4 l of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. For electron microscopy 0.2% glutaraldehyde was added to the perfusate. The brain was removed from the skull, photographed and cryoprotected in graded sucrose solutions (10–25% in 0.1 M phosphate buffer), frozen in -75°C isopentane, and cut in the coronal plane at 50 μm in ten series. In a case with BDA injection in the hypothalamus (Fig. 2), the brainstem and upper thoracic spinal cord were cut in five series. In cases with fluorescent dye injections, one series was mounted and used to map labeled neurons and terminals. After plotting, sections were stained with thionin and returned to the microscope to place architectonic borders.
Immunocytochemical procedures
In BDA experiments, one series of sections was treated in a Vector ABC Elite solution (PK-6100) overnight and 2–5 min in a 3,3'-diaminobenzidene tetrachloride (DAB-Plus, Zymed Laboratories).
To simultaneously see if some of the fast blue labeled neurons in the amygdala projecting to the hypothalamus were inhibitory, we conducted standard immunocytochemical procedures in separate series for the calcium binding proteins calbindin and parvalbumin. These markers label distinct classes of inhibitory neurons in the amygdala (e.g., [19,20]). The tissue was washed with 0.1 M PBS (pH 7.4) and preblocked with 10% goat serum (with 0.2% Triton-X) for 1 hour, and incubated for 2–3 days in primary antibody for parvalbumin (1:2,000; mouse monoclonal, Chemicon), or calbindin (1:2,000; mouse monoclonal, Accurate Chemical and Scientific Corp.) for 2–3 days. The tissue was then placed overnight in goat-anti-mouse IgG conjugated with the fluorescent probe Cyanoindocarbocyanine (Cy3, Chemicon, 1:800), or Alexa 488 (Molecular Probes, 1:200) with 0.1% Triton-X and 1% normal goat serum), and rinsed in PBS.
Tissue processing for electron microscopy
BDA treated sections were processed for electron microscopy. To determine the relationship of BDA labeled boutons from prefrontal area 32 to inhibitory interneurons in the hypothalamus, BDA treated sections were processed for pre-embedding immunocytochemistry for GABA (1:1,000; mouse monoclonal, Sigma, St. Louis, MO) according to the method of Pickel et al. [61]. Tissue sections were mounted, viewed under the light microscope and imaged with a CCD camera while wet. Small blocks of sections with BDA label, or BDA and GABA label in the same section in hypothalamic areas were cut under a dissecting microscope, postfixed in 0.5% osmium tetroxide in phosphate buffer (pH 7.4), washed in PBS and dehydrated in ascending series of alcohol, cleared in propylene oxide and embedded in Araldite at 60°C. Ultrathin sections (70 nm) were cut with a diamond knife (Diatome, Fort Washington, PA) using an ultramicrotome (Ultracut, Leica Wein, Austria) and collected on single slot grids. Sections were then stained with saturated uranyl acetate and lead citrate for transmission electron microscopy to view and photograph labeled synapses.
Data Analysis
Light and fluorescence microscopy
We viewed tissue under bright-field or fluorescence illumination. We mapped and recorded electronically the injection site as well as projection neurons and axonal terminations. Mapping of retrogradely labeled neurons or axon terminals was conducted using a microscope-computer interface and digital plotter using software developed in our laboratory, as described previously (e.g., [44]), or a commercial system (Neurolucida, MicroBrightField, Colchester, VT). On the side ipsilateral to the injection site we mapped: projection neurons in the prefrontal cortex and the amygdala that were retrogradely labeled after hypothalamic injections of the tracers fast blue or BDA; and axon terminals and boutons in the brainstem, spinal cord, and prefrontal cortex, labeled after injection of the bidirectional tracer BDA in the hypothalamus. Maps were obtained from one series of sections, using every section through the hypothalamus, the amygdala, the brainstem, and the thoracic spinal cord, and every other section through the prefrontal cortex. The nomenclature for the prefrontal cortex is according to the map of Barbas and Pandya [62], as modified from the map of Walker [63]. The terminology for the hypothalamus in macaque monkeys varies significantly in the literature, as summarized in a previous study [5]. As in our previous study, the terminology here relies on a combination of classic and modern studies in the human and monkey [26,64-67], including maps delineated on the basis of neurochemical features [68,69].
Electron microscopy
Synapses formed between hypothalamic neurons and labeled boutons from axons emanating from prefrontal area 32 were viewed on a JEOL CX-100 microscope at X10,000 and photographed. Photographic negatives were scanned and imported in an image analysis software system (MetaMorph, Universal Imaging). The cross-sectional area of synaptic boutons and the synaptic length were measured and data were imported into Excel for statistical analyses. We used classic criteria for identifying synapses and profiles [48]: aggregation of synaptic vesicles in the presynaptic bouton; rigid apposition of the presynaptic and postsynaptic membranes and associated widening of the extracellular space; and the presence of pre- and postsynaptic membrane specializations. Asymmetric synapses (type I) were classified by thickened postsynaptic densities and rounded vesicles; and symmetric (type II) synapses by thin postsynaptic densities and pleomorphic vesicles. In heavily labeled boutons the type of the vesicles could not be identified, so we used the postsynaptic density to classify synapses. Dendritic shafts contain mitochondria, microtubules and/or rough endoplasmic reticulum, while dendritic spines lack these organelles. Estimates of unlabeled synapses were made from photographs containing at least one labeled bouton (total area sampled, 1,607.5 μm2).
Authors' contributions
All authors participated in surgical procedures and mapping pathways; SS conducted the electron microscopic analysis; NR-C, drafted results from the light microscopic analyses; TG and SS drafted the figures; HB designed the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Dr. Alan Peters for help with EM and helpful discussions, Dr. Ron Killiany for conducting the MRI, Dr. Claus Hilgetag for help with statistical analyses, Mr. Piro Lera for help with graphics, and Ms. Ola Alade, Ms. Marcia Feinberg and Ms. Karen Trait for technical assistance, Dr. P. Sehgal for veterinary care, and Ms. Linda Fernsten for surgical assistance. Supported by NIH grants from NINDS and NIMH.
Contributor Information
Helen Barbas, Email: barbas@bu.edu.
Subhash Saha, Email: sgsaha@bu.edu.
Nancy Rempel-Clower, Email: REMPELCL@grinnell.edu.
Troy Ghashghaei, Email: ttroy@med.unc.edu.
References
- Kling A, Steklis HD. A neural substrate for affiliative behavior in nonhuman primates. Brain Behav Evol. 1976;13:216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descarte's Error: Emotion, Reason, and the Human Brain. 1. New York: G. P. Putnam's Sons; 1994. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: Anatomical and physiological observations. Prog Brain Res. 1990;85:147–166. doi: 10.1016/s0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. In: Paxinos G, editor. In The Rat Nervous System. New York: Academic Press; 1995. pp. 107–135. [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16:328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- De Olmos J. Amygdaloid nuclear gray complex. In: Paxinos G, editor. In The Human Nervous System. San Diego: Acedemic Press, Inc; 1990. pp. 583–710. [Google Scholar]
- Amaral DG. Amygdalohippocampal and amygdalocortical projections in the primate brain. In: Schwarcz R, Ben-Ari Y, editor. In Excitatory Amino Acids and Epilepsy Advances in Experimental Medicine and Biology. Vol. 203. New York: Plenum Press; 1985. pp. 3–17. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Rempel-Clower N, Xiao D. Anatomic basis of functional spcialization in prefrontal cortices in primates. In: Grafman J, editor. In Handbook of Neuropsychology. Amsterdam: Elsevier Science B.V; 2002. pp. 1–27. [Google Scholar]
- Holstege G. Subortical limbic system projetions to caudal brainstem and spinal cord. In: Paxinos G, editor. In The human nervous system. San Diego: Academic Press; 1990. pp. 261–286. [Google Scholar]
- Kemppainen S, Pitkanen A. Distribution of parvalbumin, calretinin, and calbindin-D28k immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Amaral DG. Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. Eur J Neurosci. 1998;10:2924–2933. doi: 10.1111/j.1460-9568.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99:613–626. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;301:1–23. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotions: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamus. In: Paxinos G, editor. In The Human Nervous System. Boston: Academic Press; 1990. pp. 389–413. [Google Scholar]
- Elmquist JK, Saper CB. Activation of neurons projecting to the paraventricular hypothalamic nucleus by intravenous lipopolysaccharide. J Comp Neurol. 1996;374:315–331. doi: 10.1002/(SICI)1096-9861(19961021)374:3<315::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Veazey RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). II. Efferent connections. J Comp Neurol. 1982;207:135–156. doi: 10.1002/cne.902070204. [DOI] [PubMed] [Google Scholar]
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. doi: 10.1002/cne.903410105. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Fibre degeneration following lesions of the amygdaloid complex in the monkey. J Anat. 1961;95:515–531. [PMC free article] [PubMed] [Google Scholar]
- Jacobson S, Trojanowski JQ. Amygdaloid projections to prefrontal granular cortex in rhesus monkey demonstrated with horseradish peroxidase. Brain Res. 1975;100:132–139. doi: 10.1016/0006-8993(75)90248-6. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam M-M. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Domesick VB. A cingulo-amygdaloid projection in the rhesus monkey. Brain Res. 1973;61:369–373. doi: 10.1016/0006-8993(73)90540-4. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Lipton PA, Alvarez P, Eichenbaum H. Crossmodal associative memory representations in rodent orbitofrontal cortex. Neuron. 1999;2:349–359. doi: 10.1016/s0896-6273(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system Neurons and their supporting cells. 3. New York: Oxford University Press; 1991. [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Descending motor pathways and the spinal motor system: limbic and non-limbic components. Prog Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. Theories of basal forebrain organization and the "emotional motor system". Prog Brain Res. 1996;107:461–484. [PubMed] [Google Scholar]
- Kennard MA. Focal autonomic representation in the cortex and its relation to sham rage. J Neuropathol Exp Neurol. 1945;4:295–304. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Ann Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine regulation of prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Van Bockstaele EJ, Chan J, Cestari DM. GABAergic neurons in rat nuclei of solitary tracts receive inhibitory-type synapses from amygdaloid efferents lacking detectable GABA-immunoreactivity. J Neurosci Res. 1996;44:446–458. doi: 10.1002/(SICI)1097-4547(19960601)44:5<446::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Malone EF. The nuclei tuberis laterales and the so-called ganglion opticum basale. The Johns Hopkins Hosp Rep. 1914;Monograph No. 6 [Google Scholar]
- Le Gros Clark WE. The topography and homologies of the hypothalamic nuclei in man. J Anat. 1936;70:203–214. [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH, Haymaker W. Hypothalamic nuclei and fiber connections. In: Haymaker W, Anderson E, Nauta WJH, editor. In The Hypothalamus. Springfield, Illinois: Thomas; 1969. pp. 136–209. [Google Scholar]
- Veazey RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). I. Cytoarchitectonic organization. J Comp Neurol. 1982;207:114–134. doi: 10.1002/cne.902070203. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Price DL, Blackstone CD, Huganir RL, Martin LJ. Non-NMDA glutamate receptors are present throughout the primate hypothalamus. J Comp Neurol. 1995;353:539–552. doi: 10.1002/cne.903530406. [DOI] [PubMed] [Google Scholar]
- Manning KA, Wilson JR, Uhlrich DJ. Histamine-immunoreactive neurons and their innervation of visual regions in the cortex, tectum, and thalamus in the primate Macaca mulatta. J Comp Neurol. 1996;373:271–282. doi: 10.1002/(SICI)1096-9861(19960916)373:2<271::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]


