Abstract
γ-Hydroxybutyrate (GHB), an anesthetic adjuvant analog of γ-aminobutyrate (GABA), depresses cell excitability in hippocampal neurons by inducing hyperpolarization through the activation of a prominent inwardly rectifying K+ (Kir3) conductance. These GABA type B (GABAB)-like effects are clearly shown at high concentrations of GHB corresponding to blood levels usually reached during anesthesia and are mimicked by the GABAB agonist baclofen. Recent studies of native GABAB receptors (GABABRs) have favored the concept that GHB is also a selective agonist. Furthermore, cloning has demonstrated that GABABRs assemble heteromeric complexes from the GABABR1 and GABABR2 subtypes and that these assemblies are activated by GHB. The surprisingly high tissue content, together with anti-ischemic and protective effects of GHB in the heart, raises the question of a possible influence of GABAB agonists on excitable cardiac cells. In the present study, we provide electrophysiological evidence that GHB activates an inwardly rectifying K+ current in rat ventricular myocytes. This effect is mimicked by baclofen, reversibly inhibited by GABAB antagonists, and prevented by pertussis toxin pretreatment. Both GABABR1 and GABABR2 are detected in cardiomyocytes by Western blotting and are shown to coimmunoprecipitate. Laser scanning confocal microscopy discloses an even distribution of the two receptors in the sarcolemma and along the transverse tubular system. Hence, we conclude that GABABRs are distributed not only in neuronal tissues but also in the heart, where they can be activated and induce electrophysiological alterations through G-protein-coupled inward rectifier potassium channels.
A structural analog of γ-aminobutyrate (GABA) synthesized locally in the brain, γ-hydroxybutyrate (GHB), exhibits GABA-like neuropharmacological properties and was primarily used to induce sedation, sleep, and eventually anesthesia in various animals and humans (1). Systemic GHB application has been shown to produce absence-like seizures (2) and inhibit neurotransmitter release (3). Although the role for GHB as neurotransmitter remains controversial, it is recognized that it has strong neuromodulatory properties (3–7). Binding assays have cast some doubt on the hypothesis that GHB acts directly through postsynaptic GABA type B (GABAB) receptors (GABABRs) to produce absence seizures (8). However, several reports suggest a direct interaction of GHB with native GABABRs; these comprise binding studies (9), as well as electrophysiological studies on dopaminergic (10, 11), thalamocortical (12), and hippocampal neurons (13). Moreover, following the cloning of the GABAB receptor that functions as a heterodimer coupling GABABR1 and GABABR2 (14–18), it has been demonstrated that GHB acts like an agonist toward recombinant heterologously expressed GABABRs (19).
Millimolar concentrations of GHB are required to cause dose-dependent hyperpolarizations through the activation of an outward K+ conductance that could be inhibited by GABAB receptor antagonists (10–13, 19). In contrast, the GABAB agonist baclofen induces quite similar effects, with an EC50 of 3–5 μM for GABABR-activated inwardly rectifying K+ currents (20, 21). As a matter of fact, several studies have borne out the conclusion that inwardly rectifying K+ channels of subfamily 3 (Kir3) are prominent postsynaptic effectors of GABAB receptors in neuronal tissue (19–25).
Surprisingly, GHB has also been found in nonneural tissue such as the heart, in which its concentration may be 5- to 6-fold that of brain (26). In addition, experimental data argue in favor of anti-ischemic cardioprotective effects when energy supplies are limited (3, 27, 28). Taken together, these observations raise the question of a possible role of GHB in cardiomyocytes, although very little evidence for any physiological function for GABAB mechanisms has been reported in peripheral organs (29). Pharmacological doses required to induce anesthesia in humans (100–200 mg/kg) raise GHB blood levels from the micromolar (3) to the millimolar range (30, 31), in keeping with concentrations that are able to produce significant electrophysiological changes in neurons (10–13, 19). We were interested in looking for electrophysiological alterations induced by these GHB concentrations in other excitable cells, namely mammalian cardiomyocytes.
In isolated rat ventricular myocytes, we provide electrophysiological evidence that GHB can activate an inwardly rectifying K+ current. Because GHB-binding sites are reportedly absent from the heart (4), we assumed that this effect could be produced through GABABRs present in rat ventricular cell membranes. Indeed, this effect was mimicked by baclofen, reversibly inhibited by GABAB antagonists, and prevented by pertussis toxin pretreatment. Furthermore, both GABABR1 and GABABR2 were detected in cardiomyocytes by Western blotting and confocal imaging. Therefore, these results show that GABABRs not only are distributed in cardiac sarcolemmal membranes but also can elicit drug-induced electrophysiological changes in mammalian cardiomyocytes.
Materials and Methods
Electrophysiology.
Ventricular cardiomyocytes from Wistar rats (250 g) were isolated as described (32). Whole-cell patch-clamp recording was performed with soft glass capillaries pulled to a tip resistance of 1.5–2 MΩ (Sutter Instruments, Novato, CA). Signals were recorded at room temperature (22°C) with an Axopatch 200A (Axon Instruments, Foster City, CA) interfaced to a personal computer through a 125-kHz Labmaster board (Axon Instruments). Membrane currents were sampled at 1–2 kHz and low-pass filtered at 2 kHz. Series resistance (Rs = 7.0 ± 1.0 MΩ) was compensated by ≈80%. Voltage commands, data acquisition, and analysis were performed by using pClamp 6.0. Solutions used were (in mM) 130 NaCl, 5.4 KCl, 1.1 MgCl2, 2 CaCl2, 1.8 NaH2PO4, 20 Hepes, 11 glucose, and 20 taurine (pH adjusted to 7.4 with NaOH) for the bath and 110 potassium aspartate, 20 KCl, 1 MgCl2, 10 Hepes, 5 EGTA, 0.4 Na2GTP, 5 Mg2ATP, and 5 disodium creatine phosphate (pH adjusted to 7.2 with NaOH), for the pipette. A series of cells were tested after a 4-h incubation at 35°C in the bath solution supplemented with 300 ng⋅ml−1 pertussis toxin. Ca2+ current was inhibited by 2 mM Co2+ and the transient outward current, by 3 mM 4-aminopyridine. In some experiments 1 mM Ba2+ was used to inhibit the background rectifier current. GHB (sodium salt), (±)-baclofen, and pertussis toxin were obtained from Sigma. In most cases, 2 mM GHB was used because this concentration correlates with blood levels clinically reached by this hypnotic during anesthesia (30, 31). The specific antagonist GABABR CGP36742 was from Ciba–Geigy. Cells were voltage-clamped to a holding potential (Vh) of −60 mV. Test potentials of 900-ms duration and ranging from −110 to +20 mV were delivered in 10-mV increments at a frequency of 0.2 Hz; 100-ms prepulses to −20 mV were applied to inactivate sodium current. The steady-state current (Iss) was taken as the current measured at the end of the pulses and normalized to membrane capacitance. Iss–V curves were then used to estimate the value of the slope conductance for the inward rectifier current. Slope conductances (Gss) for voltages negative to the reversal potential were calculated from linear regressions fitted to the experimental data.
Western Blot, Immunoprecipitation, and Northern Blot Analysis.
For Western blot analysis, whole-cell lysates were prepared from frozen hippocampus and freshly isolated adult cardiomyocytes in NET buffer, which contains (in mM) 150 NaCl, 5 EDTA, 50 Tris⋅HCl (pH 8.0), supplemented with 1% Nonidet-P40, 50 mM NaF, 0.1 mM phenylmethylsulfonyl fluoride, and 10 μg⋅ml−1 leupeptin. After 10 min of incubation on ice, the lysate was spun down for 30 min at 12,000 × g. The lysate was then diluted and subjected to immunoprecipitation, using either the Ab174.1 or the AbC22 polyclonal antibodies. Ab174.1 was directed against carboxyl-terminal epitopes of GABABR1a/b (33), whereas AbC22 was raised against amino acids 806–907 of the carboxyl-terminal sequence of GABABR2 (17). Immunoblots with or without coimmunoprecipitation were subsequently probed with either antibody according to previously described techniques (32).
To detect GABABR isoform mRNAs, approximately 2 million freshly isolated cardiac cells were purified on a Percoll gradient consisting of two density layers (1.06 and 1.08 g⋅ml−1) centrifuged for 10 min at 400 × g to remove remaining nonmyocyte cells. Brains were rapidly removed from pentobarbital-anesthetized rats, rinsed briefly in ice-cooled PBS, and freeze-clamped with the blood remaining in situ. Total RNA was extracted from the cardiomyocytes and brain by the method of Chomczynski and Sacchi (34). RNA samples were treated with RNase-free DNase before reverse transcription, to avoid amplification from contaminating genomic DNA. Reverse transcription with PCR (RT-PCR) was performed as described (35). Oligonucleotide sequences were chosen from the 5′ end of the open reading frames of the GABABR1a and -1b sequences (14) specific for each mRNA isoform. For GABABR1a, the sense and antisense primer sequences were 5′-CAA GTC TTA TTT GAC CCT GG and 5′-TTG ACA AGG CTG GTG AAC TGG AGC, complementary to nucleotides 300–319 and 800–820, respectively, of the 1a-coding sequence. The same antisense primer was used to amplify the GABABR1b isoform, paired with an alternative sense primer, 5′-TGC TGG TGA TGG CGG CTG GGG TGG, representing nucleotides 50–73 of the 1b-coding sequence. These were predicted to yield cDNAs of 524 and 426 bp for 1a and 1b isoforms, respectively. Confirmation of the identity of the RT-PCR products was obtained by restriction digestion of the RT-PCR products for 2 h with either HindIII or Sau3AI, followed by agarose gel fractionation to determine the size of the digested fragments.
Epitope-Tagged Receptors and Confocal Microscopy.
Freshly dissociated cells were fixed for 15 min in 3% paraformaldehyde in PBS, incubated in 50 mM NH4Cl for 10 min, permeabilized with 0.3% Triton X-100 for 15 min, and then incubated for 1 h with primary antibodies. GABABR1 polyclonal guinea pig antibody (PharMingen) was coupled with Alexa Fluor 546 goat anti-guinea pig conjugate (Molecular Probes). GABABR2 polyclonal rabbit antibody (17) was coupled with Alexa Fluor 488 goat anti-rabbit conjugate (Molecular Probes). Both primary and secondary antibodies were applied sequentially for 1 h at a 1:50 dilution. Fluorescent images were recorded on a Zeiss LSM 510 laser scanning confocal system coupled to a Zeiss Axiovert inverted microscope (63×, numerical aperture = 1.2, water-immersion objective). Monophotonic molecular excitation was performed with an argon (488 nm) and a helium/neon (543 nm) laser.
Data Analysis.
Results are expressed as means ± SEM. The statistical significance of differences between two means was evaluated with paired or unpaired nonparametric tests (Wilcoxon or Mann–Whitney). A two-tailed P < 0.05 was considered statistically significant.
Results
Electrophysiological Effects of GABAB Agonists.
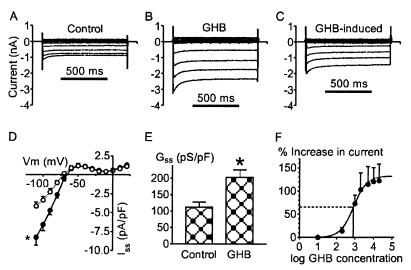
Superfusion of isolated rat ventricular myocytes with a 2 mM GHB-containing solution induced changes in the steady-state currents (Iss) elicited by hyperpolarizing steps negative to the reversal potential (Vrev ≈ −70 mV), as shown in Fig. 1. GHB-induced currents exhibited a strong inward rectifier pattern similar to that of control background currents (Fig. 1 A–C). The GHB-evoked response was characterized by a delayed time course with no effect within 1–2 min and full activation after 5–7 min. Then the effect remained stable, and it was not reversible, even after prolonged washout for 15–20 min. Analysis of current–voltage relationships obtained in control conditions and in the presence of 2 mM GHB (Fig. 1D) revealed that GHB increased the mean Iss at −110 mV from 4.14 ± 0.55 to −8.24 ± 1.06 pA/pF (P = 0.01), whereas mean Gss increased from 112.2 ± 15.6 to 202.6 ± 23.2 pS/pF (P < 0.01) (Fig. 1E). No significant change was observed for mean Vrev, in contrast to hyperpolarizations recorded in neurons after GHB or baclofen applications (10, 13, 20, 36). The concentration dependence of GHB effects on Iss recorded at 7 min is shown in Fig. 1F. The threshold for activation of GHB-induced current was around 0.2 mM, and the maximal effect was at ≈2 mM. Increasing GHB concentration to 5, 10, and 20 mM did not alter the mean response significantly. The half-maximal activation of Iss occurred at 828 μM, and the Hill coefficient was 1.2. The application of 1 mM Ba2+ induced a rapid inhibition of both the control background and GHB-induced currents; the remaining Ba2+-insensitive current was very weak and was characterized by a flat linear conductance reversing at about 0 mV (data not shown).
Figure 1.
GHB increases the whole-cell inward rectifier current in rat ventricular myocytes. (A) Current recordings in control conditions; voltage clamps from a holding potential of −60 mV to test potentials ranging from −110 to +20 mV in 10-mV steps were 900 ms in duration. (B) After exposure to 2 mM GHB for 5 min. (C) GHB-induced currents obtained by subtracting control traces from traces recorded in the presence of GHB. (D) Current–voltage relations (Iss–V; n = 7) in the absence (○) and presence (●) of 2 mM GHB. *, At −110 mV, P = 0.01. (E) Mean slope conductances (Gss) calculated from the linear portion of Iss–V relations during control and after exposure to 2 mM GHB. *, P < 0.01. (F) Concentration–response relation for GHB activation of Iss at −110 mV, expressed as the percentage increase from the control level. GHB concentration is represented in μM. Each point is the average response from three to seven cells ± SEM. The sigmoidal line is the best least-squares fit to Emax/(1 + (EC50/[GHB])n), where Emax is the maximum effect of GHB, EC50 is the half-maximally effective concentration of GHB, and n is the Hill coefficient. Emax was 132%, and EC50 was 828 μM, with a Hill coefficient of 1.2.
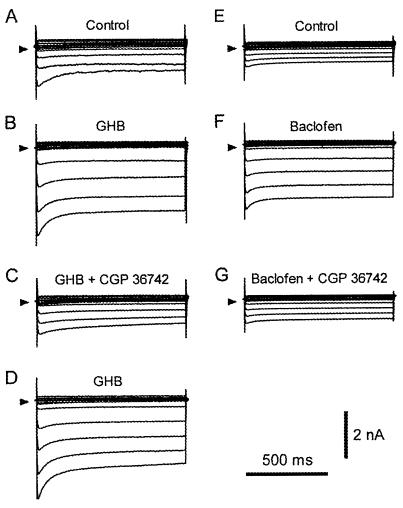
The GHB-induced electrophysiological effects in cardiomyocytes were mimicked by baclofen, a selective GABAB agonist (20, 21), and reversibly inhibited by GABAB antagonists, a response pattern already detected in neurons (10, 12, 13). After control recordings (Fig. 2A), 2 mM GHB was applied. Traces displayed in Fig. 2B were recorded 6 min later. The further application of 500 μM CGP36742, a selective GABAB receptor antagonist, abolished the action of GHB after 2 min (Fig. 2C). The inhibitory effect of CGP36742 was fully reversed 5 min after its removal while 2 mM GHB superfusion continued (Fig. 2D). Similar results were obtained in three other cardiomyocytes, and we ensured that CGP36742 did not cause any electrophysiological effect in the absence of exogenous agonist (n = 5; data not shown). The application of baclofen and CGP36742 in another set of cardiomyocytes yielded identical response patterns (Fig. 2 E–G). Baclofen, which exhibits an EC50 of 3–5 μM for GABAB receptor-activated inwardly rectifying K+ currents in neurons (20, 21), was used at 20 μM concentration. From control conditions (Fig. 2E), the background current increased 3-fold 5 min after baclofen superfusion was started (Fig. 2F). Then the further addition of 500 μM CGP36742 induced a clear, although partial, inhibition of the baclofen effect (Fig. 2G). The inhibitory effect of CGP36742 was reversible on washout within 5 min. Comparable results were obtained in three other cells. As a whole, the 20 μM baclofen-induced increases in Iss and Gss were comparable to those caused by 2 mM GHB superfusion (n = 4). Iss increased from −6.2 ± 1.8 to −11.7 ± 2.4 pA/pF, and Gss increased from 155.3 ± 40.0 to 290.5 ± 67.5 pS/pF in the presence of baclofen. GHB and baclofen were not additive, inasmuch as their coapplication showed no further increase in the current response induced by the individual agonists (data not shown).
Figure 2.
Reversibility of GHB- and baclofen-induced current changes with a specific GABAB antagonist. (A) Control conditions before GHB application. (B) Currents recorded after exposure to 2 mM GHB for 6 min. (C) Recordings obtained after a 2-min superfusion with a solution containing both 2 mM GHB and 500 μM CGP36742. (D) Washout with a 2 mM GHB solution for 5 min. (E) Control conditions before baclofen application. (F) Currents recorded after exposure to 20 μM baclofen for 5 min. (G) Recordings after a 3-min superfusion with a solution containing both 20 μM baclofen and 500 μM CGP36742. Arrows indicate the zero level.
Assuming that GHB- and baclofen-induced effects were mediated by cardiac GABAB receptor isoforms, we tested the hypothesis that, as in neurons, pertussis toxin-sensitive G proteins are required for coupling to the potassium conductance (20, 21). Accordingly, cardiomyocytes were preincubated for 4 h in a pertussis toxin-containing solution before being tested with either 2 mM GHB or 20 μM baclofen. In neither case was an increase in potassium conductance observed in these pretreated cells. Current density at −110 mV was −8.3 ± 2.5 pA/pF in control conditions and −7.8 ± 1.5 pA/pF during GHB superfusion; Gss was 195.7 ± 58.7 and 189.3 ± 50.2 pS/pF before and during GHB superfusion, respectively (n = 3). Very similar results were observed with 20 μM baclofen (n = 3).
Receptor Protein Expression and mRNA Detection.
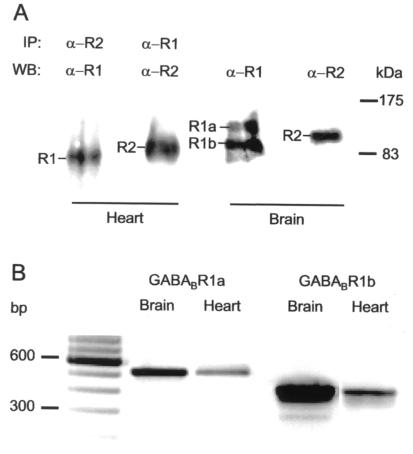
The presence of GABAB receptors was demonstrated by Western blot analysis of whole-cell lysates prepared from isolated cardiomyocytes and hippocampus, using Ab174.1 and AbC22 antibodies specific for GABABR1 and GABABR2 proteins, respectively (Fig. 3A). Immunoprecipitation with antibody incubation was required before Western blotting to obtain evidence of protein expression in cardiomyocytes. In contrast, no such procedure was needed beforehand to reveal GABAB proteins in hippocampal tissue. The existence of heteromeric (and hence functional) complexes assembling GABABR1 and GABABR2 was confirmed in coimmunoprecipitation experiments. Immunoprecipitation of GABABR2 from solubilized cell fractions with anti-GABABR2 antibody led to detection of GABABR1 with anti-GABABR1 antibody (lane 1). Conversely, GABABR2 was detected with anti-GABABR2 antibody in material immunoprecipitated with antibody specific for GABABR1 protein (lane 2). As expected (14, 17, 33), two proteins of about 100 kDa and 130 kDa denoting GABABR1a and GABABR1b isoforms (lane 3) and a band around 100 kDa signaling GABABR2 (lane 4) were identified in the hippocampus. As only one band at about 100 kDa, putatively corresponding to the GABABR1b brain isoform, was visualized in cardiomyocytes (lane 1), we wondered whether transcription follows a similar pattern in heart. Because cardiac mRNA for the GABABR1 receptor was not detected by Northern blotting (14), we used the more sensitive method of RT-PCR to detect mRNA expression. Oligonucleotide primers, designed to selectively amplify from the unique 5′ ends of the GABABR1a or -1b mRNAs, detected both splice isoforms in whole brain total RNA (Fig. 3B). The identity of the amplified cDNAs was confirmed by size fractionation of restriction digestion products (data not shown). Both products were also detected after RT-PCR of total RNA extracted from freshly isolated adult cardiac myocytes, although the yield of PCR product seemed to indicate that the levels of mRNA encoding GABABRs 1a and 1b were considerably lower in the heart than in the brain. Therefore, these data suggest a differential expression of GABAB R1 receptors in the heart in which only one GABABR1 isoform appears to be expressed at the protein level.
Figure 3.
Detection of GABABR expressions and GABABR1 transcripts in heart and brain. (A) In whole-cell lysates of rat cardiomyocytes, AbC22 directed against GABABR2 coprecipitates GABABR1 detected on the immunoblot with Ab174.1 antibody (lane 1), whereas Ab174.1 directed against GABABR1 coprecipitates GABABR2 detected with AbC22 antibody (lane 2). IP, immunoprecipitation; WB, Western blot. Thin bars point to protein bands attributed to GABABR1 (R1) and GABABR2 (R2) subtypes. α-R1 and α-R2, antibodies directed against R1 and R2, respectively. In whole-cell lysates from hippocampal cells, immunoblots confirmed the presence of GABABR1a (R1a) and GABABR1b (R1b) isoforms (lane 3) and GABABR2 (lane 4) receptor. (B) Northern blot analysis of GABABR1a and -R1b transcripts in rat cardiomyocytes (Heart) and hippocampal tissue (Brain). Receptor mRNAs were amplified by RT-PCR, using primers specific to the different 5′ ends of the two isoforms, and the products were size fractionated on 2% agarose gels for comparison with a 100-bp DNA ladder (first lane). The expected product sizes for R1a and R1b isoforms were 524 and 426 bp, respectively. Each blot is representative of three experiments.
Spatial Distribution of GABABRs in the Cardiomyocyte.
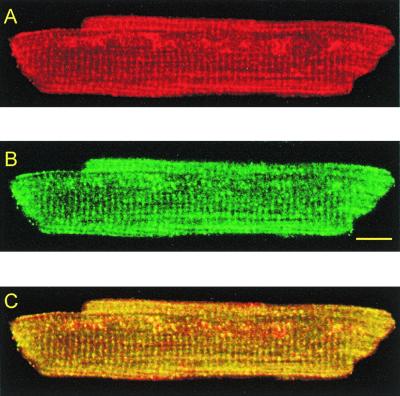
We further studied the subcellular distribution of GABABRs by laser scanning confocal microscopy, using fluorescent antibodies. GABABR1 (Fig. 4A) and GABABR2 (Fig. 4B) were colabeled with two specific primary antibodies, tagged with secondary antibodies emitting a fluorescent signal at wavelengths of 575 nm and 520 nm, respectively. The focal plane passed close to the middepth of the cell. Images disclosed an approximately rectangular array in which transverse striations showed a sarcomeric distribution, with a distance between striations of 1.85 ± 0.01 μm (n = 11) that is similar to the sarcomere length usually measured in resting cells. This transverse and longitudinal striated pattern closely resembled the picture of the transverse-axial tubular system (37). Hence, both receptors are expressed on the sarcolemma and the t-system, and this accounts for the electrophysiological results described above. The superimposition of the two fluorescent signals (Fig. 4C) resulted in a predominant yellow tone image reflecting an identical membrane distribution of the two receptors. This observation is in agreement with the functional colocalization and coexpression of GABABR1/R2 as described in neurons (17).
Figure 4.
Distribution and colocalization of GABABR1 and GABABR2 on a rat ventricular myocyte by dual-wavelength laser scanning confocal microscopy. Images are from a focal plane sampled at mid-cell depth. (A) Distribution of GABABR1 revealed by the emitted fluorescence of the secondary antibody Alexa Fluor 546. (B) Distribution of GABABR2 revealed by the emitted fluorescence of the secondary antibody Alexa Fluor 488. (C) Dual-channel image of the same cell discloses a predominant yellow tone due to the identical distribution of the fluorescent labels for GABABR1 and GABABR2. (Scale bar: 10 μm.)
Discussion
The present study demonstrates that GABAB agonists can induce significant electrophysiological alterations in isolated mammalian cardiomyocytes. Millimolar concentrations of GHB, similar to levels commonly observed during anesthesia in humans (30, 31), and micromolar doses of baclofen clearly increase the background inward rectifier conductance. This effect is reversibly inhibited by GABAB antagonists, prevented by pertussis toxin pretreatment, and appears to be mediated by GABABR1 and GABABR2 receptors colocalized within the sarcolemma and the transverse axial-tubular system of the cardiomyocyte.
Cardiac Electrophysiological Effects of GABAB Agonists.
Our findings are reminiscent of observations reported for neurons or heterologous expression vectors (10, 13, 19–25, 36), in that both GHB (at millimolar concentrations) and baclofen (at micromolar concentrations) increase an inwardly rectifying K+ conductance in a dose-dependent manner. Thus, GHB elicits specific GABAB-type responses in neurons in the same way as baclofen, but with lower affinity at GABABRs (19). We exclusively used the very specific GABAB agonist baclofen instead of GABA. The two compounds share similar binding constants and effective concentrations at GABABRs (21, 38); however, GABA also binds with equal affinity to GABAA receptors, and a specific GABAB activation with GABA can be obtained only in the presence of GABAA antagonists (20). Our assumption that GABABRs are present and behave like targets for GABAB agonists in cardiomyocytes was founded on two facts. First, specific GHB binding sites are activated by micromolar concentrations of GHB in neurons (3) but are absent in the myocardium (4). Second, EC50 values in the same millimolar range were found both at native GABAB receptors on dopaminergic neurons (10) and in our experiments when GHB-induced electrophysiological changes were measured; EC values were even much greater in heterologous expression vectors (19). Other features pertaining to the neuronal behavior of GABABR-induced inward rectifier current are also observed in cardiomyocytes; they include sensitivity to Ba2+ ions (20, 21), reversible inhibition of GHB or baclofen effects by specific GABAB antagonists (9, 10, 12, 13, 20, 36), and suppression of electrophysiological GABABR-mediated alterations by pertussis toxin pretreatment of neurons (20). Comparable responses to GABAB antagonists after GHB or baclofen applications have been obtained in heterologous expression of GABABRs and Kir3 channels (19). Indeed, the electrophysiological changes after GABAB agonist application, and the strong inwardly rectifying property of the GHB-induced current (Fig. 1C) together with its sensitivity to Ba2+, suggest that this current might be caused by the opening of Kir3 channels as described for postsynaptic inhibition after GABABR activation (20–25). Assuming that Kir3 channels might be the final effectors in cardiomyocytes could have been in contradiction to the classical notion that these channels are absent from mammalian ventricle (39). In fact, ample experimental evidence has recently been accumulated that Kir3 channel density is far from negligible in mammalian tissues, including human ventricle (40–42).
However, the long delay required (5–7 min) to reach full current activation in cardiomyocytes after GHB or baclofen is applied is unexpected if we accept the assumption of a short-cut membrane delimited pathway for current gating through pertussis toxin-sensitive G protein-coupled receptors (20). In fact, the involvement of second messenger systems affected by G protein subunits released by GABABR activation may also result in delayed responses occurring only after several minutes in hippocampal cells (20, 43). We may only speculate that such a mechanism is also present in cardiac cells.
Expression and Subcellular Distribution of GABABRs.
Western blots of whole-cell lysates from isolated cardiomyocytes exhibit bands at molecular weights slightly lower than those corresponding to brain isoforms (Fig. 3A). Besides possible artifacts, this pattern could be related to the existence of other GABABR splice variants in the myocardium. As concerns the levels of GABABR1a and -R1b transcripts, the compared yield of RT-PCR in brain versus cardiomyocytes suggests that mRNA expression is much lower in the heart. This is in keeping with the results of Northern blot analyses previously performed by Kaupmann et al. (14, 24). Indeed, by using RT-PCR techniques it has been shown lately that GABABR1a and -R1b transcripts are expressed not only in brain and heart but also in a wide range of peripheral organs (44). In the same view, the need for immunoprecipitation with antibody incubation before Western blotting, to obtain evidence of protein expression in cardiomyocytes in contrast to hippocampal neurons, supports the idea that GABABR expression is also less in the heart than in nerve tissue. Up to now there was little evidence for any physiological function for GABAB mechanisms in peripheral organs (29). In the present work, we demonstrate that GABABR1 and GABABR2 are actually colocalized, functional, and identically distributed along the sarcolemma and the t-system of cardiomyocytes (Fig. 4), following a pattern very similar to that found for other cardiac sarcolemmal proteins (32, 45).
Physiological and Clinical Significance.
Our results indicate that heteromeric GABABR1/R2 receptors are present in mammalian heart and appear to be functionally coupled to G proteins of the Gαi class. We know of no other description of a GABAB-dependent activation in nonneural excitable cells, even though GABAB binding sites have been reported in rabbit oviduct and uterus (46). Kir3 channels, which were demonstrated to be present in mammalian ventricle (40–42), are likely the main effectors of the system. As their activation plays an important role in cardiac excitability, we speculate that GABABRs are possibly involved in this property. Endogenous GHB levels relatively higher in the heart than in brain (26) might raise the question of a possible activation of cardiac Kir3 channels through a hypothetical autocrine/paracrine process, although there is as yet no experimental evidence for such a mechanism. In contrast, endogenous GHB might assume a cardioprotective function, as suggested by a previously demonstrated GHB hypoxia-sparing effect (27, 28).
In the clinical field, it is of great interest that hypnotic doses of GHB required to induce anesthesia in humans are able to produce significant electrophysiological alterations in excitable cardiac cells. Further deleterious effects on cellular mechanisms are likely to occur in the course of intoxication by GHB overdose, inasmuch as bradycardia, hypotension, and ECG abnormalities have been reported in these clinical settings (47, 48).
Acknowledgments
We are greatly indebted to Dr. M. Pucéat for helpful criticisms of the manuscript and support with molecular biology and to C. Bony for her technical assistance. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Association Française contre les Myopathies (MNM 6086, 1997), Hoechst–Marion–Roussel, and a Fondation pour la Recherche Médicale grant (to A.L.).
Abbreviations
- GHB
γ-hydroxybutyrate
- GABA
γ-aminobutyrate
- GABABR
GABA type B receptor
- Kir3
inwardly rectifying K+ channel of subfamily 3 (formerly GIRK)
- Iss
steady-state current measured at the end of a test potential
- Gss
slope conductance
- RT-PCR
reverse transcription with PCR
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Laborit H. Prog Neurobiol. 1973;1:257–274. [Google Scholar]
- 2.Snead O C. Neuropharmacology. 1991;30:161–167. doi: 10.1016/0028-3908(91)90199-l. [DOI] [PubMed] [Google Scholar]
- 3.Maitre M. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 4.Snead O C, Liu C C. Biochem Pharmacol. 1984;33:2587–2590. doi: 10.1016/0006-2952(84)90629-4. [DOI] [PubMed] [Google Scholar]
- 5.Vayer P, Mandel P, Maitre M. Life Sci. 1987;41:1547–1557. doi: 10.1016/0024-3205(87)90721-1. [DOI] [PubMed] [Google Scholar]
- 6.Cash C D. Neurosci Biobehav Rev. 1994;18:291–304. doi: 10.1016/0149-7634(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 7.Feigenbaum J J, Howard S G. Prog Neurobiol. 1996;50:1–7. doi: 10.1016/0301-0082(96)00029-9. [DOI] [PubMed] [Google Scholar]
- 8.Snead O C. Biochem Pharmacol. 1996;52:1235–1243. doi: 10.1016/0006-2952(96)00477-7. [DOI] [PubMed] [Google Scholar]
- 9.Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H. Eur J Pharmacol. 1997;321:67–75. doi: 10.1016/s0014-2999(96)00916-8. [DOI] [PubMed] [Google Scholar]
- 10.Madden T E, Johnson S W. J Pharm Exp Ther. 1998;287:261–265. [PubMed] [Google Scholar]
- 11.Erhardt S, Andersson B, Nissbrandt H, Engberg G. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;357:611–619. doi: 10.1007/pl00005215. [DOI] [PubMed] [Google Scholar]
- 12.Williams S R, Turner J P, Crunelli V. Neuroscience. 1995;66:133–141. doi: 10.1016/0306-4522(94)00604-4. [DOI] [PubMed] [Google Scholar]
- 13.Xie X, Smart T G. Eur J Pharmacol. 1992;212:291–294. doi: 10.1016/0014-2999(92)90347-7. [DOI] [PubMed] [Google Scholar]
- 14.Kaupmann K, Huggel K, Held J, Flor P J, Bischoff S, Mickel S J, McMaster G, Angst C, Bittiger H, Froestl W, et al. Nature (London) 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 15.Jones K A, Borowsky B, Tamm J A, Craig D A, Durkin M M, Dai M, Yao W, Johnson M, Gunwaldsen C, Huang L Y, et al. Nature (London) 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 16.White J H, Wise A, Main M J, Green A, Fraser N J, Disney G H, Barnes A A, Emson P, Foord S M, Marshall F H. Nature (London) 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 17.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. Nature (London) 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 18.Kuner R, Köhr G, Grünewald S, Eisenhardt G, Bach A, Kornau H C. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 19.Lingenhoehl K, Brom R, Heid J, Beck P, Froestl W, Klemens K, Bettler B, Mosbacher J. Neuropharmacology. 1999;38:1667–1673. doi: 10.1016/s0028-3908(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 20.Misgeld U, Bijak M, Jarolimek W. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- 21.Sodickson D L, Bean B P. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrengruber M U, Doupnik C A, Xu Y, Garvey J, Jasek M C, Lester H A, Davidson N. Proc Natl Acad Sci USA. 1997;94:7070–7075. doi: 10.1073/pnas.94.13.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lüscher C, Jan L Y, Stoffel M, Malenka R C, Nicoll R A. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 24.Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H, Heid J, Froestl W, Leonhard S, Pfaff T, Karschin A, et al. Proc Natl Acad Sci USA. 1998;95:14991–14996. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarolimek W, Bäurle J, Misgeld U. J Neurosci. 1998;18:4001–4007. doi: 10.1523/JNEUROSCI.18-11-04001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson T, Kaufman E, Kline J, Sokoloff L. J Neurochem. 1981;37:1345–1348. doi: 10.1111/j.1471-4159.1981.tb04689.x. [DOI] [PubMed] [Google Scholar]
- 27.Mamelak M. Neurosci Biobehav Rev. 1989;13:187–198. doi: 10.1016/s0149-7634(89)80053-3. [DOI] [PubMed] [Google Scholar]
- 28.Kolin A, Brezina A, Mamelak M, Pandula E. Int J Exp Pathol. 1993;74:275–281. [PMC free article] [PubMed] [Google Scholar]
- 29.Bowery N G. In: The GABA Receptors. Enna S J, Bowery N G, editors. Totowa, NJ: Humana; 1997. pp. 209–236. [Google Scholar]
- 30.Helrich M, McAslan T C, Skolnik S, Bessman S P. Anesthesiology. 1964;25:771–775. doi: 10.1097/00000542-196411000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Hoes M J, Vree T B, Guelen P J. Encephale. 1980;6:93–99. [PubMed] [Google Scholar]
- 32.Pucéat M, Korichneva I, Cassoly R, Vassort G. J Biol Chem. 1995;270:1315–1322. doi: 10.1074/jbc.270.3.1315. [DOI] [PubMed] [Google Scholar]
- 33.Malitschek B, Rüegg D, Heid J, Kaupmann K, Bittiger H, Froestl W, Bettler B, Kuhn R. Mol Cell Neurosci. 1998;12:56–64. doi: 10.1006/mcne.1998.0698. [DOI] [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 35.Richards S, Jaconi M, Vassort G, Pucéat M. J Cell Sci. 1999;112:1519–1528. doi: 10.1242/jcs.112.10.1519. [DOI] [PubMed] [Google Scholar]
- 36.Bon C, Galvan M. Br J Pharmacol. 1996;118:961–967. doi: 10.1111/j.1476-5381.1996.tb15493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soeller C, Cannell M B. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 38.Bowery N G. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 39.Kurachi Y. Am J Physiol. 1995;269:C821–C830. doi: 10.1152/ajpcell.1995.269.4.C821. [DOI] [PubMed] [Google Scholar]
- 40.Koumi S I, Wasserstrom J A. Am J Physiol. 1994;266:H1812–H1821. doi: 10.1152/ajpheart.1994.266.5.H1812. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z K, Boyett M R, Janvier N C, McMorn S O, Shui Z, Karim F. J Physiol (London) 1996;492:789–806. doi: 10.1113/jphysiol.1996.sp021346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koumi S, Sato R, Nagasawa K, Hayakawa H. J Membr Biol. 1997;157:71–81. doi: 10.1007/s002329900217. [DOI] [PubMed] [Google Scholar]
- 43.Premkumar L S, Chung S H, Gage P W. Proc R Soc London Ser B. 1990;241:153–158. doi: 10.1098/rspb.1990.0079. [DOI] [PubMed] [Google Scholar]
- 44.Castelli M P, Ingianni A, Stefanini E, Gessa G L. Life Sci. 1999;64:1321–1328. doi: 10.1016/s0024-3205(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 45.Frank J S, Mottino G, Reid D, Molday R S, Philipson K D. J Cell Biol. 1992;117:337–345. doi: 10.1083/jcb.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdö S L. In: GABAergic Mechanisms in the Mammalian Periphery. Erdö S L, Bowery N G, editors. New York: Raven; 1986. pp. 205–222. [Google Scholar]
- 47.Chin R L, Sporer K A, Cullison B, Dyer J E, Wu T D. Ann Emerg Med. 1998;31:716–722. [PubMed] [Google Scholar]
- 48.Li J, Stokes S A, Woeckener A. Ann Emerg Med. 1998;31:723–728. doi: 10.1016/s0196-0644(98)70231-8. [DOI] [PubMed] [Google Scholar]