Abstract
Dental caries continues to be a common chronic disease among various population groups. Patient care can be improved with detection at the earliest stage. However, current techniques do not have sufficient sensitivity and specificity. We discuss 2 new methods — optical coherence tomography (OCT) and polarized Raman spectroscopy (PRS) — that are potentially useful for early caries detection and monitoring.
OCT produces morphologic depth images of near-surface tissue structures with a resolution that is an order of magnitude greater than ultrasound imaging. Based on measurement of back-scattered near infrared light, OCT shows that sound enamel causes high-intensity back-scattering at the tooth surface that decreases rapidly with depth. In contrast, incipient lesions cause higher light back-scattering at the tooth surface and subsurface scattering indicative of porosity caused by demineralization. The scatter region within the enamel correlates well with the classical triangular shape of subsurface lesions observed in histologic sections. OCT imaging not only allows identification of incipient lesions, but also provides information on surface integrity and lesion depth.
PRS furnishes biochemical information about the tooth's composition, mineral content and crystallinity. The depolarization ratio derived from the dominant phosphate peak of hydroxyapatite in sound teeth is consistently lower than that from incipient caries. This difference is attributed to the change in enamel crystallite morphology or orientation that occurs with acid demineralization. Thus, PRS can be used to confirm suspect lesions determined by OCT and rule out false-positive signals from non-carious anomalies.
The combination of OCT and PRS provides a new detection method with high sensitivity and specificity that will improve caries management and patient care. Future studies are aimed at developing intraoral probes to validate the findings in vivo.
Dental caries is one of the most prevalent chronic diseases among children and adolescents in North America. At a symposium, “Designing dental programs for high-risk children,” held during the July 2008 International and Canadian Association for Dental Research conference in Toronto, common themes among speakers were (1) caries is preventable and (2) there is value in early caries detection and, thus, preventive approaches. With strategies to control, arrest or reverse the disease process, the economic burden, pain and suffering of placing and replacing restorations can be reduced.
An example of a prevention effort that has resulted in decreased incidence and prevalence of caries is fluoridation of community drinking water. This method is endorsed by over 90 national and international professional health organizations including Health Canada, the Canadian Public Health Association, the Canadian Dental Association, the Canadian Medical Association and the World Health Organization.1 In July 2008, Canada's chief dental officer highlighted some of the benefits of fluoridation, caries prevention being the primary one.1 This public health measure has also reached such high caries risk groups as those with low socioeconomic status and the elderly, who are particularly susceptible to root caries.
Where prevention is not successful, early detection of caries before it reaches the stage of cavitation offers another opportunity for effective dental care. Early detection and diagnosis will allow dentists to counsel and assist patients to prevent the progression of caries and, thereby, avert the need for invasive, irreversible removal and restoration of tooth structure.
Detecting early dental caries and monitoring the dynamic processes of demineralization and remineralization are challenging. Conventional diagnostic methods, such as visual observation and the use of a sharp explorer tool, rely on subjective clinical criteria: for example, colour, “softness” and resistance to removal. Routine dental radiographs cannot detect early enamel white-spot lesions on any surface. Approximately 30%–40% mineral loss is necessary before an early enamel carious lesion is visible radiographically.2 It can take 9 months or longer before demineralization appears radiographically.3
These deficiencies make it difficult to diagnose early approximal, smooth surface enamel lesions as well as occlusal caries. Accurate diagnosis of occlusal caries including assessment of lesion depth is particularly challenging due to the hypermineralized outer enamel surface (possibly as a result of fluoride treatment) that masks the underlying lesion. Conventional diagnostic techniques lack sufficient high sensitivity and specificity for early lesion detection and are not able to provide information on caries activity.
In recent years, various techniques have been explored to address the need for better detection tools to aid dentists in the diagnosis of early caries, and several of these methods have been developed into commercial products. The following is a list of various emerging techniques with corresponding commercially available or soon to be released devices in parentheses4–8:
direct digital radiography (various devices)
digital imaging fibre-optic transillumination (DIFOTI, Electro-Optical Sciences, Irvington, N.Y.]
electroconductivity measurements (Electronic Caries Monitor, Lode Diagnostics, Groningen, The Netherlands)
impedance spectroscopy (CarieScan, IDMoS, Dundee, Scotland)
quantitative light-induced fluorescence (QLF, Inspektor Pro, OMNII Oral Pharmaceuticals, West Palm Beach, Fla.)
laser fluorescence (DIAGNOdent devices, KaVo, Lake Zurich, Ill.; Midwest Caries I.D., Dentsply, York, Penn.)
photothermal radiometric and modulated luminescence methods (Canary System, Quantum Dental Technologies, Toronto, Ont.)
optical coherence tomography (OCT Dental Imaging System, Lantis Laser, Denville, N.J.)
ultrasound
near-infrared illumination
Raman spectroscopy
terahertz imaging
Despite their potential, some of these techniques and devices also suffer from subjectivity, with high intra- and inter-examiner variability; false-positive results due to stains, calculus or organic deposits; and unsuitability for detection of initial enamel caries at all tooth surfaces.9–17 Furthermore, several of these tools do not allow longitudinal monitoring of caries development or repair (i.e., caries activity) in terms of providing information about mineral loss or gain. For example, the DIAGNOdent device (KaVo) is based on fluorescence of bacterial porphyrins within lesions rather than mineral content. Thus, better, objective diagnostic tools with both high sensitivity and high specificity are still needed for early detection and monitoring of dental caries.
Shedding New Light
In this article, we do not review the various methods or products for caries detection, as these have been discussed comprehensively recently.5,17–22 Rather, our goal is to introduce 2 optical methods that might be useful in the detection of early enamel caries and to provide an overview of our research in this direction. Our approach involves combining the technique of optical coherence tomography (OCT) with polarized Raman spectroscopy (PRS).
Optical Coherence Tomography
OCT is an imaging technology that is capable of providing high-resolution (10–30 μm) morphologic depth images. It is similar in operation to ultrasound imaging, but uses light waves rather than sound waves. This technique provides image resolution that is an order of magnitude higher than that obtained by ultrasound imaging. However, where ultrasound is well suited for imaging deep-tissue structures, such as a fetus within a pregnant mother, OCT can only image the first several millimetres of tissues (2–4 mm, depending on the wavelength of light used). Thus, OCT is better suited for imaging near-surface structures.
The technique is based on coherent back-scattered light. A detailed description of the principles of OCT is beyond the scope of this article, but is available elsewhere.23,24 Briefly, an OCT system contains a Michelson interferometer that splits a light beam into 2 paths, bouncing the beams off 2 mirrors (1 fixed, 1 moving) then recombining the beams. The interference pattern created by the reflected beams generates a depth profile at a single point along the laser trajectory. This point is known as an A-scan. As the laser is moved across the surface laterally (dashed lines on Fig. 1a), adjacent A-scans are assembled to produce a 2-dimensional depth image, also known as a B-scan (Figs. 1b and 1c).
Figure 1.
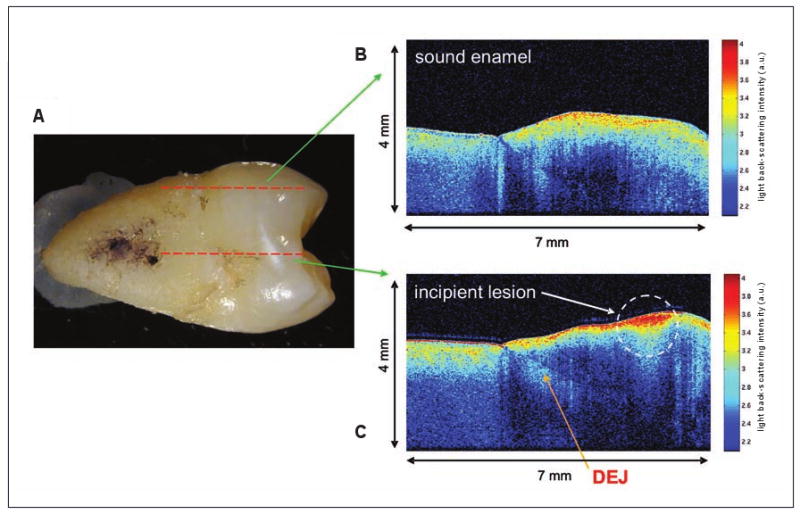
(a) Mesial surface of an extracted human premolar (tooth 15) with an incipient lesion. Dashed red lines indicate the regions scanned to produce images b and c. (b) OCT depth image from a sound enamel region. (c) OCT depth image from a region of the incipient lesion. Higher light back-scattering intensity occurs at the site of the lesion and also within the subsurface enamel. DEJ = dentinoenamel junction; a. u. = arbitrary units.
OCT light is back-scattered by changes in refractive index as the light encounters different tissue types or structures (e.g., enamel vs. dentin, healthy vs. carious regions).23,24 The light source is a low coherent laser with a wavelength in the near-infrared region (850 nm or 1310 nm), which is the region of the electromagnetic spectrum just beyond the visible red portion. At the sample, the power generated is very low, typically about 750 μW using a 850 nm wavelength or 7 mW with a 1310 nm wavelength system. Light in the near-infrared region is able to penetrate tissue better than light in the visible region of the spectrum and is non-damaging to human tissues at these intensities.
OCT is a powerful technique that is useful for examining tissues with multiple layers or demarcations. It has been used in medical imaging for the past 15 years. For example, clinical OCT systems are available in ophthalmologic clinics, where they are used diagnostically to examine the thickness of tissue layers of the retina to detect defects arising from increased intraocular pressure associated with glaucoma.25 Only in the past decade has OCT been applied in dentistry to image soft and hard tissues of the oral cavity, with many studies still at the research stage6,26–30 and the technique not yet developed into commercially available dental devices.
To illustrate the kind of information obtained from OCT imaging, we present an example from our studies. Tooth samples were collected from consenting volunteers using protocols approved by the research ethics boards of the institutions involved in the studies. In the permanent maxillary second premolar (tooth 15) shown (Fig. 1a), there is an obvious incipient carious lesion (white spot) on the mesial surface. The corresponding OCT images (Figs. 1b and 1c) reveal the morphology of the sample; the crown is oriented toward the right and the root toward the left of the image. From the depth images, the morphologic features of the crown enamel and underlying dentin as well as the transition from enamel to dentin at the dentinoenamel junction can be clearly identified. In this colour scheme, blues and greens represent low-intensity light back-scattering, while yellows and reds represent high-intensity back-scattering.
An OCT depth scan of a sound enamel surface toward the mesiolingual line angle is portrayed in Fig. 1b. OCT signal intensity is high at the air–enamel or air–root interface, with the signal dying out rapidly with depth into the tooth. On the other hand, the depth scan obtained from the area of carious lesion (Fig. 1c) shows greater signal intensity at the region of the lesion (notice red colour). In addition, higher back-scattering intensity continues beyond the surface into the enamel. Demineralization results in increased tooth porosity and, with more open spaces in the matrix, the scattered light is able to penetrate deeper into the enamel. The surface of the incipient lesion is still intact, with demineralization occurring subsurface.
The light scattering below the surface forms a triangular pattern. This shape is characteristic of subsurface lesions and is supported by a corresponding photomicrograph of a histologic section of the tooth in the region of the caries (Fig. 2). The histologic section was obtained by cutting the extracted tooth whereas OCT images are obtained non-destructively from intact teeth. The depth of this lesion was measured to be ∼427 μm on the histologic section and ∼507 μm using OCT image analysis. Although not exact, these values are within range of one another, demonstrating the potential of OCT imaging.
Figure 2.
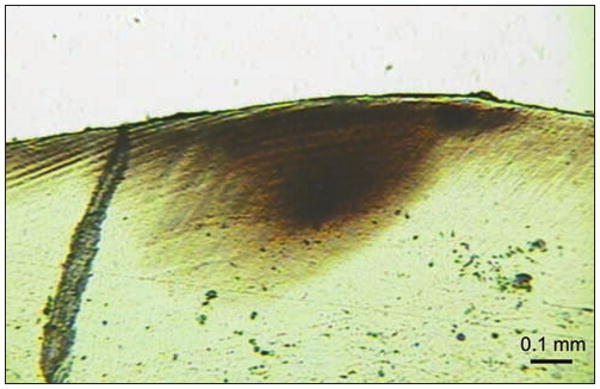
Light photomicrograph of a histologic section ∼100-μm thick that was cut from the region of the carious lesion of the tooth shown in Figure 1. Subsurface demineralization is visible and the lesion depth is ∼427 μm.
On the JCDA website, there is a link to a video clip demonstrating a series of sequential OCT scans taken across a tooth surface (www.cda-adc.ca/jcda/vol-74/issue-10/913.html).
Polarized Raman Spectroscopy
OCT imaging in regions of hypocalcification can sometimes show increased light back-scattering at the surface, which could be misinterpreted as signs of early caries. To help rule out such false-positive readings and increase the specificity of our method, we couple OCT with PRS to obtain biochemical information for confirmation of caries. PRS provides details on the molecular composition (e.g., collagen in dentin vs. predominantly inorganic apatite in enamel) and molecular structure of cells and tissues.
Like OCT, PRS measures light scattering. Although most scattered photons have the same energy and wavelength as the incoming excitation light, about 1 in 107 photons scatters at an energy different from that of the incoming light. This energy difference is proportional to the vibrational energy of the scattered molecules within the sample and is known as the Raman effect. As with other emerging optical methods, the properties of the scattered light within sound or porous carious regions are being explored to determine their use in caries detection.
In fluorescence-based techniques, there are a limited number of intrinsic fluorophores that can provide diagnostic information without the addition of external dyes. In contrast, PRS can provide information not only about bacterial porphyrins leached into carious regions, but also about the primary mineral matrix and, thus, the state of demineralization or remineralization of the tooth. This information is gathered without the need to add extrinsic dyes or agents. PRS provides information on the composition, crystallinity and orientation of the mineral matrix, all of which are affected in caries formation or remineralization.
Our studies focus on the phosphate peak from carbonated hydroxyapatite (the main component of dental hard tissues) and how its biochemical signature is affected by demineralization and remineralization. We have derived a quantitative parameter known as the depolarization ratio to follow such changes.31 This ratio is determined by calculating the intensity of the main phosphate peak from parallel- and cross-polarized Raman spectroscopic measurements, then dividing the cross-polarized value by the parallel-polarized value. In an ex vivo study based on 23 extracted teeth with 47 and 27 PRS measurements on sound and carious enamel, respectively, we could readily distinguish sound enamel from carious enamel achieving a sensitivity of 100% and a specificity of 98%.32 Using Student's t-test, the differences in the means of the 2 groups were statistically significant at p < 0.001 (Fig. 3).
Figure 3.
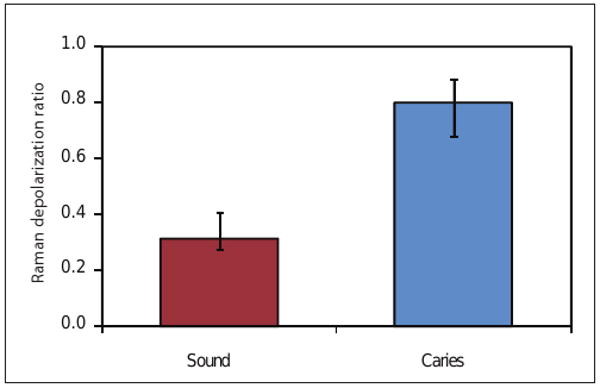
Bar graphs of the mean Raman depolarization ratio of the 959 cm-1 phosphate peak of hydroxyapatite derived from sound enamel and carious enamel surfaces. A total of 47 (sound) and 27 (caries) PRS measurements from 23 extracted teeth were used for statistical analyses (Student's t-test, p < 0.001). Error bars show standard deviation.
Future Directions
As dentists are accustomed to using dental radiographs for diagnostic purposes, OCT imaging, which shows similar dental morphology, could be readily adopted. Incipient lesions are visible on OCT images, with information available regarding lesion location, lesion depth (including proximity to the dentinoenamel junction) and surface characteristics (intact, cavitated or change in contour). Surface defects, such as cracks and fissures, should also be detectable by OCT. With PRS, it is possible to confirm that suspicious carious sites are indeed areas of demineralization.
Preliminary studies have also shown that both OCT and PRS can be used to follow demineralization and remineralization longitudinally using quantitative parameters.33,34 Studies are currently underway to examine the effects of possible confounding factors such as stain, calculus, hypocalcification and fluorosis on our optical measurements.35–37 Our next step is to validate the technology further using gold-standard methods, such as histology and transverse microradiography. In addition, we are developing an intraoral probe to validate the technology in vivo for early caries detection and monitoring. These validation studies are essential to determine the clinical utility of the technology before implementation in clinical practice.
Both OCT and PRS involve non-ionizing radiation and, therefore, can be used routinely to screen for early lesions, to monitor the caries process, to assess the success of remineralization treatments and to educate and motivate patients. Frequent use of OCT–PRS to collect data from carious sites longitudinally would provide insight into caries activity. A tool with both high sensitivity and high specificity will be a useful adjunct to assist dentists in their decision-making and treatment planning processes.
Acknowledgments
Research funding was derived from past and current grants from the Manitoba Medical Service Foundation, Canadian Institutes of Health Research (Institute of Musculoskeletal Health and Arthritis) and the United States National Institutes of Health (National Institute for Dental and Craniofacial Research; #R01DE017889). We thank the staff and students of the dental clinics at the Universities of Manitoba and Dalhousie, as well as the dentists and dental staff of numerous private dental clinics in Winnipeg and Morden, Manitoba, for assistance with tooth collection.
Biographies
The Authors

Dr. Choo-Smith is a research officer in the spectroscopy group at the National Research Council Canada's Institute for Biodiagnostics, Winnipeg, Manitoba. She is also an adjunct assistant professor in the department of restorative dentistry, faculty of dentistry, University of Manitoba, Winnipeg, Manitoba.

Dr. Dong is an associate professor in the department of restorative dentistry, faculty of dentistry, University of Manitoba, Winnipeg, Manitoba. She is also cross-appointed to the department of preventive dental science.

Dr. Cleghorn is an associate professor in the department of dental clinical sciences and director of clinical affairs at the faculty of dentistry, Dalhousie University, Halifax, Nova Scotia.

Mr. Hewko is a research council officer in the spectroscopy group at the National Research Council Canada's Institute for Biodiagnostics, Winnipeg, Manitoba. He is also cross-appointed to the department of biosystems engineering, faculty of agricultural and food sciences at the University of Manitoba, Winnipeg, Manitoba.
Footnotes
The authors have no declared financial interests in any company manufacturing the types of products mentioned in this article.
References
- 1.It's your health: fluoride and human health. Ottawa: Health Canada; 2008. [2008 Nov 3]. Available: www.hc-sc.gc.ca/hl-vs/alt_formats/pacrb-dgapcr/pdf/iyh-vsv/environ/fluor-eng.pdf. [Google Scholar]
- 2.White SC, Pharoah MJ. Oral radiology: principles and interpretation. 5th. Toronto: Mosby; 2004. Dental caries; pp. 297–313. [Google Scholar]
- 3.Lang NP, Hotz PR, Gusberti FA, Joss A. Longitudinal clinical and microbiological study on the relationship between infection with Streptococcus mutans and the development of caries in humans. Oral Microbiol Immunol. 1987;2(1):39–47. doi: 10.1111/j.1399-302x.1987.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis and management of dental caries throughout life. J Dent Educ; National Institutes of Health Consensus Development Conference statement; March 26–28, 2001; 2001. pp. 1162–8. [PubMed] [Google Scholar]
- 5.Hall A, Girkin JM. A review of potential new diagnostic modalities for caries lesions. J Dent Res. 2004;83(Spec No C):C89–94. doi: 10.1177/154405910408301s18. [DOI] [PubMed] [Google Scholar]
- 6.Fried D, Featherstone JD, Darling CL, Jones RS, Ngaotheppitak P, Buhler CM. Early caries imaging and monitoring with near-infrared light. Dent Clin North Am. 2005;49(4):771–93. doi: 10.1016/j.cden.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Jeon RJ, Han C, Mandelis A, Sanchez V, Abrams SH. Diagnosis of pit and fissure caries using frequency-domain infrared photothermal radiometry and modulated laser luminescence. Caries Res. 2004;38(6):497–513. doi: 10.1159/000080579. [DOI] [PubMed] [Google Scholar]
- 8.Ko AC, Choo-Smith LP, Hewko M, Leonardi L, Sowa MG, Dong CC, et al. Ex vivo detection and characterization of early dental caries by optical coherence tomography and Raman spectroscopy. J Biomed Opt. 2005;10(3):031118. doi: 10.1117/1.1915488. [DOI] [PubMed] [Google Scholar]
- 9.Bader JD, Shugars DA, Bonito AJ. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 2001;65(10):960–8. [PubMed] [Google Scholar]
- 10.Stookey GK, Jackson RD, Zandona AG, Analoui M. Dental caries diagnosis. Dent Clin North Am. 1999;43(4):665–77. [PubMed] [Google Scholar]
- 11.Schneiderman A, Elbaum M, Shultz T, Keem S, Greenebaum M, Driller J. Assessment of dental caries with Digital Imaging Fiber-Optic TransIllumination (DIFOTI): in vitro study. Caries Res. 1997;31(2):103–10. doi: 10.1159/000262384. [DOI] [PubMed] [Google Scholar]
- 12.Lussi A, Firestone A, Schoenberg V, Hotz P, Stich H. In vivo diagnosis of fissure caries using a new electrical resistance monitor. Caries Res. 1995;29(2):81–7. doi: 10.1159/000262046. [DOI] [PubMed] [Google Scholar]
- 13.Stookey GK. Quantitative light fluorescence: a technology for early monitoring of the caries process. Dent Clin North Am. 2005;49(4):753–70. doi: 10.1016/j.cden.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Amaechi BT, Higham SM. Quantitative light-induced fluorescence: a potential tool for general dental assessment. J Biomed Opt. 2002;7(1):7–13. doi: 10.1117/1.1427044. [DOI] [PubMed] [Google Scholar]
- 15.Shi XQ, Welander U, Angmar-Mansson B. Occlusal caries detection with KaVo DIAGNOdent and radiography: an in vitro comparison. Caries Res. 2000;34(2):151–8. doi: 10.1159/000016583. [DOI] [PubMed] [Google Scholar]
- 16.Lussi A, Megert B, Longbottom C, Reich E, Francescut P. Clinical performance of a laser fluorescence device for detection of occlusal caries lesions. Eur J Oral Sci. 2001;109(1):14–9. doi: 10.1034/j.1600-0722.2001.109001014.x. [DOI] [PubMed] [Google Scholar]
- 17.Lussi A, Hibst R, Paulus R. DIAGNOdent: an optical method for caries detection. J Dent Res. 2004;83(Spec No C):C80–3. doi: 10.1177/154405910408301s16. [DOI] [PubMed] [Google Scholar]
- 18.Stookey GK. Optical methods — quantitative light fluorescence. J Dent Res. 2004;83(Spec No C):C84–8. doi: 10.1177/154405910408301s17. [DOI] [PubMed] [Google Scholar]
- 19.Longbottom C, Huysmans MC. Electrical measurements for use in caries clinical trials. J Dent Res. 2004;83(Spec No C):C76–9. doi: 10.1177/154405910408301s15. [DOI] [PubMed] [Google Scholar]
- 20.Pretty IA, Maupomé G. A closer look at diagnosis in clinical dental practice: part 5. Emerging technologies for caries detection and diagnosis. J Can Dent Assoc. 2004;70(8):540. Available: www.cda-adc.ca/jcda/vol-70/issue-8/540.html. [PubMed]
- 21.Pretty IA. Caries detection and diagnosis: novel technologies. J Dent. 2006;34(10):727–39. doi: 10.1016/j.jdent.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Zandona AF, Zero DT. Diagnostic tools for early caries detection. J Am Dent Assoc. 2006;137(12):1675–84. doi: 10.14219/jada.archive.2006.0113. [DOI] [PubMed] [Google Scholar]
- 23.Wang XJ, Milner TE, de Boer JF, Zhang Y, Pashley DH, Nelson JS. Characterization of dentin and enamel by use of optical coherence tomography. Appl Opt. 1999;38(10):2092–6. doi: 10.1364/ao.38.002092. [DOI] [PubMed] [Google Scholar]
- 24.Feldchtein FI, Gelikonov GV, Gelikonov VM, Iksanov RR, Kuranov RV, Sergeev AM, et al. In vivo OCT imaging of hard and soft tissue of the oral cavity. Opt Express. 1998;3(6):239–50. doi: 10.1364/oe.3.000239. [DOI] [PubMed] [Google Scholar]
- 25.Schuman JS, Hee MR, Arya AV, Pedut-Kloizman T, Puliafito CA, Fujimoto JG, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6(2):89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner A, Dichtl S, Hitzenberger CK, Sattmann H, Robl B, Moritz A, et al. Polarization-sensitive optical coherence tomography of dental structures. Caries Res. 2000;34(1):59–69. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 27.Colston BW, Jr, Everett MJ, Sathyam US, DaSilva LB, Otis LL. Imaging of the oral cavity using optical coherence tomography. Monogr Oral Sci. 2000;17:32–55. doi: 10.1159/000061643. [DOI] [PubMed] [Google Scholar]
- 28.Amaechi BT, Higham SM, Podoleanu AG, Rogers JA, Jackson DA. Use of optical coherence tomography for assessment of dental caries: quantitative procedure. J Oral Rehabil. 2001;28(12):1092–3. doi: 10.1046/j.1365-2842.2001.00840.x. [DOI] [PubMed] [Google Scholar]
- 29.Jones RS, Darling CL, Featherstone JD, Fried D. Imaging artificial caries on the occlusal surfaces with polarization-sensitive optical coherence tomography. Caries Res. 2006;40(2):81–9. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 30.Jones RS, Fried D. Remineralization of enamel caries can decrease optical reflectivity. J Dent Res. 2006;85(9):804–8. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko AC, Choo-Smith LP, Hewko M, Sowa MG, Dong CC, Cleghorn B. Detection of early dental caries using polarized Raman spectroscopy. Opt Express. 2006;14(1):203–15. doi: 10.1364/opex.14.000203. [DOI] [PubMed] [Google Scholar]
- 32.Ko AC, Hewko M, Sowa MG, Dong CC, Cleghorn B, Choo-Smith LP. Early dental caries detection using a fibre-optic coupled polarization-resolved Raman spectroscopic system. Opt Express. 2008;16(9):6274–84. doi: 10.1364/oe.16.006274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choo-Smith LP, Zhu R, Dong CC, Ko A, Hewko M, Cleghorn B, et al. Monitoring demineralization in an artificial caries model using Raman spectroscopy and optical coherence tomography: a pilot study. Caries Res. 2006;40(4):311. [Google Scholar]
- 34.Choo-Smith LP, Gibrayel K, Dong CC, Popescu D, Sowa MG, Cleghorn B. Monitoring remineralization in an artificial caries model using Raman spectroscopy and OCT: a pilot study. Caries Res. 2007;41(4):326. [Google Scholar]
- 35.Dong CC, Huminicki A, Sowa MG, Cleghorn B, Choo-Smith LP. Raman spectroscopic measurements of stained enamel, unstained enamel and white spot lesions. Caries Res. 2008;42(3):213–4. [Google Scholar]
- 36.Huminicki A, Dong CC, Cleghorn B, Choo-Smith LP. Does calculus confound PRS and OCT detection of early caries? J Dent Res. 2008;87(Spec Iss B):521. [Google Scholar]
- 37.Choo-Smith LP, Huminicki A, Dong CC, Hewko M, Cleghorn B, Sowa MG. Investigating regions of enamel hypocalcification using optical coherence tomography and polarized Raman spectroscopy. Caries Res. 2008;42(3):214. [Google Scholar]


