Abstract
Background
Direct observations of underwater behaviour of free-living marine mammals are rare. This is particularly true for large and potentially dangerous species such as the walrus (Odobenus rosmarus). Walruses are highly specialised predators on benthic invertebrates – especially bivalves. The unique feeding niche of walruses has led to speculations as to their underwater foraging behaviour. Based on observations of walruses in captivity and signs of predation left on the sea floor by free-living walruses, various types of feeding behaviour have been suggested in the literature. In this study, however, the underwater feeding behaviour of wild adult male Atlantic walruses (O. r. rosmarus) is documented for the first time in their natural habitat by scuba-divers. The video recordings indicated a predisposition for use of the right front flipper during feeding. This tendency towards dextrality was explored further by examining a museum collection of extremities of walrus skeletons.
Results
During July and August 2001, twelve video-recordings of foraging adult male walruses were made in Young Sound (74°18 N; 20°15 V), Northeast Greenland. The recordings did not allow for differentiation among animals, however based on notes by the photographer at least five different individuals were involved. The walruses showed four different foraging behaviours; removing sediment by beating the right flipper, removing sediment by beating the left flipper, removing sediment by use of a water-jet from the mouth and rooting through sediment with the muzzle. There was a significant preference for using right flipper over left flipper during foraging. Measurements of the dimensions of forelimbs from 23 walrus skeletons revealed that the length of the right scapula, humerus, and ulna was significantly greater than that of the left, supporting our field observations of walruses showing a tendency of dextrality in flipper use.
Conclusion
We suggest that the four feeding behaviours observed are typical of walruses in general, although walruses in other parts of their range may have evolved other types of feeding behaviour. While based on small sample sizes both the underwater observations and skeletal measurements suggest lateralized limb use, which is the first time this has been reported in a pinniped.
Background
Walruses (Odobenus rosmarus) are highly specialised benthic feeders feeding almost exclusively on bivalves, making them an important component of the benthic ecosystem [1-4]. It is mainly the soft parts of the bivalves that are found in walrus stomachs, with pieces of shells seen only rarely [1,2]. Almost 6400 bivalve siphons have been reported in a single walrus stomach [2]. This feeding pattern makes walruses unique among pinnipeds, and has led to different theories as to how the walruses are able to locate and excavate their benthic prey [5-8]. For practical reasons, these theories have been based on the appearance and physical structure of walrus feeding patches on the sea-floor [7], food remains (shells) left on the sea bottom after a walrus feed [6], and on observations of animals in captivity [8]. Indeed Kastelein & Mosterd [8] wrote: "Because it is considered dangerous to be in the water with wild walruses, the suggested excavation technique of this species has not been investigated further.". This paper is the first that gives details of the foraging behaviour of free-living Atlantic walrus (O. r. rosmarus) based on close-up underwater video recordings in Northeast Greenland.
Young Sound has a stable, recurrent summer population of walruses, predominantly adult males (app. 50 animals, [9]), that take advantage of the area's high mollusc densities. The feeding grounds are accessible to the walruses from ice break-up at the end of July until the formation of new fast-ice in October [9,10]. A sub-goal of a study in Young Sound was to quantify the role of walruses as top-predators in this coastal marine ecosystem [11-13]. A combination of sampling of emptied bivalve shells left by the feeding walruses and satellite-telemetry information on activity were used to calculate the amount of food ingested during a typical foraging trip [14]. Underwater video recordings of feeding walruses were made during the same period.
Walrus feeding can be examined in three main ways: (1) Examination of the physical structures that are visible on the sea bottom and remains of food (i.e. empty bivalve shells) after a walrus-feeding event, (2) Quantification of food and energy intake, and (3) Direct observation of the behaviour and techniques used by the walruses when localising and excavating prey. The present study uses the latter approach whereas all previous studies have been confined to the first two e.g. [3,6,14-16].
Based on stomach contents and observations of free-ranging walruses in 1939–40, Vibe [1] suggested that the walruses take excavated mussels into their mouths, since discarded mussels often occurred at walruses' breathing holes in the ice, either whole or, more often, as empty shells, but always intact and connected. He further noted that the shells of the preferred prey (the bivalves Mya and Saxicava = Hiatella) are open at the ends and therefore suggested that the walruses suck out the soft parts of the bivalve and discard the empty shells.
Kastelein and co-workers made measurements of the suction pressure in the buccal cavity [17] and described the excavation technique used by two captive walruses that were fed molluscs [8,18,19]. These walruses rooted in the bottom of the tank with their snouts and used their vibrissae to locate the prey. They also used their mouths to create a water-jet that removed the bottom sediment, thereby exposing the prey.
During the current study, we observed a preference when excavating prey for the use of one flipper over the other. The preferred use of one limb/hand (handedness) to the other is one characteristic of lateralized behaviour [20] as reported extensively for humans [21-24]. In humans, right-handedness (dextrality) is associated with skeletal asymmetries such as a longer humerus, radius and ulna and a heavier scapula on the right-hand side compared to the left [25-28]. This makes the study of morphological asymmetries of the bones of limbs of other species interesting as a prediction of behavioural lateralization. Functional activity is seen as the reason for the lateralized difference in bone size [28,29]. We therefore investigated whether bone dimensions reflected the same preferential use of one front limb in walruses, based on measurements of skeletons.
Results
Walrus foraging behaviour
A total of twelve underwater recordings of foraging walruses were made (Table 1). The walruses were feeding at water depths between 6 and 16 m. The 2.5 hours of digital videotapes of foraging walruses showed very uniform feeding behaviour. 31 dives were defined as bottom sequences; these did not necessarily correspond to complete feeding dives because of the practical limitations associated with video recording under water. The recordings did not always start exactly at the beginning of the feed or terminate when the feed ended at the time of surfacing. While at the bottom, the walruses used different techniques for locating and excavating the bivalves. Based on recorded sequences of the diving and surfacing behaviour, the walruses dived directly to the bottom to feed, and when finished they went straight to the surface for air. Bottom-time averaged 215.8 ± 81.3 seconds (n = 31), and transit time 11.3 ± 1.7 seconds (n = 4).
Table 1.
Summary of under-water recordings of foraging walruses in Young Sound, Northeast Greenland 2001.
| Date 2001 | Walrus recording number | Observation time (sec) | Observation time (%) | No. of bottom sequences |
| 26 Jul | 1 | 208 | 3.1 | 1 |
| 29 Jul | 2 | 92 | 1.4 | 1 |
| 2 Jul | 3 | 193 | 2.9 | 1 |
| 2 Jul | 4 | 408 | 6.0 | 2 |
| 3 Jul | 5 | 30 | 0.4 | 1 |
| 3 Jul | 6 | 663 | 9.8 | 2 |
| 4 Jul | 7 | 1279 | 19.0 | 5 |
| 8 Jul | 8 | 2113 | 31.3 | 10 |
| 8 Jul | 9 | 680 | 10.1 | 3 |
| 10 Jul | 10 | 943 | 14.0 | 4 |
| 15 Jul | 11 | 130 | 1.9 | 1 |
| 15 Jul | 12 | 10 | 0.1 | 1 |
Observation time of walrus recordings number 1–12 in seconds per recording, percentage of total recording time and number of bottom sequences.
They stayed almost at the same spot for the whole feeding period, with their tusks resting like a sledge on the bottom. The wear from dragging the front of their tusks along the sediment was clearly visible when observing tusks of animals lying on haul-out. The walrus usually positioned itself facing the current, and with its body at an angle of between 45 and 90 degrees to the sea bottom (although in some situations it kept its body parallel to the sea floor). The hind flippers were used for moving forwards and backwards and the front flippers as stabilisers when not used in feeding. There was a long trail of sediment in the water around and behind the animal. It was possible for the walrus to keep a small area in front of its head clear from stirred up sediment by propelling with a front flipper a stream of clear water down in front of its head to the sediment surface. This was visible in recordings showing the backward movement of particles in small eddies on both sides of the animal. In some recordings the walruses appeared to be using their eyesight; the eyes were actively kept focusing towards the feeding spot often in combination with vigorous use of the vibrissae to provide tactile information.
When feeding on the sea floor, the walruses exhibited three different methods of exposing their prey.
1) The walrus used one of its front flippers in a waving motion over the substrate. There are also recordings of the animal resting on the sea floor on one shoulder and using the free flipper to propel water. The pulse of water removed the top layer of muddy sediment, most probably uncovering the siphons or the bodies of the bivalves. The animals kept their muzzles close to the sea floor during the whole feeding period. Hence, the actual treatment of the prey (i.e. picking it from the sediment, the suction of meat from bivalves or spitting out of emptied valves) could not be seen. However, when collecting the empty mussels after a foraging dive, shells contained 2–3% of the original soft tissue attached, e.g. siphon sheath and other remnants of soft parts, indicating that the animal sucks out the soft tissue and leaves the shells behind [14] (Figure 1). (Additional file 1: Video clip 1. Walrus using its flippers and muzzle to expose bivalves).
Figure 1.
Atlantic walrus and bivalve prey. Young Sound, Northeast Greenland 2001. (Photo by Göran Ehlmé and Søren Rysgaard). I: Walrus feeding patch on the sea bottom. Young Sound, Northeast Greenland 2001. II: Sea bottom and diver collecting empty bivalve shells from walrus feeding patch. Young Sound, Northeast Greenland 2001. III: Newly eaten bivalves from one feeding patch, with remains of soft parts. Daneborg, Northeast Greenland 2001.
2) By use of its very sensitive vibrissae [30,31] the walrus felt its way through the top-layer of the sediment likely detecting the bivalve siphons. It then rooted with its snout in the sediment like a pig in the ground (Figure 2). When using the snout, both hind flippers would rest on the sea bottom. (Additional file 2: Video clip 2. Walrus using its vibrissae to detect prey).
Figure 2.
Atlantic walrus feeding on the sea bottom. Young Sound, Northeast Greenland 2001. (Photo by Göran Ehlmé and Søren Rysgaard).
3) The walrus squirted a water-jet from its mouth into the sediment. This created enough turbulence to stir up the sediment, thereby likely exposing the bivalves. (Additional file 3: Video clip 3. Walrus feeding with water-jet).
There was no significant difference between walrus video recording number 1–12 (Table 2) in the proportion of sequence counts in the behaviour categories "flipper use" and "water-jet and muzzle use" (χ2 = 2.8; DF = 8; P = 0.99).
Table 2.
Four different walrus foraging behaviours in sequence counts per under-water recording. Young Sound, Northeast Greenland 2001.
| Walrus recording number | Right flipper sequence count | Left flipper sequence count | Water jet sequence count | Muzzle use sequence count | Binomial test P-value |
| 1 | 0 | 0 | 1 | 1 | 2 |
| 2 | 4 | 0 | 0 | 4 | 0.125 |
| 3 | 5 | 0 | 1 | 6 | 0.063 |
| 4 | 13 | 0 | 0 | 11 | 0.0002* |
| 5 | 1 | 0 | 0 | 1 | 1 |
| 6 | 24 | 2 | 2 | 23 | <0.0001* |
| 7 | 21 | 6 | 1 | 18 | 0.006* |
| 8 | 58 | 8 | 5 | 44 | <0.0001* |
| 9 | 21 | 3 | 1 | 17 | 0.0003* |
| 10 | 33 | 2 | 1 | 30 | <0.0001* |
| 11 | 0 | 0 | 0 | 1 | 2 |
| 12 | 3 | 2 | 0 | 7 | 1 |
P-values marked with * are statistically significant (P ≤ 0.05).
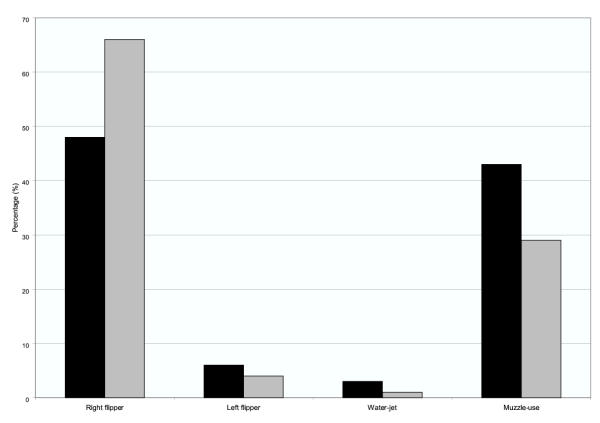
When all recordings were pooled, the walruses used their right front flipper for waving above the sediment during 66% of the bottom time and the left front flipper in only 4%. Rooting with the muzzle constituted 29%, and water jetting about 1% of the bottom time (Table 3, figure 3). When looking at the number of behavioural sequences, the walruses used the right flipper in 48%, the left flipper in 6%, the snout in 43% and water-jetting in 3% of the sequences (Table 3, figure 3). There was a significant difference among time used on the four feeding behaviours (Time: χ2r = 36.2; DF = 11; P < 0.0001; Sequences: χ2r = 29.4; DF = 11; P = 0.002). Recordings were made from different angles (0, 45, 90, 180, -90, -45 and above). However, there was no association between the location of the camera man and the walrus's chosen foraging behaviour (χ2 = 16.78; DF = 18; P = 0.54).
Table 3.
Four different walrus foraging behaviours in number of sequence counts and seconds for 12 underwater recordings combined. Young Sound, Northeast Greenland 2001.
| Feeding behaviour | Total no. of sequences | Min no. of strokes per sequences | Max no. of strokes per sequences | Total time | Min time | Max time | % foraging behaviour sequences | % foraging behaviour total time |
| Right flipper | 183 | 1 | 55 | 2881 | 1 | 73 | 48 | 66 |
| Left flipper | 23 | 1 | 12 | 166 | 2 | 21 | 6 | 4 |
| Water-jet | 12 | - | - | 37 | 1 | 17 | 3 | 1 |
| Muzzle-use | 163 | - | - | 1297 | 1 | 134 | 43 | 29 |
Observed feeding behaviours of walruses in total number and time in seconds of sequence counts with the minimum and maximum number and time of sequences from 12 recordings. Last two columns are feeding behaviours in percent for sequence counts and total time.
Figure 3.
Foraging behaviour of Atlantic walrus in percentage of behavioural sequence counts (black bars) and total time in seconds (grey bars) per walrus recording number 1–12. Young Sound, Northeast Greenland 2001.
When considering only flipper use, the walruses used their right flipper 89% of the time. Six out of the 12 recordings showed that the walrus had a preference for right flipper use, 4 showed no preference and 2 did not use their flippers in the video sequence (Table 2; Binomial two-tailed test). No walrus showed a preference for left flipper use. To obtain enough observations for the Binomial test to reveal any differences, more than 5 counts are necessary (1/2n) [32]. The 4 walrus recordings which showed no preference of flipper usage did not fulfil this requirement.
Skeletal asymmetry
No difference of variance of measures of skeletal dimensions was found between subspecies (O. r. divergens and O. r. rosmarus, respectively), age and sex so data were pooled (Subspecies difference, F-test values: Scapula 2.84; DF = 6,8; Humerus 5.05; DF = 8,2; Ulna 1.87; DF = 8,5; P > 0.05. Juvenile vs. Adult, F-test values: Scapula 2.22; DF = 3,11; Humerus 3.26; DF = 8,2; Ulna 2.29; DF = 4,9; P > 0.05. Female vs. Male, F-test values: Scapula 2.51; DF = 4,8; Humerus 1.02; DF = 8,1; Ulna 1.29; DF = 6,4; P > 0.05). On average, the right flipper was larger than the left flipper for all bone measurements except scapula height, where differences were negligible. These differences were statistically significant (Wilcoxon signed rank test) for the scapula (z = 2.83; P = 0.005), humerus (z = 2.86; P = 0.004) and ulna (z = 2.37; P = 0.018) (Table 4). The differences in mass of right- and left-hand bones were not significant (Table 4). The average difference of asymmetries was larger for adult specimens for 5 out of the 9 measurements (Tables 5 and 6).
Table 4.
Differences in size and mass between right and left bones of forelimbs in 23 walruses (Odobenus rosmarus).
| All specimens (n = 23) |
Average of differences, ∑ (R-L)/n (mm or g) | Average of differences in % of bone length or mass ∑ ((R-L)/L*100)/n | % R > L;R = L; R < L | Significance of difference (Wilcoxon signed rank test) |
| Scapula length (n = 22) |
2.63 | 1.08 | 64% 18% 18% | 0.005* |
| Scapula height (n = 22) |
0.18 | -0.02 | 41% 27% 32% | 0.68 |
| Humerus length (n = 21) |
1.00 | 0.64 | 52% 43% 5% | 0.004* |
| Radius length (n = 23) |
0.39 | 0.29 | 39% 39% 22% | 0.29 |
| Ulna length (n = 21) |
1.38 | 0.62 | 62% 29% 9% | 0.02* |
| Scapula mass (n = 19) |
2.05 | 0.66 | 37% 10% 53% | 0.67 |
| Humerus mass (n = 17) |
5.06 | 1.08 | 71% 5% 24% | 0.09 |
| Radius mass (n = 20) |
-0.10 | 1.14 | 50% 30% 20% | 0.44 |
| Ulna mass (n = 21) |
1.76 | 0.45 | 38% 33% 29% | 0.55 |
R refers to right bones. L refers to left bones. Size: mm. Mass: g. The numbers in brackets are number of examined specimens. P-values marked with * are statistically significant (P ≤ 0.05).
Table 5.
Percentage differences in size and mass between right and left bones of forelimbs from walruses (Odobenus rosmarus).
| All specimens (n = 23) | Average of differences in % of bone length or mass in adults. ∑ ((R-L)/L*100)/n | Average of differences in % of bone length or mass in juveniles. ∑ ((R-L)/L*100)/n |
| Scapula length | 1.80 (5) | 0.77 (17) |
| Scapula height | 1.13 (5) | -0.35 (17) |
| Humerus length | 0.17 (4) | 0.75 (17) |
| Radius length | 0.14 (6) | 0.35 (17) |
| Ulna length | 0.66 (6) | 0.61 (15) |
| Scapula mass | 1.24 (3) | 0.54 (16) |
| Humerus mass | 1.58 (3) | 0.97 (14) |
| Radius mass | -0.68 (3) | 1.47 (17) |
| Ulna mass | 0.35 (4) | 0.47 (17) |
The numbers in brackets are number of examined specimens.
Table 6.
Specimens of walrus (Odobenus rosmarus) examined at the Zoological Museum, Copenhagen.
| ID | Sex | Age | Year of collection | Origin |
| CN1 | Unknown | Juvenile | Unknown | Greenland/O.r.rosmarus |
| CN2 | F | Adult | 1848 | Greenland/O.r.rosmarus |
| CN3 | M | Adult | 1862 | Greenland/O.r.rosmarus |
| CN137 | Unknown | Adult | Unknown | Unknown |
| CN149 | Unknown | Juvenile | 1852 | Greenland/O.r.rosmarus |
| CN150 | M | Adult | Unknown | Greenland/O.r.rosmarus |
| CN370 | M | Adult | 1898 | Greenland/O.r.rosmarus |
| CN424 | Unknown | Juvenile | 1898 | Greenland/O.r.rosmarus |
| CN735 | M | Juvenile | Unknown | Greenland/O.r.rosmarus |
| CN736 | M | Juvenile | Unknown | Greenland/O.r.rosmarus |
| CN748 | F | Juv. (7 yr) | 1944 | Copenhagen Zoo/Unknown |
| CN827 | F | Juvenile | 1952 | Copenhagen Zoo/O.r.divergens |
| CN920 | F | Juvenile | Unknown | Copenhagen Zoo/O.r.divergens |
| CN995 | F | Juvenile | 1964 | Copenhagen Zoo/O.r.divergens |
| CN1001 | F | Juvenile | 1966 | Copenhagen Zoo/O.r.divergens |
| CN1034 | M | Juvenile | 1966 | Copenhagen Zoo/O.r.divergens |
| CN1080 | F | Juvenile | 1968 | Copenhagen Zoo/O.r.divergens |
| CN1114 | F | Juvenile | 1976 | Copenhagen Zoo/O.r.divergens |
| CN1115 | F | Juvenile | 1976 | Copenhagen Zoo/O.r.divergens |
| CN1116 | F | Juvenile | 1976 | Copenhagen Zoo/O.r.divergens |
| CN1136 | M | Juvenile | 1984 | Copenhagen Zoo/O.r.divergens |
| CN1139 | F | Juvenile | 1974 | Aalborg Zoo/O.r.rosmarus |
| CN1140 | F | Adult | 1979 | Copenhagen Zoo/O.r.divergens |
ID: Museum catalogue number, Zoological Museum, Copenhagen. Sex: M=male, F=female. Age: Juvenile / adult specimen. Based on the written data protocol made by the Zoological Museums curators with one consideration being closed or open epiphyses.
Discussion
Walrus foraging behaviour
The underwater recordings from Young Sound showed that the walruses as one of three techniques used flipper waving as a method for clearing away sediment.
The observations from the present study showed that by moving a front flipper the walrus seemed to keep an area in front of its head clear from stirred up sediment. This corresponds with previous observations [19,33] where captive Pacific walruses kept their eyes open and seemed to be using their eyesight during feeding. Anatomy studies are also supporting this behaviour [33]. One may envisage that since foraging cause turbidity of suspended material, a walrus would have an advantage in positioning itself accordingly, that is, letting the current move the sediment away and behind the animal, all the time feeding and moving in a forward leading direction. The walruses in the captivity study exhibited muzzle use and water jetting for excavation of bivalves while resting on their fore flippers. A possible reason for the differences in behaviour reported by Kastelein et al. [19,33] and ours could be the depth of buried prey. The bivalves in the pool were buried at a depth of 10 cm [8] whereas walruses in the wild need to excavate prey that is buried down to a depth of 40 cm [6,14]. The dominating sediment type in Young Sound shallow waters (<20 m) is sand [34], which could reinforce flipper use as a way of sediment removal in combination with currents. The captive walruses were normally hand-fed different kinds of fish [18], which might also explain their lack of flipper waving. Another difference between the pool-based trial [8] and our study is the need for wild-living walruses to adjust for the water current which is sometimes strong in Young Sound, however the recordings indicated that the walruses mainly used the flipper motion for removal of sediment (Video Clip 1,3).
At present our data cannot exclude the possibility that animals from other geographical regions will show other foraging behaviours than those described in the present study perhaps depending on prey density, distribution of prey or sediment type. Such spatial difference in behaviour across populations and individuals was documented in bottlenose dolphins (Tursiops truncatus) [35] and in harbour seals (Phoca vitulina) [36].
Because all 12 walrus recordings were collected in connection with a study design that required a defined feeding spot [14], our results are based on observations of walruses feeding in a localised area described as a pit [6,7]. Studies [6,7] described furrows left in the sediment from foraging walruses. Such structures were also observed in Young Sound.
In species, which exhibit behaviour where a forelimb is involved, a preference for one side might evolve first by chance and then reinforced [37,38] without this being induced by large anatomical asymmetry as seen for e.g. the fiddler crab (Uca sp.) [39]. If walruses always use only one flipper at a time it might gradually cause adaptation of a favourite side used for waving. Indeed, such lateralization has been reported in the fin preference of catfish (Ictalurus punctatus) [38].
We found that 89% of the time spent flipper waving on the sea floor was with the right flipper. Clapham et al. [40] documented lateralized behaviour when examining four different behaviours in humpback whales (Megaptera novaeangliae) one of which was flippering (the whale would raise one flipper and slap on the surface of the water). At the individual level there was a 77% preference for use of the right forelimb for flippering. Lateralized behaviour (90% right handedness) at the population level is best described for humans [23,41]. There are different views regarding the origin of handedness in humans and whether this phenomenon is related to the evolution of hemispheric asymmetry/specialization or of language, or whether it has some unrelated genetic basis [21-24]. Evolutionary laterality is still debated because of the implications of brain evolution in hominids and non-human species [42]. At present the knowledge we have of walrus brain morphology and genetics does not justify a theoretical discussion as seen for hominoids. However, here we will discuss it as a the morphometric adaptation of bone tissue to lateralized forelimb preference and function [28]. A preference for one particular side at the individual level is likely to be reinforced by use [37,38], but if the asymmetric bias occurs randomly, then handedness would approach 50:50 at the population level. Clearly, our behavioural data do not indicate a 50:50 distribution.
Lateralized limb use may be encouraged by the limbs being partially or totally freed from support of the body, which is the case of walruses in the water. The implications of these findings suggest that tool-use and object manipulation is not mandatory for development of strong limb preferences approaching handedness.
Skeletal asymmetry
Significant asymmetries of the flipper skeletons of walruses were detected in 3 of 5 length measurements. The greatest asymmetries were found in scapula length. Many of the muscles that control the forelimb attach to this bone [43], which means that a longer right scapula could indicate a greater muscle mass associated with the right flipper [27]. Although the mass measurement of scapula did not show any difference, we suggest that the asymmetry might be based on achieving more power compared to a longer reach. Given that walruses spend most of their time at sea where gravity will have less of an impact compared to a land mammal we might not expect to see a higher bone density or mass difference.
Skeletal asymmetries of the limbs have only been documented in very few other species. A study on a small sample of eleven chimpanzees (Pan troglodytes), failed to reveal clear skeletal asymmetries [44]. Falk et al. [45] found significantly larger right-side values for seven out of ten assessed dimensions in 150 rhesus monkeys (Macaca mulatta). A recent study of 441 harbour porpoise (Phocoena phocoena) revealed that the right scapula, humerus, radius and ulna were significantly longer than the left (A. Galatius unpublished data).
Latimer & Lowrance [25] found length differences of about 1% between right and left humerus, radius and ulna in a human population (our calculation on the basis of their data). The relative asymmetries are thus somewhat larger in humans compared to walruses, where the average asymmetries in these bones were 0.29% (radius), 0.64% (humerus) and 0.62% (ulna) (Table 4). Again being a land mammal gravity could account for an effect. If, as our results suggest, the asymmetries are larger in adult specimens, these values may be substantially larger in a sample of adults, since the present sample had only 6 adult specimens out of 23 (Table 5 and 6).
It has not been possible to find any other studies on asymmetry of the limbs of pinnipeds, but more research on walruses, in particular, is warranted in order to elucidate possible differences between juveniles and adults, males and females and subspecies.
Conclusions
The Atlantic walruses studied tended to exhibit one or more feeding behaviours while staying at the bottom. In contrast to previously knowledge, flipper use seemed to be of high importance combined with muzzle use. A preference for use of the right flipper rather than the left was indicated.
The observations of foraging behaviour in the wild in combination with measurements of limb skeletal asymmetry, suggest that lateralized limb use occurs in the walrus. Although based on small sample sizes this is to our knowledge the first record of a pinniped showing lateralized behaviour. More data on an individual level for several continuous years is warranted from walruses in the water as well as on haul-out.
The controlled feeding experiments by Marshall et al. [46] using a plexiglass feeding platform could be an excellent approach for studying the use of whiskers and handling of bivalves.
Methods
Walrus foraging behaviour
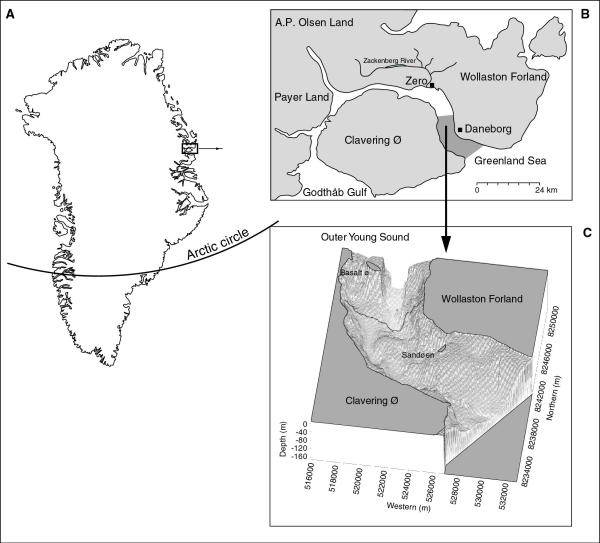
Fieldwork took place between 26 July and 15 August 2001 (i.e. during the open-water season) in Young Sound, (74°18 N; 20°15 V), a high-Arctic fjord in Northeast Greenland (Figure 4). When a foraging walrus was observed, the divers approached the animal in a rubber dinghy with an outboard 40 Hp Johnson motor. For safety reasons, scuba diving was only possible with animals foraging at water depths of less than about 30 m. The same protocol was used for every diving event with a feeding walrus. To allow the animal to get accustomed to the presence of the dinghy and the people in it, the diver did not enter the water until the walrus had completed 5–6 feeding cycles. On average, a feeding cycle consisted of a 6.7 minute dive and 1.0 minute at the surface [14]. The waiting time was used for identification and recording the diving and surfacing times and for observing any change in the feeding behaviour of the study animal.
Figure 4.
Study area: Young Sound, Northeast Greenland 2001.
The walrus, which on first approach kept a distance of about 20–50 m to the dinghy, soon appeared not to pay the boat and the diver any notice. At that moment a diver went into the water, and when the animal showed signs of beginning on a foraging dive, he followed the walrus down to the sea bottom (Figure 5). When reaching the sea floor the diver placed himself 0.5–2 m from the foraging walrus where he recorded the whole feeding session without disturbing the animal (Figure 5). After a feeding session the divers collected recently emptied bivalve shells for quantification of food intake as described by Born et al. [14].
Figure 5.
Atlantic walrus and scuba diver during a typical feeding session. Young Sound, Northeast Greenland 2001. (Photo by Göran Ehlmé and Søren Rysgaard). I: Scuba diver closing in on ventilating walrus. II: Walrus and scuba diver beginning a foraging dive. III: Walrus and scuba diver at the bottom during feeding.
The data used in the present analysis were collected during 8 days in the field period. Recordings were made for a total of 2.5 hours using a Sony VX1000 video camera and mini-digital videotapes. These tapes were subsequently run through a software program (Speed Razor Mach 4.7s in-sync) while played on a Sony Digital Component 16 Bit + 12 Bit Stereo/TBC/Timecode show-view DHR-1000 VC Digital Video Casette Recorder for further analysis. Still photos were taken of the foraging walruses and feeding patches using a NikonF100 camera in a waterproof case.
The digital videotapes had date and time codes for all recordings. These were used together with natural breaks in the behaviour to differentiate between individuals. The ID-numbers of walrus recordings #1–12 were given to animals based on the time and date of the recording. Only recordings with animals showing one of the four feeding behaviours were given an ID-number (Table 2). Animals observed at different times of day or on different dates were given new numbers even though they may have been recordings of the same animal from different days. The recordings did not give enough information to differentiate among animals based on individual marks or tusk shapes. However, based on the notes of the photographer, at least 5 different individuals were involved.
For the analyses of the recordings the methods of "focal sampling" and "continuous observations" were used [47]. The feeding behaviours seen on the video-recordings were categorised in the following way: (1) "Waving of the right-hand flipper", (2) "waving of the left flipper", (3) "Rooting with muzzle", and (4) "Using a water-jet". Flipper use was calculated as counts of strokes and total time of the entire sequence using the same flipper. Snout movement and water jetting was counted as one sequence and was timed from beginning to end.
To test for differences in choice of behaviour, the use of a flipper was counted as one sequence regardless of the number of strokes in that sequence. In this way, comparison between all four behaviours was possible. A χ2-test [32] was used to explore whether there was a difference between the proportion of sequence counts of flipper use and muzzle use between different recordings. Behavioural category "right flipper wave" was pooled with "left flipper wave" and "water-jetting" with "muzzle use" to meet the conditions for this test. A Friedmann's test [32] was used to test for consistent differences in the time spent on each behaviour across recordings. To test whether the right and left flippers were used in a ratio different from 50:50, a Binomial test (two-tailed) [32] was carried out for each recording (Table 2). Since recordings were made from both sides, in front, behind and above the walruses, the potential influence on the walrus' behaviour of the position of the diver was tested with a χ2-test. The test was done on the numbers of the different recording angels for each of the four behaviours. The critical value P < 0.05 was used in all tests.
Skeletal asymmetry
23 walrus skeletons from the collections of the Zoological Museum in Copenhagen were investigated for asymmetries in the bones of the extremities. The sample consisted of 6 adult and 17 juvenile specimens of both sexes, originating from Greenland (O. r. rosmarus) and the Bering Strait region (O. r. divergens) (see Table 6 for details on specimens studied). The semi-quantitative assessment of whether a skeleton was that of an adult or a juvenile was taken from the written data protocol made by the Zoological Museums curators with one consideration being closed or open epiphyses.
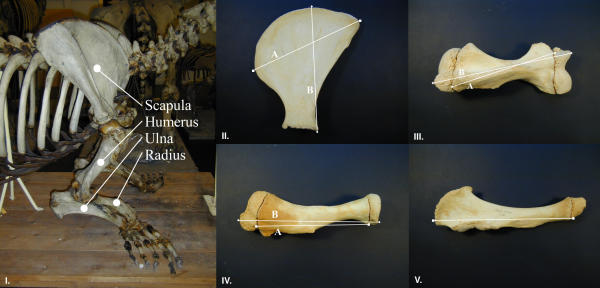
The following measurements were made (where possible) to the nearest mm on right and left bones (Figure 6) from each specimen using callipers:
Figure 6.
The bones of the forelimb of a walrus (Odobenus rosmarus) showing measurements used in the study of lateralized limb use. (Photo by Anders Galatius). I: The four measured bones are marked. II: Measurements of the scapula. A: Length, B: Height. III: Measurements of the humerus. A: Epiphyses unattached, B: Epiphyses attached. IV: Measurements of the radius. A: Epiphyses attached, B: Epiphyses unattached. V: Measurement of ulna, epiphyses unattached.
(1) The length of the scapula
(2) The height of the scapula
(3) The length of the humerus
(4) The length of the radius
(5) The length of the ulna
Percentage differences of right/left bone length were calculated as:
(Right bone measurement - Left bone measurement) / Left bone measurement * 100.
All measurements were made by the same observer. To test for variance differences between subspecies, age and sex an F-test [32] was used. Since the data were not normally distributed, a Wilcoxon non-parametric signed rank test [32] was used to test for differences in bone length and mass. The critical value P < 0.05 was used.
If epiphyses were attached to both right and left bones, these were included in the measurements. Lengths were not measured if any damage to the bone that might influence the length, was evident
The right and left scapula, humerus, radius and ulna were also weighed to the nearest gram on a digital balance. Masses were not measured if an epiphysis was only attached to one bone of a pair, or if any damage to the bone that might influence the mass was evident.
Authors' contributions
NL analysed the field data and wrote the manuscript. AG measured skeletal asymmetry and helped draft this part of the manuscript. SR and EWB designed the study of walruses underwater, raised funds for the study and helped draft the manuscript. GE participated in the design and practical part of the study and made the underwater recordings with assistance from SR. All authors read and approved the final manuscript.
Supplementary Material
Walrus using its flippers and muzzle to expose bivalves. Young Sound, Northeast Greenland 2001. (Filmed by Göran Ehlmé). The movie clip of feeding walruses has been zipped in order to reduce the size and hereby making it more suitable for readers to download when viewing. Suggestion of different software to use for viewing the clips: Windows Media player, Real Player or QuickTime player.
Walrus using its vibrissae to detect prey. Young Sound, Northeast Greenland 2001. (Filmed by Göran Ehlmé). The movie clip of feeding walruses has been zipped in order to reduce the size and hereby making it more suitable for readers to download when viewing. Suggestion of different software to use for viewing the clips: Windows Media player, Real Player or QuickTime player.
Walrus feeding with water jet. Young Sound, Northeast Greenland 2001. (Filmed by Göran Ehlmé). The movie clip of feeding walruses has been zipped in order to reduce the size and hereby making it more suitable for readers to download when viewing. Suggestion of different software to use for viewing the clips: Windows Media player, Real Player or QuickTime player.
Acknowledgments
Acknowledgements
This study was financed by the Danish Natural Science Foundation, The Danish Ministry of the Environment (Dancea), The Commission for Scientific Research in Greenland (KVUG), The Greenland Institute of Natural Resources and the Danish Environmental Research Institute. Nette Levermann had financially support from Japetus Steenstrups Legat and Aase & Jørgen Münters Fond. The study was made under a three-year multidisciplinary study (CAMP: Change in Arctic Marine Production, 1999–2001).
We thank Dr. Ron A. Kastelein, one anonymous referee, Niels Martin Schmidt, Josephine Nymand, David Nash and Mads C. Forchhammer for valuable comments and improvements to previous versions of the manuscript. The assistance and support of the following institutes and people are acknowledged: The Danish Polar Centre and the military sledge-patrol Sirius for help with logistics, NERI, Silkeborg for technical support, Mogens Andersen for providing access to the collection of the Zoological Museum of Copenhagen and Mads C. Forchhammer for advice and encouragement.
Contributor Information
Nette Levermann, Email: nlevermann@zi.ku.dk.
Anders Galatius, Email: agjorgensen@zmuc.ku.dk.
Göran Ehlme, Email: goran@waterproof.se.
Søren Rysgaard, Email: sr@dmu.dk.
Erik W Born, Email: ewb@dpc.dk.
References
- Vibe C. The Marine Mammals and the Marine Fauna in the Thule District (Northwest Greenland) with Observations on the Ice Conditions in 1939-1941. Meddelelser om Grønland. 1950;150 [Google Scholar]
- Fay FH. Ecology and Biology of the Pacific Walrus, Odobenus rosmarus divergens Illiger. North American Fauna, United States Department of the Interior, Fish and Wildlife Service. 1982;74:1–279. [Google Scholar]
- Nelson CH, Johnson KR, Barber JH. Gray whale and walrus feeding excavation on the Bering Shelf, Alaska. Journal of Sedimentary Petrology. 1987;57:419–430. [Google Scholar]
- Klaus AD, Oliver JS, Kvitek RG. The effects of gray whale, walrus, and ice gouging disturbance on benthic communities in the Bering sea and Chukchi Sea, Alaska. National Geographic Research. 1990;6:470–484. [Google Scholar]
- Fay FH. Dental function in relation to feeding behaviour of the Pacific walrus. Proceedings of the Alaska Science Conference. 1971;22:137. [Google Scholar]
- Oliver JS, Slattery PN, Oconnor EF, Lowry LF. Walrus, Odobenus rosmarus, feeding in the Bering Sea - A benthic perspective. Fishery Bulletin. 1983;81:501–512. [Google Scholar]
- Nelson CH, Johnson KR. Whales and walruses as tillers of the sea-floor. Scientific American. 1987;256:74–81. [Google Scholar]
- Kastelein RA, Mosterd P. The excavation technique for molluscs of Pacific Walruses (Odobenus rosmarus divergens) under controlled conditions. Aquatic Mammals. 1989;15:3–17. [Google Scholar]
- Born EW, Gjertz I, Reeves RR. Population assessment of Atlantic walrus (Odobenus rosmarus rosmarus L.) Norsk Polarinstitutt Meddelelser. 1995. pp. 1–99.
- Rysgaard S, Vang T, Stjerneholm M, Rasmussen B, Windelin A, Kiilsholm S. Physical conditions, carbon transport and climate change impacts in a NE Greenland fjord. Arctic, Antarctic, and Alpine Research. 2003;35 [Google Scholar]
- Born EW, Acquarone M, Griffiths D. Studies of walrus energetics and behaviour. In: CaningK and RaschM, editor. 5th Annual Report 1999. Danish Polar Center, Minestry of Research & Information Technology, Copenhagen, Denmark; 2000. pp. 72–73. (Zackenberg Ecological Research Operations, ZERO). [Google Scholar]
- Acquarone M, Born EW, Griffiths D. Studies of walrus energetics and behaviour. In: CaningK and RaschM, editor. 6th Annual Report 2000. Danish Polar Center, Minestry of Research & Information Technology, Copenhagen, Denmark; 2001. pp. 64–66. (Zackenberg Ecological Research Operations, ZERO). [Google Scholar]
- Born EW, Acquarone M, Rysgaard S, Sejr M, Ehlmé G, Levermann N, Møller T. Walrus studies. In: CaningK and RaschM, editor. 7th Annual Report 2001. Danish Polar Center, Minestry of Research & Information Technology, Copenhagen, Denmark; 2003. pp. 64–67. (Zackenberg Ecological Research Operations, ZERO). [Google Scholar]
- Born EW, Rysgaard S, Ehlmé G, Sejr M, Acquarone M, Levermann N. Underwater observations of foraging free-living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biology. 2003;26:348–357. [Google Scholar]
- Kastelein RA, Schooneman NM, Wiepkema PR. Food consumption and body weight of captive Pacific walruses (Odobenus rosmarus divergens) Aquatic Mammals. 2000;26:175–190. [Google Scholar]
- Sheffield Gay, Fay Francis H., Feder Howard, Kelly Brendan P. Laboratory digestion of prey and interpretation of walrus stomach contents. Marine Mammal Science. 2000;17:310–330. [Google Scholar]
- Kastelein RA, Muller M, Terlouw A. Oral suction of a Pacific walrus (Odobenus rosmarus divergens) in air and under water. Zeitschrift fur Saugetierkunde. 1994;59:105–115. [Google Scholar]
- Kastelein RA, Wierpkema PR. A digging trough as occupational therapy for Pacific Walruses (Odobenus rosmarus divergens) in human care. Aquatic Mammals. 1989;15:9–17. [Google Scholar]
- Kastelein RA, Wierpkema PR, Slegtenhorst C. The use of molluscs to occupy Pacific Walruses (Odobenus rosmarus divergens) in human care. Aquatic Mammals. 1989;15:6–8. [Google Scholar]
- Reiss M, Reiss G. Lateral preferences in a German population. Perceptual and Motor Skills. 1997;85:569–574. doi: 10.2466/pms.1997.85.2.569. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Hemispheric laterality in animals and the effects of early experience. Behavioral and Brain Sciences. 1981;4:1–21. [Google Scholar]
- McManus IC. Handedness, language dominance and aphasia: a genetic model. Cambridge, Cambridge University Press; 1985. pp. 1–40. (Psychological Medicine). [PubMed] [Google Scholar]
- Annett M. Handedness and cerebral dominance: The right shift theory. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10:459–469. doi: 10.1176/jnp.10.4.459. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lönnqvist J, Standertskjöld-Nordenstam CG, Kapiro J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Latimer HB, Lowrance EW. Bilateral asymmetry in length and weight of human bones. The Anatomical Record. 1965;152:217–224. doi: 10.1002/ar.1091520213. [DOI] [PubMed] [Google Scholar]
- Hiramoto Yoshisuke. Right-left differences in the lengths of human arm and leg bones. Acta Anatomica Nipponica. 1993;68:536–543. [PubMed] [Google Scholar]
- Steele J. Skeletal indicators of handedness. In: Cox and Mays, editor. Human Osteology In Archaeology and Forensic Science. London, Greenwich Medical Media Ltd.; 2000. pp. 307–323. [Google Scholar]
- Lazenby RA. Skeletal biology, functional asymmetry and the origins of "Handedness". Journal of Theoretical Biology. 2002;218:129–138. doi: 10.1006/jtbi.2002.3052. [DOI] [PubMed] [Google Scholar]
- Buskirk ER, Andersen KL, Brozek J. Unilateral Activity and Bone and Muscle Development in the Forearm. The Research Quarterly. 1956;27:127–131. [Google Scholar]
- Kastelein RA, van-Gaalen MA. The sensitivity of the vibrissae of a Pacific Walrus (Odobenus rosmarus divergens). Part 1. Aquatic Mammals. 1988;14:123–133. [Google Scholar]
- Kastelein RA, Stevens S, Mosterd P. The tactile sensitivity of the mystacial vibrissae of a Pacific Walrus (Odobenus rosmarusdivergens). Part 2: Masking. Aquatic Mammals. 1990;16:78–87. [Google Scholar]
- Zar JH. Biostatistical Analysis. Second. Prentice-Hall International, Inc.; 1984. pp. 1–717. [Google Scholar]
- Kastelein RA, Zweypfenning RC,V, Spekreijse H, Dubbeldam JL, Born EW. The anatomy of the Walrus head (Odobenus rosmarus). Part 3: The eyes and their function in Walrus ecology. Aquatic Mammals. 1993;19:61–92. [Google Scholar]
- Sejr MK, Jensen KT, Rysgaard S. Macrozoobenthic community structure in a high-arctic East Greenland fjord. Polar Biology. 2000;23:792–801. doi: 10.1007/s003000000154. [DOI] [Google Scholar]
- Shane SH, Wells RS, Wursig B. Ecology, Behavior and Social-Organization of the Bottle-Nosed- Dolphin - A Review. Marine Mammal Science. 1986;2:34–63. [Google Scholar]
- Bowen WD, Tully D, Boness DJ, Bulheier BM, Marshall GJ. Prey-dependent foraging tactics and prey profitability in a marine mammal. Marine Ecology-Progress Series. 2002;244:235–245. [Google Scholar]
- Collins RL. On inheritance of handedness.2. Selection for sinistrality in mice. Journal of Heredity. 1969;60:117–119. doi: 10.1093/oxfordjournals.jhered.a107951. [DOI] [PubMed] [Google Scholar]
- Fine ML, McElroy D, Rafi J, King CB, Loesser KE, Newton S. Lateralization of pectoral stridulation sound production in the channel catfish. Physiology & Behavior. 1996;60:753–757. doi: 10.1016/0031-9384(96)00092-3. [DOI] [PubMed] [Google Scholar]
- Dorit RL, Walker WF, Barnes RD. Zoology. Saunders College Publishing; 1991. pp. 1–1009. [Google Scholar]
- Clapham PJ, Leimkuhler E, Gray BK, Mattila DK. Do humpback whales exhibit lateralized behavior? Animal Behaviour. 1995;50:73–82. doi: 10.1006/anbe.1995.0222. [DOI] [Google Scholar]
- Annett M. Distribution of manual asymmetry. British Journal of Psychology. 1972;63:343. doi: 10.1111/j.2044-8295.1972.tb01282.x. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, StuddertKennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–263. [Google Scholar]
- Young JZ. The life of mammals, their anatomy and physiology. Second. Clarendon Press, Oxford; 1975. [Google Scholar]
- Morbeck Mary Ellen, Galloway Alison, Mowbray Kenneth M., Zihlman Adrienne L. Skeletal asymmetry and hand preference during termite fishing by Gombe chimpanzees. Primates. 1994;35:99–103. [Google Scholar]
- Falk D, Pyne L, Helmkamp RC, Derousseau CJ. Directional asymmetry in the forelimb of Macaca mulatta. American Journal of Physical Anthropology. 1988;77:1–6. doi: 10.1002/ajpa.1330770102. [DOI] [PubMed] [Google Scholar]
- Marshall CD, Maeda H, Iwata M, Furuta M, Asano S, Rosas F, Reep RL. Orofacial morphology and feeding behaviour of the dugong, Amazonian, West African and Antillean manatees (Mammalia: Sirenia): functional morphology of the muscular-vibrissal complex. Journal of Zoology. 2003;259:245–260. doi: 10.1017/S0952836902003205. [DOI] [Google Scholar]
- Altmann J. Observational study of behavior: Sampling Methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Walrus using its flippers and muzzle to expose bivalves. Young Sound, Northeast Greenland 2001. (Filmed by Göran Ehlmé). The movie clip of feeding walruses has been zipped in order to reduce the size and hereby making it more suitable for readers to download when viewing. Suggestion of different software to use for viewing the clips: Windows Media player, Real Player or QuickTime player.
Walrus using its vibrissae to detect prey. Young Sound, Northeast Greenland 2001. (Filmed by Göran Ehlmé). The movie clip of feeding walruses has been zipped in order to reduce the size and hereby making it more suitable for readers to download when viewing. Suggestion of different software to use for viewing the clips: Windows Media player, Real Player or QuickTime player.
Walrus feeding with water jet. Young Sound, Northeast Greenland 2001. (Filmed by Göran Ehlmé). The movie clip of feeding walruses has been zipped in order to reduce the size and hereby making it more suitable for readers to download when viewing. Suggestion of different software to use for viewing the clips: Windows Media player, Real Player or QuickTime player.