Abstract
Tissue invasion during metastasis requires cancer cells to negotiate a stromal environment dominated by cross-linked networks of type I collagen. Although cancer cells are known to use proteinases to sever collagen networks and thus ease their passage through these barriers, migration across extracellular matrices has also been reported to occur by protease-independent mechanisms, whereby cells squeeze through collagen-lined pores by adopting an ameboid phenotype. We investigate these alternate models of motility here and demonstrate that cancer cells have an absolute requirement for the membrane-anchored metalloproteinase MT1-MMP for invasion, and that protease-independent mechanisms of cell migration are only plausible when the collagen network is devoid of the covalent cross-links that characterize normal tissues.
Barriers to cell migration: prevailing theories
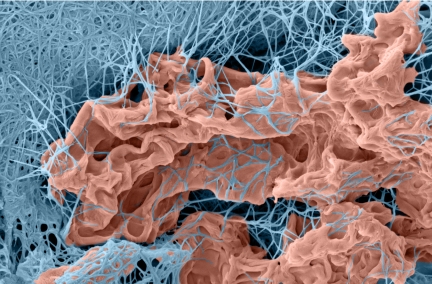
3D cancer cell invasion. Multicellular spheroids of HT-1080 fibrosarcoma cells were embedded within 3D gels of native type I collagen for 3 d. Gels were fixed in 2% glutaraldehyde/1.5% paraformaldehyde in 0.1 M sodium cacodylate buffer, freeze-fractured, and processed for SEM. Images of infiltrating cells and the surrounding ECM were digitally imaged using XStream imaging software.
SASHA MESHINCHI
After neoplastic transformation, cancer cells infiltrate local tissues and initiate metastatic programs by trafficking through a stromal extracellular matrix (ECM) dominated by cross-linked networks of type I collagen, the major extracellular protein found in mammals (Brown et al., 2003; Sabeh et al., 2004; Demou et al., 2005; Oldberg et al., 2007; Magzoub et al., 2008). To negotiate this structural barrier, cancer cells have been proposed to use either protease-dependent or protease-independent invasion schemes (Wolf et al., 2003a,b, 2007; Sabeh et al., 2004; Wilkinson et al., 2005; Carragher et al., 2006; Wyckoff et al., 2006; Gaggioli et al., 2007; Croft and Olson, 2008; Gadea et al., 2008; Li et al., 2008; Pinner and Sahai, 2008; Sanz-Moreno et al., 2008; Packard et al., 2009). Protease-dependent invasion programs rely on matrix metalloproteinase (MMP) family members to cleave impeding collagen fibrils (Sabeh et al., 2004; Fisher et al., 2006; Hotary et al., 2006; Itoh and Seiki, 2006; Li et al., 2008; Packard et al., 2009). Alternatively, negotiation of collagenous barriers has been reported to proceed in a protease-independent fashion, whereby cancer cells use actomyosin-based mechanical forces to physically displace matrix fibrils while coordinately adopting an amoeboid-like cell shape similar to that observed in myeloid cell populations (Friedl and Wolf, 2003; Wolf et al., 2003a,b, 2007; Wilkinson et al., 2005; Carragher et al., 2006; Wyckoff et al., 2006; Gadea et al., 2008; Pinner and Sahai, 2008; Sanz-Moreno et al., 2008). Indeed, in recent clinical trials, the failure of MMP inhibitors to prevent cancer progression suggests that protease-independent mechanisms of invasion may be physiologically relevant in vivo (Friedl and Wolf, 2003; Wilkinson et al., 2005; Sahai et al., 2007; Wolf et al., 2007). However, descriptions of proteinase-independent, amoeboid-like cancer cell behavior are largely drawn from in vitro assays using model, three-dimensional (3D) ECM constructs that may not recapitulate the key structural characteristics displayed by native type I collagen networks in vitro or in vivo (Sabeh et al., 2004; Demou et al., 2005; Hotary et al., 2006; Packard et al., 2009). As such, the relative importance of protease-dependent and -independent invasion modalities mobilized during cancer cell trafficking through interstitial barriers remains a subject of considerable debate.
MT1-MMP, synthetic MMP inhibitors, and the 3D cancer cell invasion program
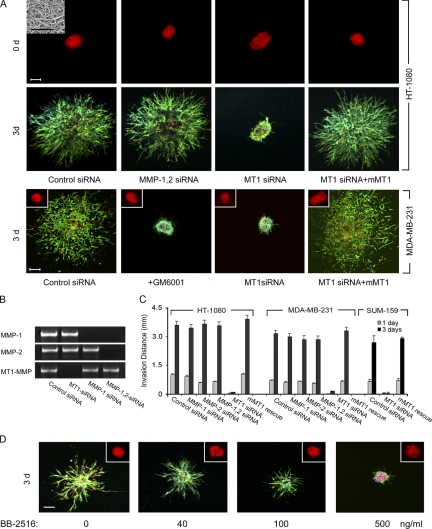
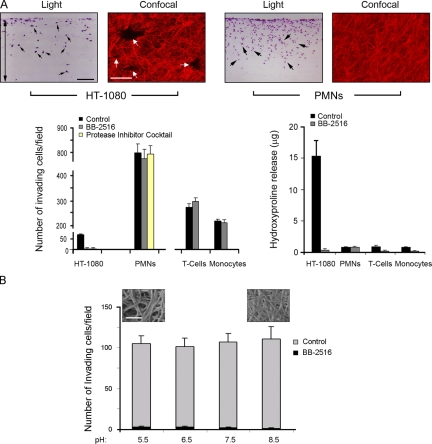
To recreate an in vivo–like environment for migrating cancer cells, multicellular spheroids of HT-1080 fibrosarcoma cells were embedded within 3D gels of native type I collagen (Hotary et al., 2003; Sabeh et al., 2004; Li et al., 2008). HT-1080 cells subsequently activate a tissue-invasive program and infiltrate the surrounding ECM in a “starburst” pattern (Fig. 1 A). Migrating HT-1080 cells express at least three distinct type I collagenolytic systems under these conditions: the secreted metalloenzymes, MMP-1 and MMP-2, as well as the membrane-anchored proteinase, MT1-MMP (Fig. 1 B) (Sabeh et al., 2004; Li et al., 2008). After siRNA-dependent silencing of both MMP-1 and MMP-2 in combination, HT-1080 spheroids retain full invasive activity (Fig. 1, A–C). In contrast, MT1-MMP silencing blocks virtually all invasive activity over a 3-d culture period (Fig. 1 A). Invasive activity is, however, rescued when MT1-MMP–silenced cells are electroporated with an expression vector for mouse MT1-MMP, an orthologue that escapes targeting by the human-specific MT1-MMP siRNA (Fig. 1, A and C). Similar results are obtained with MT1-MMP–silenced MDA-MB-231 breast cancer cells or the breast cancer stem cell–enriched SUM-159 line (Fig. 1, A and C) (Korkaya et al., 2008).
Figure 1.
Regulation of cancer cell–invasive phenotype in 3D type I collagen by MT1-MMP. (A) Di-I–labeled multicellular spheroids (150–200 µm in diameter) of HT-1080 cells or MDA-MB-231 cells were prepared in hanging droplets (Kelm et al., 2003) after electroporation with a control siRNA, MMP-1 and MMP-2 siRNAs, MT1 siRNA (50–100 nM; siRNA sequences can be found in Li et al. [2008] and Sabeh et al. [2004]), or cotransfected with MT1 siRNA and either mouse (m)MT1-MMP or a control expression vector (0.5 µg each in PCR3.1 Uni; gift of M. Seiki, University of Tokyo, Tokyo, Japan; Sabeh et al., 2004; Li et al., 2008), and embedded in 3D type I collagen gels (prepared from acid extracts of rat tail tendon as described previously [Sabeh et al., 2004] and visualized by SEM in the left-hand panel, top corner inset; bar = 5 µm) at a final concentration of 2.2 mg/ml in the absence or presence of 10 µM GM6001. Invasion was monitored by fluorescence microscopy at 0 d (top row of panels) and by confocal microscopy at 3 d after staining with Alexa-488 phalloidin and DAPI (bottom row of panels; Di-I, red; Alexa-488 phalloidin, green; DAPI, blue). For MDA-MB-231 cells (bottom row of panels), spheroid at 0 d are shown in the insets. Bar = 100 µm. (B) MT1-MMP, MMP-1, and MMP-2 siRNAs inhibited expression of the targeted MMPs in HT-1080 cells, but had no effect on the expression of nontargeted MMPs as shown by RT-PCR. (C) Invasion distance (quantified as described previously; [Hotary et al., 2000; Sabeh et al., 2004]) from the inoculation site into the surrounding matrix was monitored at 1 d and 3 d for HT-1080, MDA-MB-231, or SUM-159 cells. In the presence of a protease inhibitor cocktail directed against serine, aspartyl, cysteinyl, and matrix metallo-proteinases (Wolf et al., 2003a), HT-1080 invasion was inhibited by 99 ± 0%. Results are expressed as mean ± SEM (n = 4). (D) HT-1080 cell spheroids were embedded in type I collagen gels in the absence or presence of the indicated doses of BB-2516 for 3 d. Insets show the spheroid at 0 d after Di-I labeling and the migration of the cells after 3 d is shown in the bottom row of companion panels. Bar = 100 µm. The percentage of inhibition of invasion at 40, 100, and 500 ng/ml (1.5 µM) BB-2516 was 59 ± 4%, 86 ± 2%, and 99 ± 0%, respectively.
The impact of silencing MT1-MMP alone on cancer cell–invasive activity stands in contrast with the failure of peptidomimetic MMP inhibitors (e.g., BB-2516/marimastat) to exert beneficial effects in previously reported clinical cancer trails (Overall and Kleifeld, 2006). Importantly, however, the peak plasma concentrations recorded in most patients (i.e., ∼10–40 ng/ml) (Sparano et al., 2004) falls below the concentration required to completely block MT1-MMP–dependent invasive activity in 3D assays (i.e., ∼500 ng/ml; Fig. 1 D). Thus, the ineffectiveness of MMP inhibitors to exert palliative effects in cancer patients may reflect a requirement for drug concentrations in excess of those achievable in the clinical setting, and does not necessarily support the existence of protease-independent invasive activity in vivo.
Type I collagen cross-links dictate the rules of invasion
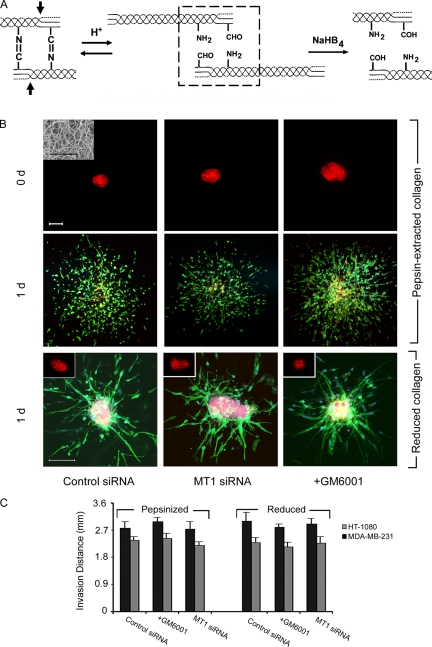
The inability of MT1-MMP–silenced HT-1080 or MDA-MB-231 cells to adopt an invasive, amoeboid phenotype-type when embedded in 3D type I collagen gels contradicts recent reports using identical cell lines (Wolf et al., 2003a,b, 2007; Demou et al., 2005; Carragher et al., 2006). However, matrices used in these studies were reconstituted from pepsin-extracted type I collagen (e.g., Vitrogen), a proteolytic process that removes the nonhelical telopeptides situated at the N- and C-terminal ends of native collagen molecules (Fig. 2 A) (Wolf et al., 2003a,b, 2007; Demou et al., 2005; Carragher et al., 2006; Gadea et al., 2008; Sanz-Moreno et al., 2008). These collagen telopeptides play important roles in fibrillogenesis and contain critical lysine residues which, after lysyl oxidase-dependent oxidation in vivo, support the intermolecular covalent cross-links necessary for stabilizing gel architecture (Gelman et al., 1979; Brennan and Davison, 1980; Eyre et al., 1984; Woodley et al., 1991; Christiansen et al., 2000; Sato et al., 2000). It is therefore possible that the ability of cancer cells to display proteinase-independent, amoeboid activity may be affected by the structural integrity of the reconstituted collagen gel used for in vitro assay.
Figure 2.
MT1-MMP–independent tumor cell invasion through telopeptide-excised or cross-link–deficient type I collagen gels. (A) Schematic of type I collagen intermolecular cross-links. In vivo, lysyl oxidase generates aldehyde moieties within the N- and C-terminal telopeptide domains of type I collagen, which are arrayed across from ϵ-amino groups (boxed region) that spontaneously condense to conform Schiff base, aldimine cross-links in vivo. N- and C-terminal telopeptides are removed during pepsin extraction (arrows). Under acidic extraction conditions, Schiff base formation is reversed to generate the starting aldehyde and amine groups. After treatment with sodium borohydride, aldehydes are converted to alcohols that no longer support cross-linked formation. (B) Di-I-labeled multicellular spheroids of HT-1080 cells, electroporated with control or MT1 siRNA, were embedded in 3D gels prepared from either pepsin-extracted bovine dermis (Vitrogen; 2.2 mg/ml) or pepsin-extracted rat tail tendon and monitored by fluorescence microscopy at 0 d (top row of panels) and after 1 d (second row of panels; Di-I, red; Alexa-488 phalloidin, green; DAPI, blue). SEM of pepsin-extracted collagen gels is shown in the inset of a 0-d spheroid electroporated with control siRNA (left-hand panel in the first row; bar = 5 µm). GM6001 (10 µM) was added to the cultures at 0 d in the right-hand column of panels. Bar, 100 µm. In the bottom row of panels, Di-I-labeled HT-1080 spheroids were embedded in borohydride-reduced, rat tail type I collagen gels (2.2 mg/ml) at 0 d (insets) and invasion monitored 1 d later after electroporation with a control siRNA, MT1-MMP siRNA or after culture in the presence of GM6001. Collagen aldehyde content before and after borohydride reduction was determined spectrophotometrically (Williams et al., 1978; Gelman et al., 1979). After the 1-d culture period, cells were stained with Alexa-488 phalloidin and DAPI as described above. Bar, 100 µm. (C) HT-1080 or MDA-MB-231 spheroids were cultured for 1 d in 3D pepsinized or reduced type I collagen and invasion distance into the surrounding matrix quantified. HT-1080 invasion in the presence of a protease inhibitor cocktail (Wolf et al., 2003a) in pepsinized or reduced collagen gels was inhibited by 0 ± 0% and 8 ± 1%, respectively. Results are expressed as mean ± SEM (n = 4). Acid-extracted, pepsin-extracted, and reduced collagen fibrils are equally trypsin resistant (not depicted).
Following gel formation under conditions identical to those used for full-length, native type I collagen, pepsin-extracted collagen form fibrillar networks indistinguishable from those obtained with telopeptide-intact collagen as assessed by SEM (Fig. 2 B). However, whereas control collagen gels are insoluble in high salt buffers, collagen matrices prepared from pepsin-extracted material rapidly solubilize, a physicochemical behavior characteristic of cross-linked versus noncross-linked collagen gels, respectively (Gelman et al., 1979). HT-1080 spheroids embedded in pepsin-extracted collagen gels infiltrate the matrix in a single-cell fashion with the invasive front displaying a mix of both spheroid- and elongated-shaped cells (Fig. 2 B). Within 24 h, migrating HT-1080 cells invade the pepsin-extracted gels to distances that require a 72-h incubation period in full-length type I collagen matrices (Fig. 2 B vs. Fig. 1 C). Neither MT1-MMP silencing, the addition of the pan-specific MMP inhibitor GM6001, nor the inclusion of a broad-spectrum cocktail of proteinase inhibitors (Wolf et al., 2003a) exert inhibitory effects on either HT-1080 or MDA-MB-231 cell invasion (Fig. 2, B and C).
Type I collagen telopeptides potentially regulate invasion by either affecting the linear or lateral stages of the fibrillogenic process, or by supporting collagen cross-link formation (Gelman et al., 1979; Woodley et al., 1991; Christiansen et al., 2000; Sato et al., 2000). Intact collagen can be acid-extracted in almost pure form from tissues of young animals because a subset of the lysyl oxidase-catalyzed, collagen cross-links formed in vivo are acid-labile, Schiff base adducts (Fig. 2 A) (Eyre et al., 1984). At low pH these cross-links are reversibly broken, resulting in the formation of aldehyde-bearing collagen molecules that are fully soluble in low ionic strength, acidic buffers (Fig. 2 A) (Eyre et al., 1984). The Schiff base adducts that interconnect collagen bundles are reformed spontaneously when collagen gels are reconstituted at neutral pH, but this cross-linking process can be prevented by chemically reducing the aldehyde moieties before gel formation (Fig. 2 A) (Gelman et al., 1979). When HT-1080 cells are embedded in 3D gels of aldehyde-reduced type I collagen, the cells exhibit the more rapid speed of infiltration observed in pepsin-extracted collagen gels and neither MT1-MMP silencing nor the addition of GM6001 significantly inhibits HT-1080 or MDA-MB-231 invasion (Fig. 2, B and C). As the pore size of collagen gels prepared from intact and pepsin-extracted collagen are similar (Demou et al., 2005), we conclude that MT1-MMP–independent invasion only proceeds when the structural pores formed in collagen gel networks are no longer stabilized by the covalent cross-links that define fibril architecture and structural rigidity (Zaman et al., 2006).
Crossing an authentic stromal barrier: the human mammary gland interstitium
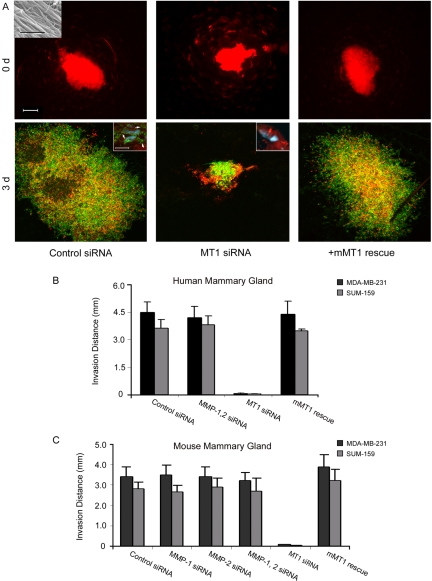
Reconstituted gels of native type I collagen provide important insights into the structural and mechanical properties that regulate cancer cell behavior, but the precise architecture of the fibrillar networks assembled in vivo cannot be duplicated fully in vitro (Christiansen et al., 2000; Raub et al., 2007, 2008). To define the relative roles of protease-dependent and -independent invasion programs in live tissue, we implanted MDA-MB-231 breast cancer cell spheroids into explants of the normal human mammary gland, and transplanted the composites atop the live chick chorioallantoic membrane (Sabeh et al., 2004). In this xenograft model, cancer cell spheroids embedded within the mammary gland rapidly infiltrate the surrounding tissues during a 72-h culture period (Fig. 3 A). Although both MMP-1 and MMP-2 can modulate breast cancer behavior in vivo (Tester et al., 2004; Wyatt et al., 2005; Gupta et al., 2007), silencing both proteases in tandem does not affect MDA-MB-231 or SUM-159 invasion (Fig. 3 B). MT1-MMP knockdown, in contrast, potently inhibits the ability of the breast cancer cells to infiltrate or degrade surrounding breast tissue (Fig. 3, A and B). The inhibitory effects exerted by the human-specific MT1-MMP siRNA are reversed by expressing mouse MT1-MMP in the siRNA-treated MDA-MB-231 or SUM-159 cells (Fig. 3, A and B). Hence, MT1-MMP not only supports 3D cancer cell invasion through reconstituted gels of cross-linked collagen, but also within the stromal environment of the mammary gland.
Figure 3.
MT1-MMP drives cancer cell invasion in human mammary gland explants. (A) MDA-MB-231 Di-I–labeled spheroids (comprised of cells electroporated with a control siRNA, MT1-MMP siRNA or MT1-MMP siRNA together and a mouse MT1-MMP expression vector) were injected (30-gauge needle) into human mammary gland explants (6 × 8 x 6 mm) and the position of the cancer cell aggregates recorded by fluorescence microscopy at 0 d. Bar = 20 µm. Inset shows SEM of type I collagen fibrils in mammary gland explant. Bar = 5 µm. The inoculated explants were then cultured atop the live chick CAM for 3 d. Invasive behavior of MDA-MB-231 cells was monitored by fluorescence microscopy after labeling of mammary gland tissues with Alexa-488 phalloidin and DAPI. Bar = 200 µm. Immunofluorescent staining of mammary tissue cross sections at d 3 (inserts) reveal tracts of denatured collagen detected with monoclonal antibody HU177 (Hotary et al., 2003; Sabeh et al., 2004) (green; arrows) surrounding invasive tumor cells (red), but not MT1-MMP–silenced cancer cells (bottom panels; bar = 10 µm). (B) Invasion distance for MDA-MB-231 or SUM-159 spheroids (electroporated with control siRNA, MMP-1 and MMP-2 siRNAs, MT1-MMP siRNA or MT1-MMP siRNA in combination with a mouse MT1-MMP expression vector) was quantified after a 3-d culture period in human mammary gland explants as described. Results are expressed as mean ± SEM (n = 3). (C) Invasion distance for MDA-MB-231 or SUM-159 spheroids (electroporated with control siRNA, MMP-1 and MMP-2 siRNAs, MT1-MMP siRNA or MT1-MMP siRNA in combination with a mouse MT1-MMP expression vector) was quantified after a 3-d culture period in mouse mammary gland explants as described. Results are expressed as mean ± SEM (n = 3).
Figure 4.
Collagen-invasive and degradative activities of cancer cells versus leukocytes. Light micrographs of cross sections of type I collagen gels (2.2 mg/ml) traversed by HT-1080 or human PMNs prepared as described (Huber and Weiss, 1989) for 3 d or 1 d, respectively. Collagen-invasive cells are H&E stained and marked with black arrows. Double-headed arrow marks the boundaries of the underlying collagen gel. Black bar = 100 µm. Laser confocal micrographs of HT-1080 cells cultured atop 3D gels of rhodamine-labeled type I collagen for 3 d demonstrate that invasion is associated with the formation of well-demarcated tunnels (white arrows; white bar = 50 µm). In contrast, PMNs stimulated with zymosan-activated plasma (Huber and Weiss, 1989) invade rhodamine-labeled collagen gels without perturbing matrix architecture (far right-hand panel). Invasion and collagen-degradative activities of HT-1080, PMNs, T cells, and monocytes (Reddy et al., 1995) were quantified in the absence or presence of BB-2516 (1.5 µM) as described previously (bar graphs, bottom panel) (Sabeh et al., 2004). PMN invasion was also assessed in the presence of the protease inhibitor cocktail prepared as described (Wolf et al., 2003a). Results are expressed as mean ± SEM (n = 4). (B) Acid-extracted, rat tail collagen gels were prepared at pH 5.5, 6.5, 7.5, and 8.5 as described (Raub et al., 2008) and the invasive activity of HT-1080 cells into the 3D gels was quantified in the absence or presence of BB-2516 (1.5 µM) after a 3-d culture period. Collagen at pH 5.5 and 8.5 as visualized by SEM are shown in the insets (n = 3; bar = 250 nm).
Cancer cells in vivo: never say never…
A requirement for MT1-MMP activity during stromal invasion in vitro or ex vivo contrasts with recent reports documenting amoeboid movement of breast cancer cells in the in vivo setting, particularly within the mouse mammary gland (Wyckoff et al., 2006, 2007; Xue et al., 2006; Sahai, 2007; Sahai et al., 2007). The density of collagen fibrils in the mouse mammary gland is lower than that displayed in humans (Parmar and Cunha, 2004), but we can likewise document a requirement for MT1-MMP in mouse explants (Fig. 3 C). In vivo models of real-time movement, however, monitor the short-term trafficking of cancer cells (i.e., observation periods ranging from 20 min to 4 h) from primary sites established 1–3 months in advance of microscopic analyses (Sahai, 2007; Sanz-Moreno et al., 2008). Under these conditions, rapid motility and protease-independent, amoeboid-like activity might be accommodated under specific circumstances.
Cancer cells could display rapid, protease-independent cell movement in vivo by co-opting preexisting matrix tunnels generated by tumor-infiltrating fibroblasts, smooth muscle cells, or endothelial cells that bore through tissues in an MT1-MMP–dependent process (Hiraoka et al., 1998; Chun et al., 2004; Sabeh et al., 2004; Filippov et al., 2005; Gaggioli et al., 2007). Likewise, damaged blood vessels or lymphatics as well as zones of necrosis could also present migrating cancer cells with “pre-cleared” passageways (Nagano et al., 2008). Indeed, rapid rates of cancer cell migration noted in several studies seem to occur within sites wherein the extensive loss of ECM networks has already occurred (Condeelis and Segall, 2003). In contrast, early phases of the tumor cell invasion program proceed through collagen-rich areas whose penetration would appear to be more consistent with the slower advance of proteolytically active neoplastic cell populations (Li et al., 2000; Curino et al., 2005; Provenzano et al., 2006, 2008a; Kedrin et al., 2008).

In vivo, cancer cells migrating away from the primary inoculation site potentially move into a nascent matrix deposited by infiltrating stromal cells which could contain defects in collagen cross-linking (Kauppila et al., 1998, 1999; Demou et al., 2005). Protease-independent invasion programs may, therefore, be accommodated by an immature, wound-like environment at sites distant from the neoplastic point-of-origin.
Given that only small numbers of carcinoma cells display rapid movement in vivo (Wyckoff et al., 2006, 2007; Xue et al., 2006; Sahai, 2007; Sahai et al., 2007), the issue arises as to the precise origin of this cell type. Cancer cell–macrophage hybrids have been characterized that may acquire the potential to access the myeloid-specific amoeboid phenotype (Vignery, 2005; Pawelek and Chakraborty, 2008). Indeed, cancer cell populations used in our studies are only able to traverse cross-linked type I collagen barriers by mobilizing MT1-MMP, even when collagen fiber pore size is increased from ∼8 µm2 to 80 µm2 by decreasing the polymerization pH of the collagen gels from pH 8.5 to pH 5.5 (Raub et al., 2008) (Fig. 4, A and B). In contrast, myeloid cells such as polymorphonuclear leukocytes, T cells, and monocytes, can rapidly infiltrate identical matrices without altering collagen architecture or structure via a process resistant to MMP-specific or broad-spectrum protease inhibitors (Fig. 4 A) (Huber and Weiss, 1989; Mandeville et al., 1997; Wolf et al., 2003b; Lammermann et al., 2008). Should cancer cell–myeloid cell hybrids acquire a similar ability to traffic through collagen networks without mobilizing proteolytic machinery—an as yet unconfirmed potential—neoplastic cells may prove able to access MT1-MMP–independent programs to negotiate matrix barriers.

In the in vivo setting, caution must be exercised in assuming that ECM pores of sufficient size or structural malleability do not exist at select sites in vivo that accommodate protease-independent trafficking (Even-Ram and Yamada, 2005). For example, cancer cell trafficking within lymph nodes likely occurs through stromal cell–lined conduits that support rapid movement through matrix-free zones (Bajenoff et al., 2006). Further, tissues devoid of interstitial collagen networks, e.g., the brain (Pluen et al., 2001), may well be traversed by tumor cells without necessitating proteolytic remodeling while still requiring ROCK-regulated molecular motors to squeeze a migrating cell through matrix openings smaller than the short axis of its nucleus (Beadle et al., 2008). Nevertheless, our findings with the normal adult mammary gland, as well as those we described previously using human dermal explants (Rosenthal et al., 1998; Sabeh et al., 2004; Hotary et al., 2006), support a model wherein the type I collagen architecture of the normal interstitium presents itself as a structural barrier to cancer cell traffic.
3D matrix architecture defines the mode of cancer cell invasion
In reconstituted gels of native type I collagen, pore size is limited to ∼1 µm in diameter (Demou et al., 2005). The motile activity of MT1-MMP–inhibited spheroids into collagen pores was restricted under our experimental conditions to extensions of filopodia-like structures or motile cell fragments (Mayer et al., 2004). Thus, the network of rigid pores stabilized by covalent cross-links between collagen fibrils pose a significant structural constraint to the mobility of cancer cells. Current evidence suggests that a migrating cancer cell has limited ability to mechanically negotiate a rigid pore whose diameter is significantly smaller than that of the cell's nucleus, the largest and most rigid of its intracellular organelles (Lutolf et al., 2003; Demou et al., 2005; Lutolf and Hubbell, 2005; Nakayama et al., 2005; Raeber et al., 2005, 2007; Ehrbar et al., 2007; Wolf and Friedl, 2008). As collagen gel microstructure can be affected by temperature, pH, ionic strength, ion stoichiometry, and monomer concentration (Roeder et al., 2002; Raub et al., 2007, 2008), minor but significant changes in experimental conditions could affect results obtained by different laboratories. However, fiber diameters generated under our standard in vitro conditions (∼60 nm as assessed by SEM) are similar to those deposited at tumor sites in vivo (Oldberg et al., 2007). Further, the pore sizes generated under our in vitro conditions are consistent with the recent demonstration that 100-nm beads injected into type I collagen-rich tissues in vivo fail to diffuse significant distances from the injection site (McKee et al., 2006; Nagano et al., 2008). Though a subset of studies describing cancer cell amoeboid activity have used acid-extracted collagen, the degree of collagen cross-linking or pore size was not assessed (Wyckoff et al., 2006). These caveats aside, type I collagen auto-assembly in vitro and cell-mediated assembly in vivo are mechanistically distinct processes (Raub et al., 2007, 2008). In vivo, collagen fibrils can be larger in size, incorporate a variety of other ECM components, and display distinct mechanical properties (Raub et al., 2007, 2008). Therefore, due caution should be exercised when interpreting data from in vitro assays using reconstructed collagen matrices. Nevertheless, breast cancer cells embedded in human mammary gland explants remain reliant on MT1-MMP–dependent proteolysis, leading us to conclude that carcinoma cells migrating into physiologically relevant tissues are confronted with matrix barriers similar to those generated in vitro from full-length, covalently cross-linked fibrils of type I collagen.
Extending the caveat emptor of using cross-link–free matrix barriers
Independent of the mechanisms used by cancer cells to traverse stromal barriers, increasing attention has also focused on the reported ability of cancer cells to undergo a mesenchymal-amoeboid transition as they cross the basement membrane, a specialized ECM barrier that surrounds or underlies epithelial cells, muscle, nerves, and the endothelium (Wang et al., 2003; Zaman et al., 2006; Gadea et al., 2007; Kitzing et al., 2007; Sahai, 2007; Wolf et al., 2007; Fackler and Grosse, 2008; Rowe and Weiss, 2008). However, conclusions regarding proteinase-independent, amoeboid-type trafficking across basement membranes have been similarly based on artificial ECM composites such as Matrigel, a noncovalently cross-linked extract of basement membrane macromolecules whose structural organization fails to recapitulate that observed in vivo (Even-Ram and Yamada, 2005; Hotary et al., 2006; Rowe and Weiss, 2008; Sodek et al., 2008). Using authentic basement membranes recovered from mouse or human tissues, cancer cells likewise use MT1-MMP activity to traverse these cross-linked barriers (Hotary et al., 2006).
What's more to be done?
At the end of the day, it is unlikely that the complex milieu of the in vivo environment can be duplicated fully in vitro. Changes in oxygen tension, pH, contact guidance, and cancer cell–stromal cell crosstalk, as well as the growth factor/cytokine milieu within this microenvironment could induce changes in cancer cell behavior that are unique to the neoplastic site (Anderson et al., 2006; Provenzano et al., 2008b; Doyle et al., 2009). Dissecting the relative role of protease-dependent and -independent trafficking mechanisms in vivo will require the marriage of sophisticated 4D intravital microscopic techniques with conditional knockout models and/or the selective silencing of MT-MMP family members in primary populations of human cancer cells embedded within the humanized mouse mammary gland (Proia and Kuperwasser, 2006). Although such studies are likely forthcoming (Alexander et al., 2008; Kedrin et al., 2008), a reductionist's approach to modeling cancer cell invasion in vitro suggests that the frequently assumed “given” of cancer cell mesenchymal-amoeboid transitions that allow for protease-independent invasion must be viewed cautiously as investigators attempt to extend current insights into the in vivo setting. Indeed, when confronting rigid structural barriers whose pore size is not permissive for passive cell movement, cancer cells appear to be almost entirely reliant on MT1-MMP activity, and perhaps the closely related membrane-anchored collagenase MT2-MMP (Hotary et al., 2000, 2006). Certainly, a “one size fits all” model—even regarding the dominant role proposed for MT1-MMP in tumor cell invasion—seldom stands the test of time when applied to the complex in vivo environment. No doubt, conditions will exist in vivo where the cancer cell will use actomyosin-generated forces to either displace impeding barriers or alter its nuclear dimensions to “squeeze” through narrow pores. However, as important roles for MT-MMP family members in cancer invasion, growth, and systemic spread continue to be defined (Taniwaki et al., 2007; Hu et al., 2008; Husemann et al., 2008; McGowan and Duffy, 2008; Szabova et al., 2008; Devy et al., 2009), carefully controlled in vitro and in vivo models should allow for new insights into the mechanisms controlling cancer cell migration through the ECM.
Acknowledgments
This work was supported by the National Institutes of Health (grant CA071699) and the Breast Cancer Research Foundation.
References
- Alexander S., Koehl G.E., Hirschberg M., Geissler E.K., Friedl P. 2008. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model.Histochem. Cell Biol. 130:1147–1154 [DOI] [PubMed] [Google Scholar]
- Anderson A.R., Weaver A.M., Cummings P.T., Quaranta V. 2006. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment.Cell. 127:905–915 [DOI] [PubMed] [Google Scholar]
- Bajenoff M., Egen J.G., Koo L.Y., Laugier J.P., Brau F., Glaichenhaus N., Germain R.N. 2006. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes.Immunity. 25:989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle C., Assanah M.C., Monzo P., Vallee R., Rosenfeld S.S., Canoll P. 2008. The role of myosin II in glioma invasion of the brain.Mol. Biol. Cell. 19:3357–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M., Davison P.F. 1980. Role of aldehydes in collagen fibrillogenesis in vitro.Biopolymers. 19:1861–1873 [DOI] [PubMed] [Google Scholar]
- Brown E., McKee T., diTomaso E., Pluen A., Seed B., Boucher Y., Jain R.K. 2003. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation.Nat. Med. 9:796–800 [DOI] [PubMed] [Google Scholar]
- Carragher N.O., Walker S.M., Scott Carragher L.A., Harris F., Sawyer T.K., Brunton V.G., Ozanne B.W., Frame M.C. 2006. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function.Oncogene. 25:5726–5740 [DOI] [PubMed] [Google Scholar]
- Christiansen D.L., Huang E.K., Silver F.H. 2000. Assembly of type I collagen: fusion of fibril subunits and the influence of fibril diameter on mechanical properties.Matrix Biol. 19:409–420 [DOI] [PubMed] [Google Scholar]
- Chun T.H., Sabeh F., Ota I., Murphy H., McDonagh K.T., Holmbeck K., Birkedal-Hansen H., Allen E.D., Weiss S.J. 2004. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix.J. Cell Biol. 167:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Segall J.E. 2003. Intravital imaging of cell movement in tumours.Nat. Rev. Cancer. 3:921–930 [DOI] [PubMed] [Google Scholar]
- Croft D.R., Olson M.F. 2008. Regulating the conversion between rounded and elongated modes of cancer cell movement.Cancer Cell. 14:349–351 [DOI] [PubMed] [Google Scholar]
- Curino A.C., Engelholm L.H., Yamada S.S., Holmbeck K., Lund L.R., Molinolo A.A., Behrendt N., Nielsen B.S., Bugge T.H. 2005. Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy.J. Cell Biol. 169:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demou Z.N., Awad M., McKee T., Perentes J.Y., Wang X., Munn L.L., Jain R.K., Boucher Y. 2005. Lack of telopeptides in fibrillar collagen I promotes the invasion of a metastatic breast tumor cell line.Cancer Res. 65:5674–5682 [DOI] [PubMed] [Google Scholar]
- Devy L., Huang L., Naa L., Yanamandra N., Pieters H., Frans N., Chang E., Tao Q., Vanhove M., Lejeune A., et al. 2009. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis.Cancer Res. 69:1517–1526 [DOI] [PubMed] [Google Scholar]
- Doyle A.D., Wang F.W., Matsumoto K., Yamada K.M. 2009. One-dimensional topography underlies three-dimensional fibrillar cell migration.J. Cell. Biol. 184:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrbar M., Rizzi S.C., Schoenmakers R.G., Miguel B.S., Hubbell J.A., Weber F.E., Lutolf M.P. 2007. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions.Biomacromolecules. 8:3000–3007 [DOI] [PubMed] [Google Scholar]
- Even-Ram S., Yamada K.M. 2005. Cell migration in 3D matrix.Curr. Opin. Cell Biol. 17:524–532 [DOI] [PubMed] [Google Scholar]
- Eyre D.R., Paz M.A., Gallop P.M. 1984. Cross-linking in collagen and elastin.Annu. Rev. Biochem. 53:717–748 [DOI] [PubMed] [Google Scholar]
- Fackler O.T., Grosse R. 2008. Cell motility through plasma membrane blebbing.J. Cell Biol. 181:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov S., Koenig G.C., Chun T.H., Hotary K.B., Ota I., Bugge T.H., Roberts J.D., Fay W.P., Birkedal-Hansen H., Holmbeck K., et al. 2005. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells.J. Exp. Med. 202:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.E., Pop A., Koh W., Anthis N.J., Saunders W.B., Davis G.E. 2006. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling.Mol. Cancer. 5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Wolf K. 2003. Tumour-cell invasion and migration: diversity and escape mechanisms.Nat. Rev. Cancer. 3:362–374 [DOI] [PubMed] [Google Scholar]
- Gadea G., de Toledo M., Anguille C., Roux P. 2007. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices.J. Cell Biol. 178:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea G., Sanz-Moreno V., Self A., Godi A., Marshall C.J. 2008. DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells.Curr. Biol. 18:1456–1465 [DOI] [PubMed] [Google Scholar]
- Gaggioli C., Hooper S., Hidalgo-Carcedo C., Grosse R., Marshall J.F., Harrington K., Sahai E. 2007. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells.Nat. Cell Biol. 9:1392–1400 [DOI] [PubMed] [Google Scholar]
- Gelman R.A., Williams B.R., Piez K.A. 1979. Collagen fibril formation. Evidence for a multistep process.J. Biol. Chem. 254:180–186 [PubMed] [Google Scholar]
- Gupta G.P., Nguyen D.X., Chiang A.C., Bos P.D., Kim J.Y., Nadal C., Gomis R.R., Manova-Todorova K., Massague J. 2007. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis.Nature. 446:765–770 [DOI] [PubMed] [Google Scholar]
- Hiraoka N., Allen E., Apel I.J., Gyetko M.R., Weiss S.J. 1998. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins.Cell. 95:365–377 [DOI] [PubMed] [Google Scholar]
- Hotary K., Allen E., Punturieri A., Yana I., Weiss S.J. 2000. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3.J. Cell Biol. 149:1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K.B., Allen E.D., Brooks P.C., Datta N.S., Long M.W., Weiss S.J. 2003. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix.Cell. 114:33–45 [DOI] [PubMed] [Google Scholar]
- Hotary K., Li X.Y., Allen E., Stevens S.L., Weiss S.J. 2006. A cancer cell metalloprotease triad regulates the basement membrane transmigration program.Genes Dev. 20:2673–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Yao J., Carroll D.K., Weremowicz S., Chen H., Carrasco D., Richardson A., Violette S., Nikolskaya T., Nikolsky Y., et al. 2008. Regulation of in situ to invasive breast carcinoma transition.Cancer Cell. 13:394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A.R., Weiss S.J. 1989. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall.J. Clin. Invest. 83:1122–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann Y., Geigl J.B., Schubert F., Musiani P., Meyer M., Burghart E., Forni G., Eils R., Fehm T., Riethmuller G., Klein C.A. 2008. Systemic spread is an early step in breast cancer.Cancer Cell. 13:58–68 [DOI] [PubMed] [Google Scholar]
- Itoh Y., Seiki M. 2006. MT1-MMP: a potent modifier of pericellular microenvironment.J. Cell. Physiol. 206:1–8 [DOI] [PubMed] [Google Scholar]
- Kauppila S., Stenback F., Risteli J., Jukkola A., Risteli L. 1998. Aberrant type I and type III collagen gene expression in human breast cancer in vivo.J. Pathol. 186:262–268 [DOI] [PubMed] [Google Scholar]
- Kauppila S., Bode M.K., Stenback F., Risteli L., Risteli J. 1999. Cross-linked telopeptides of type I and III collagens in malignant ovarian tumours in vivo.Br. J. Cancer. 81:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedrin D., Gligorijevic B., Wyckoff J., Verkhusha V.V., Condeelis J., Segall J.E., van Rheenen J. 2008. Intravital imaging of metastatic behavior through a mammary imaging window.Nat. Methods. 5:1019–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm J.M., Timmins N.E., Brown C.J., Fussenegger M., Nielsen L.K. 2003. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types.Biotechnol. Bioeng. 83:173–180 [DOI] [PubMed] [Google Scholar]
- Kitzing T.M., Sahadevan A.S., Brandt D.T., Knieling H., Hannemann S., Fackler O.T., Grosshans J., Grosse R. 2007. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion.Genes Dev. 21:1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H., Paulson A., Iovino F., Wicha M.S. 2008. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion.Oncogene. 27:6120–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T., Bader B.L., Monkley S.J., Worbs T., Wedlich-Soldner R., Hirsch K., Keller M., Forster R., Critchley D.R., Fassler R., Sixt M. 2008. Rapid leukocyte migration by integrin-independent flowing and squeezing.Nature. 453:51–55 [DOI] [PubMed] [Google Scholar]
- Li C.Y., Shan S., Huang Q., Braun R.D., Lanzen J., Hu K., Lin P., Dewhirst M.W. 2000. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models.J. Natl. Cancer Inst. 92:143–147 [DOI] [PubMed] [Google Scholar]
- Li X.Y., Ota I., Yana I., Sabeh F., Weiss S.J. 2008. Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase.Mol. Biol. Cell. 19:3221–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M.P., Hubbell J.A. 2005. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering.Nat. Biotechnol. 23:47–55 [DOI] [PubMed] [Google Scholar]
- Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B., Hubbell J.A. 2003. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics.Proc. Natl. Acad. Sci. USA. 100:5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzoub M., Jin S., Verkman A.S. 2008. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin.FASEB J. 22:276–284 [DOI] [PubMed] [Google Scholar]
- Mandeville J.T., Lawson M.A., Maxfield F.R. 1997. Dynamic imaging of neutrophil migration in three dimensions: mechanical interactions between cells and matrix.J. Leukoc. Biol. 61:188–200 [DOI] [PubMed] [Google Scholar]
- Mayer C., Maaser K., Daryab N., Zanker K.S., Brocker E.B., Friedl P. 2004. Release of cell fragments by invading melanoma cells.Eur. J. Cell Biol. 83:709–715 [DOI] [PubMed] [Google Scholar]
- McGowan P.M., Duffy M.J. 2008. Matrix metalloproteinase expression and outcome in patients with breast cancer: analysis of a published database.Ann. Oncol. 19:1566–1572 [DOI] [PubMed] [Google Scholar]
- McKee T.D., Grandi P., Mok W., Alexandrakis G., Insin N., Zimmer J.P., Bawendi M.G., Boucher Y., Breakefield X.O., Jain R.K. 2006. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector.Cancer Res. 66:2509–2513 [DOI] [PubMed] [Google Scholar]
- Nagano S., Perentes J.Y., Jain R.K., Boucher Y. 2008. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors.Cancer Res. 68:3795–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Amano M., Katsumi A., Kaneko T., Kawabata S., Takefuji M., Kaibuchi K. 2005. Rho-kinase and myosin II activities are required for cell type and environment specific migration.Genes Cells. 10:107–117 [DOI] [PubMed] [Google Scholar]
- Oldberg A., Kalamajski S., Salnikov A.V., Stuhr L., Morgelin M., Reed R.K., Heldin N.E., Rubin K. 2007. Collagen-binding proteoglycan fibromodulin can determine stroma matrix structure and fluid balance in experimental carcinoma.Proc. Natl. Acad. Sci. USA. 104:13966–13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall C.M., Kleifeld O. 2006. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy.Nat. Rev. Cancer. 6:227–239 [DOI] [PubMed] [Google Scholar]
- Packard B.Z., Artym V.V., Komoriya A., Yamada K.M. 2009. Direct visualization of protease activity on cells migrating in three-dimensions.Matrix Biol. 28:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H., Cunha G.R. 2004. Epithelial-stromal interactions in the mouse and human mammary gland in vivo.Endocr. Relat. Cancer. 11:437–458 [DOI] [PubMed] [Google Scholar]
- Pawelek J.M., Chakraborty A.K. 2008. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis.Nat. Rev. Cancer. 8:377–386 [DOI] [PubMed] [Google Scholar]
- Pinner S., Sahai E. 2008. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE.Nat. Cell Biol. 10:127–137 [DOI] [PubMed] [Google Scholar]
- Pluen A., Boucher Y., Ramanujan S., McKee T.D., Gohongi T., di Tomaso E., Brown E.B., Izumi Y., Campbell R.B., Berk D.A., Jain R.K. 2001. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors.Proc. Natl. Acad. Sci. USA. 98:4628–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia D.A., Kuperwasser C. 2006. Reconstruction of human mammary tissues in a mouse model.Nat. Protoc. 1:206–214 [DOI] [PubMed] [Google Scholar]
- Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. 2006. Collagen reorganization at the tumor-stromal interface facilitates local invasion.BMC Med. 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P.P., Inman D.R., Eliceiri K.W., Knittel J.G., Yan L., Rueden C.T., White J.G., Keely P.J. 2008a. Collagen density promotes mammary tumor initiation and progression.BMC Med. 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P.P., Inman D.R., Eliceiri K.W., Trier S.M., Keely P.J. 2008b. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization.Biophys. J. 95:5374–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber G.P., Lutolf M.P., Hubbell J.A. 2005. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration.Biophys. J. 89:1374–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber G.P., Lutolf M.P., Hubbell J.A. 2007. Mechanisms of 3-D migration and matrix remodeling of fibroblasts within artificial ECMs.Acta Biomater. 3:615–629 [DOI] [PubMed] [Google Scholar]
- Raub C.B., Suresh V., Krasieva T., Lyubovitsky J., Mih J.D., Putnam A.J., Tromberg B.J., George S.C. 2007. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy.Biophys. J. 92:2212–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub C.B., Unruh J., Suresh V., Krasieva T., Lindmo T., Gratton E., Tromberg B.J., George S.C. 2008. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties.Biophys. J. 94:2361–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V.Y., Zhang Q.Y., Weiss S.J. 1995. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages.Proc. Natl. Acad. Sci. USA. 92:3849–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder B.A., Kokini K., Sturgis J.E., Robinson J.P., Voytik-Harbin S.L. 2002. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure.J. Biomech. Eng. 124:214–222 [DOI] [PubMed] [Google Scholar]
- Rosenthal E.L., Johnson T.M., Allen E.D., Apel I.J., Punturieri A., Weiss S.J. 1998. Role of the plasminogen activator and matrix metalloproteinase systems in epidermal growth factor- and scatter factor-stimulated invasion of carcinoma cells.Cancer Res. 58:5221–5230 [PubMed] [Google Scholar]
- Rowe R.G., Weiss S.J. 2008. Breaching the basement membrane: who, when and how? Trends Cell Biol. 18:560–574 [DOI] [PubMed] [Google Scholar]
- Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S., et al. 2004. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP.J. Cell Biol. 167:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E. 2007. Illuminating the metastatic process.Nat. Rev. Cancer. 7:737–749 [DOI] [PubMed] [Google Scholar]
- Sahai E., Garcia-Medina R., Pouyssegur J., Vial E. 2007. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility.J. Cell Biol. 176:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Moreno V., Gadea G., Ahn J., Paterson H., Marra P., Pinner S., Sahai E., Marshall C.J. 2008. Rac activation and inactivation control plasticity of tumor cell movement.Cell. 135:510–523 [DOI] [PubMed] [Google Scholar]
- Sato K., Ebihara T., Adachi E., Kawashima S., Hattori S., Irie S. 2000. Possible involvement of aminotelopeptide in self-assembly and thermal stability of collagen I as revealed by its removal with proteases.J. Biol. Chem. 275:25870–25875 [DOI] [PubMed] [Google Scholar]
- Sodek K.L., Brown T.J., Ringuette M.J. 2008. Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration.BMC Cancer. 8:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparano J.A., Bernardo P., Stephenson P., Gradishar W.J., Ingle J.N., Zucker S., Davidson N.E. 2004. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196.J. Clin. Oncol. 22:4683–4690 [DOI] [PubMed] [Google Scholar]
- Szabova L., Chrysovergis K., Yamada S.S., Holmbeck K. 2008. MT1-MMP is required for efficient tumor dissemination in experimental metastatic disease.Oncogene. 27:3274–3281 [DOI] [PubMed] [Google Scholar]
- Taniwaki K., Fukamachi H., Komori K., Ohtake Y., Nonaka T., Sakamoto T., Shiomi T., Okada Y., Itoh T., Itohara S., et al. 2007. Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice.Cancer Res. 67:4311–4319 [DOI] [PubMed] [Google Scholar]
- Tester A.M., Waltham M., Oh S.J., Bae S.N., Bills M.M., Walker E.C., Kern F.G., Stetler-Stevenson W.G., Lippman M.E., Thompson E.W. 2004. Pro-matrix metalloproteinase-2 transfection increases orthotopic primary growth and experimental metastasis of MDA-MB-231 human breast cancer cells in nude mice.Cancer Res. 64:652–658 [DOI] [PubMed] [Google Scholar]
- Vignery A. 2005. Macrophage fusion: are somatic and cancer cells possible partners? Trends Cell Biol. 15:188–193 [DOI] [PubMed] [Google Scholar]
- Wang H.R., Zhang Y., Ozdamar B., Ogunjimi A.A., Alexandrova E., Thomsen G.H., Wrana J.L. 2003. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation.Science. 302:1775–1779 [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Paterson H.F., Marshall C.J. 2005. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion.Nat. Cell Biol. 7:255–261 [DOI] [PubMed] [Google Scholar]
- Williams B.R., Gelman R.A., Poppke D.C., Piez K.A. 1978. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results.J. Biol. Chem. 253:6578–6585 [PubMed] [Google Scholar]
- Wolf K., Friedl P. 2008. Mapping proteolytic cancer cell-extracellular matrix interfaces.Clin. Exp. Metastasis. ePub DOI 10.1007/s10585-008-9190-2 [DOI] [PubMed] [Google Scholar]
- Wolf K., Mazo I., Leung H., Engelke K., von Andrian U.H., Deryugina E.I., Strongin A.Y., Brocker E.B., Friedl P. 2003a. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis.J. Cell Biol. 160:267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Muller R., Borgmann S., Brocker E.B., Friedl P. 2003b. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases.Blood. 102:3262–3269 [DOI] [PubMed] [Google Scholar]
- Wolf K., Wu Y.I., Liu Y., Geiger J., Tam E., Overall C., Stack M.S., Friedl P. 2007. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion.Nat. Cell Biol. 9:893–904 [DOI] [PubMed] [Google Scholar]
- Woodley D.T., Yamauchi M., Wynn K.C., Mechanic G., Briggaman R.A. 1991. Collagen telopeptides (cross-linking sites) play a role in collagen gel lattice contraction.J. Invest. Dermatol. 97:580–585 [DOI] [PubMed] [Google Scholar]
- Wyatt C.A., Geoghegan J.C., Brinckerhoff C.E. 2005. Short hairpin RNA-mediated inhibition of matrix metalloproteinase-1 in MDA-231 cells: effects on matrix destruction and tumor growth.Cancer Res. 65:11101–11108 [DOI] [PubMed] [Google Scholar]
- Wyckoff J.B., Pinner S.E., Gschmeissner S., Condeelis J.S., Sahai E. 2006. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo.Curr. Biol. 16:1515–1523 [DOI] [PubMed] [Google Scholar]
- Wyckoff J.B., Wang Y., Lin E.Y., Li J.F., Goswami S., Stanley E.R., Segall J.E., Pollard J.W., Condeelis J. 2007. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors.Cancer Res. 67:2649–2656 [DOI] [PubMed] [Google Scholar]
- Xue C., Wyckoff J., Liang F., Sidani M., Violini S., Tsai K.L., Zhang Z.Y., Sahai E., Condeelis J., Segall J.E. 2006. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis.Cancer Res. 66:192–197 [DOI] [PubMed] [Google Scholar]
- Zaman M.H., Trapani L.M., Sieminski A.L., Mackellar D., Gong H., Kamm R.D., Wells A., Lauffenburger D.A., Matsudaira P. 2006. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis.Proc. Natl. Acad. Sci. USA. 103:10889–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]