Abstract
Among the five phytochromes in Arabidopsis thaliana, phytochrome A (phyA) plays a major role in seedling deetiolation. Mutant analyses have identified more than 10 positive components acting downstream of phyA to inhibit hypocotyl elongation. However, their sites of action and their hierarchical relationships are poorly understood. Here, we investigated the genetic and molecular relationship between two homologous proteins, FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and FHY1-LIKE (FHL), and two transcription factors, LONG AFTER FAR-RED LIGHT1 (LAF1) and LONG HYPOCOTYL IN FAR-RED1 (HFR1). Analyses of double and triple mutants showed that LAF1, a myb factor, and HFR1, a basic helix-loop-helix factor, independently transmit phyA signals downstream of FHY1 and FHL. Coimmunoprecipitation experiments showed that phyA, FHY1, FHL, LAF1, and HFR1 are components of protein complexes in vivo. In vitro pull-down assays demonstrated direct interactions between partner proteins with the N-terminal region of FHY1, as well as that of FHL, interacting with the LAF1 N-terminal portion and the HFR1 C-terminal region. These results suggest that, in addition to assisting phyA nuclear accumulation, FHY1 and FHL are required to assemble photoreceptor/transcription factor complexes for phyA signaling.
INTRODUCTION
Plants use the intensity, direction, duration, and wavelength composition of light in the environment as cues to inform themselves of the time and place (Neff et al., 2000). Light affects all phases of a plant's life cycle, from seed germination, seedling deetiolation, and shade avoidance responses to flowering. These light-triggered physiological processes are mediated by several groups of photoreceptors, including phytochromes (phyA to phyE), cryptochromes (cry1 and cry2), and phototropins (Phot1 and Phot2). The Arabidopsis thaliana genome encodes five members of the phytochrome family detecting light in the red (R) and far-red (FR) regions (Sharrock and Quail, 1989; Quail, 2002; Gyula et al., 2003). Among them, phyA plays an important role in the transition from heterotrophic to phototrophic growth (Dehesh et al., 1993; Neff and Chory, 1998). This developmental transition, called deetiolation, occurs at the most vulnerable stage of a plant's life cycle. It is thought that, upon activation by FR, phyA initiates a signaling cascade culminating in alterations in gene expression, which underpin the morphological changes of light-exposed seedlings. The nature of this signaling cascade and the characterization of participating components is a subject of intense investigation.
A powerful approach to understanding phyA signaling is through the analysis of mutants that are either unresponsive or reduced in responsiveness to continuous FR (FRc). The two most prominent morphological features accompanying FR-triggered deetiolation are inhibition of hypocotyl elongation and unfolding and expansion of cotyledons. Mutants defective in phyA signaling should be blind to FRc and develop long hypocotyls and folded cotyledons as found in etiolated seedlings. These morphological features have been used to screen for phyA signaling mutants deficient in positive regulatory components (Dehesh et al., 1993; Whitelam et al., 1993; Hudson et al., 1999; Chen et al., 2004). So far, more than 15 such mutants have been isolated and characterized: phytochrome A signal transduction 1 (pat1), far-red insensitive 219 (fin219), far-red insensitive 2 (fin2), far-red impaired response 1 (far1), far-red elongated hypocotyl3 (fhy3), long hypocotyl in far-red1 (hfr1), long after far-red light1 (laf1), elongated hypocotyl 5 (hy5), long after far-red light 3 (laf3), long after far-red light 6 (laf6), phytochrome signaling 2 (psi2), etc. (Whitelam et al., 1993; Oyama et al., 1997; Genoud et al., 1998; Hoecker et al., 1998; Hudson et al., 1999; Bolle et al., 2000; Fairchild et al., 2000; Fankhauser and Chory, 2000; Hsieh et al., 2000; Soh et al., 2000; Ballesteros et al., 2001; Desnos et al., 2001; Møller et al., 2001; Hare et al., 2003). Phenotypic analysis showed that only mutants defective in the phyA photoreceptor have hypocotyl lengths in FRc equivalent to those of wild-type etiolated seedlings (Neff et al., 2000). All other mutants deficient in components downstream of the photoreceptor have intermediate hypocotyl lengths between those of the wild type and phyA mutants (Wang and Deng, 2003).
Most of the genes encoding phyA signaling components have been characterized. Many of them encode transcription factors, while the biochemical functions of other encoded proteins are unknown (Ni et al., 1998; Fairchild et al., 2000; Ballesteros et al., 2001; Duek and Fankhauser, 2003). The observation that these mutants have hypocotyl lengths shorter than those of phyA photoreceptor mutants suggests that phyA signals are distributed through several downstream pathways. This view is supported by the findings that hfr1-201 hy5-1, fhy1-1 fhy3-1, fhy1-1 far1, fhy3-1 far1, and FHY1-LIKE (FHL) RNA interference (RNAi)/fhy1-3 double mutants have additive phenotypes compared with the single parental mutants (Kim et al., 2002; Wang and Deng, 2002; Zhou et al., 2005). It is not known whether these downstream components regulate different phyA pathways or cooperate to form a phyA signaling network. In addition, there is recent evidence for a positive feedback mechanism suggesting that phyA signaling is complex and might encompass different regulatory layers (Lin et al., 2007).
A major issue in phyA-mediated responses is to define the number and nature of signaling pathways downstream of the photoreceptor and the hierarchical relationship of their components. Based on relative hypocotyl lengths of mutants in FR, it is reasonable to assume that FHY1 and its homolog, FHL, operate near the top of the cascade in phyA signaling (Zhou et al., 2005). This notion is supported by the observations that nuclear translocation of phyA is attenuated in fhy1-1 and fhy1-1/FHL RNAi lines and (Hiltbrunner et al., 2005, 2006) and the C-terminal part of FHY1 is sufficient to assist phyA nuclear translocation (Genoud et al., 2008). Although FHY1/FHL were found to preferentially interact with Pfr phyA in vitro (Hiltbrunner et al., 2005, 2006), recent studies showed that in fact, these two homologous proteins prefer the Pr form in vivo. Indeed, FHY1/FHL and FHY3 may possibly protect phyA in a signaling complex (Saijo et al., 2008; Shen et al., 2009). In addition to these findings, it was reported that phyA mutant seedlings display perfect inhibition of negative gravitropism under FR, while 88% of fhy1-3 fhI-1 seedlings grow within ±25° of the vertical. Moreover, the phyA mutant, but not fhy1-3 fhI-1, is impaired in blue light (B)–dependent inhibition of negative gravitropism (Rösler et al., 2007). These results suggest that an as yet unidentified branched pathway, not involving FHY1 and FHL, plays a role in phyA signal transduction.
HFR1 and LAF1 are two well-characterized transcriptional activators of the phyA signaling network (Fairchild et al., 2000; Soh et al., 2000; Ballesteros et al., 2001; Duek and Fankhauser, 2003). Mutants deficient in HFR1 or LAF1 are hyposensitive to FRc, but their hypocotyl lengths are shorter than those of fhy1-3 and fhy1-3/FHL RNAi. Furthermore, we recently reported that the hfr1 laf1 double mutant also has an additive phenotype of the two single mutants, indicating that LAF1 and HFR1 regulate largely independent pathways (Jang et al., 2007). Seedling hypocotyls of the hfr1 laf1 double mutant are still shorter than those of fhy1-3 and fhy1-3/FHL RNAi (see Supplemental Figure 1 online). These differences in hypocotyl lengths suggest that HFR1 and LAF1 likely operate close to the bottom of the phyA signaling cascade consistent with their biochemical functions. However, it is not known whether they mediate phyA signals by a direct photoreceptor interaction, through FHY1 and FHL, or via some other intermediary components upstream and whether these factors are targets of multiple signal inputs.
Here, we generated double and triple mutants, analyzed their hypocotyl phenotypes under various FRc fluencies, and performed in vitro and in vivo pull-down assays using FHY1 and FHL as bait proteins. Based on these molecular genetic and biochemical analyses, we concluded that FHY1 and FHL mediate interactions between phyA and transcription factors (TFs), such as HFR1 and LAF1, to assemble protein complexes important for phyA signaling.
RESULTS
HFR1 and LAF1 Transmit phyA Signals Downstream of FHY1 and FHL
To examine genetic relationships between fhy1 and hfr1 and fhy1 and laf1, we first generated fhy1-3 hfr1-201 double mutant and fhy1-3/LAF1 RNAi (LAF1 Ri) lines (for convenience, these lines will be referred to hereafter as the fhy1-3/LAF1 Ri double mutant). Because fhy1-3 and laf1 are in different genetic backgrounds (Ballesteros et al., 2001; Zeidler et al., 2001), we used RNAi to knockdown LAF1 expression (Figure 1C). We also generated fhy1-3/FHL RNAi (FHL Ri) lines and confirmed that hypocotyl lengths of these lines were not significantly different from those of the fhy1-3 fhl-1 double mutant at different fluence rates tested (see Supplemental Figure 2 online; Rösler et al., 2007).
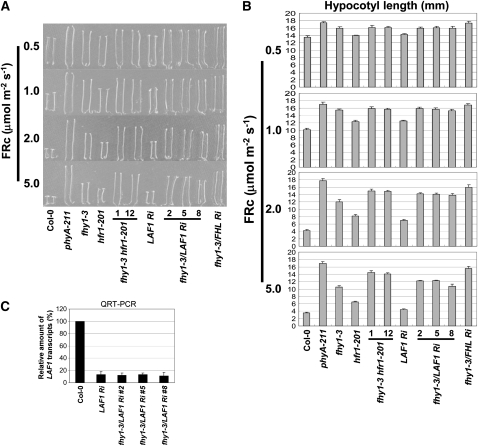
Figure 1.
fhy1-3 hfr1-201 and fhy1-3/LAF1 Ri Double Mutants Show an Additive Phenotype at Higher FRc Fluences.
(A) Seedling morphology at various FRc fluences (μmol m−2 s−1). Two fhy1-3 hfr1-201 lines (1 and 2) and three fhy1-3/LAF1 Ri lines (2, 5, and 8) were examined with Columbia-0, phyA-211, fhy1-3/FHL Ri, fhy1-3, LAF1 Ri, and hfr1-201 control lines.
(B) Determination of hypocotyl length. Data show average hypocotyl length ± sd (n = 20).
(C) LAF1 transcript levels. Average value ± sd (n = 3). QRT-PCR, quantitative RT-PCR.
We found no apparent difference in hypocotyl lengths between fhy1-3, fhy1-3 hfr1-201, and fhy1-3/LAF1 Ri at low FRc fluences. On the other hand, hypocotyl lengths of the double mutants were clearly longer than those of single mutants (fhy1-3, hfr1-201, and LAF1 Ri) at higher fluences (Figures 1A and 1B), indicating additive effects of the mutations. At these high FRc fluences, hypocotyl lengths of fhy1-3 hfr1-201 and fhy1-3/LAF1 Ri were still shorter than those of fhy1-3/FHL Ri, indicating that HFR1 and LAF1 have nonoverlapping functions. These additive phenotypes can be explained by two possible models: (1) FHY1 operates largely independently of and parallel to HFR1 and LAF1; and (2) at low FRc fluences, HFR1 and LAF1 function downstream of FHY1, but at higher FRc fluences, these factors can also transmit phyA signals downstream of FHL, a homolog of FHY1.
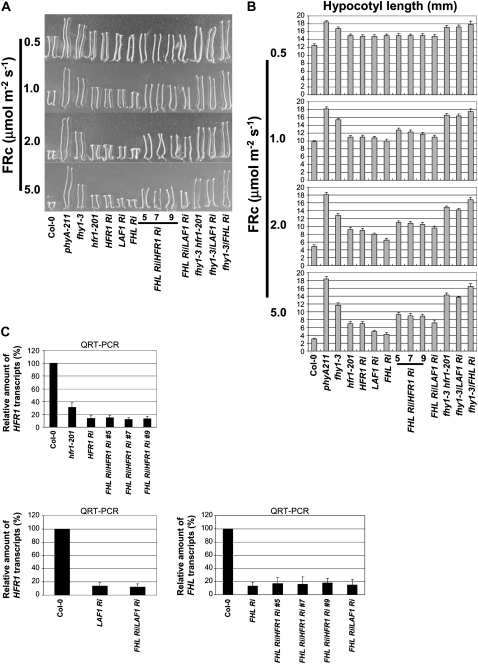
Previous experiments have provided evidence that FHL is homologous and functionally redundant to FHY1 (Zhou et al., 2005). Similar partial functional redundancy of homologous proteins has been shown for FHY3/FAR1 (Wang and Deng, 2002; Lin et al., 2007). Therefore, we favored the second model and analyzed the effects of reduced HFR1 and LAF1 expression levels in the FHL Ri mutant background. We confirmed that expression of all the target transcripts was reduced by RNAi technology (Figure 2C). Analysis of mutant hypocotyls showed that FHL Ri/HFR1 Ri and FHL Ri/LAF1 Ri had additive effects, indicating independent actions of FHL and HFR1 and LAF1 (Figures 2A and 2B). At lower FR fluencies, FHL Ri/HFR1 Ri and FHL Ri/LAF1 Ri displayed similar hypocotyl lengths as hfr1-201 or LAF1 Ri, but at higher FRc fluences, the hypocotyl lengths of the double mutants were longer than those of the single mutants (Figure 2B). These results suggest that at high FRc fluences, phyA signals could be transmitted through either FHY1 or FHL to downstream components, such as HFR1 and LAF1.
Figure 2.
FHL Ri/HFR1 Ri and FHL Ri/LAF1 Ri Double Mutants Show an Additive Phenotype at Higher FRc Fluences.
(A) Seedling morphology at various FRc fluences (μmol m−2 s−1). Three FHL Ri/HFR1 Ri lines (5, 7, and 9) and one fhy1-3/LAF1 Ri line were tested with additional LAF1 Ri, HFR1 Ri, FHL Ri, fhy1-3 hfr1-201, fhy1-3/LAF1 Ri control lines.
(B) Hypocotyl length measurements. Data show average hypocotyl length ± sd (n = 20).
(C) Top, HFR1 transcript levels; bottom left panel, LAF1 transcript levels; bottom right panel, FHL transcript levels. Data show average value ± sd (n = 3).
To obtain definitive evidence that FHY1/FHL operate upstream of the two TFs, we generated triple mutant lines of fhy1-3/FHL Ri/HFR1 Ri and fhy1-3/FHL Ri/LAF1 Ri by RNAi technology. Quantitative PCR experiments confirmed that these triple mutant lines were indeed deficient in HFR1 and LAF1 transcripts and that the greatly reduced FHY1 and FHL expression in fhy1-3/FHL Ri lines did not significantly affect HFR1 but increased LAF1 transcript levels by 1.8-fold (Figure 3C). Note that FHL transcript levels in the fhy1-3 mutant were approximately threefold higher than those in the wild type. Nevertheless, in this mutant background, the FHL RNAi technology was effective in reducing FHL transcript levels (Figure 3C). Phenotypic analysis showed that reducing HFR1 or LAF1 levels in fhy1-3/FHL Ri did not alter seedling hypocotyl lengths in FRc (Figures 3A and 3B), indicating that fhy1-3/FHL Ri is epistatic to hfr1 and laf1. Therefore, in phyA signal transduction cascade, HFR1 and LAF1 function downstream of FHY1/FHL to inhibit hypocotyl elongation in FRc.
Figure 3.
Phenotypes of fhy1-3/FHL Ri/HFR1 Ri and fhy1-3/FHL Ri/LAF Ri Triple Mutants under Various FRc Fluences.
(A) Seedling morphology at various FRc fluences (μmol m−2 s−1). Three fhy1-3/FHL Ri/HFR1 Ri lines (1, 5, and 7) and two fhy1-3/FHL Ri/LAF Ri lines (1 and 2) were examined with control lines.
(B) Hypocotyl length measurement. Data show average hypocotyl length ± sd (n = 20).
(C) Top, HFR1 transcript levels; bottom left panel, LAF1 transcript levels; bottom right panel, FHL transcripts levels. Data show average value ± sd (n = 3).
hfr1 and laf1 Abrogate Hypersensitivity of FHY1 Overexpression
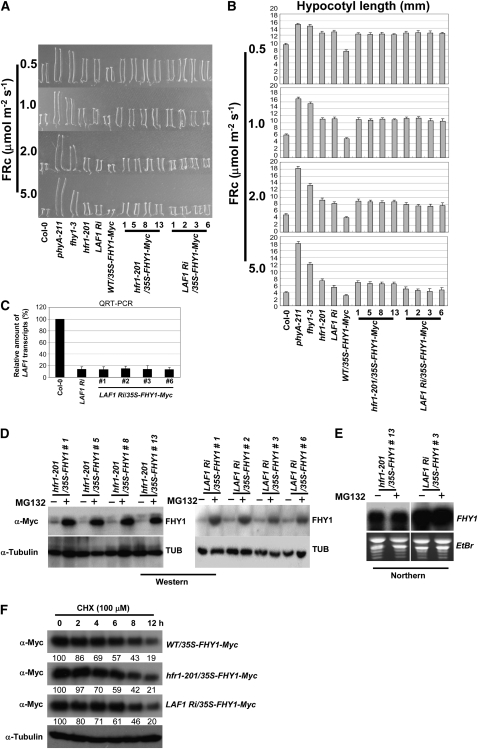
We have previously reported that overexpression of FHY1 (35S-FHY1-Myc) can confer FRc hypersensitivity in wild-type seedlings, and this phenotype was most obvious at low FRc fluences (Figures 4A and 4B). If HFR1 and LAF1 function downstream of FHY1 in phyA signaling cascades, this FRc hypersensitivity should be abrogated by hfr1-201 and laf1 mutations. Figures 4A and 4B show that this was indeed the case. Seedlings of hfr1-201/35S-FHY1-Myc displayed similar FRc hyposensitivity to hfr1-201 seedlings. Similarly, the FRc hypersensitivity of 35S-FHY1-Myc seedlings was converted to hyposensitivity by RNAi-mediated reduction of LAF1 transcripts (Figure 4C). RNA and protein gel blot analysis confirmed that the FHY1-Myc transcript was expressed in the transgenic lines, and FHY1 protein accumulated at high levels when proteasome activity was inhibited by MG132 (Figures 4D and 4E).
Figure 4.
FR Hypersensitivity Conferred by FHY1 Overexpression Is Blocked by hfr1-201 and LAF1 Ri.
(A) Seedling morphology at various FRc fluences (μmol m−2 s−1).
(B) Hypocotyl length measurement. Data show average hypocotyl length ± sd (n = 20, except where specified). The hfr1-201/35S-FHY1-Myc and LAF1 Ri/35S-FHY1-Myc lines were significantly longer than WT/35-FHY1-Myc lines (P < 0.01, Student's t test at 5.0 μmol m−2 s−1, n > 80).
(C) LAF1 transcript levels. Data show average value ± sd (n = 3).
(D) Protein gel blot analysis. hfr1-201/35S-FHY1-Myc (left panel) or LAF1 Ri/35S-FHY1-Myc (right panel) seedlings were treated with or without MG132. FHY1-Myc proteins were detected with anti-Myc antibody, and tubulin levels were used as loading controls.
(E) RNA gel blot analysis of FHY1-Myc plants. Total RNAs were isolated from same samples used for protein gel blot analyses. Ethidium bromide (EtBr) staining was used to monitor loading of RNAs (5 μg per lane).
(F) Protein stability of FHY1-Myc was not dramatically affected by reducing HFR1 and LAF1 transcript levels. Relative amounts of blotted protein were quantitatively compared with the initial amount by percentage. Four-day-old etiolated seedlings were treated with MG132 followed by cycloheximide (CHX) and then exposed to FRc (2.0 μmol m−2 s−1). Proteins were extracted at the indicated time and analyzed by protein gel blots using anti-Myc antibody.
To confirm that the attenuation of hypersensitivity shown was a consequence of affected downstream targets, we monitored FHY1 stability in the hfr1-201 and LAF1 Ri genetic backgrounds. We found that in all tested lines, FHY1-Myc accumulated to similar levels with MG132 treatment and decayed with a similar half-life (Figure 4F). This result shows that the reduced hypersensitivity in hfr1-201/35S-FHY1-Myc and LAF1 Ri/35S-FHY1-Myc is due to a reduction in HFR1 and LAF1 transcript levels and not to significant changes in FHY1 protein stability.
Direct Interaction of FHY1 and FHL with HFR1 and LAF1 in Vitro
Our results so far indicate that HFR1 and LAF1 function downstream of FHY1/FHL in phyA signaling, but it is not known whether they transmit phyA signals directly from FHY1 and FHL or through one or more as yet unidentified intermediary components. To investigate this point, we examined possible direct association between FHY1/FHL and HFR1 and LAF1 by in vitro assays. For these assays, FHY1, FHL, HFR1, LAF1, and their deletion derivatives were expressed in Escherichia coli as glutathione S-transferase (GST) or maltose binding protein (MBP) fusion proteins that were then purified by affinity chromatography (Figure 5A). To study in vitro interactions, a GST fusion protein (bait) was mixed with an MBP fusion protein (target), and the former was pulled down by glutathione beads. The presence of the target MBP fusion protein in the pulled-down fraction was monitored by protein gel blots with antibody to MBP.
Figure 5.
FHY1 and FHL Interact with HFR1 and LAF1 in Vitro.
(A) Schematic diagrams of fusion proteins used. All fusion proteins carry at their N termini, MBP (M) or GST (G). FHY1 contains seven putative α-helical regions that are filled with diagonal lines and numbered consecutively from the N terminus. FHL contains six putative α-helical regions that are lined and numbered from the N terminus. The basic helix-loop-helix region of HFR1 located in the middle of the protein is dotted. The R2R3 domain of LAF1 is shown in the N-terminal region with gray dots.
(B) In vitro pull-down assays. Left panel: N-terminal regions of FHY1 and FHL interact with HFR1 in vitro. GST or GST-HFR1 (GHFR1) was used as bait and incubated with 1 μg of the indicated prey protein. Middle panel: N-terminal regions of FHY1 and FHL interact with LAF1 in vitro. GST or GST-LAF1 (GLAF1) was used as bait and incubated with the same set of MBP target fusion proteins. Right panel: amounts of MFHY1 and MFHL proteins and deletion derivatives added to the assays (INPUT). After incubation, GST, GST-HFR1 (GHFR1), and GST-LAF1 (GLAF1) were retrieved using glutathione beads, and pulled-down proteins were detected by antibodies to MBP.
(C) C-terminal region of HFR1 mediates FHY1 and FHL interactions. GST-FHY1 (GFHY1) and GST-FHL (GFHL) were used as baits and incubated with MBP-HFR1 (MHFR1) or its deletion derivatives. The amounts of MHFR1, MHFR1-N, and MHFR1-C proteins are shown on the blot (INPUT).
(D) The R2R3 domain of LAF1 mediates FHY1 and FHL interactions. GST-FHY1 (GFHY1) and GST-FHL (GFHL) were used as baits and incubated with 1 μg of MBP-LAF1 (MLAF1) or a deletion mutant (MLAF1-N). The amounts of MLAF1 and MLAF1-N proteins are shown on the blot (INPUT). After incubation, GST-FHY1 and GST-FHL were retrieved using glutathione beads, and pulled-down proteins were detected by antibodies to MBP.
(E) In vitro signal complex assembly assay. Two micrograms of HIS-PHYA full-length protein were incubated with GST, GST-FHY1 (1 μg), and/or MBP-HFR1 (1 μg) as indicated. GST-FHY1 or MBP-HFR1 was retrieved using glutathione beads or MBP amylose resin, respectively. Pulled-down proteins were detected with anti-PHYA antibody.
Using the above protocol, we mapped the domains responsible for the interactions between FHY1/FHL and HFR1 and LAF1. Figure 5B shows that GST-HFR1 was able to pull down full-length FHY1 and the N-terminal portion of FHY1 (FHY1-N) but not the C-terminal portion of FHY1 (FHY1-C), and similar results were obtained with FHL. Neither FHY1 nor FHL was recovered when the negative control protein GST was used as bait. Note that both the full-length and truncated versions of MBP-FHY1 were expressed in E. coli (Figure 5B, input), and truncated forms that likely represented N-terminal derivatives of MBP-FHY1 were also pulled down (Figure 5B, lane MFHY1). When GST-LAF1 was used as bait, it interacted with full-length FHY1 and FHY1-N and also with FHL and FHL-N (Figure 5B).
Next, we used GST fusions containing full-length FHY1 and FHL as bait proteins to define the interacting region on HFR1. GST alone was used as a negative control. Figure 5C shows that both FHY1 and FHL associated mostly with the C-terminal fragment of HFR1, which included the helix-loop-helix domain. Similar experiments with LAF1 showed that FHY1 and FHL interacted with the N-terminal fragment of LAF1, which contained the R2R3 domain responsible for DNA binding (Figure 5D). In both cases, GST alone did not interact with HFR1 nor LAF1, indicating the specificity of the assays. Taken together, our results show that there is direct association of FHY1/HFR1, FHL/HFR1, FHY1/LAF1, and FHL/LAF1, and the N-terminal fragment of FHY1 and FHL, the C-terminal region of HFR1, and the N-terminal portion of LAF1 mediate this interaction.
We also tested whether FHY1 was able to simultaneously interact with PHYA and/or HFR1 using an in vitro protein complex assembly assay. Although HIS-PHYA did not interact with MBP-HFR1 and MBP alone, addition of GST-FHY1 allowed the assembly of a PHYA/FHY1/HFR1 complex. MBP-HFR1 pulled down PHYA only in the presence of GST-FHY1 but not in its absence (Figure 5E). This result shows that FHY1 is required for PHYA/HFR1 interaction in vitro and suggests that FHY1 may be necessary for the assembly of phyA signaling complexes in vivo.
FHY1/FHL Interact with HFR1 and LAF1 in Vivo
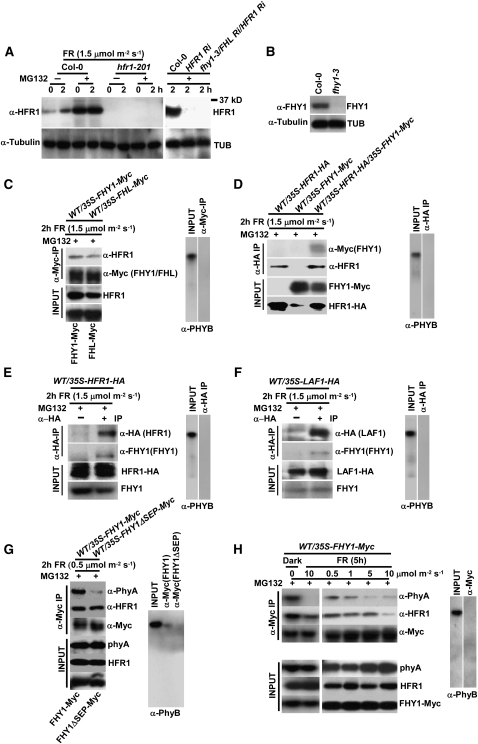
To test whether the in vitro interactions between HFR1 and LAF1 with FHY1/FHL can be recapitulated in vivo, we generated a specific antibody to HFR1. Figure 6A shows that the HFR1 antibody was indeed specific. In wild-type seedlings, HFR1 expression was induced by FR, but the protein was unstable as its accumulation level could be further increased by MG132 treatment. Also, the specificity of the antibody against FHY1 was verified by protein gel blots using fhy1-3 as a control (Figure 6B).
Figure 6.
In Vivo Interactions of FHY1, FHL, HFR1, and LAF1 Proteins.
(A) Endogenous HFR1 levels. Four-day-old etiolated seedlings were treated with or without 50 μM MG132 and exposed to FRc light for 0 or 2 h. Protein gel blots were analyzed with polyclonal anti-HFR1 antibody. Tubulin levels were used as a loading control.
(B) Specificity of FHY1 polyclonal antibody analyzed by protein gel blots.
(C) Coimmunoprecipitation of FHY1-Myc and FHL-Myc with endogenous HFR1. Seedlings were treated as in (A). Extracts were treated with anti-Myc antibody to immmunoprecipitate FHY1-Myc or FHL-Myc. The immunoprecipitates were analyzed with anti-HFR1 antibody. The immunoprecipitates were detected with anti-PHYB antibody as a negative control to verify binding specificity. Input protein levels of endogenous HFR1, FHY1-Myc, and FHL-Myc were detected in whole crude extracts using anti-HFR1 and anti-Myc antibodies.
(D) Coimmunoprecipitation of FHY1-Myc and HFR1-HA. Seedlings were treated as in (A). Extracts were treated with anti-HA antibody to immmunoprecipitate HFR1-HA. The immunoprecipitates were analyzed with anti-Myc antibody.
(E) Coimmunoprecipitation of endogenous FHY1 and HFR1-HA. Seedlings were treated as in (A). Extracts were mixed with anti-HA antibody to immunoprecipitate HFR1-HA. The immunoprecipitates were analyzed with anti-FHY1 antibody.
(F) Coimmunoprecipitation of endogenous FHY1 and LAF1-HA. Seedlings were treated as in (A). Extracts were mixed with anti-HA antibody to immunoprecipitate LAF1-HA. The immunoprecipitates were analyzed with anti-FHY1 antibody to detect the presence of endogenous FHY1.
(G) Coimmunoprecipitation of FHY1-Myc with endogenous phyA or HFR1. Seedlings were treated as in (A). Extracts were mixed with anti-Myc antibody to immunoprecipitate FHY1-Myc or FHY1ΔSEP-Myc. The immunoprecipitates were monitored with anti-PHYA or anti-HFR1 antibodies to detect the presence of phyA or HFR1.
(H) Coimmunoprecipitation of signaling complexes upon dark to FRc light transition. Seedlings were treated as in (A) but exposed to various FRc fluences for 5 h.
We used wild-type seedlings expressing 35S-FHY1-Myc or 35S-FHL-Myc to determine the association of HFR1 with FHY1/FHL in vivo. Figure 6C shows that HFR1 could be coimmunoprecipitated with FHY1 and also with FHL, indicating HFR1/FHY1 and HFR1/FHL associations in seedlings exposed to FRc. We also used 35S-HFR1-HA/35S-FHY1-Myc double overexpression lines or 35S-HFR1-HA overexpression lines to confirm FHY1/HFR1 interaction. The results show that HFR1-HA could be coimmunoprecipitated with FHY1-Myc or endogenous FHY1 (Figures 6D and 6E) and provide evidence that HFR1 interacts with FHY1 and FHL in vivo.
Because we were unsuccessful in generating a specific antibody against LAF1, we used transgenic seedlings expressing LAF1-HA (Jang et al., 2007) for our experiments. Protein gel blot analysis showed that FHY1 was coimmunprecipitated along with LAF1-HA, and its presence in the immunoprecipitate was dependent on the anti-HA antibody (Figure 6F). Our results indicate that LAF1 also interacts with FHY1 in vivo.
We used wild-type seedlings expressing 35S-FHY1-Myc or 35S-FHY1ΔSEP-Myc to examine the association of phyA and HFR1 with FHY1 in vivo. Compared with FHY1, the FHY1ΔSEP mutant contains a 35–amino acid deletion in the C-terminal region (168 to 202 amino acids). Because of the extreme instability of HFR1 (Jang et al., 2005, 2007), we performed the coimmunoprecipitation experiments with MG132-treated seedlings, which accumulated HFR1 to detectable levels. Figure 6G shows that in extracts of MG132-treated seedlings, phyA and HFR1 could be coimmunoprecipitated with FHY1, while FHY1ΔSEP interacted only with HFR1 in vivo. Recently, Genoud et al. (2008) showed that a 36–amino acid segment in the FHY1 C terminus (for convenience, it will be referred hereafter as SEP) is essential for phyA binding. Although this segment is absent from FHY1ΔSEP, the latter still binds HFR1. Our results, together with those of others (Hiltbrunner et al., 2005, 2006; Genoud et al., 2008; Shen et al., 2009), provide evidence that FHY1 interaction with phyA and HFR1 occurs in separate and distinct domains. Therefore, FHY1 might be required for the assembly of a signaling complex in addition to its function in aiding photoreceptor nuclear translocation. All the coimmunoprecipitation experiments were done using phyB as a negative control.
FHY1 Dissociates from phyA and HFR1 in Response to FRc
We assumed that light intensity may influence the affinity of interaction between proteins in phyA signaling because phyA/FHY1 interaction was affected by FRc in vitro (Hiltbrunner et al., 2005, 2006), and the requirement of FHL is only uncovered in the absence of FHY1 and more apparent at higher FR fluences. Accordingly, we explored the interaction dynamics between FHY1 with phyA and with HFR1. We used MG132-treated WT/35S-FHY1-Myc plants and performed coimmunoprecipitation assays to see whether FRc fluences affect the interaction. Figure 6H shows that, in treated seedling extracts, FHY1-Myc strongly interacted with endogenous phyA and HFR1 in darkness but dissociated from phyA upon exposure to various FRc fluences. Moreover, the interaction between FHY1 and phyA was more transient than that between FHY1 and HFR1. The preferential association of FHY1/FHL with the phyA Pfr form in vitro has been noted by Hiltbrunner et al. (2005, 2006). By coimmunoprecipitation of seedling extracts, Saijo et al. (2008) and Shen et al. (2009) showed that FHY1/FHL preferentially interact with the phyA Pr form in vivo, and this observation is confirmed by our results here (Figures 6G and 6H). In addition, we detected dissociation of the phyA/FHY1 complex in increasing FRc fluences in vivo (Figure 6H), a phenomenon similar to the dissociation of FHY1/FHL from phyA in response to R/FR pulse in vitro (Hiltbrunner et al., 2005, 2006).
Localization of HFR1 and LAF1 Is Independent of FHY1 and FHL
The lack of a nuclear localization signal (NLS) in phyA is overcome by its association with FHY1 and FHL (Hiltbrunner et al., 2005, 2006; Genoud et al., 2008). Although both HFR1 and LAF1 possess an NLS, it is possible that their nuclear translocation may also depend on FHY1 and FHL as well. Hence, we constructed fhy1-3/35S-HFR1-CFP (for cyan fluorescent protein), fhy1-3/35S-LAF1-CFP, fhy1-3/FHL Ri/35S-HFR1-CFP, and fhy1-3/FHL Ri/35S-LAF1-CFP lines to investigate whether FHY1 and FHL would affect HFR1 and LAF1 localization. As expected, expression of these TFs did not complement fhy1-3 and fhy1-3/FHL Ri mutant phenotypes (Figures 7A, 7B, and 7E). Furthermore, because of their instability (Seo et al., 2003; Jang et al., 2005), both HFR1-CFP and LAF1-CFP were detectable in overexpression lines only after MG132 treatment (Figures 7C and 7D). In these lines, nuclear localization of HFR1 and LAF1 was not affected by FHY1 and FHL deficiency (Figure 7F). Therefore, the extreme FRc hyposensitivity of fhy1-3 and fhy1-3/FHL Ri mutants cannot be attributed to changes in protein levels nor localization of HFR1 and LAF1. These findings further support the notion that FHY1 and FHL act upstream of HFR1 and LAF1 in phyA signaling, and their action is crucial for the assembly of phyA/HFR1 and phyA/LAF1 signaling complexes.
Figure 7.
Nuclear Localization of HFR1 and LAF1 Occur Independently of FHY1 and FHL.
(A) Seedling morphology at various FRc fluences.
(B) Data show average hypocotyl length ± sd (n = 20, except where specified). No significant differences were found between fhy1-3/FHL Ri and fhy1-3/FHL Ri/35-HFR1-CFP (P > 0.05, Student's t test at 5.0 μmol m−2 s−1, n > 80). As before, no significant differences were found between fhy1-3/FHL Ri and fhy1-3/FHL Ri/35S-LAF1-CFP (P > 0.05, Student's t test at 5.0 μmol m−2 s−1, n > 80).
(C) and (D) Protein gel blot analysis. Indicated seedlings were treated with or without MG132 (25 μM) for 12 h. HFR1-CFP and LAF1-CFP proteins were detected with anti-GFP antibody, and tubulin levels were used as loading controls.
(E) RNA gel blot analysis. Total RNAs were isolated from the same samples used for protein gel blot analysis. Full-length HFR1 and LAF1 cDNAs were used as probes. Ethidium bromide (EtBr) staining was used to monitor loading of RNAs (5 μg per lane).
(F) Nuclear localization of HFR1-CFP and LAF1-CFP in wild-type, fhy1-3, and fhy1-3/FHL Ri lines. a, WT/35S-HFR1-CFP; b, fhy1-3/35S-HFR1-CFP; c, fhy1-3/FHL Ri/35S-HFR1-CFP; d, WT/35S-LAF1-CFP; e, fhy1-3/35S-LAF1-CFP; f, fhy1-3/FHL Ri/35S-LAF1-CFP. CFP, CFP channel image; YFP, YFP channel image; DIC, differential interference contrast in light microscopy mode. Bars = 5 μm.
[See online article for color version of this figure.]
Overexpression of 35S-YFP-NLS-SEP Does Not Fully Rescue the Long Hypocotyl Phenotype of the fhyl-3 fhl-1 Double Mutant
Genoud et al. (2008) recently reported that the FR hyposensitivity of fhy1 could be rescued by an artificial FHY1 protein harboring a SV40 nuclear localization signal and a SEP domain (C-terminal 36 amino acids; 167 to 202). Furthermore, they showed that the SEP domain is sufficient to assist translocation of phyA into the nucleus. However, we found that FHL, in addition to FHY1, is also required for proper transmission of phyA signals especially at higher fluences of FRc, and the N-terminal region of both FHY1 and FHL can interact with downstream TFs, such as HFR1 and LAF1. Furthermore, fhy1-3 mutants compensate for the lack of FHY1 by expressing 3 times more FHL transcripts compared with the wild type (Figure 3C; Zhou et al., 2005). Because Genoud et al. (2008) proposed a model where only the NLS and SEP domains of FHY1 account for the full FHY1 function, we designed a new construct (35S-yellow fluorescent protein [YFP]-NLS-SEP) and tested its ability to rescue the extreme long hypocotyl phenotype of the fhy1 fhl double mutant. We argued that, if the sole function of FHY1 is to assist phyA translocation, then fhy1-3/fhl-1/35S-YFP-NLS-SEP lines would become more sensitive to FRc and display a hypocotyl phenotype closer to that of the fhl mutant. We obtained 26 lines with high transgene expression levels and analyzed four of them for their hypocotyl phenotypes. Contrary to the expectation, overexpression of YFP-NLS-SEP was unable to fully rescue the long hypocotyl phenotype of fhy1-3 fhl-1. Indeed, all four independent lines displayed hypocotyl length similar to that of fhy1-3 (Figures 8A and 8B). This partial complementation seen in fhy1-3/fhl-1/YFP-NLS-SEP lines was probably due to the absence of FHL, the latter can compensate for FHY1 function in the single fhy1 mutant.
Figure 8.
Overexpression of YFP-NLS-SEP Is Not Sufficient to Rescue the Long Hypocotyl Phenotype of fhy1-3 fhl-1.
(A) Seedling morphology at various FRc fluences.
(B) Data show average hypocotyl length ± sd (n = 20, except where specified).
(C) fhy1-3/fhl-1/35S-YFP-NLS-SEP seedlings were grown for 10 d and sampled for protein gel blot analysis (left panel) and RNA gel blot analysis (right panel). Proteins were detected with anti-GFP antibody. Tubulin levels were used as loading controls. Total RNAs were isolated from same samples used for protein gel blot analysis. Ethidium bromide (EtBr) staining was used to monitor loading of RNAs (5 μg per lane).
(D) Coimmunoprecipitation of YFP-NLS-SEP with endogenous PHYA. Seedlings were grown in darkness for 5 d, and extracts were treated with anti-GFP antibody to immmunoprecipitate YFP-NLS-SEP. Protein gel blots of the immunoprecipitates were analyzed with anti-PHYA antibody. Anti-PHYB antibody was used as a negative control to verify binding specificity.
(E) Nuclear localization of YFP-NLS-SEP in fhy1-3 fhl-1 line. a, observed root with 10×; b, 40×; c, 63×. YFP, YFP channel image; Dic, differential interference contrast in light microscopy mode. Box = 40 μm2, bars = 10 μm.
[See online article for color version of this figure.]
To fully characterize these lines, we determined protein and transcript levels in fhy1-3/fhl-1/35S-YFP-NLS-SEP (Figure 8C) and confirmed the interaction between YFP-NLS-SEP and PHYA by coimmunoprecipitation. In these experiments, we selected line fhy1-3/fhl-1/35S-YFP-NLS-SEP #9 and a nonexpression line (fhy1-3/fhl-1/35S-YFP-NLS-SEP #8) as a negative control (Figure 8D). We found that YFP-NLS-SEP was mainly localized in the nucleus (Figure 8E), confirming that the construct was functional. Our results confirm those of Genoud et al. (2008) with respect to the role of NLS and SEP domains in phyA interaction and nuclear translocation. In addition, they also suggest that the FHY1 N-terminal region is required for the full function of this protein in the phyA signaling cascade.
Overexpression of 35S-FHY1ΔSEP-Myc Obstructs phyA Signaling
We have previously shown that overexpression of FHY1ΔSEP (deletion of C-terminal 35 amino acids) in the fhy1-3 mutant background not only failed to complement the fhy1-3 phenotype but in fact promoted additional hypocotyl elongation under FRc (see Supplemental Figure 3A online; Zeidler et al., 2004). This deletion mutant does not contain the phyA interaction site recently identified by Genoud et al. (2008), and indeed it did not bind phyA in vivo (Figure 6G). Because FHY1/FHL homo- and heterodimerization occur via their C-terminal domain and the FHY1ΔSEP and SEP domains hardly interact with FHY1 in vitro (Zhou et al., 2005), the extreme FRc hyposensitivity elicited by FHY1ΔSEP expression could not be attributed to the sequestration of FHY1 and FHL. One interpretation of this dominant-negative phenotype is that the FHY1ΔSEP mutant may compete with FHL, which still operates in fhy1-3, for downstream TFs (e.g., HFR1 and LAF1), thereby mimicking an FHL Ri phenotype. Moreover, expressing FHY1ΔSEP in the wild-type background would lead to competition not only with FHL but also with FHY1, producing nonfunctional FHY1ΔSEP/TF complexes. Indeed, hypocotyl lengths of WT/35S-FHY1ΔSEP-Myc lines were similar to those of hfr1-201 but shorter than those of fhy1-3 and phyA-211 (Figures 9A and 9B). Protein gel blot analyses verified the expression of FHY1ΔSEP-Myc (Figures 9C and 9D), and we also confirmed that HFR1 and LAF1 transcript levels were not substantially changed in WT/35S-FHY1ΔSEP-Myc lines (see Supplemental Figure 3B online). Our results suggest that the dominant-negative phenotype (FRc hyposensitivity) of WT/35S-FHY1ΔSEP-Myc lines is likely due to its interference with the transient assembly of phyA/FHY1/HFR1 or phyA/FHY1/LAF1 protein complexes by titrating out HFR1 and LAF1.
Figure 9.
Overexpression of FHY1ΔSEP-Myc Obstructs phyA Signaling in Response to FRc.
(A) Seedling morphology at various FRc fluences.
(B) Data show average hypocotyl length ± sd (n = 20, except where specified).
(C) WT/35S-FHY1ΔSEP-Myc seedlings were treated with or without MG132 (25 μM) for 12 h. Proteins were detected with anti-Myc antibody. Tubulin levels were used as loading controls.
(D) RNA gel blot analysis of transgenic plants expressing FHY1ΔSEP-Myc. Total RNAs were isolated from same samples used for protein gel blot analysis. Ethidium bromide (EtBr) staining was used to monitor loading of RNAs (5 μg per lane).
(E) Schematic diagram of proposed phyA signaling cascade. FHY1 and FHL genes are activated by FHY3/FAR1, and then FHY1 and FHL are distributed in cytosol and nucleus in the dark. Upon exposure to FRc, FHY1 and FHL interact with phyA in the cytosol and aid its nuclear translocation. In the nucleus, FHY1 and FHL directly interact with LAF1, HFR1, and probably an unidentified factor X and phyA. This complex allows close proximity of phyA and TFs under FRc light. Unknown factor Y also transmits phyA signal independent of FHY1/FHL (FHY3/FAR1 are possible candidates). The cytosolic component A mediates the branched phyA signal (PKS1, NDPK2, or another unidentified molecule).
DISCUSSION
FHY1/FHL Is Upstream of HFR1 and LAF1 in the phyA Signaling Network
A major aim of this work was to define the hierarchical and molecular relationship between FHY1/FHL and the two TFs HFR1 and LAF1. We have approached this issue first by epistasis analysis of double mutants (fhy1-3 hfr1-201, fhy1-3/LAF1 Ri, FHL Ri/HFR1 Ri, and FHL Ri/LAF1 Ri) and by analysis of 35S-FHY1-Myc–overexpressing lines made deficient in HFR1 and LAF1 transcripts. Using hypocotyl length as an indicator of FRc sensitivity, we have obtained several lines of evidence to support the conclusion that FHY1/FHL operate upstream of HFR1 and LAF1. (1) Hypocotyl lengths of fhy1-3 and fhy1-3/FHL Ri are longer than those of hfr1-201 and LAF1 Ri, suggesting that FHY1 and FHY1/FHL are likely upstream of HFR1 and LAF1. (2) Hypocotyl lengths of fhy1-3/FHL Ri are still longer than fhy1-3 hfr1-201 or fhy1-3/LAF1 Ri. (3) The FRc hypersensitivity conferred by FHY1-Myc overexpression can be abrogated by hfr1-201 and LAF1 Ri. Moreover, seedlings of hfr1-201/35S-FHY1-Myc and LAF1 Ri/35S-FHY1-Myc are hyposensitive to FRc with hypocotyl lengths similar to those of hfr1-201 and LAF1 Ri lines. Also, we confirmed that the hypocotyl lengths of fhy1-3/FHL Ri are not affected when HFR1 and LAF1 transcript levels are reduced in hfr1-201 and LAF1 Ri, respectively. This observation suggests that phyA signals transduced by FHY1/FHL are distributed downstream to one or more factors, in addition to HFR1 and LAF1. One such factor may be HY5 because hypocotyl lengths of the hy5 mutant in FRc are similar to those of hfr1 and laf1 but shorter than those of fhy1-3 and fhy1-3/FHL Ri. Moreover, HY5 and HFR1 have been shown to regulate independent phyA pathways (Kim et al., 2002).
FHY1 and FHL Mediate Assembly of phyA Signaling Complexes
The finding that phyA interacts with FHY1 and FHL raised the question of how, after nuclear translocation, phyA would transmit its signal to downstream TFs such as HFR1 and LAF1. We have addressed this issue by performing detailed analysis of FHY1 and FHL interacting partners. We have previously shown that FHY1 and its homolog, FHL, can form homodimers and heterodimers with each other by direct interaction between their C-terminal regions (Zhou et al., 2005). Using similar in vitro pull-down assays, we show here that the N-terminal domains of FHY1 and FHL directly bind to LAF1 and HFR1. Further deletion analyses implicated the N-terminal region of LAF1 and the C-terminal region of HFR1 in this binding. These protein–protein associations are physiologically relevant, as the interacting proteins can be recovered in the same immunocomplex in seedling extracts.
The interaction between FHY1/FHL and HFR1/LAF1 raised the question of whether the former proteins would be required for nuclear accumulation of the two TFs. We demonstrate here that, unlike phyA, HFR1 and LAF1 possess an NLS that is sufficient for their nuclear localization. Therefore, we hypothesized that FHY1/FHL may mediate the assembly of nuclear signaling complexes, including the activated photoreceptor and the TFs. This hypothesis is supported by several findings reported here. (1) FHY1 can act as a bridge in vitro to assemble a protein complex consisting of PHYA and HFR1. (2) phyA/FHY1/HFR1 and phyA/FHY1/LAF1 complexes can be detected in vivo under FRc. (3) The inability of 35S-YFP-NLS-SEP to complement the long hypocotyl phenotype of fhy1-3 fhl-1 double mutant in four independent lines suggests that the FHY1 SEP domain (C-terminal 36 amino acids) alone cannot account for all aspects of FHY1 function. (4) An FHY1 deletion mutant, FHY1ΔSEP-Myc, which is unable to interact with phyA, still retains its ability to bind HFR1.
Expression of 35S-FHY1ΔSEP-Myc in the wild-type background produces a dominant-negative phenotype similar to that of hfr1-201 under FRc. This mutant phenotype can be explained by the sequestering of HFR1/LAF1 into nonproductive complexes by FHY1ΔSEP-Myc. These in vitro and in vivo interactions along with molecular genetic results suggest that FHY1 and its homolog FHL mediate the assembly of phyA and HFR1 (LAF1) to form multicomplexes and that there is a dynamic association of these components depending on FRc fluences. We also found that FHY1 association with phyA is less stable than that with HFR1 under FRc conditions. FHY1 preferentially interacts with phyA Pr in darkness, but the strength of interaction decreases with increasing FRc fluences, probably owing to the photolabile nature of phyA.
Several groups have addressed the mode of interaction between FHY1 and phyA (Hiltbrunner et al., 2005, 2006; Saijo et al., 2008; Shen et al., 2009). Hiltbrunner et al. (2005, 2006) reported that FHY1/FHL interact with the Pfr form of phyA by in vitro pull-down assay after a 5-min pulse of R/FR and by yeast two-hybrid assay under FRc or R for 3 d. On the other hand, Saijo et al. (2008) showed that FHY1 interacts with underphosphorylated phyA by coimmunoprecipitation using extracts derived from FRc-grown seedlings. Recently, Shen et al. (2009) demonstrated that FHY1/FHL interact with the Pr form of phyA by coimmunoprecipitation using transgenic seedlings extracts. In their experiments, fhy1-1 seedlings expressing 35S:GFP-FHY1 or 35S:GFP-FHL were grown in FRc for 3 d or in darkness for 3 d and then subjected to a 5-min pulse of R/FR treatment. Several factors may account for the differences between these results and the results reported here. (1) Different growth conditions were used by different groups. For example, we used 5- to 6-d-old etiolated transgenic seedlings prior to exposure to FRc. (2) We, as well as Hiltbrunner et al. (2005, 2006), used FRc fluence of ∼10 μmol m−2 s−1, whereas Shen et al. (2009) used 110 μmol m−2 s−1. (3) We treated etiolated transgenic seedlings with MG132 to increase TF levels, and this might possibly affect certain cellular processes during the dark/FRc transition. At this moment, we do not know whether growth conditions, light fluences, dark/light transition, and MG132 would affect the assembly of the phyA/FHY1 complex. However, we found the interaction between phyA and YFP-NLS-SEP to be quite strong in the darkness in seedlings without MG132 treatment (Figure 8D), confirming that FHY1 prefers the Pr form of phyA in vivo (Saijo et al., 2008; Shen et al., 2009). Saijo et al. (2008) suggested that FHY1 and FHY3 are able to assemble with underphosphorylated phyA as a signal complex preventing photoreceptor modification by the SPA1/COP1 complex. We have extended their observation by identifying downstream TFs as additional members of this complex.
Genoud et al. (2008) provided strong evidence that FHY1 has a major function in phyA nuclear translocation, since a constitutively nuclear phyA photoreceptor could rescue both fhy1-1 and fhy3 mutants under different FRc fluences. Moreover, they showed that a 36–amino acid segment in the FHY1 C-terminal region is sufficient for phyA binding and full complementation of fhy1-3, and expression of this domain without NLS or with Nuclear Export Signal resulted in a dominant-negative phenotype under higher FRc fluences, probably due to anchoring of phyA in the cytoplasm. Our results suggest additional roles for FHY1 in phyA signaling. By analyzing protein interactions in vivo and in vitro, we found that FHY1 could act as a platform for assembly of signaling complexes between phyA and downstream TFs. Although we used a slightly modified version of the Genoud et al. (2008) construct, we confirmed its functionality and showed that expression of YFP-NLS-SEP in the fhy1-3 fhl-1 mutant was not sufficient for full complementation of fhy1-3 fhl-1 hyposensitivity to FRc (Figures 8A and 8B). The partial complementation might be due to the absence of FHL. Note that FHL could compensate for FHY1 in the single fhy1 mutant (Figure 3C; Zhou et al., 2005). The existence of additional functions of FHY1/FHL is further supported by our findings that fhy1-3/35S-FHY1ΔSEP lines displayed a dominant-negative phenotype with hypocotyl lengths longer than those of fhy1-3 but similar to those of fhy1-3 hfr1-201 (see Supplemental Figure 1A online), and WT/35S-FHY1ΔSEP-Myc lines also showed a dominant-negative phenotype with hypocotyl lengths similar to those of hfr1-201 (Figures 9A and 9B).
Previously, we reported that two well-characterized TFs, HFR1 and LAF1, bind to each other and increase their protein stabilities. Furthermore, this interaction created new interdependent functions in addition to their independent roles in response to FRc (Jang et al., 2007). These results raised the question whether FHY1/FHL would affect the stability of HFR1 and LAF1. We used WT/35S-HFR1-CFP, WT/35-LAF1-CFP, fhy1-3/FHL Ri/35S-HFR1-CFP, and fhy1-3/FHL Ri/35S-LAF1-CFP lines to test whether fhy1 and fhl mutations might affect HFR1 and LAF1 protein levels. Although we could not rule out possible side effects of overexpression of HFR1 and LAF1, under our experimental conditions, no significant changes were found (see Supplemental Figure 3C online). We further showed that the N-terminal region of FHY1 and FHL interacted with the basic helix-loop-helix region of HFR1 and the R2R3 region of LAF1. However, we could not determine whether this interaction involves the monomeric form of HFR1 and LAF1 or in the HFR1/LAF1 heterodimer. The exact mechanism for these interactions remains an important issue for future clarification.
A Model for the phyA Signaling Network under FRc
Based on the data presented here, as well published results of other groups, we propose a working model for the phyA signaling network that results in FRc inhibition of hypocotyl elongation (Figure 9E). In this model, FHY3 and FAR1 trigger FHY1 and FHL transcription (Lin et al., 2007). In darkness, FHY1 and FHL can interact with phyA in the cytosol and with TFs in the nucleus. Upon FRc irradiation, activated phyA is translocated into the nucleus assisted by FHY1/FHL (Hiltbrunner et al., 2005, 2006; Genoud et al., 2008). Association between FHY1 and phyA under FRc prevents early targeting of phyA by SPA1/COP1 and stabilizes the photoreceptor (Saijo et al., 2008). Nuclear FHY1 and FHL, along with activated phyA, can associate with other downstream factors (HFR1, LAF1, and X, an unknown component) to form nuclear phyA/TF signaling complexes. At present, it is not known whether FHY1 and FHL interact with phyA as dimers or monomers while also interacting with HFR1 or LAF1 as monomers (Figure 5; Zhou et al., 2005). It is also not known how phyA signals are transduced from the activated photoreceptor to the TFs in such complexes. Our model shows that factor(s) (designated “X” in Figure 9E) other than LAF1 and HFR1 are also involved in transducing phyA signals downstream of FHY1/FHL. Since both LAF1 and HFR1 are TFs, it is reasonable to assume that “X” would belong to the same functional category as well. An attractive candidate for “X” is the bZIP TF HY5, which is a positive regulator of phyA signaling and known to function independently from HFR1 (Oyama et al., 1997; Kim et al., 2002).
Available data suggest the presence of unknown downstream factors “Y,” which are able to transmit phyA signals independently of FHY1/FHL. This hypothesis could be explained by several findings. (1) The overexpression of YFP-NLS-SEP in fhy1-3 fhl-1 showed a hypocotyl phenotype close to fhy1-3, probably a consequence of alternative signal transduction (Figure 8). (2) The absence of FHY1 possibly affects FHY3/FAR1 activity toward FHL expression. (3) FHY3/FAR1 are responsible for the expression of FHY1/FHL (Lin et al.. 2007). (4) FHY3 preferentially interacts with underphosphorylated phyA (Saijo et al., 2008). Based on these observations, we consider FHY3, and its partner FAR1, to be suitable candidates for factor “Y.”
We propose that FHY1/FHL and FHY3/FAR1 may associate in a combinatorial manner with downstream TFs and generate different protein complexes for phyA signaling under different environmental conditions. Since FHY1 and FHL are distributed between nucleus and cytoplasm, it is possible that FHY1/FHL act as transient anchors for TFs before phyA is transported into nucleus. Recently, Shen et al. (2009) have shown that phyA could phosphorylate FHY1 in an R/FR reversible manner and both FHY1 and FHL interact with the Pr form of phyA. These results suggest that phyA directly regulates FHY1/FHL levels, thereby influencing signal transmission downstream. The characterization and regulation of such signaling complexes and their dynamics will be a major challenge for the future.
METHODS
Plant Material, Growth Conditions, and Generation of Transgenic Lines
The fhy1-3, fhl-1 (Zeidler et al., 2001; Zhou et al., 2005), fhy1-3 fhl-1 (Rösler et al., 2007), hfr1-201 (Soh et al., 2000), FHL Ri (Zhou et al., 2005), and phyA-211 (Reed et al., 1994) mutants and all transgenic lines used in this study were in the Columbia background. Sterilized seeds were germinated on Murashige and Skoog (MS) medium without sucrose. After 4 d of incubation in darkness at 4°C, seeds were treated as described by Bolle et al. (2000). To analyze seedling morphology, mutants and wild-type seeds were kept in darkness or exposed to various continuous FR fluences (0.5, 1.0, 2.0, and 5.0 μmol m−2 s−1) for 5 d, except in the case of fhy1-3 fhl-1 in which hypocotyl lengths were measured after 4 d (Rösler et al., 2007). Transgenes were introduced into plants by Agrobacterium tumefaciens (EHA105 strain)–mediated infiltration using the floral dip method (Clough and Bent, 1998; Zhang et al., 2006).
Construction of Double and Triple Mutants
fhy1-3 hfr1-201 and fhy1-3/FHL Ri double mutants were constructed by genetic crosses between fhy1-3 (Basta resistance) and hfr1-201 (kanamycin resistance) or FHL Ri (kanamycin resistance; Zhou et al., 2005). F2 seeds were screened on MS medium containing Basta and kanamycin. For RNAi-mediated gene silencing against HFR1 and LAF1, DNA fragments were amplified by PCR using two oligonucleotides sets: 5′-CACCGCTCAAGTCTCAGAGCTTAC-3′ and 5′-ACGTCGTTGTTGATGGAGAA-3′ for LAF1-RNAi and 5′-CACCTGCGTAAGCTACAGCAACTC-3′ and 5′-CGTGAAGAGACTGAGGAGAA-3′ for HFR1-RNAi. The LAF1-RNAi fragment (500 bp) and the HFR1-RNAi fragment (350 bp) were cloned into pENTR/D, followed by LR reaction with pH7-GWIWG2(I) (Plant Systems Biology/VIB-Ghent University) vector harboring a hygromycin resistance marker to create pH7-RNAi-LAF1 and pH7-RNAi-HFR1. These clones were introduced into fhy1-3 and FHL Ri mutants to create fhy1-3 LAF1 Ri, FHL Ri HFR1 Ri, and FHL Ri LAF1Ri double mutant lines. These RNAi constructs were also introduced into fhy1-3/FHL Ri double mutants to produce fhy1-3/FHL Ri/HFR1 Ri and fhy1-3/FHL Ri/LAF1 Ri triple mutants.
Construction of Overexpression Lines
The Gateway system was used to generate the following full-length cDNA fused to various tag-encoded sequences: FHY1-Myc, FHL-Myc, FHY1ΔSEP-Myc, HFR1-HA, HFR1-CFP, LAF1-CFP, and YFP-NLS-SEP (SV40 NLS sequence from Genoud et al., 2008). These sequences were placed downstream of a 35S promoter, and the resulting clones were used to transform the wild type, hfr1-201, and LAF1 Ri, fhy1-3, fhy1-3/FHL Ri, and fhy1-3/fhl-1 mutants to generate WT/35S-FHY1-Myc, hfr1-201/35S-FHY1-Myc, LAF1 Ri/35S-FHY1-Myc, WT/35S-FHL-Myc, WT/35S-FHY1ΔSEP-Myc, WT/35S-HFR1-CFP, WT/35S-LAF1-CFP, fhy1-3/35S-HFR1-CFP, fhy1-3/35S-LAF1-CFP, fhy1-3/FHL Ri/35S-HFR1-CFP, fhy1-3/FHL Ri/35S-LAF1-CFP, WT/35S-FHY1-Myc/35S-HFR1-HA, and fhy1-3/fhl-1/35S-YFP-NLS-SEP transgenic lines (see Supplemental Table 1 online). The WT/35S-HFR1-HA lines and WT/35S-LAF1-HA lines were described by Jang et al. (2005, 2007).
RNA Extraction, Quantitative RT-PCR, and RNA and Protein Gel Blot Analyses
Total RNA was extracted from Arabidopsis thaliana seedlings using RNeasy plant mini kits (Qiagen) or Trizol reagent (Invitrogen). Reverse transcription was performed using Ready-To-go You Prime first-strand beads (GE Healthcare) according to the manufacturer's instructions. Quantitative PCR was performed using the Applied Biosystems 7900HT real-time PCR system with four sets of primers: 5′-TTCTTGCTGGACCACTGTTC-3′ and 5′-ACGTCGTTGTTGATGGAGAA-3′ for LAF1, 5′-GTCGGATCACTTGCTGTGAA-3′ and 5′-CGTGAAGAGACTGAGGAGAA-3′ for HFR1, 5′-ATGATAGTTGCTGTGTGGAATCTC-3′ and 5′-CATCATGAGTGTAGAAAAGTACTGC-3′ for FHL, and 5′-GCACCCTGTTCTTCTTACCG-3′ and 5′-AACCCTCGTAGATTGGCACA-3′ for ACTIN. Relative amounts of HFR1, LAF1, and FHL transcripts were obtained from calibrating HFR1, LAF1, and FHL threshold cycles with ACTIN threshold cycles. Calculations were performed by the equation 2(-ΔΔCT), where CT was the cycle number at which the fluorescence reached the threshold point for detection. All experiments were performed with three independent replicates to compensate for possible loading errors.
RNA gel blot analysis was as described by Zeidler et al. (2001) using as probes a fragment encoding the entire open reading frame of FHY1, HFR1, or LAF1 (see Supplemental Table 1 online). For protein gel blot analyses, 2-week-old seedlings of WT/35S-FHY1-Myc, WT/35S-FHY1ΔSEP-Myc, hfr1-201/35S-FHY1-Myc, LAF1 Ri/35S-FHY1-Myc, fhy1-3/35S-HFR1-CFP, fhy1-3/35S-LAF1-CFP, fhy1-3/FHL Ri/35S-HFR1-CFP, and fhy1-3/FHL Ri/35S-LAF1-CFP lines were treated with MG132 (25 μM) or mock treatment for 12 h and then were ground with Tissuelyzer (Qiagen) with 6× SDS sample buffer for 1 min, and extracts were resolved on 8 to 10% SDS-PAGE after boiling at 100°C. fhy1-3/fhl-1/35S-YFP-NLS-SEP lines were subjected to the same procedure without MG132 treatment. Blots were detected with anti-Myc (1:2500; Santa Cruz Biotechnology), anti-GFP (1:5000; Sigma-Aldrich), and antitubulin (1:2500; Santa Cruz Biotechnology) antibodies. Posttranslational decay assay was similar to that described by Jang et al. (2005, 2007). Dark-grown seedlings were incubated with MG132 (50 μM) for 12 h, washed, and then transferred to MS liquid medium with 100 μM cycloheximide for 3 h. Treated seedlings were exposed to FRc (2.0 μmol m−2 s−1). Proteins were extracted at the indicated time and analyzed by protein gel blots using anti-Myc antibody (1:2500; Santa Cruz Biotechnology) followed by mouse secondary antibody (1:5000; Amersham Bioscience). Relative amounts of proteins were calculated in percentage by a densitometer (Bio-Rad).
Confocal Microscopy
Confocal microscopy was performed as described by Jang et al. (2005).
Construction of Escherichia coli Expression Vectors and Protein Purification
Full-length and deletion mutants of PHYA, FHY1, FHL, LAF1, and HFR1 were previously described (Seo et al., 2003; Zhou et al., 2005; Jang et al., 2005, 2007). E. coli, BL21 (DE3) strain, harboring expression vectors was incubated at 37°C for 2 h, shifted to 22°C, and incubated for an additional 12 h after Isopropyl β-D-1-thiogalactopyranoside (1 mM) induction. Extraction and purification of all GST fusion and MBP fusion recombinant proteins from E. coli was previously described (Jang et al., 2005, 2007; Zhou et al., 2005). Extraction and purification of HIS fusion recombinant protein was previously described (Seo et al., 2003). For immunization of HFR1 and FHY1 polyclonal antibodies, affinity-purified GST-FHY1 and MBP-HFR1 proteins were further separated by PAGE. Proteins were isolated from gel using gel eluter (Bio-Rad) and boosted into rabbits three times. Whole serum of rabbits harboring anti-HFR1 antibody or anti-FHY1 antibody was used for protein gel blot analysis.
In Vitro Pull-Down Assay and Coimmunoprecipitation
For in vitro pull-down assays, 1 to 2 μg of target proteins were incubated with 2 μg of GST-fused or MBP-fused bait proteins and glutathione sepharose 4B or MBP amylase bead (25 μL) for 2 h at 4°C. Pulled-down proteins were extensively washed with buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.6% Triton X-100) before the samples were resolved on 8% SDS-PAGE and analyzed by protein gel blots using anti-MBP (1:5000; Santa Cruz Biotechnology) or anti-PHYA antibody followed by a mouse secondary antibody (1:5000; Amersham Bioscience). For coimmunoprecipitation, 4- or 6-d-old transgenic Arabidopsis seedlings grown in darkness (WT/35S-FHY1-Myc, WT/35S-FHL-Myc, WT/35S-FHY1ΔSEP-Myc, WT/35S-HFR1-HA/35S-FHY1-Myc, WT/35S-HFR1-HA, and WT/35S-LAF1-HA) were treated with MG132 (25 to 50 μM) for 6 h and exposed to FRc (0 to 10 μmol m−2 s−1) for 2 to 5 h. Samples were ground in liquid nitrogen and suspended in IP buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.6% Triton X-100, 1 mM DTT, 50 μM MG132, and 1× Protease Inhibitor cocktail). Five micrograms of anti-Myc or anti-HA monoclonal antibody (1:2500; Santa Cruz Biotechnology) were used to immunoprecipitate tagged proteins, and agarose-protein A (50 μL) was added to precipitate protein-antibody immunocomplexes. Immunoprecipitates were then analyzed using anti-PHYA, anti-HFR1, or anti-FHY1 antibody followed by rabbit or mouse secondary antibody (1:5000; Amersham Bioscience). Immunoprecipitates were also analyzed using the phyB-specific antibody (1:2500; Santa Cruz Biotechnology) to test the specificity of the reactions.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: FHY1 (AT2G37678), FHL (AT5G02200), LAF1 (AT4G25560), HFR1 (AT1G02340), and PHYA (AT1G09570).
Supplemental Data
The following materials are available in the online version of this article.
Supplemetal Figure 1. LAF1 Ri/hfr1-201 Double Mutants Are Shorter Than fhy1-3 and fhy1-3/FHL Ri.
Supplemental Figure 2. fhy1-3/FHL Ri Double Mutants Show Similar Hypocotyl Length to Those of the fhy1-3/fhl-1 Double Mutants.
Supplemental Figure 3. Dominant-Negative Phenotype of fhy1-3/35S-FHY1ΔSEP under FRc.
Supplemental Table 1. List of Primers and Vectors.
Supplementary Material
Acknowledgments
We thank A. Nagatani for monoclonal antibodies to phyA and M. Zeidler for the fhy1-3 fhl-1 line. S.W.Y. was supported by a postdoctoral fellowship from the Korean Research Foundation, I.-C.J. was supported by a Human Frontier Science Program Postdoctoral Fellowship (LT00309/2004-C), and R.H. was funded in part by a Gulbenkian Postdoctoral Fellowship. This work was supported by National Institutes of Health Grant GM44640 to N.-H.C.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Nam-Hai Chua (chua@mail.rockefeller.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Ballesteros, M.L., Bolle, C., Lois, L.M., Moore, J.M., Vielle-Calzada, J.P., Grossniklaus, U., and Chua, N.H. (2001). LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev. 15 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle, C., Koncz, C., and Chua, N.H. (2000). PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 14 1269–1278. [PMC free article] [PubMed] [Google Scholar]
- Chen, M., Chory, J., and Fankhauser, C. (2004). Light signal transduction in higher plants. Annu. Rev. Genet. 38 87–117. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Dehesh, K., Franci, C., Parks, B.M., Seeley, K.A., Short, T.W., Tepperman, J.M., and Quail, P.H. (1993). Arabidopsis HY8 locus encodes phytochrome A. Plant Cell 5 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos, T., Puente, P., Whitelam, G.C., and Harberd, N.P. (2001). FHY1: A phytochrome A-specific signal transducer. Genes Dev. 15 2980–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duek, P.D., and Fankhauser, C. (2003). HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signaling. Plant J. 34 827–836. [DOI] [PubMed] [Google Scholar]
- Fairchild, C.D., Schumaker, M.A., and Quail, P.H. (2000). HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 14 2377–2391. [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., and Chory, J. (2000). RSF1, an Arabidopsis locus implicated in phytochrome A signaling. Plant Physiol. 124 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud, T., Millar, A.J., Nishizawa, N., Kay, S.A., Schafer, E., Nagatani, A., and Chua, N.H. (1998). An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud, T., Schweizer, F., Tscheuschler, A., Debrieux, D., Casal, J.J., Schäfer, E., Hiltbrunner, A., Fankhauser, C. (2008). FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 4 e1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyula, P., Schafer, E., and Nagy, F. (2003). Light perception and signalling in higher plants. Curr. Opin. Plant Biol. 6 446–452. [DOI] [PubMed] [Google Scholar]
- Hare, P.D., Møller, S.G., Huang, L.F., and Chua, N.H. (2003). LAF3, a novel factor required for normal phytochrome A signaling. Plant Physiol. 133 1592–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner, A., Tscheuschler, A., Viczian, A., Kunkel, T., Kircher, S., and Schafer, E. (2006). FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 47 1023–1034. [DOI] [PubMed] [Google Scholar]
- Hiltbrunner, A., Viczian, A., Bury, E., Tscheuschler, A., Kircher, S., Toth, R., Honsberger, A., Nagy, F., Fankhauser, C., and Schafer, E. (2005). Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol. 15 2125–2130. [DOI] [PubMed] [Google Scholar]
- Hoecker, U., Xu, Y., and Quail, P.H. (1998). SPA1: A new genetic locus involved in phytochrome A-specific signal transduction. Plant Cell 10 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, H.L., Okamoto, H., Wang, M., Ang, L.H., Matsui, M., Goodman, H., and Deng, X.W. (2000). FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 14 1958–1970. [PMC free article] [PubMed] [Google Scholar]
- Hudson, M., Ringli, C., Boylan, M.T., and Quail, P.H. (1999). The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev. 13 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, I.C., Yang, J.Y., Seo, H.S., and Chua, N.H. (2005). HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, I.C., Yang, S.W., Yang, J.Y., and Chua, N.H. (2007). Independent and interdependent functions of LAF1 and HFR1 in phytochrome A signaling. Genes Dev. 21 2100–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.M., Woo, J.C., Song, P.S., and Soh, M.S. (2002). HFR1, a phytochrome A signaling component, acts in a separate pathway from HY5, downstream of COP1 in Arabidopsis thaliana. Plant J. 30 711–719. [DOI] [PubMed] [Google Scholar]
- Lin, R., Ding, L., Casola, C., Ripoll, D.R., Feschotte, C., and Wang, H. (2007). Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318 1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller, S.G., Kunkel, T., and Chua, N.H. (2001). A plastidic ABC protein involved in intercompartmental communication of light signaling. Genes Dev. 15 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M.M., and Chory, J. (1998). Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M.M., Frankhauser, C., and Chory, J. (2000). Light: An indicator of time and place. Genes Dev. 14 257–271. [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95 657–667. [DOI] [PubMed] [Google Scholar]
- Oyama, T., Shimura, Y., and Okada, K. (1997). The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail, P.H. (2002). Phytochrome photosensory signaling networks. Nat. Rev. Mol. Cell Biol. 3 85–93. [DOI] [PubMed] [Google Scholar]
- Reed, J.W., Nagatani, A., Elich, T.D., Fagan, M., and Chory, J. (1994). Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler, J., Klein, I., and Zeidler, M. (2007). Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl. Acad. Sci. USA 25 10737–10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo, Y., Zhu, D., Li, J., Rubio, V., Zhou, Z., Shen, Y., Hoecker, U., Wang, H., and Deng, X.W. (2008). Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell 31 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, H.S., Yang, J.Y., Ishikawa, M., Bolle, C., Ballesteros, M.L., and Chua, N.H. (2003). LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 26 995–999. [DOI] [PubMed] [Google Scholar]
- Shen, Y., Zhou, Z., Feng, S., Li, J., Tan-Wilson, A., Qu, L.J., Wang, H., and Deng, X.W. (2009). Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell 21 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock, R.A., and Quail, P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3 1745–1757. [DOI] [PubMed] [Google Scholar]
- Soh, M.S., Kim, Y.M., Han, S.J., and Song, P.S. (2000). REP1, a basic helix-loop-helix protein, is required for a branch pathway of phytochrome A signaling in Arabidopsis. Plant Cell 12 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., and Deng, X.W. (2002). Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J. 21 1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., and Deng, X.W. (2003). Dissecting the phytochrome A-dependent signaling network in higher plants. Trends Plant Sci. 8 172–178. [DOI] [PubMed] [Google Scholar]
- Whitelam, G.C., Johnson, E., Peng, J., Carol, P., Anderson, M.L., Cowl, J.S., and Harberd, N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler, M., Bolle, C., and Chua, N.H. (2001). The phytochrome A specific signaling component PAT3 is a positive regulator of Arabidopsis photomorphogenesis. Plant Cell Physiol. 42 1193–1200. [DOI] [PubMed] [Google Scholar]
- Zeidler, M., Zhou, Q., Sarda, X., Yau, C.P., and Chua, N.H. (2004). The nuclear localization signal and the C-terminal region of FHY1 are required for transmission of phytochrome A signals. Plant J. 40 355–365. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Henriques, R., Lin, S.S., Niu, Q.W., and Chua, N.H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protocols 1 641–646. [DOI] [PubMed] [Google Scholar]
- Zhou, Q., Hare, P.D., Yang, S.W., Zeidler, M., Huang, L.F., and Chua, N.H. (2005). FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 43 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.