Abstract
Tillering in rice (Oryza sativa) is one of the most important agronomic traits that determine grain yields. Previous studies on rice tillering mutants have shown that the outgrowth of tiller buds in rice is regulated by a carotenoid-derived MAX/RMS/D (more axillary branching) pathway, which may be conserved in higher plants. Strigolactones, a group of terpenoid lactones, have been recently identified as products of the MAX/RMS/D pathway that inhibits axillary bud outgrowth. We report here the molecular genetic characterization of d27, a classic rice mutant exhibiting increased tillers and reduced plant height. D27 encodes a novel iron-containing protein that localizes in chloroplasts and is expressed mainly in vascular cells of shoots and roots. The phenotype of d27 is correlated with enhanced polar auxin transport. The phenotypes of the d27 d10 double mutant are similar to those of d10, a mutant defective in the ortholog of MAX4/RMS1 in rice. In addition, 2′-epi-5-deoxystrigol, an identified strigolactone in root exudates of rice seedlings, was undetectable in d27, and the phenotypes of d27 could be rescued by supplementation with GR24, a synthetic strigolactone analog. Our results demonstrate that D27 is involved in the MAX/RMS/D pathway, in which D27 acts as a new member participating in the biosynthesis of strigolactones.
INTRODUCTION
Shoot branching plays an important role in determining the diversity of plant architectures. In higher plants, branches are derived from shoot apical meristems (SAMs). The primary SAM provides the main axis of the plant body, while the secondary SAMs in the axils of leaves generate axillary meristems (AMs) (McSteen and Leyser, 2005). Formation of branches generally comprises two distinct steps: the formation of AMs in the leaf axils and the outgrowth of axillary buds (Shimizu-Sato and Mori, 2001). However, after initiation, an AM can arrest its growth and form a dormant bud, which will be released in response to particular environmental and/or developmental signals (Wang and Li, 2008).
In some plant species, the outgrowth of axillary buds may be inhibited by the primary shoot, a phenomenon known as apical dominance (Sachs and Thimann, 1964; Cline, 1991). The plant hormone auxin has long been implicated in participating in this process (Thimann and Skoog, 1934; Cline, 1991; Leyser, 2003). Indole-3-acetic acid (IAA) is the most abundant natural plant auxin and is synthesized mainly in the shoot apex and young leaves. It is transported along the shoot-root axis from cell to cell in a polar manner, which is essential for inhibiting the outgrowth of axillary buds (Ljung et al., 2001; Leyser, 2003; Sieberer and Leyser, 2006). However, a large body of evidence suggests that auxin cannot directly enter the axillary buds and that a second messenger is required to inhibit the outgrowth of axillary buds (Shelagh and John, 1975; Morris, 1977; Pilate et al., 1989; Prasad et al., 1993; Booker et al., 2003). Cytokinin is the first reported second messenger candidate, which is synthesized in roots and transported acropetally in the xylem to promote directly the outgrowth of axillary buds (Van Dijck et al., 1988; Cline, 1991; Eklof et al., 1997; Kapchina-Toteva et al., 2000; Nordstrom et al., 2004). Exogenous application of cytokinin to axillary buds promotes their outgrowth (Sachs and Thimann, 1964; Cline, 1991). Increased cytokinin levels lead to reduced apical dominance in Arabidopsis thaliana (Tantikanjana et al., 2001; Jung et al., 2005). It is plausible that auxin suppresses the outgrowth of axillary buds by influencing the supply of cytokinin to axillary buds (Eklof et al., 2000; Li and Bangerth, 2003; Nordstrom et al., 2004; Tanaka et al., 2006).
Recent studies on a series of branching mutants, more axillary growth (max) of Arabidopsis, ramosus (rms) mutants of pea (Pisum sativum), decreased apical dominance (dad) mutants of petunia (Petunia hybrida), have revealed an additional carotenoid-derived hormone as a second messenger of auxin action on the regulation of AM outgrowth (Beveridge et al., 1994, 1996, 2000; Napoli, 1996; Stirnberg et al., 2002; Sorefan et al., 2003; Booker et al., 2004, 2005; Simons et al., 2007). Genetic analysis and grafting experiments on these mutants have shown that this kind of carotenoid derivative, synthesized in the root and transported acropetally or synthesized locally, represses branch outgrowth (Beveridge et al., 1997; Morris et al., 2001; Turnbull et al., 2002; Sorefan et al., 2003). In Arabidopsis, four MAX loci, MAX1 to MAX4, have been identified and proved to be involved in a common signaling pathway, known as the MAX pathway (Booker et al., 2005). MAX1, a cytochrome P450 family member, acts downstream of MAX3 and MAX4, two carotenoid cleavage deoxygenase (CCD) family proteins, CCD7 and CCD8, in the biosynthesis of the signal (Sorefan et al., 2003; Booker et al., 2004, 2005; Lazar and Goodman, 2006). By contrast, MAX2 encodes an F-box protein, which is responsible for perceiving and transducing the signal (Stirnberg et al., 2002, 2007). In pea, RMS4, RMS5, and RMS1 are orthologous of MAX2, MAX3, and MAX4, respectively (Sorefan et al., 2003; Johnson et al., 2006).
Although the outgrowth behaviors between dicotyledonous and monocotyledonous AMs are apparently different, the fact that orthologs of MAX2 to MAX4 have also been identified in rice (Oryza sativa) suggests that monocots and dicots share a conserved MAX-involved carotenoid-derived branching signal pathway (Wang and Li, 2008). Rice plants defective in D3, HTD1/D17, and D10, which correspond to Arabidopsis MAX2, MAX3, and MAX4, respectively, give rise to more tillers and reduced plant height (Ishikawa et al., 2005; Zou et al., 2006; Arite et al., 2007), indicating their similar functions in suppressing the branch development in monocotyledonous plants. Thereafter, this carotenoid-derived branching inhibiting signal pathway was generally known as the MAX/RMS/D pathway.
Recently, two groups have independently reported that the MAX/RMS/D pathway is involved in the production and signaling of strigolactones (Gomez-Roldan et al., 2008; Umehara et al., 2008). Strigolactones, synthesized from carotenoids, are a group of terpenoid lactones that have been found in root exudates of diverse plant species (Cook et al., 1972; Bouwmeester et al., 2003; Matusova et al., 2005; Humphrey and Beale, 2006; Lopez-Raez et al., 2008). Mutations of CCD7 or CCD8 in pea, rice, and Arabidopsis results in reduced strigolactones production. By contrast, the signaling mutant d3, which is defective in the ortholog of Arabidopsis MAX2, accumulates higher levels of strigolactones. Furthermore, application of strigolactones inhibits shoot branching in the ccd mutants of pea, rice, and Arabidopsis, but it has no effect on rms4/max2/d3 signaling mutants (Gomez-Roldan et al., 2008; Umehara et al., 2008). These data strongly suggest that strigolactones act as a new class of phytohormones involved in regulating plant branching.
Strigolactones have been identified previously as seed germination stimulants of root parasitic plants (Cook et al., 1972; Bouwmeester et al., 2003; Humphrey and Beale, 2006) and hyphal branching signals when plants interact with mycorrhizal fungi (Akiyama et al., 2005). However, the strigolactone biosynthesis and signaling pathways still remain to be elucidated. Identification of new branching mutants and isolation of their genes in multiple plant systems will facilitate the elucidation of the biosynthesis and signaling pathways of this new type of hormone in plants.
Tillering in rice is one of the most important agronomic traits that determine grain yields and a model system for elucidating molecular mechanisms that regulate axillary buds (Wang and Li, 2005). In this study, we characterize a rice dwarf 27 (d27) mutant that is defective in the outgrowth of axillary buds. Map-based cloning and in-depth analysis of D27 revealed that it encodes a novel chloroplast-located iron-containing protein. Our results demonstrate that D27 regulates tiller bud outgrowth through the MAX/RMS/D pathway and participates in the biosynthesis of strigolactones.
RESULTS
Phenotypes of the Rice Tillering Dwarf Mutant d27
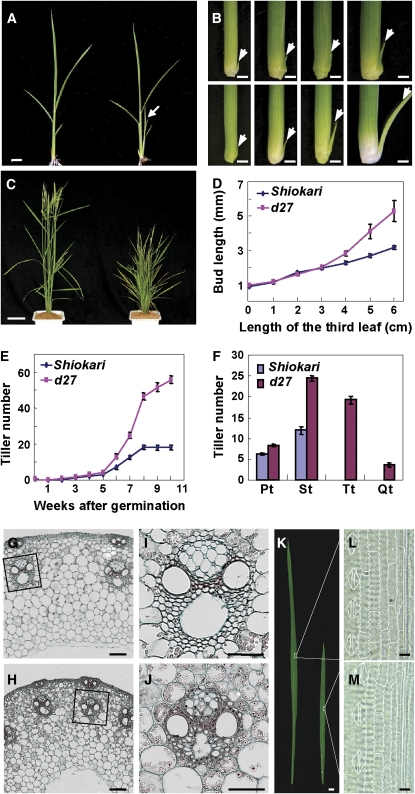
The rice d27 mutant is a classic rice mutant described previously (Ishikawa et al., 2005; Arite et al., 2007). To elucidate the molecular mechanism that determines rice tiller number, we further characterized d27 in depth. At the seedling stage, d27 exhibits accelerated tiller production (Figure 1A). Kinetic analysis of the tiller bud development demonstrated that the increased tiller number is ascribed to accelerated tiller bud outgrowth rather than to more tiller bud formation (Figures 1B and 1D; see Supplemental Figure 1 online). At the mature stage, the d27 mutant plant showed a high tillering and severe dwarf phenotype (Figure 1C). Kinetic analysis showed that the final tiller number of d27 is three times that of the wild type (Figure 1E), which results from the outgrowth of higher-order tillers (Figure 1F). Further histological analysis suggested that the cell number and size are both reduced in d27 mutant culms and leaves, leading to severe dwarfism in the mutant plant (Figures 1G to 1M).
Figure 1.
Morphological Comparison between Wild-Type and d27 Plants.
(A) The phenotype of wild-type (Shiokari) (left) and d27 seedling (right). Arrow indicates the first tiller in d27, which is absent in wild type at this stage. Bar = 1 cm.
(B) Developmental process of tiller buds at the axil of the first leaves in the wild type (top panel) and d27 (bottom panel), showing the accelerated tiller bud elongation in d27. The pictures were photographed when the third leaf was 0, 2, 4, and 6 cm in length. Arrows indicate the examined tiller buds. Bars = 1 mm.
(C) Phenotype of wild-type (left) and d27 (right) plants at the heading stage. Bar = 10 cm.
(D) Kinetic analyses of the elongation of tiller buds at the axil of the first leaves indicated in (B). Each value represents the mean ± se of 15 replicates.
(E) Kinetic comparison of tiller numbers between wild-type and d27 plants at different developmental stages. Each value represents the mean ± se of 15 replicates.
(F) The types of tillers at the heading stage. Pt, primary tillers; St, secondary tillers; Tt, tertiary tillers; Qt, quaternary tillers. Each value represents the mean ± se of 15 replicates.
(G) to (J) Cross sections of the wild type ([G] and [I]) and d27 ([H] and [J]) culms. Bars = 100 μm.
(I) and (J) are magnifications of indicated regions in (G) and (H), respectively. Bars = 50 μm.
(K) Morphological comparison of flag leaves between the wild type (left) and d27 (right). Bar = 1 cm.
(L) and (M) Micrographs of cleared flag leaves from the wild type (L) and d27 (M). The regions indicated in (K) are shown. Bars = 100 μm.
Cloning and Characterization of D27
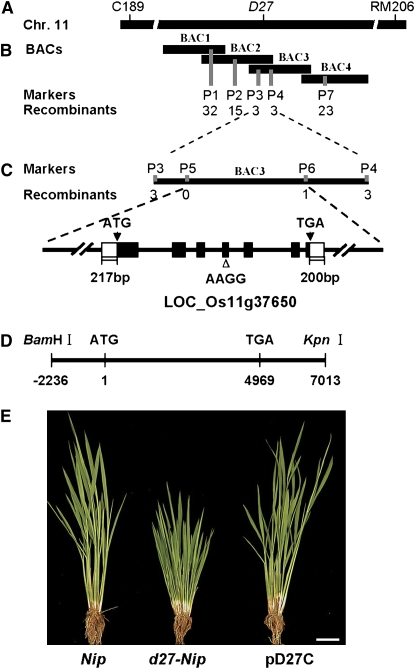
To isolate the D27 gene, we took a map-based cloning approach. D27 was primarily delimited in an interval of ∼3.0 centimorgans between the two molecular markers C189 and RM206 on the long arm of chromosome 11 (Figure 2A). To fine-map the D27 locus, we generated a large F2 mapping population derived from a cross between d27-ZF802 and its isogenetic lines ZF802. Of 21,000 F2 plants, 5200 mutant plants were used for fine-mapping, and the D27 locus was located between the two cleaved amplified polymorphic sequence (CAPS) markers P1 and P7 (Figure 2B). Screening with newly developed molecular markers P1 to P7 (see Supplemental Table 1 online), D27 was further placed within an 18-kb DNA region between the P3 and P6 markers and cosegregated with the P5 marker (Figure 2C). Within this region, there are two open reading frames (ORFs). Sequencing of these two ORFs of d27-ZF802 revealed a 4-bp deletion at the fourth exon of a putative gene, ORF LOC_Os11g37650, and this deletion results in a frame shift and generates a premature translation termination product (see Supplemental Figure 2 online).
Figure 2.
Map-Based Cloning of D27.
(A) The D27 locus was mapped on the long arm of chromosome 11 between markers C189 and RM206.
(B) A BAC contig spanning the D27 locus. The numerals indicate the number of recombinants identified from 5300 F2 mutant plants. BAC1, AC137588; BAC2, AC104847; BAC3, AC136148; BAC4, AC146334.
(C) Fine-mapping of D27 with markers developed based on the AC136148 sequence. The D27 gene was narrowed to an 18-kb genomic DNA region between CAPS markers P3 and P6 and cosegregated with marker P5. LOC_Os11g37650 is the candidate for D27.
(D) The complementation plasmid containing the entire D27 (pD27C).
(E) Phenotypic comparison among Nipponbare, d27-Nipponbare, and the transgenic line harboring pD27C. Bar = 5 cm.
The identity of D27 was further confirmed by a genetic complementation test. The plasmid pD27C, containing a 9.25-kb genomic DNA fragment consisting of a 2236-bp upstream sequence, the entire D27 gene including seven exons and six introns, and a 2044-bp downstream region (Figure 2D), was introduced into a d27-Nipponbare mutant. All four transgenic lines of pD27C complement the d27 phenotype (Figure 2E). Therefore, ORF LOC_Os11g37650 is the rice D27 gene, and its 4-bp deletion is responsible for the altered phenotype of d27.
D27 Encodes a Novel Iron-Containing Protein
Sequence analysis of 5′- and 3′-rapid amplification of cDNA ends (RACE) products indicated that the full length of D27 cDNA is 1254-bp long, with an ORF of 837 bp, a 217-bp 5′-untranslated region, and a 200-bp 3′-untranslated region (see Supplemental Figure 2 online). Sequence comparison between genomic DNA and cDNAs revealed that D27 is composed of seven exons that encodes a 278–amino acid polypeptide (Figure 2C; see Supplemental Figure 2 online). The 4-bp deletion in d27 results in a premature translational product (Figure 3A; see Supplemental Figure 2 online). The BLASTP (Altschul et al., 1997) analysis revealed that D27 shares no homology with any functionally identified protein and contains no conserved domain. However, analysis of multiple alignment against the National Center for Biotechnology Information database and The Institute for Genomic Research (TIGR) plant transcript assemblies showed that D27 has homologies in many plant species, from lower plants to higher plants (see Supplemental Figure 3 online), suggesting that D27 may play a basic role in plants.
Figure 3.
Characterization of the D27 Protein.
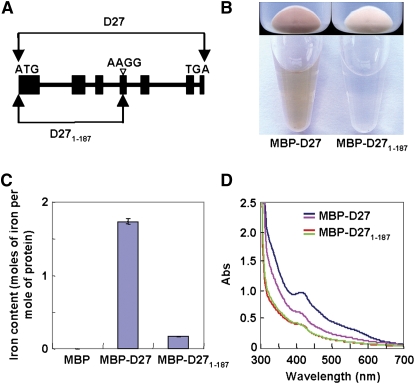
(A) Schematic representation of D27 and D271-187 (premature D27).
(B) Escherichia coli strains (top panel) expressing MBP-D27 and MBP-D271-187 and purified MBP-D27 and MBP-D271-187 proteins (bottom panel), showing the brown color of D27 proteins.
(C) Iron contents bound to MBP, MBP-D27, and MBP- D271-187. Values are means ± se of three independent experiments.
(D) UV visible absorbance spectra of purified MBP-D27 and MBP-D271-187 proteins before (blue and red) and after (purple and green) reduction with dithionite.
Interestingly, when we tried to express and purify recombinant D27, we found that the bacterial cells expressing the maltose binding protein (MBP)-D27 fusion protein were strikingly brown in color, as was the purified MBP-D27 fusion protein (Figure 3B). This result suggested that D27 is very likely to have a cofactor. To explore this possibility, we analyzed the recombinant D27 with inductively coupled plasma mass spectrometry (ICP-MS) and found that the recombinant MBP-D27 protein contains ∼1.7 mole of iron per mole of protein, in contrast with an extremely low level of iron bound to the C-terminal truncated polypeptide, MBP-D271-187 (Figure 3C, Table 1), which is equivalent to the mutated form of D27. The binding of iron to D27 was further confirmed by characterizing the absorbtion spectrum of the MBP-D27 fusion protein, which showed a specific peak at 420 nm, a characteristic for the presence of iron (Figure 3D). Furthermore, when the recombinant MBP-D27 protein was treated with the reducing agent dithionite, the peak at 420 nm exhibited a dramatic decrease (Figure 3D), indicating that D27 is indeed an iron-containing protein. Moreover, the purified recombinant D27 protein contained no significant amount of other metals (Table 1), suggesting that the binding of iron to D27 is specific. Taken together, all these results indicate that D27 is an authentic iron-containing protein in plants.
Table 1.
Metal Contents of MBP-D27 Protein Determined by ICP-MS Analysis
| Moles of Metal per Mole of Protein
|
|||
|---|---|---|---|
| Metal | MBP | MBP-D27 | MBP-D271-187 |
| Iron | 0.01 ± 0.01 | 1.74 ± 0.04 | 0.17 ± 0.01 |
| Calcium | 0.55 ± 0.03 | 0.46 ± 0.01 | 0.49 ± 0.01 |
| Chromium | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Copper | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Nickel | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Magnesium | <0.001 | <0.001 | <0.001 |
| Manganese | <0.001 | <0.001 | <0.001 |
| Zinc | <0.001 | <0.001 | <0.001 |
Values are means ± se of three independent experiments.
Expression Patterns of D27 and Subcellular Localization of the D27 Protein
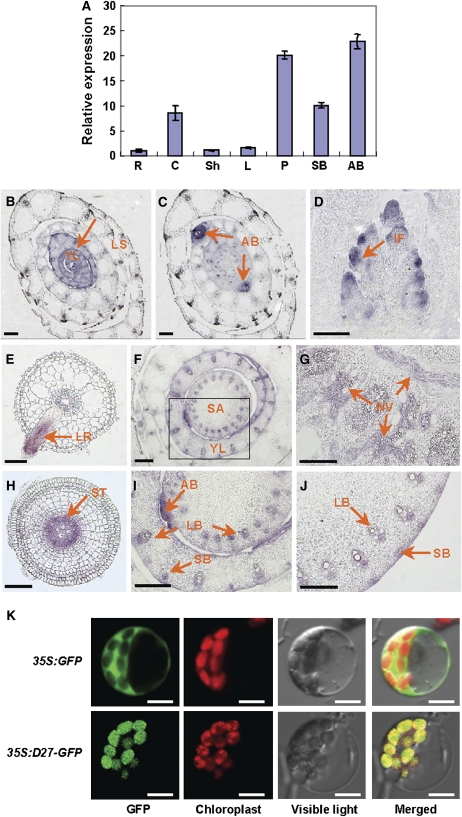
Real-time PCR analysis revealed that the D27 expression level is high in axillary buds and young panicles, medium in shoot bases and culms, and low in roots, sheaths, and leaves (Figure 4A). The tissue-specific expression pattern of D27 was further examined using mRNA in situ hybridization. D27 was predominantly expressed in young leaves (Figure 4B), axillary buds (Figure 4C), inflorescence promodia (Figure 4D), lateral roots (Figure 4E), and crown roots (Figure 4H). Furthermore, D27 expression was detected in vascular cells at the shoot apex of the main stem and young leaves (Figures 4F and 4I), in the nodal vascular anastomosis (Figure 4G), and in large and small vascular bundles of the internodes (Figure 4J).
Figure 4.
Expression Patterns of D27 and Subcellular Localization of the D27 Protein.
(A) D27 expression levels revealed by real-time PCR in various organs, including roots (R), culms (C), sheaths (Sh), leaves (L), panicles (P), shoot bases (SB), and axillary buds (AB). Values are means ± se of three independent experiments.
(B) to (J) D27 expression patterns revealed by mRNA in situ hybridization. The cross sections of the vegetative shoot apexes show the expression of D27 in young leaves (B) and axillary buds (C). The longitudinal section of an inflorescence meristem at the secondary branch differentiation stage shows the expression of D27 in the inflorescence promodia (D). The cross sections of roots show the D27 expression in lateral roots (E) and the steles of crown roots (H). The cross sections of the unelongated stem internodes ([F] and [I]), nodes (G), and internodes (J) of elongated culms indicate the expression of D27 in vasculature tissues. (I) is the magnification image of the squared region in (F). Arrows indicate the expression sites of D27. YL, young leaf; LS, leaf shealth; AB, axillary bud; IF, inflorescence; SA, shoot apex; NV, nodal vascular anastomosis; LR, lateral root; LB, large vascular bundle; SB, small vascular bundle; ST, stele. Bars = 100 μm in (B) to (E) and (H) and 200 μm in (F), (G), (I), and (J).
(K) Subcellular localization of 35S:GFP (top panel) and 35S:D27-GFP (bottom panel) in rice protoplast cells. Bars = 5 μm.
To determine the subcellular localization of the D27 protein, we performed a transient expression experiment of D27 in rice leaf protoplasts. The C terminus of D27 was fused with green fluorescent protein (GFP) under the control of cauliflower mosaic virus (CaMV) 35S promoter, and the construct was transferred into rice leaf protoplasts by the polyethylene glycol–mediated method. In contrast with the control, which was ubiquitous in protoplast cells, the D27-GFP fusion protein was predominantly localized in chloroplasts (Figure 4K).
Enhanced Polar Auxin Transport in d27
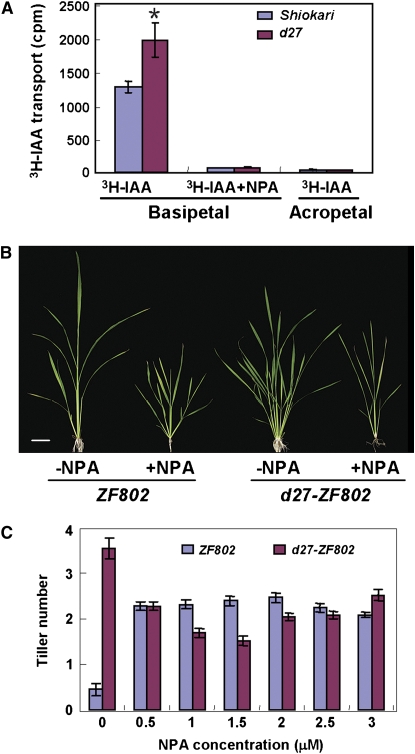
Shoot branching has been reported to be correlated to polar auxin transport (PAT) in Arabidopsis and pea (Morris, 1977; Beveridge et al., 2000; Bennett et al., 2006; Dai et al., 2006). We therefore investigated whether D27 is involved in PAT in rice. By comparing the basipetal and acropetal IAA transport in uppermost internodes between the wild-type and d27 plants, we found that basipetal PAT in d27 was significantly elevated, whereas acropetal PAT of 3H-IAA and basipetal transport of 3H-IAA treated with the PAT inhibitor N-1-naphthylphtalamic acid (NPA) showed no significant difference between the wild-type and mutant plants (Figure 5A).
Figure 5.
Comparison of PAT and Tillering Responses upon NPA Treatments between Wild-Type and d27 Plants.
(A) Comparison of PAT between the wild type (Shiokari) and d27 in the uppermost internodes. The acropetal auxin transport measurement is used as a negative control. Values are means ± se of three independent experiments. The asterisk represents significance difference between the wild type (Shiokari) and d27 determined by the Student's test at P < 0.05.
(B) The phenotypic comparison between wild-type (ZF802) and d27 plants upon 5-week 1.5 μM NPA treatment. Bar = 5 cm.
(C) Comparison of tillering upon 5-week NPA treatment between wild-type (ZF802) and d27 seedlings. Each value represents the mean ± se of 15 seedlings.
To investigate whether the increased auxin transport is related to the d27 mutant phenotype, we further examined the effect of NPA on d27 seedlings in hydroponic culture. Two-week-old seedlings were treated with various concentrations of NPA. As shown in Figures 5B and 5C, the tiller number of d27 mutant plants was largely rescued when grown in the presence of 1.5 μM NPA for 5 weeks. Furthermore, when 2-week-old wild-type and d27 seedlings were treated with as low as 0.5 μM NPA, the outgrowth of tiller buds of the wild type was significantly promoted, which is consistent with the long-established concept that too little auxin transport also leads to an increased shoot branching (Chatfield et al., 2000). By contrast, the treatment of NPA showed a remarkable inhibition to the tiller outgrowth of the d27 seedlings, suggesting that the tillering phenotype of d27 may be correlated with an enhanced PAT.
D27 May Function through the MAX/RMS/D Pathway
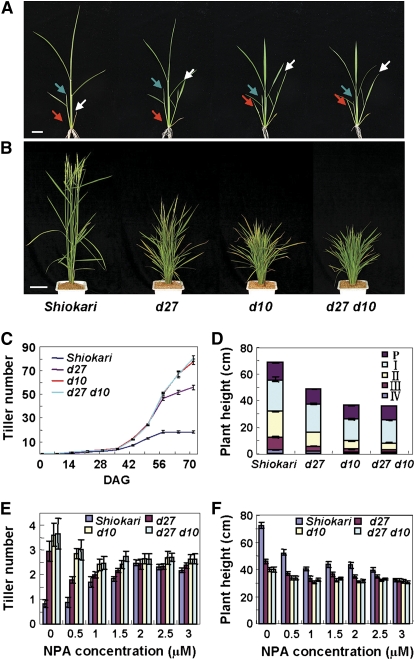
In rice, one class of tillering dwarf mutants with an increased tiller number and reduced plant height has been previously reported (Kinoshita and Takahashi, 1991). Among them, d3, d17/htd1, and d10 are found to function as their orthologs of MAX2/RMS4, MAX3/RMS5, and MAX4/RMS1/DAD1 in Arabidopsis, pea, and Petunia, respectively (Stirnberg et al., 2002; Sorefan et al., 2003; Booker et al., 2004; Foo et al., 2005; Ishikawa et al., 2005; Snowden et al., 2005; Johnson et al., 2006; Zou et al., 2006; Arite et al., 2007). The phenotype of d27 and the involvement of D27 in PAT prompted us to test whether D27 is a new member of the MAX/RMS/D pathway in rice. We therefore generated a d27 d10 double mutant and compared the phenotypes of single and double mutants of d27, d10, and d27 d10. As shown in Figure 6, d10 exhibits similar phenotype to d27, but has more tillers and a more severe dwarf stature than d27 (Figures 6A and 6B). Phenotypic analysis revealed that the d27 d10 double mutant showed similar tiller number and plant height to d10 (Figures 6A to 6D). Further investigation on the responses of d27, d10, and d27 d10 to the treatment of different NPA concentrations indicated that the d27 d10 double mutant has a similar response to d10 (Figures 6E and 6F). These results strongly suggested that D27 participates in the MAX/RMS/D pathway.
Figure 6.
Phenotypic Analyses of the d27 d10 Double Mutant.
(A) and (B) Phenotypes of wild-type, d27, d10, and d27 d10 at the seeding stage (A) and at the heading stage (B). Red, white, and blue arrows indicate first, second, and third tillers, respectively. Bars = 2 cm in (A) and 10 cm in (B).
(C) Kinetic tillering analyses of d27, d10, and d27 d10 plants at different developmental stages. DAG, days after germination. Each value represents the mean ± se of 15 seedlings.
(D) Dwarf phenotype of d27, d10, and d27 d10 plants. Internode length was measured after harvest. P, panicle; I to IV, nodes numbered from top to bottom. Each value represents the mean ± se of 15 seedlings.
(E) and (F) Comparison of tiller number (E) and plant height (F) of 13-week-old plants in response to NPA treatment at various concentrations. Each value represents the mean ± se of 15 seedlings.
D27 Participates in the Biosynthesis of Strigolactones
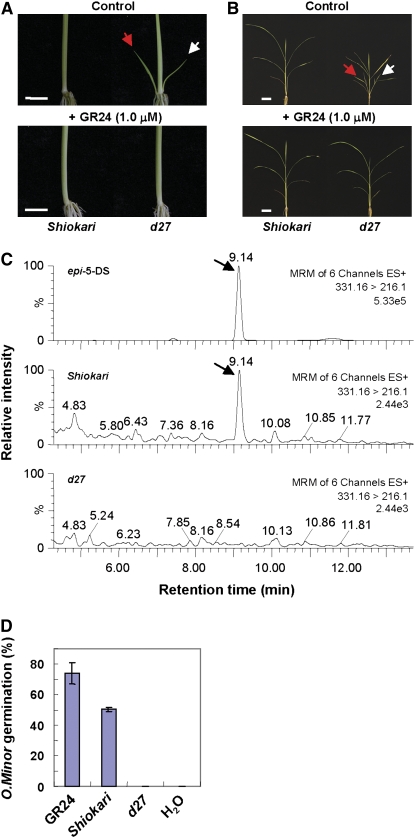
Recent studies have shown that the proposed novel hormones that inhibit plant branching and are derived from the MAX/RMS/D pathway are strigolactones or their downstream metabolites. MAX1, MAX3/RMS5/D17, and MAX4/RMS1/D10 are involved in the biosynthesis of strigolactones, while MAX2/RMS4/D3 is involved in strigolactone signaling (Gomez-Roldan et al., 2008; Umehara et al., 2008). To further understand the role of D27 in the MAX/RMS/D pathway in rice, we investigated whether d27 is deficient in strigolactone production or signaling. By applying 1.0 μM GR24, a synthetic strigolactone analog that acts as native strigolactones, to wild-type and d27 seedlings in a hydroponic culture, we found that the exogenous supplement of GR24 was able to fully inhibit tiller bud outgrowth of 3-week-old d27 seedlings (Figure 7A), and continuous treatment for 7 weeks fully restored the tillering dwarf phenotype of d27 (Figure 7B).
Figure 7.
Analysis of Strigolactones in Wild-type and d27 Plants.
(A) Three-week-old wild-type (Shiokari) and d27 seedlings treated with (bottom panel) or without (top panel) 1.0 μM GR24. Red and white arrows indicate first and second tillers, respectively. Bars = 1.0 cm.
(B) Seven-week-old wild-type and d27 seedlings treated with (bottom panel) or without 1.0 μM (top panel) GR24. Red and white arrows indicate first and second tillers, respectively. Bars = 5.0 cm.
(C) LC-MS/MS chromatograms for the standard epi-5DS (top trace) and root exudates from wild-type (middle trace) and d27 (bottom trace) seedlings. Arrows indicate the detection of epi-5DS.
(D) Germination rate of O. minor seeds 5 d after treatments with water, GR24, or extracts of wild-type or d27 root exudates. Each value represents the average of three replicates ± se.
We further analyzed and compared the strigolactones produced in the root exudates of wild-type and d27 seedlings by liquid chromatography–quadruple/time-of-flight tandem mass spectrometry (LC/MS-MS). Our results clearly showed that 2′-epi-5-deoxystrigol (epi-5DS), an identified strigolactone in the hydroponic culture media of rice seedlings, was produced in the wild-type cultivar Shiokari root exudates but was undetectable in d27 (Figure 7C). Moreover, we performed a highly sensitive germination assay using Orobanche minor seeds to estimate the strigolactone production in d27 root exudates. In agreement with LC/MS-MS data, the germination-stimulating activity of d27 root exudates dramatically decreased in comparison with the wild type (Figure 7D; see Supplemental Figure 4 online). These results indicate that D27 is required for the production of strigolactones in rice.
DISCUSSION
In higher plants, the degree and pattern of branching are major determinants of plant architecture. Although there has been significant progress in the characterization of branching mutants and the understanding of corresponding regulatory genes has been achieved recently, the molecular mechanisms underlying plant branching remain to be elucidated. A rice tiller is a specialized grain-bearing branch that grows independently of the main culm by its own adventitious roots. Tillering in rice is not only an important agronomic trait for grain yield, but also an ideal system for studying branching in higher plants, especially for monocotyledonous species. In this article, we reported the cloning of D27 and functional characterization of its mechanism underlying the axillary tiller development, showing that D27 suppresses the outgrowth of tiller buds by producing strigolactones.
D27 Is a Novel Iron binding Protein That Localizes in Chloroplasts
Although the bioinformatic analysis shows that D27 encodes a novel protein with no homology to any functionally known proteins, D27 homologs are found from algae (cyanophyta) to higher plants, but not in animals or fungi (see Supplemental Figure 3 online), suggesting that D27 may be a plant-specific protein. Analysis of the transient expression in rice protoplasts demonstrates that the D27 protein is localized in chloroplasts, similar to MAX3 and MAX4/D10 (Booker et al., 2004; Auldridge et al., 2006; Arite et al., 2007).
Our data also showed that D27 is an iron-containing protein and that the truncated D27 loses the ability to bind iron (Figure 3C). Bacterial cells expressing truncated MBP-D271-187 are colorless, and the purified protein does not have a 420-nm peak. By contrast, the full-length MBP-D27 fusion protein is brown and has the characteristic absorbance at 420 nm (Figures 3B and 3D), suggesting that the C terminus of D27 may contain an iron binding domain.
D27 Suppresses the Outgrowth of Rice Tiller Buds through the MAX Pathway
The development of shoot branching occurs in two steps, the initiation of the AM and the outgrowth of axillary buds. Unlike the elusive molecular mechanism that regulates AM initiation, the outgrowth of axillary buds is well understood due to the recent breakthrough in the MAX/RMS/D pathway. Studies on a number of mutants that display excess axillary branches, max in Arabidopsis (Stirnberg et al., 2002, 2007; Sorefan et al., 2003; Booker et al., 2004), rms in pea (Beveridge et al., 1994, 1996, 2000; Foo et al., 2001, 2005; Morris et al., 2001; Sorefan et al., 2003), and dad in petunia (Napoli, 1996; Snowden et al., 2005; Simons et al., 2007), have revealed the existence of a carotenoid-derived AM outgrowth regulating pathway. Although the outgrowth behaviors between dicotyledonous and monocotyledonous axillary buds are different (for reviews, see McSteen and Leyser, 2005; Wang and Li, 2008), they appear to share a conserved branching signal pathway because orthologs of MAX2/RMS4, MAX3/RMS5, and MAX4/RMS1 have also been identified in rice; they are D3, HTD/D17, and D10, respectively (Ishikawa et al., 2005; Zou et al., 2006; Arite et al., 2007). Rice plants harboring individual loss-of-function mutations in these genes lead to more tillers and reduced plant height, a similar phenotype to those in Arabidopsis and pea, indicating their conserved functions in suppressing branch development in monocotyledonous plants.
The more tillers phenotype of d27 is ascribed to the extensive outgrowth of tiller buds, especially to the higher-order tiller buds, which are dormant in the wild-type plants (Figures 1A to 1F). The comparable morphology of d27 to that of the rice tillering dwarf mutant d3, htd1/d17, or d10 prompted us to test the hypothesis that D27 is also involved in the MAX/RMS/D pathway. The analysis of the double mutant d27 d10 confirms the hypothesis. In the phenotypes tested, including tillering behavior, plant height, and response to NPA treatment, d27 d10 resembles d10 (Figure 6), suggesting that D27 may function the same as D10 in the MAX/RMS/D pathway. In agreement with this, D27 is expressed in roots and shoots, especially in the vasculature tissue of the plants (Figures 4B to 4J), an expression pattern similar to those of D10 and HTD1/D17. These results are consistent with a role in the biosynthesis of strigolactones. Further determination of strigolactone-related products in d27 and d10 will facilitate the understanding of the genetic relationship between D27 and D10.
D27 Is Required for the Biosynthesis of Strigolactones
The MAX/RMS/D pathway has been proven to interact with classic plant hormones auxin and cytokinin, but all the evidence obtained so far has demonstrated that the MAX/RMS/D -dependent branching signals are not attributed to any known hormones. Recent studies uncover the role of strigolactones or their metabolites acting as a new class of branching hormones, the signal derived from the MAX/RMS/D pathway (Gomez-Roldan et al., 2008; Umehara et al., 2008). Although previous studies have shown that strigolactones are derived from the carotenoid pathway and function as important signals in establishing the interaction between plants and mycorrhizal fungi (Akiyama et al., 2005; Matusova et al., 2005), the biosynthetic and signaling pathways of strigolactones are poorly understood. MAX1, MAX3/RMS5/D17, and MAX4/RMS1/D10 are essential components for the biosynthesis of strigolactones, whereas MAX2/RMS4/D3 is involved in the perception of the signal. Our studies provide direct evidence that D27 is a new component of the MAX/RMS/D pathway and plays an essential role in biosynthesizing strigolactones. First, the d27 phenotype can be restored to the wild type upon supplemention with GR24 (Figures 7A and 7B). Second, the d27 root extract contains undetectable strigolactone, which is normally produced in the wild-type root extract (Figure 7C). Third, unlike the wild type, the d27 root exudates failed to stimulate the seed germination of O. minor (Figure 7D; see Supplemental Figure 4 online). Based on the findings that the D27 protein is localized in chloroplasts and contains iron and the fact that the complex structure of strigolactones should undergo a number of enzymatic reactions, including hydroxylation, epoxydation, oxidation, etc., to achieve its biosynthesis (Matusova et al., 2005), we hypothesize that D27 may participate in a redox reaction involved in the biosynthesis of strigolactones. Further biochemical experiments are required to confirm this possibility in the future.
Roles of Strigolactones and Auxin in Regulating Shoot Branching
The discovery of strigolactones as a product of the MAX/RMS/D pathway provides an opportunity to elucidate mechanisms of shoot branching in higher plants. Currently, how strigolactones and auxin interact to regulate shoot branching is still vague, and two models have been proposed based on different experimental systems. One is the PAT hypothesis, which proposes that the MAX/RMS/D pathway acts by regulating PIN-dependent auxin transport in the stem, which inhibits auxin transport from buds (Bennett et al., 2006; Ongaro and Leyser, 2008; Leyser, 2009). The other proposes that strigolactones function as secondary messengers of auxin that repress the bud outgrowth directly (Beveridge et al., 2000; Brewer et al., 2009; Ferguson and Beveridge, 2009). Our work on the rice tillering mutant d27 has revealed that the mutation in D27 leads to a deficiency in strigolactone biosynthesis and an increase in PAT, which are consistent with previous studies on Arabidopsis and pea (Beveridge et al., 2000; Bennett et al., 2006). However, it is still unclear whether the enhanced PAT is a direct consequence or a feedback effect of the deficiency in strigolactones. Moreover, it should be pointed out that, in contrast with the complete rescue of the mutant phenotypes by the treatment with GR24 (Figure 7), the dwarf phenotype of d27 is completely recovered to the wild type by treatment with NPA (Figures 5B and 5C). These results suggest that the action of strigolactones or auxin may not be in a simple linear pathway. A full elucidation of the actions of strigolactones and their interactions with auxin and other branching signals awaits the identification of more novel MAX/RMS/D-dependent branching mutants and corresponding inhibiting signals.
METHODS
Plant Materials
The d27 mutant is in a Shiokari background. In this study, we also generated d27-ZF802 and d27-Nipponbare mutants by backcrossing the d27 mutant plants with indica cultivar ZF802 and japonica variety Nipponbare. The d10 mutant also has a Shiokari background. The d27 and d10 mutants were provided by Takamure Itsuro of Hokkaido University. Rice (Orzya sativa) plants were cultivated in the experimental field at the Institute of Genetics and Developmental Biology in Beijing in the natural growing seasons. For NPA (at indicated concentrations) and 1 μM GR24 treatment, germinated seeds were grown on a nylon net floating on hydroponic solution in the greenhouse.
Mapping of D27
To map the D27 locus, the d27-ZF802 mutant was crossed to the wild type (ZF802), and the genomic DNA from 5200 F2 progeny with the mutant phenotype was extracted with a modified CTAB method described previously (Mou et al., 2000). To fine-map D27, the CAPS markers were generated based on single nucleotide polymorphisms identified in the sequence. The molecular lesion of d27-ZF802 was identified by PCR amplification of the D27 genomic region from the wild-type and d27-ZF802 mutant plants and comparison of their sequences using ClustalW within Lasergene version 5.0 software (DNASTAR). The primer sequences are listed in Supplemental Table 1 online.
Complementation of d27
The BAC clone OSJNBa0029K08 was digested with BamHI and KpnI to generate a 9.25-kb genomic DNA fragment. The DNA fragment was ligated to the BamHI and KpnI digested pCAMBIA1300 vector (CAMBIA), forming pD27C, which contains a 2236-bp upstream sequence, the entire D27 gene, and a 2044-bp downstream region. The pD27C plasmid was introduced into Agrobacterium tumefaciens EHA105 by electroporation, and the rice d27-Nipponbare mutant was transformed according to a published method (Hiei et al., 1994). The phenotype was scored in T1 transformants and T2 progeny.
RT-PCR, RACE-PCR, and Real-Time PCR Analyses
Total RNA was prepared using a TRIzol kit according to the user manual (Invitrogen). One microgram of total RNA was treated with DNase I and used for cDNA synthesis with an RT kit (Promega). The 5′- or 3′-RACE of D27 was performed using a SMART RACE cDNA amplification kit according to the manufacturer's instructions (Clontech). Real-time PCR experiments were performed using gene-specific primers in a total volume of 10 μL with 1 μL of the RT reactions, 1 μM gene-specific primers D27EF and D27ER, and 5 μL SYBR Green Master mix (Applied Biosystems) on an ABI 7900 real-time PCR machine (Applied Biosystems) according to the manufacturer's instructions. The rice Ubiquitin gene was used as the internal control. The relative expression levels of D27 in various organs were compared with that in the root, after normalization with Ubiquitin transcript and averaged from three biological replicates. The primer sequences used for the above studies are listed in Supplemental Table 2 online.
Histological Analysis and mRNA in Situ Hybridization
Tissues of rice were fixed with 4% (w/v) paraformaldehyde at 4°C overnight, followed by a series of dehydration and infiltration, and embedded in paraffin (Paraplast Plus; Sigma-Aldrich). The tissues were sliced into 8- to 10-μm sections with a microtome (Leica RM2145), affixed to microscope slides, and stained with Safranin O and Fast Green (Fisher) according to Ruzin (1999). Sections were observed under bright field through a microscope (Leica DMR) and photographed using a Micro Color CCD camera (Apogee Instruments).
To investigate the morphology of the leaf blade epidermal cells, samples were cleared in benzyl-benzoate-four-and-half fluid as previously described (Herr, 1982).
RNA in situ hybridization was performed as described previously (Li et al., 2007) with minor modification. Briefly, the 14- to 760-bp region of the D27 gene was amplified by gene-specific primers D27IF and D27IR with BamHI and KpnI adaptors (see Supplemental Table 2 online) and subcloned into the BamHI- and KpnI-digested pBluescript II SK+ vector (Stratagene). The construct was used as the template to generate sense and antisense RNA probes. Digoxigenin-labeled RNA probes were prepared using a DIG Northern Starter Kit (Roche) according to the manufacturer's instructions. Slides were observed under bright field through a microscope (Leica DMR) and photographed with a Micro Color CCD camera (Apogee Instruments).
Subcellular Localization of D27
To generate CaMV35S-GFP, a HindIII-NotI fragment containing GFP was amplified by primers GFPF and GFPR (see Supplemental Table 2 online) using CaMV35SΩ-sGFP (S65T)-NOS-3′ cassette vector (Niwa et al., 1999). The resultant fragment was cloned into the pET28a (Novagen) vector to generate pET28a-GFP. The β-glucuronidase fragment of the pBI221 vector (Clontech) was replaced by the BamHI-NotI fragment of pET28a-GFP to generate the CaMV35S-GFP construct. A BamHI-HindIII fragment containing the coding region of D27 amplified by the primers D27F and D27R (see Supplemental Table 2 online) was subcloned into the BamHI and HindIII sites of CaMV35S-GFP to generate CaMV35S:D27-GFP. The plasmids CaMV35S-GFP and CaMV35S:D27-GFP were introduced into rice leaf protoplasts as described (Bart et al., 2006). After overnight incubation in the dark, the GFP signal and chlorophyll autofluorescence were examined under a confocal microscope at excitation wavelengths of 488 and 647 nm, respectively (FluoView 1000; Olympus).
PAT Assay
The PAT was assayed according to the method described previously with some minor modifications (Okada et al., 1991; Li et al., 2007). Briefly, the apical or basal ends of the 20-mm excised segments from the uppermost internode at the early heading stage (for basipetal or acropetal transport assays, respectively) were incubated in 10 μL of half-strength Murashige and Skoog liquid medium containing 0.35% phytogel and 0.1 μM 3H-labeled IAA (American Radiolabeled Chemicals) in 1.5-mL Eppendorf tubes in the dark at room temperature. NPA (10 μM) was added to the medium as indicated to block IAA active transport so that the IAA diffusion levels could be compared between wild-type and mutant plants. After a 3-h incubation, 5-mm sections from the nonsubmerged ends of segments were excised and transferred into Eppendorf tubes containing 2 mL of scintillation liquid. After an 18-h incubation in 2 mL of scintillation liquid, the radioactivity of each tube was counted by a liquid scintillation counter (1450 MicroBeta TriLux; Perkin-Elmer).
Expression and Purification of MBP-D27 Fusion Proteins in Escherichia coli
The D27 cDNAs corresponding to full length and amino acids 1 to 187 were each amplified by the primer sets listed in Supplemental Table 2 online and cloned into the EcoRI and BamHI sites of the E. coli expression vector pMAL-c2 (New England Biolabs). Expression of MBP, MBP-D27, and MBP-D271-187 in BL21 Rosetta cells (Stratagene) was induced with 0.1 mM isopropyl-1-thio-d-galactopyranoside at 16°C for 18 h. Fusion proteins were purified using amylose-affinity chromatography (New England Biolabs) according to the manufacturer's protocols and quantified by the Bio-Rad protein assay reagent.
Metal Quantitation
The purified recombinant MBP, MBP-D27, and MBP-D271-187 proteins were digested with 40% nitric acid on a heating block, after cooling the metal contents of the digests were determined using a Thermo ICP-MS XII. The optical spectra of recombinant D27-MBP and MBP-D271-187 (∼10 to 20 mg/mL protein in 50 mM Tris, pH 8.0, and 50 mM NaCl) were measured from the near UV to the near IR (200 to 800 nm) on a Beckman Coulter DU800. Chemical reduction of D27-MBP and MBP-D271-187 proteins was achieved by adding 2 mM dithionite to the protein solution.
LC/MS-MS Analysis of epi-5DS and Germination Assay of Orobanche minor
The strigolactone epi-5DS measurement and the O. minor germination assay were performed according to the method described by Yoneyama et al. (2008). The hydroponic culture media were collected and extracted twice with ethyl acetate. The ethyl acetate phase was washed with 0.2 M K2HPO4, dried over anhydrous Na2SO4, and concentrated in vacuo. The extracts were dissolved in 50% (v/v) acetonitrile and were subjected to LC/MS-MS analysis using a system consisting of a triple quadruple tandem mass spectrometer (Quattro Premier XE; Waters MS Technologies) and an Acquity Ultra Performance Liquid Chromatograph (Acquity UPLC; Waters) equipped with a reverse phase column (BEH-C18, 2.1 × 50 mm, 1.7 μm; Waters). The mobile phase was changed from 30% (v/v) acetonitrile to 40% and 70% (v/v) linearly in 6 and 15 min after the injection, respectively, at a flow rate of 0.4 mL min−1. The column temperature was set to 25°C. MS parameters were set to the following values: desolvation gas flow 800 L·h−1, capillary voltage 3800 V, cone voltage 30 V, desolvation temperature 350°C, source temperature 120°C, collision energy 15 V, using MRM 331.16 > 216.10 transition for the epi-5DS detection, and 5 pg/μL epi-5DS in 50% (v/v) acetonitrile was used as reference for the qualification of epi-5DS in the root exudate sample.
To obtain strigolactone-containing exudates for germination assays, the 1.5 liters of hydroponic culture media were concentrated using Oasis HLB columns (Waters) and eluted with 5 mL acetone. The exudates for each bioassay were prepared by mixing 100 μL of the concentrated eluates in acetone and 900 μL of water and evaporating the acetone in a vacuum centrifuge. Deionized water and GR24 were used as negative and positive controls, respectively.
Accession Numbers
The GenBank accession number for the rice Dwarf27 sequence reported in this article is FJ641055. Sequence data from this article can be found in the GenBank database and TIGR plant transcript assemblies database (boldfaced) under the following accession numbers or plant TA identifier. GenBank identification numbers and TIGR numbers are as follows: Acaryochloris marina (Am): YP_001515237.1; Arabidopsis thaliana (At): NP_680560.1; NP_564838.1; NP_973748.1; NP_563673.1; TA47796_3702; Chlamydomonas reinhardtii (Cr): XP_001702558.1; XP_001697941.1; Fragaria vesca (Fv): DY667171; Glycine max (Gm): BI470614; Lactuca perennis (Lp): DW093521; Manihot esculenta (Me): TA7061_3983; Medicago truncatula (Mt): TA29020_3880; Ostreococcus lucimarinus (Ol): XP_001420448.1; XP_001421321.1; XP_001419261.1; XP_001420823.1; Oryza sativa (Os): NP_001060847.1 (Os08g0114100); NP_001054553.1 (Os05g0131100); EAZ41303.1 (OsJ_25811); D27, ABA94460.1; EEC68482.1; EAY81567.1 (OsI_36731); EEC78459.1 (OsI_18326); Ostreococcus tauri (Ot): CAL57302.1; CAL55718.1; CAL57640.1; CAL54767.1; Physcomitrella patens (Pp): XP_001755220.1; XP_001752784.1; XP_001763276.1; XP 001,763,362.1; XP_001767010.1; Picea sitchensis (Ps): ABK22858.1; ABK23534.1; Phaeodactylum tricornutum (Pt): EEC48282.1; EEC51126.1; Selaginella moellendorffii (Sm): DN838054; Synechococcus sp PCC 7335 (Sy): YP_002711663.1; Triticum aestivum (Ta): TA111626_4565; Thermosynechococcus elongates (Te): NP_682732.1; Taraxacum officinale (To): DY820710; TA1119_50225; Triphysaria versicolor (Tv): DR172918; TA4072_64093; Vitis vinifera (Vv): CAO40130.1; CAO62908.1; CAO22611.1; Zea mays (Zm): NP_001144840.1; ACG26781.1; ACG28622.1; Zingiber officinale (Zo): TA7516_94328.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Comparison of Tiller Bud Formation between the Wild Type and d27.
Supplemental Figure 2. D27 cDNA Sequence and Its Deduced Amino Acid Sequence.
Supplemental Figure 3. Multiple Sequence Alignment of the Deduced Amino Acid Sequence of D27 with Its Homologs.
Supplemental Figure 4. Germination of O. minor Seeds 5 d after Treatment with Root Exudate Extracts of Shiokari, d27, d10, Water (Negative Control), or GR24 (Positive Control).
Supplemental Table 1. List of PCR-Based Molecular Markers Developed in This Study.
Supplemental Table 2. Primer Sequences Used for D27 Analyses.
Supplementary Material
Acknowledgments
We thank Zhijie Liu and Neil Shaw (Institute of Biophysics, Chinese Academy of Sciences) for advice on protein purification, Jindong Zhao (Institute of Hydrobiology, Chinese Academy of Sciences, and Peking University) for suggestions on protein analysis, Takamure Itsuro (Hokkaido University) for providing d27 and d10 mutants, and Dun Li (Stony Brook University) for the improvement of the English language. We also thank Koichi Yoneyama and Xiaonan Xie (Utsunomiya University) for sharing information on strigolactone analysis and kindly providing GR24 and Kohki Akiyama (Osaka Prefecture University) for providing epi-5DS. This work was supported by grants from the Ministry of Science and Technology of China (2006AA10A101) and the National Natural Science Foundation of China (90817108 and 30830009).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Yonghong Wang (yhwang@genetics.ac.cn).
Online version contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Akiyama, K., Matsuzaki, K., and Hayashi, H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J.H., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arite, T., Iwata, H., Ohshima, K., Maekawa, M., Nakajima, M., Kojima, M., Sakakibara, H., and Kyozuka, J. (2007). DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 51 1019–1029. [DOI] [PubMed] [Google Scholar]
- Auldridge, M.E., Block, A., Vogel, J.T., Dabney-Smith, C., Mila, I., Bouzayen, M., Magallanes-Lundback, M., DellaPenna, D., McCarty, D.R., and Klee, H.J. (2006). Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 45 982–993. [DOI] [PubMed] [Google Scholar]
- Bart, R., Chern, M., Park, C.J., Bartley, L., and Ronald, P.C. (2006). A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, T., Sieberer, T., Willett, B., Booker, J., Luschnig, C., and Leyser, O. (2006). The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 16 553–563. [DOI] [PubMed] [Google Scholar]
- Beveridge, C.A., Ross, J.J., and Murfet, I.C. (1994). Branching mutant rms-2 in Pisum sativum (grafting studies and endogenous indole-3-acetic acid levels). Plant Physiol. 104 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge, C.A., Ross, J.J., and Murfet, I.C. (1996). Branching in pea (action of genes Rms3 and Rms4). Plant Physiol. 110 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge, C.A., Symono, G.M., Murfet, I.C., Ross, J.J., and Rameau, C. (1997). The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol. 115 1251–1258. [Google Scholar]
- Beveridge, C.A., Symons, G.M., and Turnbull, C.G. (2000). Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 123 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker, J., Auldridge, M., Wills, S., McCarty, D., Klee, H., and Leyser, O. (2004). MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 14 1232–1238. [DOI] [PubMed] [Google Scholar]
- Booker, J., Chatfield, S., and Leyser, O. (2003). Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker, J., Sieberer, T., Wright, W., Williamson, L., Willett, B., Stirnberg, P., Turnbull, C., Srinivasan, M., Goddard, P., and Leyser, O. (2005). MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 8 443–449. [DOI] [PubMed] [Google Scholar]
- Bouwmeester, H.J., Matusova, R., Zhongkui, S., and Beale, M.H. (2003). Secondary metabolite signalling in host-parasitic plant interactions. Curr. Opin. Plant Biol. 6 358–364. [DOI] [PubMed] [Google Scholar]
- Brewer, P.B., Dun, E.A., Ferguson, B.J., Rameau, C., and Beveridge, C.A. (2009). Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 150 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield, S.P., Stirnberg, P., Forde, B.G., and Leyser, O. (2000). The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 24 159–169. [DOI] [PubMed] [Google Scholar]
- Cline, M.G. (1991). Apical dominance. Bot. Rev. 57 318–358. [Google Scholar]
- Cook, C.E., Whichard, L.P., Wall, M.E., Egley, G.H., Coggon, P., Luhan, P.A., and McPhail, A.T. (1972). Germination stimulants. II. The structure of strigol-a potent seed germination stimulant for witchweed (Striga lutea Lour.). J. Am. Chem. Soc. 94 6198–6199. [Google Scholar]
- Dai, Y., Wang, H., Li, B., Huang, J., Liu, X., Zhou, Y., Mou, Z., and Li, J. (2006). Increased expression of MAP KINASE KINASE7 causes deficiency in polar auxin transport and leads to plant architectural abnormality in Arabidopsis. Plant Cell 18 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklof, S., Astot, C., Blackwell, J., Moritz, T., Olsson, O., and Sandberg, G. (1997). Auxin-cytokinin interactions in wild-type and transgenic tobacco. Plant Cell Physiol. 38 225–235. [Google Scholar]
- Eklof, S., Astot, C., Sitbon, F., Moritz, T., Olsson, O., and Sandberg, G. (2000). Transgenic tobacco plants co-expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin- and cytokinin-overproducing phenotypes. Plant J. 23 279–284. [DOI] [PubMed] [Google Scholar]
- Ferguson, B.J., and Beveridge, C.A. (2009). Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 149 1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo, E., Bullier, E., Goussot, M., Foucher, F., Rameau, C., and Beveridge, C.A. (2005). The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo, E., Turnbull, C.G., and Beveridge, C.A. (2001). Long-distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiol. 126 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan, V., et al. (2008). Strigolactone inhibition of shoot branching. Nature 455 189–194. [DOI] [PubMed] [Google Scholar]
- Herr, J.M. (1982). An analysis of methods for permanently mounting ovules cleared in four-and-a-half type clearing fluids. Stain Technol. 57 161–169. [DOI] [PubMed] [Google Scholar]
- Hiei, Y., Ohta, S., Komari, T., and Kumashiro, T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6 271–282. [DOI] [PubMed] [Google Scholar]
- Humphrey, A.J., and Beale, M.H. (2006). Strigol: Biogenesis and physiological activity. Phytochemistry 67 636–640. [DOI] [PubMed] [Google Scholar]
- Ishikawa, S., Maekawa, M., Arite, T., Onishi, K., Takamure, I., and Kyozuka, J. (2005). Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 46 79–86. [DOI] [PubMed] [Google Scholar]
- Johnson, X., Brcich, T., Dun, E.A., Goussot, M., Haurogne, K., Beveridge, C.A., and Rameau, C. (2006). Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 142 1014–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J.H., Yun, J., Seo, Y.H., and Park, C.M. (2005). Characterization of an Arabidopsis gene that mediates cytokinin signaling in shoot apical meristem development. Mol. Cells 19 342–349. [PubMed] [Google Scholar]
- Kapchina-Toteva, V.V., van Telgen, H.J., and Yakimova, E. (2000). Role of phenylurea cytokinin CPPU in apical dominance release in in vitro cultured Rosa hybrida L. J. Plant Growth Regul. 19 232–237. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., and Takahashi, M. (1991). The one hundredth report of genetic studies on rice plant. J. Fac. Agric. Hokkaido Univ. 65 1–61. [Google Scholar]
- Lazar, G., and Goodman, H.M. (2006). MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc. Natl. Acad. Sci. USA 103 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser, O. (2003). Regulation of shoot branching by auxin. Trends Plant Sci. 8 541–545. [DOI] [PubMed] [Google Scholar]
- Leyser, O. (January 2, 2009). The control of shoot branching: An example of plant information processing. Plant Cell Environ. http://dx.doi.org/10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed]
- Li, C., and Bangerth, F. (2003). Stimulatory effect of cytokinins and interaction with IAA on the release of lateral buds of pea plants from apical dominance. J. Plant Physiol. 160 1059–1063. [DOI] [PubMed] [Google Scholar]
- Li, P., Wang, Y., Qian, Q., Fu, Z., Wang, M., Zeng, D., Li, B., Wang, X., and Li, J. (2007). LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res. 17 402–410. [DOI] [PubMed] [Google Scholar]
- Ljung, K., Bhalerao, R.P., and Sandberg, G. (2001). Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 28 465–474. [DOI] [PubMed] [Google Scholar]
- Lopez-Raez, J.A., Charnikhova, T., Gomez-Roldan, V., Matusova, R., Kohlen, W., De Vos, R., Verstappen, F., Puech-Pages, V., Becard, G., Mulder, P., and Bouwmeester, H. (2008). Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 178 863–874. [DOI] [PubMed] [Google Scholar]
- Matusova, R., Rani, K., Verstappen, F.W., Franssen, M.C., Beale, M.H., and Bouwmeester, H.J. (2005). The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 139 920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen, P., and Leyser, O. (2005). Shoot branching. Annu. Rev. Plant Biol. 56 353–374. [DOI] [PubMed] [Google Scholar]
- Morris, D.A. (1977). Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.). Planta 136 91–96. [DOI] [PubMed] [Google Scholar]
- Morris, S.E., Turnbull, C.G., Murfet, I.C., and Beveridge, C.A. (2001). Mutational analysis of branching in pea. Evidence that Rms1 and Rms5 regulate the same novel signal. Plant Physiol. 126 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou, Z., He, Y., Dai, Y., Liu, X., and Li, J. (2000). Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli, C. (1996). Highly branched phenotype of the Petunia dad1-1 mutant is reversed by grafting. Plant Physiol. 111 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, Y., Hirano, T., Yoshimoto, K., Shimizu, M., and Kobayashi, H. (1999). Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 18 455–463. [DOI] [PubMed] [Google Scholar]
- Nordstrom, A., Tarkowski, P., Tarkowska, D., Norbaek, R., Astot, C., Dolezal, K., and Sandberg, G. (2004). Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 101 8039–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro, V., and Leyser, O. (2008). Hormonal control of shoot branching. J. Exp. Bot. 59 67–74. [DOI] [PubMed] [Google Scholar]
- Pilate, G., Sossountzov, L., and Miginiac, E. (1989). Hormone levels and apical dominance in the aquatic fern Marsilea drummondii A. Br. Plant Physiol. 90 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, T.K., Li, X., and Cline, M.G. (1993). Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea nil. Ann. Bot. (Lond.) 71 223–229. [Google Scholar]
- Ruzin, S.E. (1999). Plant Microtechnique and Microcopy. (New York: Oxford University Press).
- Sachs, T., and Thimann, K.V. (1964). Release of lateral buds from apical dominance. Nature 201 939–940. [Google Scholar]
- Shelagh, M.H., and John, R.H. (1975). Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. timing of bud growth following decapitation. Planta 123 137–143. [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato, S., and Mori, H. (2001). Control of outgrowth and dormancy in axillary buds. Plant Physiol. 127 1405–1413. [PMC free article] [PubMed] [Google Scholar]
- Sieberer, T., and Leyser, O. (2006). Plant science. Auxin transport, but in which direction? Science 312 858–860. [DOI] [PubMed] [Google Scholar]
- Simons, J.L., Napoli, C.A., Janssen, B.J., Plummer, K.M., and Snowden, K.C. (2007). Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol. 143 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden, K.C., Simkin, A.J., Janssen, B.J., Templeton, K.R., Loucas, H.M., Simons, J.L., Karunairetnam, S., Gleave, A.P., Clark, D.G., and Klee, H.J. (2005). The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan, K., Booker, J., Haurogne, K., Goussot, M., Bainbridge, K., Foo, E., Chatfield, S., Ward, S., Beveridge, C., Rameau, C., and Leyser, O. (2003). MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 17 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg, P., Furner, I.J., and Ottoline Leyser, H.M. (2007). MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 50 80–94. [DOI] [PubMed] [Google Scholar]
- Stirnberg, P., van De Sande, K., and Leyser, H.M. (2002). MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129 1131–1141. [DOI] [PubMed] [Google Scholar]
- Tanaka, M., Takei, K., Kojima, M., Sakakibara, H., and Mori, H. (2006). Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45 1028–1036. [DOI] [PubMed] [Google Scholar]
- Tantikanjana, T., Yong, J.W., Letham, D.S., Griffith, M., Hussain, M., Ljung, K., Sandberg, G., and Sundaresan, V. (2001). Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene. Genes Dev. 15 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann, K.V., and Skoog, F. (1934). On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc. R. Soc. Lond. B. Biol. Sci. 114 317–339. [Google Scholar]
- Turnbull, C.G., Booker, J.P., and Leyser, H.M. (2002). Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 32 255–262. [DOI] [PubMed] [Google Scholar]
- Umehara, M., Hanada, A., Yoshida, S., Akiyama, K., Arite, T., Takeda-Kamiya, N., Magome, H., Kamiya, Y., Shirasu, K., Yoneyama, K., Kyozuka, J., and Yamaguchi, S. (2008). Inhibition of shoot branching by new terpenoid plant hormones. Nature 455 195–200. [DOI] [PubMed] [Google Scholar]
- Van Dijck, R., De Proft, M., and De Greef, J. (1988). Role of ethylene and cytokinins in the initiation of lateral shoot growth in bromeliads. Plant Physiol. 86 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., and Li, J. (2005). The plant architecture of rice (Oryza sativa). Plant Mol. Biol. 59 75–84. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and Li, J. (2008). Molecular basis of plant architecture. Annu. Rev. Plant Biol. 59 253–279. [DOI] [PubMed] [Google Scholar]
- Yoneyama, K., Xie, X., Sekimoto, H., Takeuchi, Y., Ogasawara, S., Akiyama, K., Hayashi, H., and Yoneyama, K. (2008). Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 179 484–494. [DOI] [PubMed] [Google Scholar]
- Zou, J., Zhang, S., Zhang, W., Li, G., Chen, Z., Zhai, W., Zhao, X., Pan, X., Xie, Q., and Zhu, L. (2006). The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 48 687–698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.