Abstract
Floral organ identities are specified by a few transcription factors that act as master regulators. Subsequently, specification of organ axes programs the distribution of distinct tissue types within the organs that themselves develop unique identities. The C-class, AGAMOUS-clade MADS box genes are primary promoters of the gynoecium, which is divided into a distal style and a subtending ovary along the apical-basal axis. We show that members of a clade of B3 domain transcription factors, NGATHA1 (NGA1) to NGA4, are expressed distally in all lateral organs, and all four have a redundant and essential role in style development. Loss of all four genes results in gynoecia where style is replaced by valve-like projections and a reduction in style-specific SHATTERPROOF1 (SHP1) expression. In agreement, floral misexpression of NGA1 promotes ectopic style and SHP1 expression. STYLISH1, an auxin biosynthesis inducer, conditionally activated NGA genes, which in turn promoted distal expression of other STY genes in a putative positive feedback loop. Inhibited auxin transport or lack of YABBY1 gene activities resulted in a basally expanded style domain and broader expression of NGA genes. We speculate that early gynoecium factors delimit NGA gene response to an auxin-based signal, elicited by STY gene activity, to restrict the activation of style program to a late and distal carpel domain.
INTRODUCTION
The primary molecular genetic program underlying the identity of the concentric whorls of sepals, petals, stamens, and carpels of the flower is well characterized. Floral organ identity is governed by transcriptions factors acting in specific combinations defined as A, B, and C class to promote identity and in some cases antagonize genes of other classes that promote an alternative fate (Coen and Meyerowitz, 1991; Honma and Goto, 2001; Jack, 2004). In the gynoecium, expression of the AG-like genes AGAMOUS (AG), SHATTERPROOF1 (SHP1), and SHP2 have redundant roles in carpel tissue promotion (Pinyopich et al., 2003). However, little is known about the mechanism by which AG-like activities are translated into gynoecium tissue differentiation.
The Arabidopsis thaliana gynoecium is one of the most complex organs of the plant, reflecting its multifunctional role of housing the female gametophyte, acting as a conduit for pollen tube growth, and developing into a fruit containing the seed (Ferrandiz et al., 1999; Balanza et al., 2006). Structurally, the gynoecium consists of two congenitally fused carpels with distinct apical-basal, medio-lateral, and abaxial-adaxial pattern elements (Bowman et al., 1999). Along the apical-basal axis, a distal stigma caps a short style above an ovary of two locules and an intervening medial replum connected to the flower receptacle by a short gynophore (Figure 1). The inner-to-outer axis (adaxial-abaxial) consists of a central transmitting tissue within a septum flanked by ovules encapsulated by the valves that have a distinct adaxial-abaxial histology. The style, septum, and ovules initiate after the gynoecium has first formed an ∼200-μm open cylinder (floral stage 9; Smyth et al., 1990; Figure 1A). Thus, the gynoecium passes through an early phase of growth before differentiating the distal and internal marginal tissues (Alvarez and Smyth, 2002).
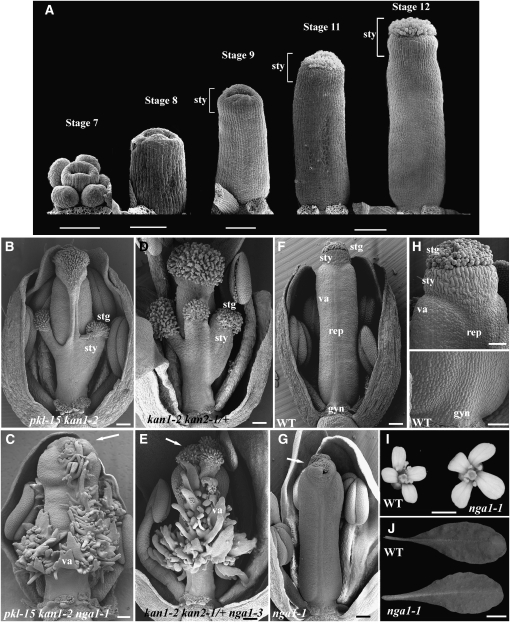
Figure 1.
nga1-1 Mutant Phenotypes.
(A) Scanning electron micrographs of Arabidopsis wild-type gynoecium development in progressive stages. The style becomes evident during stage 9 of flower development. All gynoecia are orientated medially. Floral stages after Smyth et al. (1990).
(B) to (H) Scanning electron micrographs of medially orientated mature flowers with some outer floral organs removed to reveal the gynoecium.
(B) pkl-15 kan1-2 gynoecium with basal stigma and style tissue extensions.
(C) pkl-15 kan1-2 nga1-1 gynoecium with external valve and placenta outgrowths extending from the medial and basal positions of the ovary and a reduced style.
(D) kan1-1 kan2-1/+ gynoecium with basal and medial style and stigmatic tissues extensions.
(E) kan1-1 kan2-1/+ nga1-3 gynoecium revealing valve-like tissue as in (C).
(F) Wild-type flower showing the proximal-distal elements in the gynoecium and bilateral symmetry of the ovary.
(G) nga1-1 gynoecium showing disruptions in style growth.
(H) Magnified view of the distal part of the wild-type gynoecium including the stigma and style (top) and basal region including the gynophore (bottom).
(I) Mature wild-type and nga1-1 flowers. Note the larger petals of nga1-1 mutants.
(J) Wild-type and nga1-1 leaves. nga1-1 leaves are more serrated.
Arrows mark disrupted style fusion and reduced stigmatic papillae. stg, stigmatic papillae; sty, style tissue; va, valve; rep, replum; gyn, gynophore. Bars = 100 μm in (A) (stage 11 and 12) and 50 μm for stages 7 to 9, 100 μm in (B) to (G), 50 μm in (H), 1 mm in (I), and 1 cm in (J).
Mutant screens and reverse genetic approaches have identified a number of genes that function in specifying tissue types and growth during gynoecium development. These can be principally categorized into genes that function specifically in the gynoecium and include the YABBY domain gene CRABS CLAW (CRC), the MADS domain genes SHP1 and SHP2, the transmitting tract factors NO TRANSMITTING TRACT and HECATE1-3, and a regulator of flower meristem determinacy KNUCKLES (Bowman and Smyth, 1999; Liljegren et al., 2000; Payne et al., 2004; Crawford et al., 2007; Gremski et al., 2007). Other genes that function more broadly in plant development were also co-opted for a particular function in the gynoecium. These include the meristem genes CUPSHAPED COTYLEDON1 (CUC1) and CUC2, SHOOTMERISTEMLESS (Ishida et al., 2000; Scofield et al., 2007), as well as the organ growth and polarity genes and their homologs FILAMENTOUS FLOWER, JAGGED, KANADI1, ETTIN, AINTEGUMENTA, LEUNIG, SPATULA, and PICKLE (Elliott et al., 1996; Sessions et al., 1997; Eshed et al., 1999; Sawa et al., 1999; Siegfried et al., 1999; Heisler et al., 2001; Ohno et al., 2004; Dinneny et al., 2006; Azhakanandam et al., 2008; Sitaraman et al., 2008). The organ growth and polarity genes generally perform roles that are an extension of their roles outside the gynoecium. Thus, in agreement with its evolutionary origin, the carpel program overlies the molecular genetic program regulating lateral organ development.
Another general process having a patterning role in the gynoecium is auxin synthesis and flux. For example, the YUCCA4 gene, which encodes an enzyme involved in auxin biosynthesis, is apparently activated by the transcription factor STYLISH1 (STY1) (Cheng et al., 2006; Sohlberg et al., 2006). STY1 is expressed at the distal part of the gynoecium, and its overexpression results in expansion of style tissue into the valve domain, while its loss of function results in style reduction (Kuusk et al., 2002, 2006). Plants mutant in the auxin efflux protein PIN-FORMED1 (PIN1) (Galweiler et al., 1998) or its polar localization regulator, the PINOID kinase (PID) (Bennett et al., 1995; Christensen et al., 2000; Benjamins et al., 2001), exhibit defects in apical-basal gynoecium patterning. pin1 and pid mutants exhibit increased basal gynophore and apical style/stigma regions and a reduced ovary. pin1 mutants are defective in polar auxin transport (PAT), and a phenocopy can be produced by treatment with PAT inhibitors (Okada et al., 1991). This suggests that apical-basal patterning of the gynoecium is dependent on an auxin gradient, whereby distal auxin biosynthesis and basal transport create a decreasing, instructional gradient with high levels of auxin inducing style and stigma differentiation (Nemhauser et al., 2000). The dramatic role of an auxin gradient(s) in gynoecium patterning suggests that dynamic programs that may create only subtle boundaries and gradients in the development of other organs (e.g., leaves) are exploited to program distinct tissues within the gynoecium.
Here, we describe plants lacking activities of four NGATHA (NGA) transcription factors in which style and stigma are lost and replaced by outgrowths with valve identity. Members of this clade were distally expressed in all organs, and their floral overexpression resulted in ectopic style/stigma morphogenesis. NGA gene activity can be conditionally activated by STY1, which promotes auxin biosynthesis (Sohlberg et al., 2006), suggesting that STY1 may act on NGA indirectly through an auxin-related signal. NGA gene activity then promotes AGAMOUS clade and SHI/STY family members in a presumptive positive feedback loop to program style and stigma morphogenesis.
RESULTS
Identification of the nga1 Mutation
Mutant screens in a crc-1 background helped identifying genes regulating organ polarity, growth, and flower meristem determinacy (Eshed et al., 1999; Bowman et al., 2001; Prunet et al., 2008). Both crc pickle and crc kanadi double mutants have adaxial tissues developing in abaxial positions, implicating these genes in regulating carpel polarity. The gynoecium of gym-5 kan1-2 and kan1-2 kan2-1/+ have style-like outgrowths that arise from the base and medial replum of the gynoecium (Figures 1B and 1D). Mutagenesis in the gym-5 kan1-2 background identified two independent alleles of ngatha1 (nga1-1 and nga1-2). A third allele was identified in the kan1-2 kan2-1/+ background (nga1-3). In both backgrounds, extensions of style-like tissue were replaced by a proliferation of outgrowths with valve-like identity. In addition, the distal stigma and style were comparatively reduced and the style was split in the medial plane (Figures 1C and 1E). This phenotype was clearly distinct from that produced when the carpel polarity gene, CRC, was reduced in the same backgrounds (see Supplemental Figures 1A to 1C online; Eshed et al., 1999).
In an otherwise wild-type background, nga1 gynoecia had an occasional misshapen or split style in the medial plane and reduced stigmatic papillae (Figure 1G) but were otherwise fully fertile. Gynoecium phenotypes of double mutant combinations between nga1 and other mutations effecting gynoecium development, including ettin, sty1, spatula, and crc, were largely additive (see Supplemental Figures 1D to 1M online). Other nga1-1 phenotypes in the flower included an enlargement of the petals (Figure 1I), apparently due to increased petal cell number (see Supplemental Figures 1N and 1O online). In the vegetative phase, nga1 leaves were more serrated than their wild-type counterparts (Figure 1J).
NGA1 Is Part of a Small Clade of B3 Domain–Containing Proteins That Have a Redundant Role in Style Morphogenesis and Organ Development
The NGA1 gene was identified by map-based cloning as At2g46870 (Figure 2B; see Supplemental Figure 2A online). A 10.5-kb genomic fragment spanning the locus complemented the nga1-1 mutation in the nga1-1 kan1-1 background. NGA1 encodes a RAV-B3 domain–containing protein (Kagaya et al., 1999) and is a member of a clade containing four genes (NGA1 to NGA4; Alvarez et al., 2006) that has a sister clade of three genes. None of the seven genes possess an AP2 domain characteristic of the RAV genes (NGAL; Figure 2A; see Supplemental Figure 2B and Supplemental Data Set 1 online; Swaminathan et al., 2008).
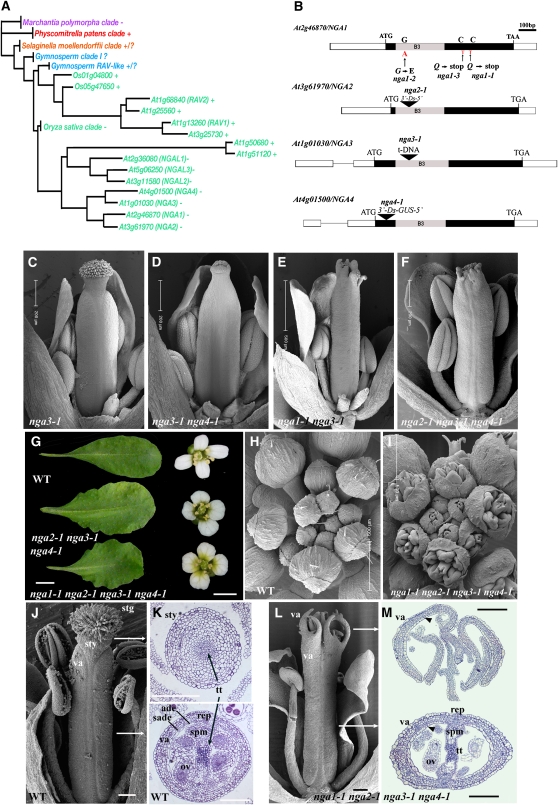
Figure 2.
Redundant Genetic Interactions among Members of the Monophyletic NGA Clade.
(A) Cladogram showing phylogenetic relationships of the RAV clade of B3 transcription factors in land plants (angiosperms,green; gymnosperms, blue; lycophyte, orange; moss, red; liverwort, purple). A complete cladogram with detailed analysis is presented in Supplemental Figure 2 online. Some members have an amino AP2 domain (+), while other members lack this domain (−). That the Physcomitrella and at least some of the Selaginella genes possess an AP2 domain suggests that it has been lost in some clades of flowering plant genes.
(B) A scheme of the NGA genes. Rectangles are exons and lines are introns. Filled rectangles denote the coding region for which the start and stop codons, the ethyl methanesulfonate-generated alleles, the positions of insertions, and the highly conserved 120–amino acid B3 domains are marked.
(C) to (F) Gynoecium phenotypes of lines with a progressive reduction in NGA levels. Genotypes are as labeled.
(G) Leaf and flower phenotypes of nga2-1 nga3-1 nga4-1 triple and nga quadruple mutant plants relative to the wild type. The mature sixth leaf was taken in each case.
(H) and (I) Scanning electron micrographs of wild-type (H) and nga quadruple mutant (I) inflorescences from an aerial perspective.
(J) Scanning electron micrograph of a medial view of a wild-type gynoecium at maturity.
(K) Light microscope sections through the distal style (top) and basal ovary (bottom) of a mature wild-type gynoecium taken at approximately the locations demarked by the lines in (J). The radially elongated adaxial epidermal and longitudinally elongated subepidermal cells are unique to the valve.
(L) Scanning electron micrograph of a medial view of a mature nga quadruple mutant gynoecium where the style and stigma are absent and replaced by outgrowths with valve identity.
(M) Light microscope sections though the distal (top) and central ovary (bottom) regions of a mature nga quadruple mutant gynoecium. The approximate, relative locations of the sections are marked by white arrows in (L). The outgrowths of tissue in the distal region have a valve identity with the typical adaxial epidermal and subepidermal cell layers (arrowhead) (K). The ovary (bottom) exhibits an apparently wild-type structure.
stg, stigmatic papillae; sty, style tissue; va, valve; ade, adaxial epidermis; sade, subadaxial epidermis; rep, replum; gyn, gynophore; tt, transmitting tissue; ov, ovule; spm, septum. Bar are as marked in (C) to (F), (H), and (I). Bars = 1 cm in (G) for leaves and 1 mm for petals, and 100 μm in (J) to (M).
Isolation of mutant alleles in all NGA genes and examination of multiple mutant combinations illustrated their redundancy in carpel patterning (Alvarez et al., 2006; Figures 2C to 2F and 2J to 2M). All four genes play a quantitative role in gynoecium and lateral organ development (Figures 2G to 2I; see Supplemental Figures 3A and 3B online). Progressive reduction in the activity of these genes resulted in reduced style and stigma development (Figures 2C to 2F and 2J to 2M), leaves that were wider and more serrated than nga1-1 single mutants (Figure 2G; see Supplemental Figure 3B online), and shorter but wider sepals and petals (Figures 2G to 2I; see Supplemental Figure 3A online). Genetic analyses suggested that the relative contribution of the mutations to the severity of phenotype was nga1-1>nga3-1>nga4-1>nga2-1. The loss of NGA gene activity culminated in the nga1-1 nga2-1 nga3-1 nga4-1 quadruple mutant (termed nga quadruple mutant hence), where effects on growth in all aerial organs were most severe (Figures 2G to 2M; see Supplemental Figure 3 online). In the gynoecium, the style and stigma tissues were replaced by projections with valve morphology (Figures 2J to 2M; see Supplemental Figures 4A to 4E online). The solid, wild-type style has a distinct crenellated epidermis with wax deposits and open stomata with an internal, central core of transmitting tissue (Figures 1H, 2J, and 2K). The ovary valve wall consists of an abaxial epidermis with small irregular rectangular cells interspersed with immature stomata that open during fruit development. This overlays three cell layers of chlorenchyma followed by a distinct adaxial subepidermal layer of longitudinally elongate cells and a radially elongated inner epidermis (Figure 2K; see Supplemental Figures 4A and 4B online) that becomes lignified in fruit development (Liljegren et al., 2000). These characteristic adaxial epidermal cell layers were observed in distal projections of the nga quadruple mutant, with more basal sections revealing an essentially normal ovary (Figure 2M; see Supplemental Figures 4D and 4E online). The nga quadruple mutant gynoecium is indistinguishable from the wild type until stage 9 of flower morphogenesis (see Supplemental Figures 5A to 5D online), coincident with the time of style and stigmatic papillae initiation (Smyth et al., 1990). These observations suggest that the NGA genes have a specific role in style morphogenesis rather than having an indirect or pleiotropic function.
NGA Gene Activity Is Required for Distal Carpel Identity
The complete loss of style and stigmatic papillae in nga quadruple mutants implies an essential role for the NGA genes in promoting these tissues. Since members of the AGAMOUS clade, AG, SHP1, and SHP2, play a central, redundant role in specifying carpel identity (Pinyopich et al., 2003), we examined the effects of NGA gene activity on expression of SHP1. To facilitate this analysis, we used an artificial microRNA that simultaneously targets the four NGA transcripts based on shared homology of a 21-nucleotide sequence (Alvarez et al., 2006). Overexpression of amiR-NGA164a using the constitutive viral 35S promoter mimicked the nga quadruple mutant phenotype (see Supplemental Figures 3C to 3E online). SHP1 expression is exclusively in the gynoecium and in the style and is initiated in the stage 6-7 gynoecium in the medial domain that will become the abaxial replum. During stage 9-10, a new expression domain that marks the top of the gynoecium and the developing style is initiated and maintained until stage 12 (Flanagan et al., 1996; Bowman et al., 1999; Figures 3A and 3C). This distal domain of SHP1 expression is missing in 35S:amir-NGA164a gynoecia (Figures 3B and 3D). These observations suggest that NGA gene activity is upstream of SHP1 style expression. If a lack of AG-like gene expression is responsible for the reduction of style and stigma tissues, then artificially elevated AG expression could rescue components of the nga gynoecium phenotype. LEAFY (LFY) is an activator of AG (Busch et al., 1999), and plants homozygous for an activated form of LFY (LFY-VP16) that drives high levels of AG have flowers that are composed of only carpel tissue topped by abundant style and stigma (Figure 3E; see Supplemental Figures 5E to 5H online). Similarly, overexpression of the tomato (Solanum lycopersicum) AGAMOUS gene (TAG1) (Pnueli et al., 1994) results in flowers with sepals homeotically converted to carpels with extensive marginal style and stigmatic tissue (Figure 3G) (as is observed with overexpression of the Arabidopsis AG gene [Mizukami and Ma, 1992] but without the frequent cosuppression of the transgene and endogenous AG gene). Combining 35S:amiR-NGA164a with either the LFY-VP16 or the 35S:TAG1 background resulted in plants where style and stigmatic tissues were eliminated, both in the carpelloid sepals and in the central gynoecium (Figures 3F and 3H). Thus, NGA genes are essential for all style and stigma development in the flower, a role that cannot be substituted for by solely elevating AG-like gene activity.
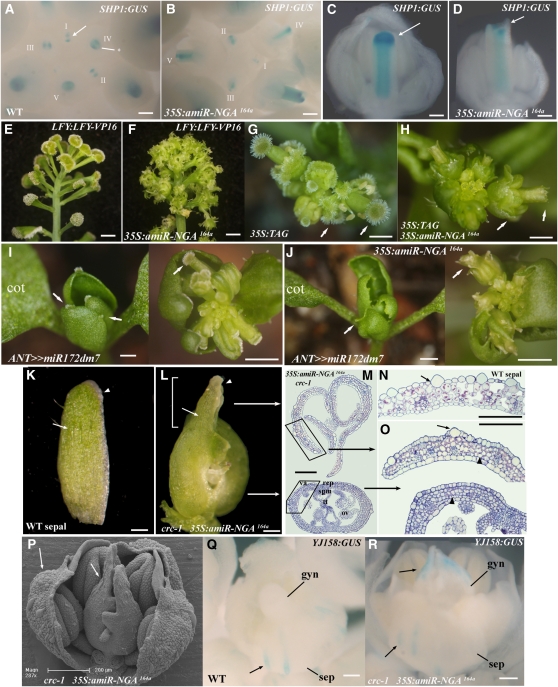
Figure 3.
NGA Gene Activity Is Necessary for Normal and Ectopic Style Tissues.
(A) to (D) Histochemical localization of SHP1:GUS in developing wild-type and 35S:amiR-NGA164a gynoecia.
(A) and (B) Aerial view of wild-type (A) and 35S:amiR-NGA164a (B) inflorescences showing SHP1:GUS staining at the tips of developing gynoecia. Developmentally consecutive flowers are labeled using roman numerals. In the wild type, SHP1:GUS expression is initiated in the medial ridge, the progenitors of the placenta and septum (arrow), and later expands to the lateral and medial regions of the initiating style (asterisk).
(C) Medial view of a stage 10-11 wild-type gynoecium showing intense SHP1:GUS staining in the lateral domain of the developing style (arrow).
(D) Medial view of a stage 10-11 35S:amiR-NGA164a gynoecium. SHP1:GUS expression is absent from the equivalent domain (arrow).
(E) and (F) Side view of inflorescences of plants homozygous for the LFY:LFY-VP16 transgene alone (E) and hemizygous for 35S:amiR-NGA164a (F).
(E) LFY:LFY-VP16 flowers are composed of carpel tissue topped by enlarged stigmas.
(F) 35S:amiR-NGA164a LFY:LFY-VP16 flowers lack style and stigma.
(G) and (H) Aerial view of inflorescences of plants overexpressing the tomato AGAMOUS (TAG) gene alone (G) and together with 35S:amiR-NGA164a (H).
(G) In 35S:TAG flowers, the sepals display extensive style and stigma tissue formation (arrow).
(H) In 35S:TAG 35S:amiR-NGA164a plants, the ectopic and normal style and stigma tissues are lost (arrows).
(I) and (J) Aerial views of plants overexpressing a modified version of miR172d, miR172dm7 (I) and miR172dm7 together with amiR-NGA164a (J).
(I) ANT>>miR-172dm7 plants produce small leaves that are topped by stigmatic papillae (left; arrows) and flowers typically composed entirely of carpels (right; arrow).
(J) ANT>>miR-172dm7 35S:amiR-NGA164a plants lacking ectopic and normal style (arrows).
(K) to (R) Sepal-like identity in carpel tips lacking both CRC and NGA gene activities.
(K) A wild-type sepal. Note the longitudinally elongate epidermal cells (arrow) and the pale band of marginal cells (arrowhead).
(L) Gynoecium from 35S:amiR-NGA164a crc-1 flower. The distal regions of the carpels (bracketed) include large longitudinally elongated epidermal cells (arrow) and marginal band of pale cells (arrowhead) not found in normal carpels.
(M) Sections though the upper and lower 35S:amiR-NGA164a crc-1 gynoecium in positions represented by the two arrows in (L). The upper gynoecium lacks carpel tissues in contrast with the base where tissues similar to those of a wild-type ovary are found. The lamina tissue in the upper gynoecium has features of both sepal and valve detailed in (N).
(N) Section through a wild-type sepal. The sepal has distinct large longitudinally elongated cells at the abaxial epidermis (arrow).
(O) Magnified image of the upper and lower 35S:amiR-NGA164a crc-1 gynoecium (boxed in [M]) with sepal-like cells at the upper domain (arrow). Mutant upper valves also lack the characteristic adaxial subepidermal cell layer of carpels (arrowhead).
(P) Scanning electron micrograph of a 35S:amiR-NGA164a crc-1 flower with a sepal, petals, and stamens removed. Large abaxial epidermal cells can be observed in the upper carpel domain and in the sepals (arrows).
(Q) Staining of YJ-158:GUS is limited to the long abaxial epidermal cells of wild-type sepals (arrow) and is not observed at any stage in the wild-type gynoecium.
(R) In 35S:amiR-NGA164a crc-1 gynoecia, staining from YJ-158:GUS is also found in the long epidermal sepal-like cells in the upper gynoecium (arrows).
cot, cotyledon; va, valve; rep, replum; tt, transmitting tissue; ov, ovule; spm, septum; gyn, gynoecium; sep, sepal. Bars = 100 μm in (A) to (D) and (K) to (R), 5 mm in (E), (F), (I), and (J), and 3 mm in (G) and (H).
Vegetative stigmatic papillae development is evident on the cauline leaves of plants overexpressing miR172 (Aukerman and Sakai, 2003). miR172 targets five APETALA2 family genes that repress flowering and includes the floral homeotic gene APETALA2 (AP2) (Aukerman and Sakai, 2003; Chen, 2004). A modified version of miR172d, miR172dm7, produces a more severe phenotype (see Supplemental Figure 6 online) when expressed using the AINTEGUMENTA (ANT) promoter (Elliott et al., 1996; Schoof et al., 2000), including severe curling and significant stigma development at the tips of the first leaves as well as flowers composed only of carpels (Figure 3I; see Supplemental Figure 6 online). Vegetative stigma development was abolished when amiR-NGA164a was coexpressed with miR172dm7 (ANT>>miR172dm7 35S:amiR-NGA164a; Figure 3J; >> denotes transactivation; Moore et al., 1998), suggesting that the AP2 family genes targeted by miR172 restrict the carpel program that recruits NGA activity for stigma development.
Extending the use of amiR-NGA164a for other genetic analyses, we found that the 35S:amir-NGA164a combinations with spt-2, sty1-1, gym-5 kan1-2, kan1-1 kan2-1/+, kan1-1 kan2-1 (Eshed et al., 2001), and amiR-ARF164b (Alvarez et al., 2006) were largely additive (see Supplemental Figures 7A to 7K online). Notably, however, in 35S:amir-NGA164a crc-1 gynoecia, the unfused carpels comprising the distal part of the gynoecium were sepal-like in appearance, including long, large epidermal cells and lighter colored marginal cells (Figures 3K to 3P). Sections through the distal region of the 35S:amir-NGA164a crc-1 gynoecium revealed a reduction of valve-like histological features in the upper gynoecium and the presence of large, sepal-like, epidermal cells, but with a lack of significant airspaces that characterize the spongy mesophyll of wild-type sepals. By contrast, the basal fused carpels of the 35S:amir-NGA164a crc-1 gynoecium retained all characteristics of a wild-type ovary (Figures 3M to 3O). In wild-type flowers, expression of the enhancer trap line YJ158 (Eshed et al., 2004) is restricted to the large epidermal cells of the sepals (Figure 3Q). In 35S:amiR-NGA164a crc-1 flowers, YJ158 expression was observed in cells in the unfused carpels of the upper gynoecium from stage 9, as well as in the sepals, confirming a sepal-like identity in the distal region of 35S:amiR-NGA164a crc-1 carpels (Figure 3R; see Supplemental Figures 7L to 7Q online). Thus, coincident loss of CRC and the NGA genes allows the distal gynoecium to pursue a partial sepal identity program.
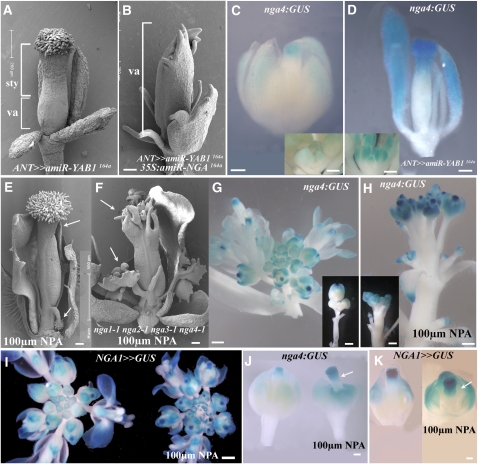
NGA4 and NGA1 Expression Is Confined to the Distal Region of Lateral Organs
The nga1-1 mutation enhances abaxial growth in the kan1-2 background (Figures 1B to 1D), suggesting that the NGA genes may have an abaxial polarity function, while the reduction of style and stigmatic tissues in nga quadruple mutants (Figure 2L) implies the NGA genes regulate tissue specification and growth. Establishing the expression domain of these genes can help resolve their function. The nga4-1 allele is a Ds gene trap (Figure 2B) that is referred to as nga4:β-glucuronidase (GUS). Expression of this line is observed at the tips of the cotyledons and leaves and at leaf hydathodes (Figures 4A to 4C). In flowers, nga4:GUS is similarly expressed at the distal region of all floral organs (Figure 4D), with expression evident only after organ initiation. No nga4:GUS expression is observed before late stage 6-7 of flower development (Figures 4E and 4G), at which time expression is first observed in the developing sepals and stamens (Figure 3F). Only during stage 9 is nga4:GUS expression observed at the tip of the developing gynoecium coincident with the time of style initiation (Figure 1A; see Supplemental Figures 5A to 5D online). By stage 12, nga4:GUS expression is observed at the distal domain of all floral organs (Figures 4H and 4I) and in the gynoecium becomes confined to the style and stigma. Likewise, nga4:GUS expression is observed in the ectopic style and stigma tissues of kan1-2 kan2-1/+ gynoecia (Figure 4J). Evidence that this expression domain marks the distal end of the gynoecium, and not merely the style and stigma, comes from staining at the apex of the nga quadruple mutant gynoecium where the style and stigma are lost (Figure 4K).
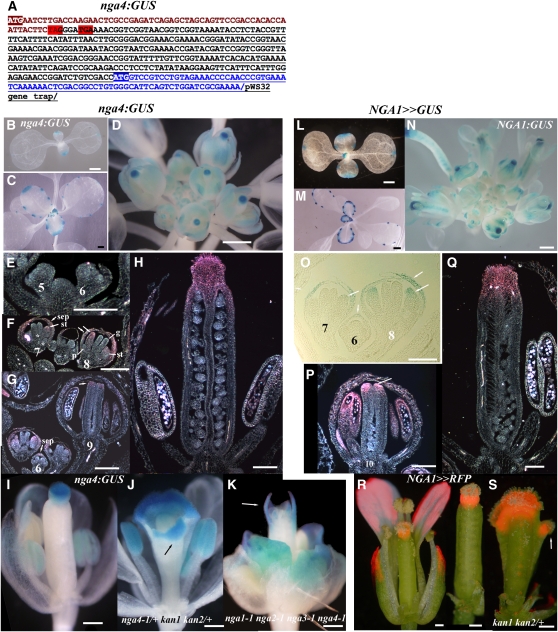
Figure 4.
Expression of NGA4 and NGA1 Is Late and Distal in All Floral Organs.
(A) nga4:GUS fusion structure based on sequenced RT-PCR products. NGA4 sequence is in brown, pWS32 gene sequence trap is underlined, and that of the GUS gene is also in blue. Two stop codons, highlighted in red, truncate the nga4-1 protein. Intact translation from the ATG of the GUS gene is highlighted.
(B) to (K) Expression of nga4:GUS in seedlings (B), leaves (C), and flowers of wild type ([D] to [I]), nga4-1/+ kan1-2 kan2-1/+ (J), and nga quadruple mutants (K).
(L) to (Q) NGA1>>GUS expression in seedlings (L), leaves (M), and flowers ([N] to [Q]) of the wild type.
(R) and (S) NGA1>>RFP (dsRED) localization in wild-type (R) and kan1-2 kan2-1/+ (S) gynoecia.
pe, petal; st, stamen; gy, gynoecium; se, sepal. Bars = 1 mm in (B) to (E) and (L) to (N), 200 μm in (I) to (K), (R), and (S), and 100 μm in (E) to (H) and (O) to (Q).
To examine NGA1 expression, a 5-kb fragment upstream of the NGA1 start codon was used in the transactivation system (see Methods). The NGA1:LhG4 driver line, which complemented nga-1 upon transactivation of OP:NGA1, was subsequently used to drive either GUS or dsRED reporters. Expression driven by the NGA1 promoter was similar to that of nga4:GUS, being confined to the tips of the cotyledons and leaves and leaf hydathodes (Figures 4L and 4M), and late in the distal part of the flower organs (Figure 4N). In the flowers, initial expression during stage 6 is restricted to the sepals and during floral stages 7-8 is seen in stamens too. During late stage 9, NGA1 expression is observed at the distal part of the gynoecium, at the time of style initiation (Figure 1A) and continues to be expressed in the style and stigma up to stage 13 of gynoecium development (Figures 4P to 4R). Like NGA4, NGA1 is expressed in the ectopic style and stigmatic tissues of kan1-2 kan2-1/+ gynoecia consistent with the role of NGA1 in maintaining their identity (Figures 1B to 1E and 4S). These observations suggest that NGA genes can function as specific tissue identity factors in the gynoecium and general distal factors in all tissues. To investigate this, we performed overexpression studies with all the NGA genes.
NGA Gene Overexpression Promotes Ectopic Style Tissue Development in the Flower
All four NGA genes were cloned behind an array of the lac operator (OP) sequences followed by a TATA box for overexpression analyses by select transactivating driver lines, which contain the LacIH17-GAL4 (LhG4) chimeric transcription factor expressed under the control of a tissue-specific promoter. Transactivation of each gene by the ANT:LhG4 driver resulted in reduced cotyledon and leaf growth, cotyledon fusion, and occasional leaf fusion (see Supplemental Figure 8 online). In this experimental system, the NGA1 gene had the most potent effects, followed by NGA4 and NGA3, with NGA2 giving the weakest phenotype (largely reflecting the contribution of each gene in the loss-of-function analysis).
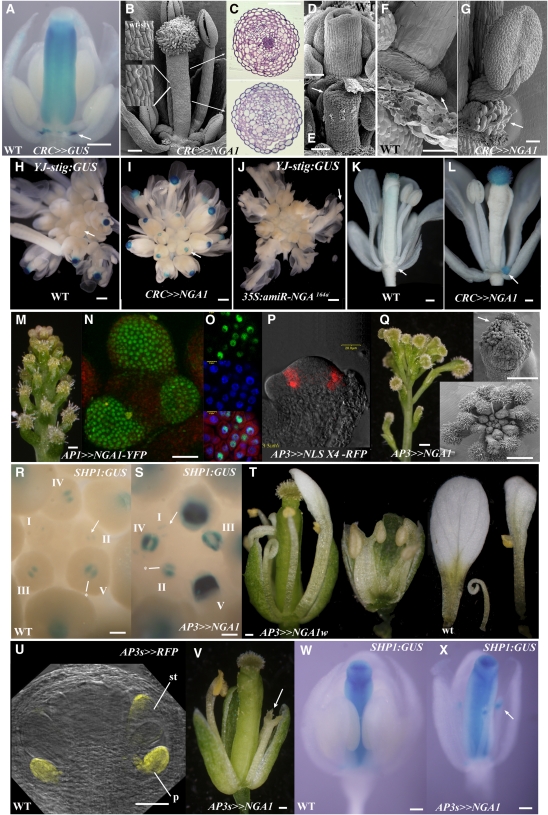
To test the function of the NGA genes in a carpel-specific context, effects of NGA1 expression by the CRC:LhG4 driver line was studied. This line is expressed throughout the carpel valve anlagen during gynoecium initiation in stage 5 flowers as well as the developing nectaries but not in the other floral organs (Bowman and Smyth, 1999; Alvarez et al., 2006). Stigmatic papillae were initiated precociously in CRC>>NGA1 gynoecia and histologically the mature gynoecium appeared to be composed primarily of style and stigma tissues (Figures 5B to 5E; see Supplemental Figures 4F and 4G online). Having an additional copy of the OP:NGA1 transgene resulted in more extensive production of stigmatic papillae in a basally expanded domain (see Supplemental Figures 9A and 9B online). Notably, tissue in nectary positions was topped by stigmatic papillae-like cells (Figures 5F and 5G). The YJ-STIG∷GUS marker is expressed specifically in maturing stigmatic papillae of wild-type gynoecia (Figure 5H). In CRC>>NGA1, staining was apparent earlier in the distal region of the gynoecium (Figure 5I) and eliminated in 35S:mirNGA164a gynoecia that lack style and stigmatic tissues (Figure 5J). Strong YJ-STIG:GUS expression was observed in the papillae-like cells in nectary positions of CRC>>NGA1 flowers (Figures 5K and 5L), confirming that ectopic NGA1 expression in this domain drives stigmatic papillae development.
Figure 5.
NGA1-Mediated Promotion of Ectopic Style within the Flower.
(A) Expression of CRC>>GUS in a stage 12 flower is observed in the style, ovary, and nectaries (arrow).
(B) Scanning electron micrograph of a stage 13 CRC>>NGA1 gynoecium that lacks overt evidence of ovary development and is topped by abundant stigma. The epidermal cells (insets) are similar to that of wild-type style (top inset).
(C) Sections through a CRC>>NGA1 gynoecium at positions marked by the arrows in (B). The top section is similar to that of the wild-type style (see Figure 1), while the bottom section lacks any evidence of wild-type ovary tissues.
(D) and (E) Scanning electron micrographs of a stage 9 gynoecial cylinder. In the wild type (D), there is no evidence of stigmatic papillae development, while in the CRC>>NGA1 gynoecium (E), precocious stigmatic papillae differentiation is apparent (arrow).
(F) Wild-type nectary tissue arising from the base of the lateral stamen (arrow).
(G) The structure developing in the nectary position at the base of the lateral stamens in mature CRC>>NGA1 flowers has papillae-like projections (arrow).
(H) to (L) Expression of the YJ-STIG:GUS marker.
(H) Wild-type inflorescence with staining in the maturing stigma starting at stage 11 flowers.
(I) CRC>>NGA1 inflorescence with staining of younger flowers than the wild type.
(J) 35S:amiR-NGA164a inflorescence with no staining in any maturing flowers (arrow).
(K) A stage 13 wild-type flower with staining in the stigmatic papillae only.
(L) A stage 13 CRC>>NGA1 flower with additional expression in the nectary position (arrow).
(M) to (O) Morphology (M) and nuclear localization of fluorescence in AP1>>NGA1-YFP inflorescences ([N] and [O]).
(M) Normal flowers are replaced by attenuated floral meristems topped by stigmatic papillae.
(N) and (O) Fluorescence detected by a two-photon excitation microscope (N) or confocal laser scanning microscopy (O) where conuclear localization of the NGA1-YFP (green) and 4',6-diamidino-2-phenylindole (blue) is shown in the bottom panel.
(P) RFP fluorescence in a longitudinal section of a stage 3 AP3>>NLSx4-RFP flower.
(Q) AP3>>NGA1 flowers. Light microscope image of inflorescence (left), an aerial scanning electron microscope view (bottom inset), and a flower (top inset) showing early, ectopic differentiation of stigmatic papillae on and interior to the sepals (arrow).
(R) and (S) Expression of SHP1:GUS in developing wild-type (R) and AP3>>NGA1 (S) gynoecia. Developmentally consecutive flowers from the time of SHP1:GUS appearance are labeled using roman numerals.
(T) A range of phenotypes obtained when the weak OP:NGA1w line is driven by the AP3 promoter. Growth of the petals and stamens is reduced and petals-stamen and sepal-petal-stamen fusions occasionally occur. A wild-type petal is a control.
(U) RFP fluorescence (appearing yellow) in a stage 7 flower marks the AP3short (AP3s) expression domain, which is initiated in stage 6-7 petal and stamen primordia.
(V) An AP3s>>NGA1 flower with sepal removed. Petal and stamen growth is significantly reduced, and filamentous structures topped by stigmatic papillae are observed in the third whorl (arrow).
(W) and (X) Expression of SHP1:GUS in stage 12 wild-type (W) and AP3s>>NGA1 (X) flowers. In the mutant, ectopic expression is observed in third whorl filamentous structures (arrow).
Bars = 100 μm in (A) to (C), (K), (L), and (Q) (flower) to (T), 50 μm in (D) to (G) and (V) to (X), 1 mm in (H) to (J), 20 μm in (N) and (O), and 1 mm in (M) and (Q).
We subsequently expressed NGA1 at high levels in the flower outside of the gynoecium. Expression of the NGA1 alone, or NGA1 fused to yellow fluorescence protein (YFP) at the C terminus (NGA1-YFP), under control of the flower meristem driver AP1:LhG4 (Alvarez et al., 2006; AP1>>NGA1:YFP) had reduced floral meristems topped by stigmatic papillae (Figures 5M to 5O). Fluorescence of NGA1:YFP was nuclear localized, consistent with the proposed DNA binding function of B3 domain proteins (Yamasaki et al., 2004; Figures 5N and 5O). AP3:LhG4 (AP3) driver expression is restricted to the sepal margins, petal, and stamen anlagen and primordia (Alvarez et al., 2006). In AP3>>NGA1 plants, all tissues arising internal to the sepals develop into a cylinder of carpelloid style tissue (Figure 5Q) and were accompanied by expanded and elevated expression levels of SHP1:GUS (Figures 5R and 5S). This included an ectopic, precocious differentiation of stigmatic papillae at the sepal margins of young stage 7 flowers (Figure 5Q). Expression of NGA1 in stamen and carpel anlagen and primordia using the AG promoter similarly resulted in a cylinder of style tissue, but in this case, interior to the petals (see Supplemental Figures 9C and 9D online). To investigate the organ fusion and style tissue development induced by NGA1 overexpression, we used a weak NGA1 overexpression line (OP:NGA1weak, NGA1w; see Supplemental Figure 8 online) and a short version of the AP3 promoter (AP3short:LhG4; AP3s) that initiates expression in petal and stamen primordia only at stages 6 to 7 (Figure 5U). In AP3>>NGA1w flowers, sepal-petal-stamen fusion events as well as reduced organ growth were observed, but organ identity was less affected (Figure 5T). In AP3s>>NGA1, petal growth was repressed, while third whorl organs were filamentous and topped by stigma-like tissue (Figure 5V) that ectopically expressed SHP1 (Figures 5W and 5X) and CRC (see Supplemental Figures 9E to 9G online).
These observations suggest that NGA gene activity elicits different effects depending on the timing and level of expression. Early NGA gene expression can disrupt the organ separation program as well as reduce organ growth. Low to intermediate levels of NGA activity within the organs reduces growth, while, in the flower, high levels promote a program of style/stigma tissue morphogenesis.
These NGA1-based observations were also confirmed for other NGA genes. Smaller petals were produced in AP3>>NGA4 and AP3>>NGA3 flowers, while in AP3>>NGA4/NGA4 flowers, filamentous third whorl organs topped by stigmatic papillae and ectopically expressing SHP1 were observed (see Supplemental Figures 9H to 9N online). In addition, ANT>>NGA3 and ANT>>NGA4 plants exhibited organ separation defects in the inflorescence and the flower (see Supplemental Figures 9O to 9R online). Lastly, since no function has been assigned to members of the B3 encoding NGA-like sister clade (Figure 2A), we assayed one of these genes, NGAL1 (At2g36080), by overexpression. ANT>>NGAL1, AP1>>NGAL1, and AP3>>NGAL1 phenotypes were similar to those elicited by NGA1 overexpression (see Supplemental Figure 10 online), suggesting that NGAL1 targets may overlap with those of the NGA genes.
STYLISH1 Activity Acts as a Strong Facultative Promoter of NGA Gene Activity
The loss of style tissues in NGA loss-of-function plants, the expression domain of NGA1 and NGA4, and the strong promotion of style tissues by NGA1 suggest that activation of the NGA genes in the distal gynoecium during stage 9 promotes a style and stigma program. Overexpression of members of the STY gene family also promote ectopic style tissue formation in the valve (Kuusk et al., 2002). Since STY1 expression initiates at the apex of stage 6 gynoecia (Kuusk et al., 2002), preceeding NGA1 and NGA4, it represents a potential upstream regulator of the NGA genes. As the sty1-1 phenotype is relatively weak (see Supplemental Figure 1H online), reflecting significant redundancy between STY1 and related family members (Kuusk et al., 2006), we pursued possible links between STY gene activity and NGA gene activation by overexpressing STY1. Overexpression of OP:STY1 driven by the CRC promoter resulted in a minute gynoecium that appeared histologically undifferentiated relative to the wild type (Figures 6A to 6C; see Supplemental Figures 4H and 4I online). This gynoecium lacked the ectopic style formation observed when NGA1 was expressed with the same promoter (Figure 5B), and the apical stigmatic papillae was reduced (Figures 6A to 6C), as was nga4:GUS expression relatively to nga4-1/+ gynoecia (cf. Figure 6D with 6L). CRC>>STY1/amiR-NGA164a plants lacked stigmatic tissue, but other elements of the CRC>>STY1 gynoecium histology remained unchanged (cf. Figures 6E with 6F). These results are in contrast with results from other circumstances of STY1 activation, in which strong induction of ectopic style were the common theme (Kuusk et al., 2002; Sohlberg et al., 2006; Staldal et al., 2008).
Figure 6.
NGA Genes Exhibit a Strong but Facultative Response to STY1 Activity.
(A) CRC>>STY1 flower with a severely affected gynoecium, whereas other floral organs are largely unaffected. The apical stigmatic papillae are reduced in their growth (arrow).
(B) Scanning electron micrograph of the CRC>>STY1 gynoecium surface. The epidermis is composed of small cells without significant cuticular outgrowths and numerous intervening immature stomates.
(C) Transverse sections through the CRC>>STY1 gynoecium at the positions marked by arrows in (B). No tissues with the characteristic histology of the mature wild-type gynoecium are apparent, although a rudimentary medial ridge (arrow) is observed.
(D) Expression of nga4:GUS in CRC>>STY1 gynoecium is reduced compared with the wild-type (L).
(E) Gynoecium of a plant cotransactivating STY1 and amiR-NGA164a by the CRC promoter (CRC>>STY1/amiR-NGA164a). Stigmatic papillae are lacking (arrow), but the abnormal histology of the epidermal surface appears largely unchanged (F).
(G) to (I) Scanning electron micrographs of a gynoecium with magnified views of the epidermis marked by the arrows.
(G) In the wild type, the style and ovary have distinct cuticular morphologies.
(H) In CRCw>>STY1 gynoecia, the entire ovary surface has style-like epidermal characteristics.
(I) The style-like epidermal features are abolished in CRCw>>STY1/amiR-NGA164a gynoecium, but it does not revert to the wild type.
(J) and (K) Expression of CRCw>>GUS in a stage 12 flower (J) is apparent in the ovary and nectaries, whereas in a stage 14 flower (K), it is mostly in the nectaries.
(L) to (P) Expression of nga4:GUS in different genotypes.
(L) In the wild-type gynoecium, nga4:GUS is restricted to the style.
(M) In CRCw>>STY1 stage 12, staining is observed in the ovary and nectaries (arrows).
(N) By stage 14-15, CRCw>>STY1 flower staining is restricted to the gynoecium base and nectaries.
(O) and (P) In CRCw>>NGA1 gynoecia (O) or CRC>>NGA1 flowers (P), nga4:GUS staining is not observed in the style-converted gynoecium nor in the papilliae that develop at nectary positions.
(Q) to (U) The effects of ectopic STY1 expression outside the gynoecium.
(Q) A diminutive AP3>>STY1 flower showing reduced growth of all organs.
(R) AP3s>>STY1 flower. Stamen and petal growth is abnormal, and stigma and style tissue is observed on the staminoid organs.
(S) SHP1:GUS is ectopically activated in the carpelloid stamens of AP3s>>STY1 flowers (arrow).
(T) AP3s>>STY1/amiR-NGA164a flower with comparatively normal stamens.
(U) Ectopic SHP1:GUS is not observed in the third whorl organs of the AP3s>>STY1/amiR-NGA164a flower.
Bars = 100 μm in (A), (D), (E), (J) to (P), and (R) to (U), 50 μm in (B), (C), and (F), 200 μm in lower-magnification images of (G) to (I), and 20 μm in the adjacent higher-magnification images.
The differential style promotion by STY1 may represent temporal or quantity-specific responses to this protein. The CRCw:LhG4 promoter line (CRCw) is expressed at the same domain as the CRC promoter, but a lower level (Figures 6J and 6K). Strikingly, in CRCw>>STY1 flowers, the entire ovary surface acquired style epidermal histology that was eliminated in CRCw>>STY1/amiR-NGA164a flowers, indicating that it is NGA dependent (Figures 6G to 6I; see Supplemental Figures 11A to 11C online). Consistent with this, nga4:GUS expression was observed to be broadly, ectopically expressed in the CRCw>>STY1 gynoecium and nectary positions (Figures 6L to 6N). Significantly, ectopic expression of nga4:GUS was not observed in CRC>>NGA1 or CRCw>>NGA1 gynoecia (Figures 6O and 6P), except occasionally and then weakly in nectary positions (see Supplemental Figures 11D to 11F online). These observations indicate that STY1 can function as a strong promoter of NGA gene activity in a level-dependent manner, while NGA4 expression is highly responsive to STY1, but not to NGA1, or to the presence of differentiating style and stigma tissues per se.
To further compare the effects of NGA and STY gene activity, STY1 was transactivated by the same driver lines used for NGA1 overexpression outside the gynoecium. In AG>>STY1 flowers, the stamens were converted into broad, lobe-like structures that had an epidermal morphology similar to style tissue (see Supplemental Figure 11G online). In the AP3>>STY1 background, the flower was greatly reduced in size, and second and third whorl organ development was strongly suppressed (Figure 6Q). In AP3s>>STY1 flowers, a suppressed second whorl was followed by abnormal third whorl organs with occasional stigmatic papillae that ectopically expressed gynoecium markers SHP1:GUS (Figures 6R and 6S) and CRC:GUS (see Supplemental Figures 11H and 11I online). This ectopic carpel tissue and SHP1:GUS expression was significantly reduced when NGA gene activity was compromised in AP3s>>STY1/mirNGA164a flowers (Figures 6T and 6U), indicating that STY1 promotes ectopic carpel tissue through the NGA gene pathway.
Reduced YABBY Gene Activity and Auxin Transport Result in Earlier and Expanded NGA Activity
Extensive NGA-dependent style tissue development when STY1 is ectopically expressed (Figures 6G to 6I) demonstrates that STY1 can activate the NGA genes. However, there is a significant temporal separation between the stage 6 onset of STY1 (Kuusk et al., 2002) and the stage 9 activation of NGA gene expression (Figure 4). Activation of NGA activity in response to STY1 may be prevented by factors acting before stage 9. Signature features of reduced activity in such NGA gene repressors would be earlier, broader NGA activity and an expanded style domain. Candidates include the YAB1 genes FILAMENTOUS FLOWER (FIL) and YAB3 (Sawa et al., 1999; Siegfried et al., 1999), as the style domain of fil-8 yab3-2 double mutants is basally extended. Regulation of auxin accumulation, movement, and signaling is another candidate, since STY1 is a presumptive activator of auxin biosynthesis, and chemical or genetic inhibition of PAT causes proportionally expanded style and stigma (Staldal et al., 2008) and strong expression of the auxin efflux carrier auxin transport PIN1 (PIN1:PIN1-GFP; Benková et al., 2003) is seen in the developing gynoecium (see Supplemental Figures 12E and 12F online). To investigate a possible role for YAB1 genes and auxin transport in NGA gene regulation, we examined NGA gene expression and action in the absence of YAB1 or in the presence of the PAT inhibitor, 1-N-naphtylphtalamic acid (NPA).
Plants overexpressing the synthetic microRNA amiR-YAB1164a, which targets FIL and YAB3, using the ANT promoter faithfully mimic the fil-8 yab3-2 double mutant phenotype (Goldshmidt et al., 2008). The gynoecia of ANT>>amiR-YAB1164a plants have an expanded style (Figure 7A), which is lost and replaced by valve tissue in ANT>>amiR-YAB1164a/35S∷amiR-NGA164a plants (Figure 7B), directly implicating expanded NGA gene activity in the expanded style of fil-8 yab3-2 flowers. Consistent with this, nga4:GUS expression was stronger, initiated earlier, and basally expanded in all floral organs of ANT>>amiR-YAB1164a plants (Figures 7C and 7D; see Supplemental Figures 12A and 12B online). While these observations imply that YAB1 genes are effective negative regulators of NGA gene activity, the negative regulation is likely reciprocal. FIL expression is excluded from the distal gynoecium upon style initiation in the wild type (Siegfried et al., 1999; see Supplemental Figure 12C online), but remains throughout 35S:amiR-NGA164a gynoecia (see Supplemental Figure 12D online).
Figure 7.
Expanded NGA Activity upon Reduction in YAB1 Gene Activity or Arrest of Auxin Transport.
(A) In ANT>>amiR-YAB1164a flowers, the style is basally expanded and constitutes more than half of the gynoecium.
(B) In ANT>>amiR-YAB1164a 35S:amiR-NGA164a gynoecia, style development is abolished and valve tissue extends to the distal tip of the gynoecium.
(C) nga4:GUS localization in a stage 12 wild-type flower and younger flowers (inset). Expression is observed in the distal regions of the sepals and the gynoecium.
(D) In a stage 12 ANT>>amiR-YAB1164a flower, nga4:GUS expression is distally expanded and in young flowers (inset) is more intense and earlier compared with (C).
(E) to (K) The effects of 100 μm NPA treatment on proximo-distal tissue distribution and marker gene expression.
(E) Scanning electron micrograph of a wild-type gynoecium after exposure to NPA treatment. The style is more extensive and carpelloid outgrowths topped by stigmatic papillae arise from near the base.
(F) nga quadruple mutant gynoecium treated with NPA. Style and stigma tissues are missing, but other effects, including the basal-gynoecium projections, are apparent.
(G) and (H) Expression of nga4:GUS in wild-type inflorescence (G) is weak; thus, in the inset, older flowers have been dissected away to expose expression in the young flowers. After NPA treatment (H), expression is more intense, earlier, and basally expanded.
(I) NGA1>>GUS expression in untreated (left) and NPA-treated inflorescences. Expression is stronger and earlier after the NPA treatment.
(J) nga4:GUS expression in flowers before (left) and after (right) an NPA treatment. The treated flower shows a significant basal expansion in nga4:GUS expression in the sepals and the gynoecium.
(K) NGA1>>GUS expressing flowers. The NPA-treated right flower exhibits a strong, basal extension in GUS expression.
sty, style; va, valve. Bars = 100 μm in (A) to (F), (J), and (K) and 1 mm in (G) to (I) and the inset in (C) and (D).
Treatment of wild-type inflorescences with 100 μm NPA resulted in a basally expanded style and outgrowths topped with stigmatic papillae emanating from the base of the gynoecium (Figure 7E). An equivalent treatment of the nga quadruple mutant failed to rescue the style defects, whereas outgrowths at the base of the gynoecium formed (Figure 7F). This suggests that a basipetal expansion of NGA gene activity and consequent style development underlie the response to NPA application. Consistent with this, nga4:GUS and NGA1>>GUS expression in NPA-treated inflorescences was earlier, stronger, and basally expanded relative to untreated controls (Figures 7G to 7K). That nga4:GUS expression was expanded in NPA-treated nga quadruple mutant gynoecia, in the absence of style and stigma tissue development, indicted that changes in NGA4 expression reflect a response to altered auxin distribution and not simply a reflection of the style tissue domain (see Supplemental Figures 12G and 12H online). Together, these results imply that both YAB1 and effective auxin transport are involved in restricting the spatial and temporal domain of NGA gene activity.
SHI/STY Family Members Are under Positive NGA Gene Regulation
To gain additonal understanding as to the processes regulated by NGA gene activity, we analyzed RNA extracted from wild-type (n = 7), nga quadruple mutants (n = 2), and 35S:amiR-NGA164a (n = 2) inflorescences and hybridized to ATH1 affymetrix expression arrays (see Methods). Genes with fold change of >1.5 and false discovery rate (FDR) P value < 0.05 were considered significantly modified, resulting in 447 downregulated and 236 upregulated genes in NGA mutants (see Supplemental Data Set 2 online).
Gene Ontology annotation analysis of genes downregulated in NGA mutants revealed enrichment for plastid genes (P < 1E-8). Given the green/yellow tinge to nga quadruple mutant petals (Figure 2G; see Supplemental Figure 3A online), we suspected this indicated a delay in plastid maturation. To test this, the transcriptome of wild-type stage 12 and 15 petals was obtained from AtGenExpress collection (Schmid et al., 2005) and analyzed. Of the annotated plastid genes downregulated in the nga quadruple mutant, genes that increase with organ age (i.e., maturation genes) were highly enriched (45 of 61, P < 0.001, χ2 test). That is, the plastid-annotated transcripts of nga quadruple mutants match a younger stage of development, implying a general delay in maturation.
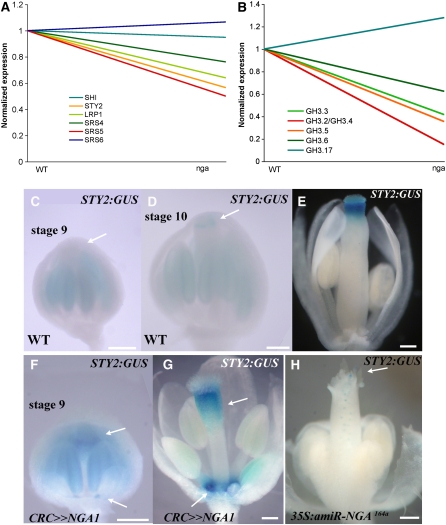
Notably, among the genes significantly downregulated in the mutant, there were two SHI/STY family members, STY2 and SRS5. Examination of all SHI/STY family members on the array (Figure 8A) shows that LRP1 and SRS4 are also reduced, but to a lesser degree (fold change of 1.56 and 1.31, respectively). Since the auxin biosynthesis flavin monooxygenase YUC4 and a number of GH3-like genes are upregulated in response to STY1 activity (Sohlberg et al., 2006), we also examined their expression. Expression of the YUCCA clade members was not significantly reduced in our microarray studies. However, there was a significant downregulation in the expression of GH3-2/GH3-4 and GH3-5 and to a lesser significance GH3-3 and GH3-6 (fold change of 2.4 and 1.6, respectively; Figure 8B), the majority of which were upregulated on STY1 induction (Sohlberg et al., 2006). Since these GH3-like genes encode auxin-inducible indole-3-acetic acid-amido synthetase proteins shown to catalyze conjugation of indole-3-acetic acid to amino acids in vitro (Staswick et al., 2005), their reduced expression suggests altered levels of auxin in the nga quadruple mutant inflorescence.
Figure 8.
NGA Activity Promotes Expression of Members of the STY Family.
(A) and (B) Normalized expression of six members of the STY gene family, including STY2 (A) and six members of the auxin-responsive GH3 genes (B) in wild-type inflorescences compared with lines lacking activities of the four NGA genes.
(C) to (H) Expression of STY2:GUS in wild-type ([C] to [E]), CRC>>NGA1 ([F] and [G]), and 35S:amiR-NGA164a (H) flowers. In the wild type, STY2:GUS is not expressed in the style of stage 9 gynoecia (C) (arrow) but becomes apparent in the style of stage 10 flowers ([D]; arrow). In stage 12-13 wild-type gynoecia (E), expression is confined to the style. In stage 9 CRC>>NGA1 flowers (F), expression can be observed at the top of the gynoecium and the nectary positions (arrows).
(G) By stage 13, expression is strong in the nectary positions and is basally expanded at the top of the gynoecium in CRC>>NGA1 flowers (arrows).
(H) A stage 12, 35S:amiR-NGA164a flower where STY2:GUS expression is restricted to low levels at the tips of the valve-like outgrowths (arrow).
Bars = 100 μm.
To confirm these results, we examined the expression of a STY2 marker line in backgrounds with altered NGA gene activity. STY2 has a redundant role with STY1, and expression of STY2:GUS (Kuusk et al., 2002) is activated distally in the stage 9 gynoecium (Figures 8C and 8D) and later confined to the style (Figure 8E; see Supplemental Figure 13A online). In CRC>>NGA1 flowers, STY2:GUS is activated earlier in the gynoecium and in nectary positions (Figures 8F and 8G; see Supplemental Figure 13B online), while being almost abolished in the gynoecium of 35S:amiR-NGA164a plants (Figure 8H; see Supplemental Figure 13C online). By comparison, STY2:GUS ovary expression, while expanded in CRCw>>STY1 ovaries, is not observed in the nectary positions (see Supplemental Figures 13E and 13F online). Together with the evidence that STY1 can activate NGA gene activity (Figure 6), these observations suggest a scenario whereby STY1 promotes NGA gene activity, which in turn, can activate other members of the SHI/STY gene family.
DISCUSSION
The distinct identities of the sepals, petals, stamens, and gynoecium that compose the flower are the result of a complex orchestration of specific growth and tissue differentiation programs. Style development occurs at the distal domain of the carpels, a floral organ specified by unique action of the C-class genes along with their SEPALLATA partners (Coen and Meyerowitz, 1991; Pelaz et al., 2000; Honma and Goto, 2001; Pinyopich et al., 2003). The mechanism by which by this program is realized downstream of the carpel identity program is unclear and limits our understanding of the processes of morphogenesis in plants. Here, we showed that activities of four NGA genes, which function in all aerial lateral organs, are required for style and stigma morphogenesis in the gynoecium. This role is due to late, distal NGA gene expression that is consequent, at least in part, of activation by earlier expression of SHI/STY gene family members (Kuusk et al., 2002, 2006). NGA gene activity also appears to promote the activities of several members of the SHI/STY family, suggesting a positive feedback loop between these two families of transcription factors. An additional surprising observation is that the NGA genes, though they are expressed in all organs of the flower where they affect development, can act as carpel organ identity genes, promoting the activity of at least one member of the AGAMOUS clade, SHP1. That loss of NGA gene activity in conjunction with that of CRC results in the distal gynoecium acquiring sepal identity likely reflects this role. In total, these observations imply a central role for NGA genes in a positive-feedback program that promotes and maintains style morphogenesis through the concerted self-reinforcing action of STY, NGA, and AG gene activity. The basis for the activation of this program appears to be the timing of NGA gene expression, which appears to be contingent on developmental capacity to respond to its activators, such as STY1. Factors that prevent precocious NGA gene activation appear to include YAB1 gene activity and effective auxin transport, and these could be interrelated.
The NGA Genes Are Essential for Stigma and Style Morphogenesis
Progressively lower levels of NGA gene activity results in a progressive reduction in style and stigma formation, until evidence of these tissues is eliminated in nga quadruple mutants (Figure 2). All other tissues of the ovary are present in the quadruple mutant, indicating that the role of the NGA genes is specific to the style and stigma. Reduced NGA gene activity also results in style and stigma tissues being eliminated in the carpel-converted organs of LFY:LFY-VP16, 35S:TAG and ANT>>miR172dm7 plants where these tissues develop ectopically from the sepals and leaves, respectively (Figure 3). This suggests that NGA gene activity is essential for style and stigma tissues in all contexts and that elevated levels of AG cannot compensate for their loss. Ectopic expression of NGA1 activity in the flower promotes ectopic and precocious style and stigma development and differentiation (Figure 5), whereas the capacity for STY1 overexpression to promote style and stigma development is conditional and NGA dependent (Figure 6). In this respect, while the disrupted style phenotypes of leunig, crc, seuss, and spatula could be rescued by STY1 overexpression, and those of spatula, sty, leunig, crc, seuss, and jagged could be rescued by NPA application (Staldal et al., 2008), neither STY1 overexpression (Figure 6) nor NPA application (Figure 7) could rescue a reduction in NGA activity. Thus, the NGA gene pathway is pivotal for style and stigma development and is likely used by all known promoters of these tissues.
NGA Activity in Flowers Is Intimately Linked to Style Formation and the Carpel Genes
The loss of style and delayed petal maturation in nga quadruple mutants suggests a differentiation function for NGA activity in all aerial organs (Figure 2; see Supplemental Figure 11 online). Thus, the essential role for the NGA gene function in style development can be viewed as a dramatic manifestation of a differentiation role played in all organs. Loss of NGA activity leads to the absence of SHP1 expression from the top of the gynoecium (Figure 3), reduced NGA activity in conjunction with reduced CRC results in the distal part of the gynoecium acquiring partial sepal identity (Figure 3), and ectopic overexpression of NGA1 results in ectopic style and stigma morphogenesis throughout the flower, which is accompanied by ectopic SHP1 and CRC expression (Figure 5; see Supplemental Figure 12 online). This suggests a direct link between NGA and style/stigma promotion through carpel gene activation. Notably, ectopic NGA activity is level dependent in this respect. Lower levels of floral ectopic NGA activity expression affect organ growth, presumably consistent with their wild-type role in sepals and petals, whereas high levels promote style/stigma and ectopic carpel gene expression (Figure 5; see Supplemental Figure 9 online). Since ectopic overexpression of NGA genes in the leaves does not result in ectopic carpel tissues (see Supplemental Figure 8 online), the NGA gene carpel function must be mediated by flower specific cofactors that are, in part, negatively regulated by the five AP2-related genes targeted by miR172 (Chen, 2004; Figure 3). Such cofactors presumably include members of both the SEPALLATA and AG MADS box gene families, which are essential for carpel development in the flower (Pelaz et al., 2000; Honma and Goto, 2001; Ditta et al., 2004). The nature of these interactions awaits elucidation.
A question arises as to why there is such an intimate molecular connection between NGA activity and style/stigma morphogenesis. A likely scenario is that NGA style program has evolved features that parallel the activity of floral ABC selector genes in organogenesis to allow cells of the nascent style to rapidly distinguish themselves in growth and identity from the underlying ovary. High NGA levels promote AG clade members and exclude ovary factors, such as FIL from the initiating style (see Supplemental Figure 12 online) in the program of style differentiation. For many angiosperm species, the analogy of a style being consequent of a distinct organogenesis program is particularly appropriate. In maize (Zea mays), for example, the style (the silk) can develop into an enormous length of >20 cm compared with the minute ovary (Carcova et al., 2003). Since the primary mode of NGA gene regulation is transcriptional (Figures 4 and 5), understanding the basis for their activation is critical. Our results suggest that the SHI/STY genes likely have a central role in promoting and maintaining NGA gene expression.
NGA Gene Activation: Regulated Competence to Respond to an Activator
The expression of NGA1 and NGA4 occurs well after initiation in all organs, and in the gynoecium the expression is tightly correlated with style initiation at stage 9. Since STY1 overexpression promoted ectopic, NGA gene-dependent style tissue and nga4:GUS expression, the transcriptional control of the NGA genes likely involves positive regulation through STY1 and other members of the SHI/STY gene family (Kuusk et al., 2006; Figure 6). However, the temporal separation of the stage 6 STY1 and stage 9 NGA expression suggests that NGA gene activation by STY1 is context dependent. Since STY1 is an activator of YUC4, which encodes an auxin biosynthesis enzyme (Cheng et al., 2006; Sohlberg et al., 2006), a simple model is that the NGA genes respond to an auxin-related signal derived from distal STY1 activity that does not reach an NGA-activating threshold until stage 9 of gynoecium development (Figure 9). This is supported by auxin transport inhibition causing early, basally expanded NGA gene activation (Figure 7). Reduction in YAB1 (FIL/YAB3) genes, which, as well as regulating organ polarity, have been implicated in signaling (Goldshmidt et al., 2008) and growth promotion (Eshed et al., 2004), also results in earlier, stronger, and basally expanded NGA gene activity. The YAB1 genes, which are expressed from the inception of gynoecium development (stage 5) in the valve primordia, could be direct negative regulators of NGA gene activity. Alternatively, they may function to maintain a cellular environment that prevents NGA sensitivity to the STY signal. Detailed investigation into these scenarios will have to await a careful analysis of auxin flux (see Supplemental Figure 12 online) and steady state auxin levels along the developing gynoecium. Other scenarios are also possible. For instance, NGA1 to NGA4 are among the genes subject to histone H3 Lys-27 trimethylation (Zhang et al., 2007), and it may be this form of regulation that is involved in negatively regulating gene expression in young tissues.
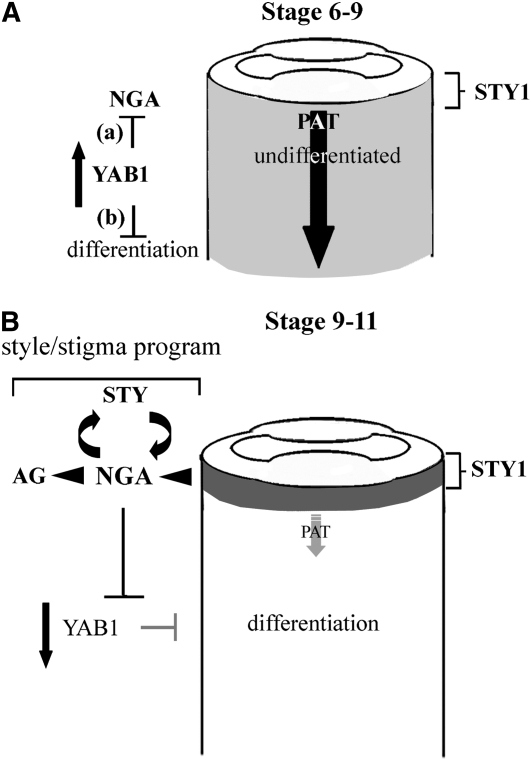
Figure 9.
Genetic Models for NGA Activation and Function in Promoting Style Development and Differentiation in Aerial Organs.
(A) During stages 6-9 of gynoecium development, early ovary factors, such as the YABBY1 genes (FIL and YAB3), function to either/or (a) suppress NGA gene activity directly or (b) suppress NGA gene activity passively by preventing the accumulation of an activating signal through an effect that has been defined here simplistically as delayed differentiation. We suggest that a distal, auxin-based signal promoted by the STY genes, including STY1, acts to induce NGA gene activity at a threshold level. This level is not reached (faint shading) because of efficient PAT (large arrow) maintained in gynoecium at that stage.
(B) In the stage 9-11 gynoecium, YAB1 efficacy is reduced as is their ability to negative regulate NGA gene activity or passively prevent NGA response to an activating signal. Under the auxin-based activator scenario, the polar transport of auxin from the distal site of STY1-mediated synthesis becomes inefficient and auxin accumulates to an NGA-activating threshold. Upon activation, NGA gene activity suppresses YAB1 while promoting AG clade and SHI/STY gene family members. These in turn maintains NGA gene activity and their own expression through positive feedback. Together, the NGA, STY, and AG clade genes constitute a developmental module for style/stigma morphogenesis.
After NGA gene activation in the stage 9 gynoecium, promotion and maintenance of distal NGA gene activity apparently comes from positive feedback between the NGA genes and SHI/STY family members (Figure 8). Under this scenario, SHI/STY-activated NGA activity suppresses earlier, ovary factors while promoting AG clade members and additional SHI/STY factors that further activate NGA gene activity in style and stigma development (Figure 9). From this perspective, the valve tissue that develops instead of style in the nga quadruple mutant (Figure 2) can be seen as a homeotic conversion.
Many molecular programs underlying gynoecium morphogenesis function in leaf development and were likely encompassed under the regulatory direction of C class activity (Sitaraman et al., 2008), consistent with Goethe's vision of the carpel as a modified leaf (Goethe, 1790). In this respect, the temporal and spatial action of the NGA genes in the gynoecium appears to be shared with the other floral organs, suggesting that NGA function has been co-opted for an essential role in style and stigma differentiation. Consistent with this, the petals of the nga quadruple mutants appear to exhibit delayed maturation. It is therefore tempting to speculate that the primary role of the NGA genes is organ differentiation, which is conserved in leaves. Under this scenario, the basic framework of gene action postulated for NGA gene activation, an interplay between the NGA and STY family members, elevated auxin levels and suppression of earlier organ growth factors, is a step in the differentiation of all lateral organs. In this respect, the numerous valve outgrowths observed in nga quadruple mutants (Figure 2) and at the margins of nga1-1 gym-5 kan1-2 and nga1-1 kan1-2 kan2-1/+ gynoecia (Figure 1) may reflect a loss of a growth suppressing function of the NGA differentiation program and not a conversion of style to valve tissue. Loss of such a program could also lead to the more serrated leaves of nga quadruple mutants (Figure 2). Investigation into the leaf role of the NGA genes will help shed light on these possibilities.
METHODS
Plant Material, Growth, Transformation, and Mutagenesis
All plants described were in the Landsberg erecta background and grown under 18-h cool white fluorescent light at 18 to 22°C. For the NGA1:LhG4 and AP3-short:LhG4 driver lines, 5 and 400 bp upstream of the translation start site, respectively, were transcriptionally fused 5′ of the chimeric LhG4 transcription factor. NGA1, NGA2, NGA3, NGA4, NGAL1, and STY1 cDNAs subcloned behind an operator array in BJ36 plasmid to generate responder lines (Moore et al., 1998). Primers used for PCR cloning are presented in Supplemental Table 1 online, and the cloning strategies for 4xNLSmRFP, pre-miR172d, and miR172dm7 are presented as Supplemental Methods online.
To complement the nga1-1 mutation, the DNA from BAC clone F14M4 was digested with BamHI and KpnI and an ∼10.5-kb genomic fragment spanning the At2g46870 locus (NGA1), including 5.7 kb of sequence upstream from the presumptive initiation ATG codon and 3.8 kb of sequence downstream of the putative stop codon was cloned into pBluescript SK+ before being subcloned into pBJ36. Transformation was performed into nga1-1 kan1-1 mutant plants to facilitate identification of complemented plants. All constructs were subcloned to the pMLBART binary plasmid and transformed to plants by floral dipping using the Agrobacterium tumefaciens GV3101 strain. For transactivation, Promoter:LhG4 driver lines were crossed to different OP:cDNA responder lines to generate transactivated F1s (marked as >> in the text). Mutant and transgene combinations were generated through conventional breeding.
Map-Based Cloning of NGA1
To map the NGA1 locus, the nga1-1 kan1-2 double mutant was crossed to a kan1-10 homozygote in the Columbia background to facilitate genotyping, and the F1 plants were allowed to self-pollinate. In the F2 population, genomic DNA was isolated from 440 plants homozygous for nga1-1 and subject to genetic mapping using cleaved-amplified polymorphic sequence and Simple Sequence Length Polymorphism (SSLP) markers. The nga1-1 mutation was localized to the lower arm of chromosome 2 on BAC F14M4. Candidate genes in the nga1-1 background were sequenced, and a mutation in the locus At2g46870 was identified. Distinct molecular lesions were subsequently identified in the At2g46870 locus of nga1-2 and nga1-3 mutant plants. Transcript ends were determined by rapid amplification of cDNA ends (RACE) using total RNA isolated from young inflorescences with TriReagent (Sigma-Genosys) and the Clontech SMART RACE cDNA amplification kit in combination with gene-specific primers (see Supplemental Table 2 online).
Transcriptome Analysis of nga Inflorescences
RNA was extracted from inflorescences of 30-d-old plants using the Qiagen RNAEasy kit. cRNA was synthesized and hybridized to Affymetrix ATH1 array according to the manufacturer's recommendations. Seven repeats of the wild type, two repeats of nga quadruple mutants, and two repeats of 35S:amiR-NGA164a were collected. Signal values were obtained and normalized using GeneChip-Robust Multi-array Average (GC-RMA), as implemented in R 2.7.2 (www.r-project.org) and Bioconductor 2.2 (www.bioconductor.org/). Average correlation values between repeats were 0.985, while the average correlation between wild-type and mutant samples was 0.972. As the average correlation between samples, the nga quadruple mutants and 35S:amiR-NGA164a plants was 0.984, and given the identical phenotype, we grouped the two genotypes together. Genes were filtered for fold change between the wild type and mutant higher than 1.5 and absolute expression value larger than log2(10). Welsh t test was performed on the filtered list, followed by FDR, using the multitest R package. Genes with FDR P value < 0.05 were selected as significantly changed. Analysis of Gene Ontology annotation enrichment was performed using DAVID (http://david.abcc.ncifcrf.gov/).
NPA Treatment
The NPA treatment was performed as described by Nemhauser et al. (2000).
Microscopy and Confocal Imaging
Tissue was prepared and sectioned to view florescent signals according to Goldshmidt et al. (2008). Confocal images were taken on an Olympus IX-70 microscope with an argon laser set at 488 nm for excitation, a 505- to 525-nm filter for GFP emission, and a 560- to 600-nm filter for Propidium Iodide (PI) emission. The 4',6-diamidino-2-phenylindole staining was observed with a 405-nm diode laser for excitation and a 450- to 490-nm filter for emission. Images were captured and processed with the FW-500 image analysis system. Two-photon imaging was performed with a Zeiss LSM 510 META NLO microscope equipped with ×20 and ×40 water immersion objectives. A Mai Tai One Box Ti:Sapphire Laser (Spectra Physics, Newport) was used for two-photon excitation. Image acquisition was performed using LSM 510 acquisition software.
For light sections and scanning electron microscopy, inflorescences were fixed in 2% glutaraldehyde in 0.025 M sodium phosphate buffer at pH 6.8 and vacuum infiltrated at room temperature for up to 1 h. For sections, tissues were washed, dehydrated in an ethanol series, and embedded in LR White resin. Sections of 2 μm for light microscopy were cut and dried onto slides. Sections were stained with toluidine blue. Scanning electron microscopy was performed using an XL30 ESEM FEG microscope (FEI).
Accession Numbers
The Arabidopsis Genome Initiative locus identifiers for NGA1 to NGA4 are At2g46870, At3g61970, At1g01030, and At4g01500, respectively. STY1, STY2, SPH1, CRC, FIL, YAB3, and KAN1 correspond to At3g51060, At4g36260, At3g58780, At1g69180, At2g45190, At4g00180, and At5g16560, respectively. Microarray data were submitted to the Geo Omnibus repository (accession number GSE15555).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. nga1-1 Genetic Interactions.
Supplemental Figure 2. Multiple Alignment of NGA1-4 Proteins and Phylogenetic Relationships of the RAV Clade of B3 Transcription Factors in Land Plants.
Supplemental Figure 3. NGA Genes Redundantly Regulate Growth of Flower Organs and Leaves.
Supplemental Figure 4. Histological Analyses of Diverse Mature Gynoecia.
Supplemental Figure 5. Style Development Requires NGA Gene Activity.
Supplemental Figure 6. Design and Overexpression of a Native and Modified miR172 MicroRNA.
Supplemental Figure 7. Genetic Combinations between nga and Mutants Effecting Gynoecium Development Produce Additive and Synergistic Interactions.
Supplemental Figure 8. Effects of NGA Gene Overexpression during Vegetative Growth.
Supplemental Figure 9. NGA Gene Overexpression Reduces Organ Growth and Promotes a Style Program within the Flower.
Supplemental Figure 10. Effect of Overexpression of NGAL1/AT2G36080.
Supplemental Figure 11. Effects of Expressing NGA1 and STY1 with the CRCw (CRC Weak) Promoter.
Supplemental Figure 12. Spatial Regulation of NGA Gene Activities.
Supplemental Figure 13. STY2:GUS Expression in Backgrounds with Different NGA Levels or STY1 Overexpression.
Supplemental Table 1. Primers for PCR-Mediated Cloning.
Supplemental Table 2. Gene-Specific Primers for RACE.
Supplemental Data Set 1. Alignment of Sequences Used for Phylogenetic Analysis.
Supplemental Data Set 2. Genes Modified in Their Expression in nga Mutant Apices.
Supplemental Methods.
Supplementary Material
Acknowledgments
We thank Vyacheslav Kalchenko of the Weizmann Veterinary Resources In Vivo Optical Imaging Unit for assistance with two-photon imaging, Eugenia Klein and the electron microscopy facility for help with scanning electron microscopy, Raya Eilam and Eyal Shimoni for help with tissue preparation techniques, and Vladimir Kiss for assistance with confocal laser scanning microscopy. The dedicated work of Galit Shahar, Anna Pistunov, and Oshri Afanzer is highly appreciated. We also thank Eva Sundberg for the STY2:GUS line, members of the Eshed lab for comments and discussions, and Cristina Ferrándiz for sharing unpublished results. This work was made possible with funding from the Israel Science Foundation (Research Grant Award No. 863-06; Y.E.), from MINERVA (Y.E.), and from the U. S. National Science Foundation (IOB 0332556; J.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Yuval Eshed (yuval.eshed@weizmann.ac.il).
Online version contains Web-only data.
References
- Alvarez, J., and Smyth, D.R. (2002). Crabs Claw and Spatula genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. Int. J. Plant Sci. 163 17–41. [Google Scholar]
- Alvarez, J.P., Pekker, I., Goldshmidt, A., Blum, E., Amsellem, Z., and Eshed, Y. (2006). Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18 1134–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhakanandam, S., Nole-Wilson, S., Bao, F., and Franks, R.G. (2008). SEUSS and AINTEGUMENTA mediate patterning and ovule initiation during gynoecium medial domain development. Plant Physiol. 146 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanza, V., Navarrete, M., Trigueros, M., and Ferrandiz, C. (2006). Patterning the female side of Arabidopsis: the importance of hormones. J. Exp. Bot. 57 3457–3469. [DOI] [PubMed] [Google Scholar]
- Benjamins, R., Quint, A., Weijers, D., Hooykaas, P., and Offringa, R. (2001). The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067. [DOI] [PubMed] [Google Scholar]
- Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertová, D., Jürgens, G., and Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. [DOI] [PubMed] [Google Scholar]
- Bennett, S.M.R., Alvarez, J., Bossinger, G., and Smyth, D.R. (1995). Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 8 505–520. [Google Scholar]
- Bowman, J.L., Baum, S.F., Eshed, Y., Putterill, J., and Alvarez, J. (1999). Molecular genetics of gynoecium development in Arabidopsis. Curr. Top. Dev. Biol. 45 155–205. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Eshed, Y., Baum, S., Emery, J.F., Floyd, S.K., Alvarez, J., Hawker, N.P., Lee, J.Y., Siegfried, K.R., Khodosh, R., Tatom-Jaurez, M., and Perea, J.V. (2001). The story of CRABS CLAW (or How we learned to love the mutagen). Flowering Newsletter 31 3–11. [Google Scholar]
- Bowman, J.L., and Smyth, D.R. (1999). CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 126 2387–2396. [DOI] [PubMed] [Google Scholar]
- Busch, M.A., Bomblies, K., and Weigel, D. (1999). Activation of a floral homeotic gene in Arabidopsis. Science 285 585–587. [DOI] [PubMed] [Google Scholar]
- Carcova, J., Andrieu, B., and Otegui, M.E. (2003). Silk elongation in maize: Relationship with flower development and pollination. Crop Sci. 43 914–920. [Google Scholar]
- Chen, X. (2004). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y., Dai, X., and Zhao, Y. (2006). Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 20 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.K., Dagenais, N., Chory, J., and Weigel, D. (2000). Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478. [DOI] [PubMed] [Google Scholar]
- Coen, E.S., and Meyerowitz, E.M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature 353 31–37. [DOI] [PubMed] [Google Scholar]
- Crawford, B.C.W., Ditta, G., and Yanofsky, M.F. (2007). The NTT gene is required for transmitting-tract development in carpels of Arabidopsis thaliana. Curr. Biol. 17 1101–1108. [DOI] [PubMed] [Google Scholar]
- Dinneny, J.R., Weigel, D., and Yanofsky, M.F. (2006). NUBBIN and JAGGED define stamen and carpel shape in Arabidopsis. Development 133 1645–1655. [DOI] [PubMed] [Google Scholar]
- Ditta, G., Pinyopich, A., Robles, P., Pelaz, S., and Yanofsky, M.F. (2004). The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 14 1935–1940. [DOI] [PubMed] [Google Scholar]
- Elliott, R.C., Betzner, A.S., Huttner, E., Oakes, M.P., Tucker, W.Q.J., Gerentes, D., Perez, P., and Smyth, D.R. (1996). AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., and Bowman, J.L. (1999). Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99 199–209. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., Perea, J.V., and Bowman, J.L. (2001). Establishment of polarity in lateral organs of plants. Curr. Biol. 11 1251–1260. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Izhaki, A., Baum, S.F., Floyd, S.K., and Bowman, J.L. (2004). Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131 2997–3006. [DOI] [PubMed] [Google Scholar]
- Ferrandiz, C., Pelaz, S., and Yanofsky, M.F. (1999). Control of carpel and fruit development in arabidopsis. Annu. Rev. Biochem. 68 321–354. [DOI] [PubMed] [Google Scholar]
- Flanagan, C.A., Hu, Y., and Ma, H. (1996). Specific expression of the AGL1 MADS-box gene suggests regulatory functions in Arabidopsis gynoecium and ovule development. Plant J. 10 343–353. [DOI] [PubMed] [Google Scholar]
- Galweiler, L., Guan, C., Muller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230. [DOI] [PubMed] [Google Scholar]
- Goethe, J.W. (1790). Versuch die Metamorphose der Pflanzen zu erklären. Gotha: Carl Wilhelm Ettinger (transl. Arber, A. [1946]. Goethe's botany. Chromca Bot. 10: 63–126.)
- Goldshmidt, A., Alvarez, J.P., Bowman, J.L., and Eshed, Y. (2008). Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell 20 1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremski, K., Ditta, G., and Yanofsky, M.F. (2007). The HECATE genes regulate female reproductive tract development in Arabidopsis thaliana. Development 134 3593–3601. [DOI] [PubMed] [Google Scholar]
- Heisler, M.G., Atkinson, A., Bylstra, Y.H., Walsh, R., and Smyth, D.R. (2001). SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development 128 1089–1098. [DOI] [PubMed] [Google Scholar]
- Honma, T., and Goto, K. (2001). Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409 525–529. [DOI] [PubMed] [Google Scholar]
- Ishida, T., Aida, M., Takada, S., and Tasaka, M. (2000). Involvement of CUP-SHAPED COTYLEDON genes in gynoecium and ovule development in Arabidopsis thaliana. Plant Cell Physiol. 41 60–67. [DOI] [PubMed] [Google Scholar]
- Jack, T. (2004). Molecular and genetic mechanisms of floral control. Plant Cell 16 S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya, Y., Ohmiya, K., and Hattori, T. (1999). RAV1, a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res. 27 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusk, S., Sohlberg, J.J., Eklund, D.M., and Sundberg, E. (2006). Functionally redundant SHI family genes regulate Arabidopsis gynoecium development in a dose-dependent manner. Plant J. 47 99–111. [DOI] [PubMed] [Google Scholar]
- Kuusk, S., Sohlberg, J.J., Long, J.A., Fridborg, I., and Sundberg, E. (2002). STY1 and STY2 promote the formation of apical tissues during Arabidopsis gynoecium development. Development 129 4707–4717. [DOI] [PubMed] [Google Scholar]
- Liljegren, S.J., Ditta, G.S., Eshed, Y., Savidge, B., Bowman, J.L., and Yanofsky, M.F. (2000). SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404 766–770. [DOI] [PubMed] [Google Scholar]
- Mizukami, Y., and Ma, H. (1992). Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identify. Cell 71 119–131. [DOI] [PubMed] [Google Scholar]
- Moore, I., Galweiler, L., Grosskopf, D., Schell, J., and Palme, K. (1998). A transcription activation system for regulated gene expression in transgenic plants. Proc. Natl. Acad. Sci. USA 95 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser, J.L., Feldman, L.J., and Zambryski, P.C. (2000). Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127 3877–3888. [DOI] [PubMed] [Google Scholar]
- Ohno, C.K., Reddy, G.V., Heisler, M.G.B., and Meyerowitz, E.M. (2004). The Arabidopsis JAGGED gene encodes a zinc finger protein that promotes leaf tissue development. Development 131 1111–1122. [DOI] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, T., Johnson, S.D., and Koltunow, A.M. (2004). KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development 131 3737–3749. [DOI] [PubMed] [Google Scholar]
- Pelaz, S., Ditta, G.S., Baumann, E., Wisman, E., and Yanofsky, M.F. (2000). B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405 200–203. [DOI] [PubMed] [Google Scholar]
- Pinyopich, A., Ditta, G.S., Savidge, B., Liljegren, S.J., Baumann, E., Wisman, E., and Yanofsky, M.F. (2003). Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424 85–88. [DOI] [PubMed] [Google Scholar]
- Pnueli, L., Hareven, D., Rounsley, S.D., Yanofsky, M.F., and Lifschitz, E. (1994). Isolation of the tomato AGAMOUS gene TAG1 and analysis of its homeotic role in transgenic plants. Plant Cell 6 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunet, N., Morel, P., Thierry, A.-M., Eshed, Y., Bowman, J.L., Negrutiu, I., and Trehin, C. (2008). REBELOTE, SQUINT, and ULTRAPETALA1 function redundantly in the temporal regulation of floral meristem termination in Arabidopsis thaliana. Plant Cell 20 901–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa, S., Watanabe, K., Goto, K., Kanaya, E., Morita, E.H., and Okada, K. (1999). FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 13 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, M., Davison, T.S., Henz, S.R., Pape, U.J., Demar, M., Vingron, M., Scholkopf, B., Weigel, D., and Lohmann, J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37 501–506. [DOI] [PubMed] [Google Scholar]
- Schoof, H., Lenhard, M., Haecker, A., Mayer, K.F.X., Jrgens, G., and Laux, T. (2000). The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644. [DOI] [PubMed] [Google Scholar]
- Scofield, S., Dewitte, W., and Murray, J.A.H. (2007). The KNOX gene SHOOT MERISTEMLESS is required for the development of reproductive meristematic tissues in Arabidopsis. Plant J. 50 767–781. [DOI] [PubMed] [Google Scholar]
- Sessions, A., Nemhauser, J.L., McColl, A., Roe, J.L., Feldmann, K.A., and Zambryski, P.C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124 4481–4491. [DOI] [PubMed] [Google Scholar]
- Siegfried, K.R., Eshed, Y., Baum, S.F., Otsuga, D., Drews, G.N., and Bowman, J.L. (1999). Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126 4117–4128. [DOI] [PubMed] [Google Scholar]
- Sitaraman, J., Bui, M., and Liu, Z. (2008). LEUNIG HOMOLOG and LEUNIG perform partially redundant functions during Arabidopsis embryo and floral development. Plant Physiol. 147 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg, J.J., Myrenas, M., Kuusk, S., Lagercrantz, U., Kowalczyk, M., Sandberg, G., and Sundberg, E. (2006). STY1 regulates auxin homeostasis and affects apical-basal patterning of the Arabidopsis gynoecium. Plant J. 47 112–123. [DOI] [PubMed] [Google Scholar]
- Staldal, V., Sohlberg, J.J., Eklund, M.D., Ljung, K., and Sundberg, E. (2008). Auxin can act independently of CRC, LUG, SEU, SPT and STY1 in style development but not apical-basal patterning of the Arabidopsis gynoecium. New Phytol. 180 798–808. [DOI] [PubMed] [Google Scholar]
- Staswick, P.E., Serban, B., Rowe, M., Tiryaki, I., Maldonado, M.T., Maldonado, M.C., and Suza, W. (2005). Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan, K., Peterson, K., and Jack, T. (2008). The plant B3 superfamily. Trends Plant Sci. 13 647–655. [DOI] [PubMed] [Google Scholar]
- Yamasaki, K., et al. (2004). Solution structure of the B3 DNA binding domain of the Arabidopsis cold-responsive transcription factor RAV1. Plant Cell 16 3448–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Clarenz, O., Cokus, S., Bernatavichute, Y.V., Pellegrini, M., Goodrich, J., and Jacobsen, S.E. (2007). Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 5 e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.