Abstract
The function of phosphatidylcholine (PC) in the bacterial cell envelope remains cryptic. We show here that productive interaction of the respiratory pathogen Legionella pneumophila with host cells requires bacterial PC. Synthesis of the lipid in L. pneumophila was shown to occur via either phospholipid N-methyltransferase (PmtA) or phosphatidylcholine synthase (PcsA), but the latter pathway was demonstrated to be of predominant importance. Loss of PC from the cell envelope caused lowered yields of L. pneumophila within macrophages as well as loss of high multiplicity cytotoxicity, while mutants defective in PC synthesis could be complemented either by reintroduction of PcsA or by overproduction of PmtA. The lowered yields and reduced cytotoxicity in mutants with defective PC biosynthesis were due to three related defects. First, there was a poorly functioning Dot/Icm apparatus, which delivers substrates required for intracellular growth into the cytosol of infected cells. Secondly, there was reduced bacterial binding to macrophages, possibly due to loss of PC or a PC derivative on the bacterium that is recognized by the host cell. Finally, strains lacking PC had low steady state levels of flagellin protein, a deficit that had been previously associated with the phenotypes of lowered cytotoxicity and poor cellular adhesion.
INTRODUCTION
Legionella pneumophila is a Gram-negative respiratory pathogen that causes severe pneumonia in immunocompromised individuals as well as occasional community-wide epidemic outbreaks resulting from single-source aerosols (Chow and Yu, 1998; Fraser et al., 1977). After deposition in human lungs, the organism grows within alveolar macrophages, which fail to degrade the bacterium, and instead allow the formation of a membrane-bound replication vacuole (Nash et al., 1984; Horwitz, 1983). A remarkable aspect of the natural history of the disease is that growth in host macrophages appears to mimic the strategy used by the microorganism to replicate within amoebae in the environment (Rowbotham, 1980). The bacterium probably has intimate interaction with unicellular eukaryotes during extracellular growth in the environment, as L. pneumophila primarily inhabits complex biofilms, such as those that develop in plumbing sources prior to aerosol contact with humans (Rogers et al., 1994). Therefore, in addition to being an intracellular parasite, the bacterium comes into contact with unique metabolites provided by eukaryotic hosts in its environmental reservoir.
Intracellular growth and formation of the replication vacuole requires the Dot/Icm secretion system, a multiprotein complex in the bacterial envelope that allows translocation of macromolecules across host cell membranes (Sexton and Vogel, 2002; Segal and Shuman, 1999; Vogel et al., 1998). L. pneumophila strains that properly assemble this complex recruit membranous material derived from host cell early secretory compartments, and this contributes to the formation of the replication vacuole containing the bacterium (Derre and Isberg, 2004; Kagan et al., 2004; Kagan and Roy, 2002; Swanson and Isberg, 1995). A number of bacterial proteins have been identified that are translocated by the Dot/Icm system across the vacuolar membrane, presumably manipulating host cell signaling and survival pathways (Chen et al., 2004; Conover et al., 2003; Luo and Isberg, 2004; Nagai et al., 2002; Shohdy et al., 2005) (Gao and Abu Kwaik, 1999).
The absence of certain components of the Dot/Icm apparatus (DotL, DotM and DotN) results in lowered plating efficiency of bacteria on solid medium, probably because a partially assembled Dot/Icm lacking these components is misregulated (Buscher et al., 2005; Conover et al., 2003). To identify proteins that may regulate the function or assembly of the Dot/Icm system, a genetic screen was performed to isolate insertion mutations that had phenotypes similar to dotL mutations. As seen with dotL mutations, strains isolated from this mutant hunt all showed lowered viability in the presence of overproduced DotA, a component of the apparatus, but showed normal viability in strains making either no or low levels of DotA (Conover et al., 2003). One of the mutants that had reduced plating efficiency in the presence of DotA overproduction had an insertion in the lidK gene (lpg1584), predicted to encode phosphatidylcholine synthase (Pcs), a recently discovered enzyme that allows the synthesis of phosphatidylcholine (PC) lipids in some bacterial species (Sohlenkamp et al., 2000; de Rudder et al., 1999). In a separate study, lidK (or pcsA to denote its activity) was shown to be one of a number of orfs from a variety of bacterial species that can promote the production of PC when overexpressed in E. coli, consistent with lidK/pcsA encoding a Pcs (Martinez-Morales et al., 2003).
PC is a major essential component of eukaryote membranes, but only a fraction of bacterial species synthesize this phospholipids (Wilderman, et al, 2002). Many of the bacterial species that produce PC are found intimately associated with eukaryotic hosts (Sohlenkamp et al., 2003). Those bacteria that synthesize PC do so via one or more enzymatic pathways, and the enzymatic activities for two of these pathways have been identified in L. pneumophila by genomic searches (Fig. 1A; (Martinez-Morales et al., 2003)). The first pathway uses a strategy also found in eukaryotes, in which phosphatidylethanolamine is subjected to a series of methylation reactions catalyzed by the phospholipid N-methyltransferase enzyme (PmtA; (Arondel et al., 1993)). The second pathway is promoted by the PcsA enzyme unique to bacteria, which condenses exogenous choline and CDP-diacylgyceride to produce PC in a single step (Fig. 1A; (de Rudder et al., 1999)). Pcs requires that the microorganism be in contact with eukaryotic hosts, or have access to eukaryotic metabolites, because choline is not a biosynthetic product of prokaryotes. Although it has been shown that L. pneumophila Orfs corresponding to both pathways are enzymatically active when expressed in E. coli, it is not clear if either contributes to PC production in L. pneumophila (Martinez-Morales et al., 2003).
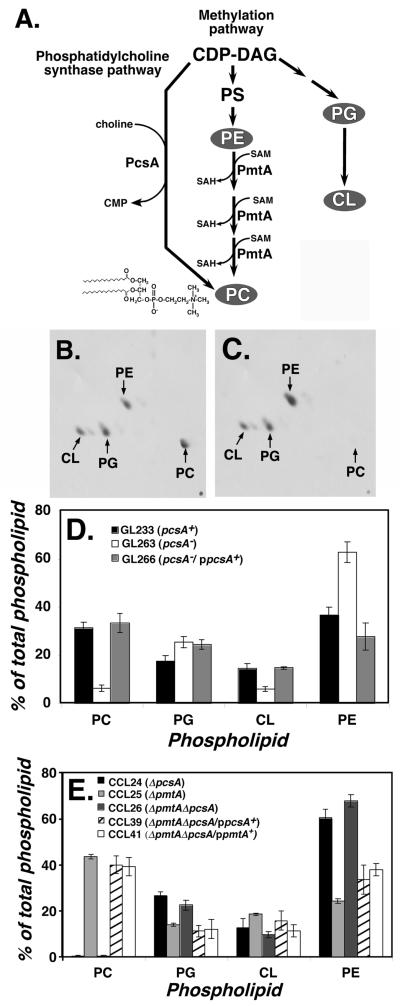
Figure 1. Knockout of pcsA causes drastic loss of PC content in L. pneumophila membranes.
(A). Pathways for phosphatidylcholine biosynthesis in bacteria. Shown are the two major pathways for synthesis of phosphatidylcholine (PC) in bacteria, catalyzed by phosphatidylcholine synthase (PcsA) and phospholipid N-methyltransferase (PmtA). L. pneumophila Philadelphia 1 ORFs encoding both of these enzymes have the predicted activities when expressed in E. coli (Martinez-Morales et al., 2003). CDP-DAG: cytidine diphospho-diacylglyceride. PS: phosphatidylserine. PG: phosphatidylglycerol. CL: cardiolipin. SAM: S-adenosylmethionine. SAH: S-adenosylhomoserine. PE: phosphatidylethanolamine. In gray are the three major lipids found in bacterial membranes in addition to PC (Lopez-Lara and Geiger, 2001). (B). Phospholipid content of L. pneumophila labeled with [1-14C]acetate was determined by thin layer chromatography (TLC) of membrane extracts (Experimental Procedures). Shown is two-dimensional TLC fractionation of GL233 (pcsA+) lipids. (C). Phospholipids from GL263 (pcsA−) prepared as above and subjected to two-dimensional TLC fractionation. (D). Complementation of PC biosynthesis defect by cloned pcsA+ gene. Displayed are relative percentages of phospholipids in various L. pneumophila strains having either an intact pcsA+ gene or the pcsA− mutation. Data shown are mean of triplicate samples. (E). Total absence of PC in ΔpcsA ΔpmtA strain and its complementation. Phospholipid profiles are displayed as in (D) for strains noted in legend. Data shown are mean of triplicate samples. PC: phosphatidylcholine. PE: phosphatidylethanolamine. PG: phosphatidylglycerol. CL: cardiolipin.
There is little information regarding why some bacteria species have PC in their envelopes. The most striking phenotype for PC-deficient mutants is in the plant pathogenic Agrobacterium tumefaciens, in which the lack of PC has the unexplained effect of reducing transcription of genes encoding components of the T DNA conjugative apparatus (Wessel et al., 2006). Mutations causing a PC deficit in other microorganisms that interact with host cells similarly cause alterations in host cell relationships. For instance, Bradyrhizobium japonicum PC mutants show inefficient symbiosis with the soybean host plant (Minder et al., 2001), while Brucella mutants unable to synthesize PC have lowered virulence in a mouse model and less efficient replication vacuole formation (Comerci et al., 2006; Conde-Alvarez et al., 2006)).
In this work, we demonstrate that the presence of PC in L. pneumophila is important for both adhesion to host cells as well as for function of the Dot/Icm system. The loss of PC results in the absence of the flagellin protein, a posttranscriptional defect that may be the major cause of altered host cell interactions observed in PC-defective mutants.
RESULTS
Mutations in lidK/pcsA gene reduce the plating efficiency of L. pneumophila in the presence of high-level expression of the dotA gene (Conover et al., 2003). Therefore, for most experiments presented here, bacterial strains were used that produced low levels of the DotA protein encoded by derivatives of the plasmid pGC36. Such strains grow within macrophages, although there is some reduction in the efficiency of effector translocation relative to wild type strains. Strains expressing low levels of DotA show normal viability on bacteriological media in the presence of a lidK/pcsA mutation (Conover et al., 2003).
Mutations in the L. pneumophila lidK/pcsA gene result in reduced phosphatidylcholine content
It has been reported that phosphatidylcholine (PC) represents approximately 30% of the L. pneumophila phospholipid (Hindahl and Iglewski, 1984). To determine if the demonstrated phosphatidylcholine synthase activity of the lidK/pcsA gene product (Martinez-Morales et al., 2003) contributes to the steady state level of this phospholipid, bacteria were grown in broth to post-exponential phase (A600=3.5), labeled with [1-14C]acetate, and total lipids were extracted and separated by 2-dimensional thin layer chromatography (Fig. 1B-E; Experimental Procedures). In cells having an intact lidK/pcsA gene, chromatograms revealed four major compounds corresponding to PC, phosphatidylethanolamine (PE), phosphatidylglycerol (PG) and cardiolipin (CL) (Fig. 1B,C), with >30% of the total phospholipid being PC (Fig. 1D; GL233), in agreement with previous results (Hindahl and Iglewski, 1984). The relative levels of the four lipids was almost identical to those found in the fully Dot/Icm+ parent strain Lp02 or in JR32, another L. pneumophila strain that is commonly used (Supplemental Data). In contrast, the lidK::Tn10 mutant showed low amounts of PC in the membrane (Fig. 1D), with approximately 6% of the total phospholipids being PC in bacteria grown to post-exponential phase (Fig. 1D). The deficit of PC in this mutant could be completely complemented in trans by a single open reading frame encoding lidK/pcsA (Fig. 1D; GL266). As the product of lidK/pcsA was demonstrated to produce PC dependent on the addition of exogenous choline (Martinez-Morales et al., 2003), and its absence in L. pneumophila results in reduced PC synthesis, this gene will be exclusively referred to as pcsA.
Although PC represented only a small fraction of the phospholipid found in the pcsA− mutant, this amount increased with storage at −80°C and subsequent recovery of CFU at 37°C. Therefore, a deletion of pcsA was constructed and analyzed soon after isolation. The amounts of PC in this strain were extremely low, indicating that continued storage selects for isolates with enhanced PC content (Fig. 1E; CCL24). As any residual PC was probably due to the presence of the phospholipid N-methlytransferase encoded by pmtA (orf lpg2158), we analyzed the effects of eliminating the second pathway of PC synthesis (Fig. 1A). In a strain containing an intact pcsA gene, deletion of pmtA resulted in at least as much PC as in the wild type control both in post-exponential phase (Fig. 1E) or in cells extracted from other growth phases (data not shown). Therefore, the pathway that utilizes phosphatidylcholine synthase encoded by the pcsA gene is the major source of PC in L. pneumophila, and the activity of PmtA is likely to be only detected in the absence of the pcsA gene. The low level of PC in the absence of lidK/pcsA was not due to choline in the medium interfering with expression or activity of the pmtA gene, as growth of wild type strains in the absence of choline resulted in similarly low levels of PC (data not shown). This indicates that the amount of flux through the PmtA pathway is probably much lower than that through the PcsA path using most growth conditions.
As the pcsA− mutants showed varying amounts of residual PC in membrane extracts, we isolated a strain lacking both pmtA and pcsA. Although isolation of the double mutant required a two-step procedure, in which the ΔpmtA allele was crossed into a ΔpcsA/ppcsA+ heterozygote followed by curing of the wild type pcsA+ gene, strains lacking both enzymes in PC biosynthesis grew as well in bacteriological medium as did the parental ΔpcsA single mutant (data not shown). The resulting double mutant (pcsA::GmR ΔpmtA) grown to postexponential phase showed no detectable levels of PC after chromatographic analysis of phospholipids (CCL26; Fig. 1E), and PC levels in this strain were returned to wild type levels by introduction of a wild type copy of pcsA in trans (CCL39; Fig. 1E). Interestingly, high PC levels could be restored in the double mutant by introduction of pmtA on a multicopy plasmid (CCL41; Fig. 1E).
L. pneumophila lacking PC results in reduced yields after infection of macrophages
Strains lacking either one or both pathways of PC biosynthesis were analyzed for their ability to grow within mouse bone marrow-derived macrophages (Fig. 2A and B). Both the ΔpcsA::GmR and the ΔpcsA::GmR ΔpmtA mutants showed lower yields of bacteria after three days incubation than the parental strain (GL233) that has a normal content of PC. In contrast, the ΔpmtA mutant was indistinguishable from the parental control (Fig. 2B), consistent with its high levels of PC (Fig. 1E). Introduction of a plasmid expressing pcsA+ in trans restored intracellular growth of both the ΔpcsA::GmR (Fig. 2A) and the ΔpcsA::GmR ΔpmtA double mutant (Fig. 2B) to levels that were indistinguishable from the parental control, indicating that defective growth was due to lack of PC. Interestingly, the double mutant harboring pmtA on a plasmid also was able to grow as well as the wild type strain (Fig. 2B), consistent with the observation that overproduction of PmtA on a plasmid restores high level PC synthesis (Fig. 1E). To determine if the defect in intracellular growth was at the level of formation of replication vacuoles, the number of bacteria per vacuole was examined 18 h after the introduction of L. pneumophila onto macrophages (Fig. 2C). The pcsA mutant was defective for formation of large replication vacuoles, and macrophages harboring the mutant accumulated vacuoles with bacteria that failed to initiate replication.
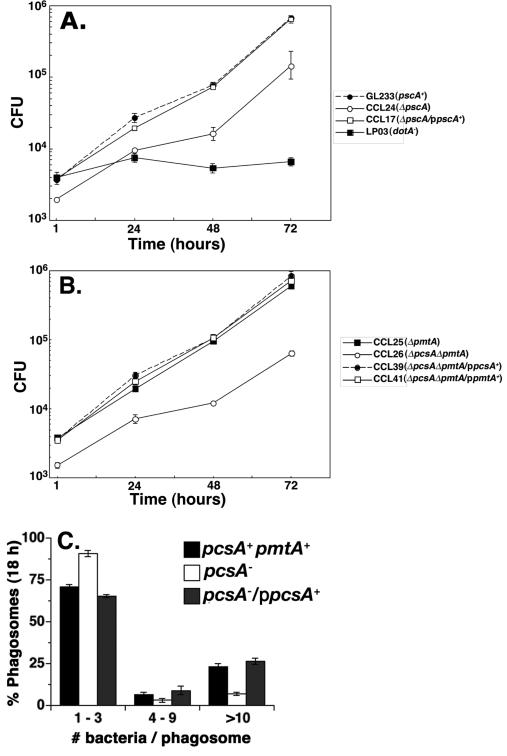
Figure 2. Defective intracellular growth in strains lacking PC.
(A). Defective growth of ΔpcsA::GmR in bone marrow-derived macrophages. Assays for viable counts over time performed as described (Experimental Procedures). Strains are: GL233, pcsA+pmtA+; CCL24, ΔpcsA; CCL17, ΔpcsA/ppcsA+; LP03, dotA−. Data shown are means ± S.D. of triplicate incubations performed in parallel. (B). Loss of pmtA is not sufficient to impair L. pneumophila intracellular growth. Strains are: CCL25, ΔpmtA; CCL26, ΔpmtA Δpcs; CCL39, ΔpmtA ΔpcsA/ ppcsA+; CCL41, ΔpmtA ΔpcsA / ppmtA+. Data shown are means ± S.D. of triplicate incubations performed in parallel. (C) Absence of PcsA results in defective replication vacuole formation. Macrophages plated on coverslips were incubated with pcsA+ (GL233), pcsA− (GL263) and pcsA−/ppcsA+ (GL266) for 18 hours and processed for immunofluorescence (Experimental procedures). The number of bacteria per macrophages was recorded for 104 phagosomes in triplicate samples for each strain. Shown are typical experiments. All experiments were repeated at least twice.
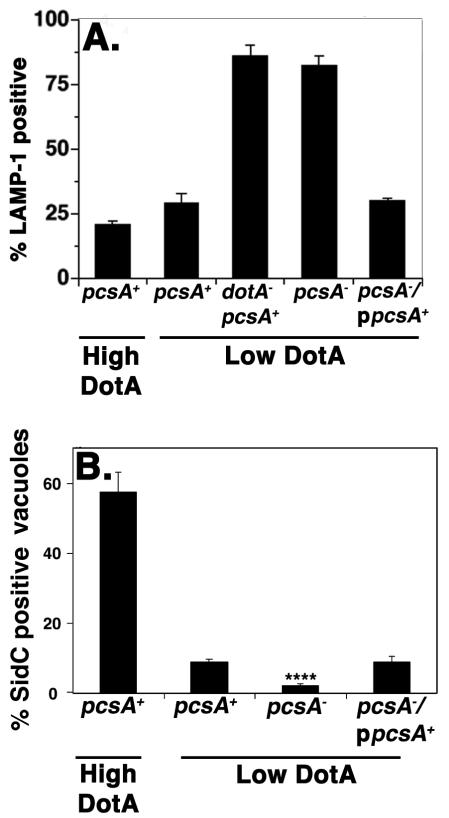
The observed depression in intracellular growth caused by a deficit in PC synthesis may result from a poorly functioning Dot/Icm translocator, which would interfere with the ability of the microorganisms to direct formation of the morphologically distinct replication vacuole. Alternatively, the bacteria could proliferate slowly once a properly constructed vacuole is formed. These possibilities can be distinguished, because mutations that impair translocator function result in strains that traffic to the macrophage endocytic network. Furthermore, interference with Dot/Icm function should result in depressed efficiency of substrate translocation into target cells. To test for defective trafficking of the L. pneumophila-containing vacuole, macrophages incubated for one hour with bacteria were assayed for colocalization of this compartment with the endocytic marker LAMP-1 (Andrews et al., 1998). About 2-3 times as many pcsA− bacteria colocalized with LAMP-1 compared to the low DotA expressing control (Fig. 3A). These levels of colocalization approached those of a strain having a defective Dot/Icm translocator (Fig. 3A; dotA-pcsA+). The defect that led to colocalization with LAMP-1 compartments was totally reversed by complementation with a plasmid that expressed pcsA+ (Fig. 3A)
Figure 3. The function of the Dot/Icm system is impaired by loss of PC.
(A). Loss of PC results in defective targeting of the Legionella-containing vacuole. Bone marrow-derived macrophages were incubated with noted L. pneumophila strains for 25 min and colocalization of the late endosome/lysosome glycoprotein marker LAMP-1 with bacteria was scored by immunofluorescence microscopy (Experimental Procedures). (B). The absence of PC results in defective translocation of a Dot/Icm substrate. Bacteria were incubated with bone marrow-derived macrophages for one hour and scored for the colocalization of SidC by immunofluorescence microscopy (Experimental Procedures. High DotA: strains contain wild type levels of DotA protein. Low DotA: Strains express attenuated amounts of DotA (Conover et al., 2003). High DotA strains used: pcsA+, LP02. Low DotA strains used: pcsA+, GL233; pcsA−; GL263; pcsA−/ppcsA+, GL266; dotA−, GL83 . ****: P<8.2 × 10−4. Data shown are the mean ± standard deviation of 3 independent experiments in which 104 phagosomes were quantified for each strain.
The lack of PC also led to a reduction in the efficiency of effector translocation (Fig. 3B). Macrophages incubated for one hour with L. pneumophila strains were fixed and probed with antibody directed against SidC, a substrate of the Dot/Icm system that localizes on the cytoplasmic face of the vacuolar membrane (Experimental Procedures; (Luo and Isberg, 2004)) Although the efficiency of translocation of SidC was relatively low in the parental pcsA+ strain that has reduced amounts of DotA protein, the lack of PC resulted in levels of SidC translocation that were far lower than the wildtype (Fig. 3B). This defect was fully complemented by the presence of pcsA in trans (P < 8.2 × 10−4; Fig. 3B) indicating that the absence of PC clearly interfered with proper Dot/Icm function.
Defective adhesion to macrophages by PC mutants
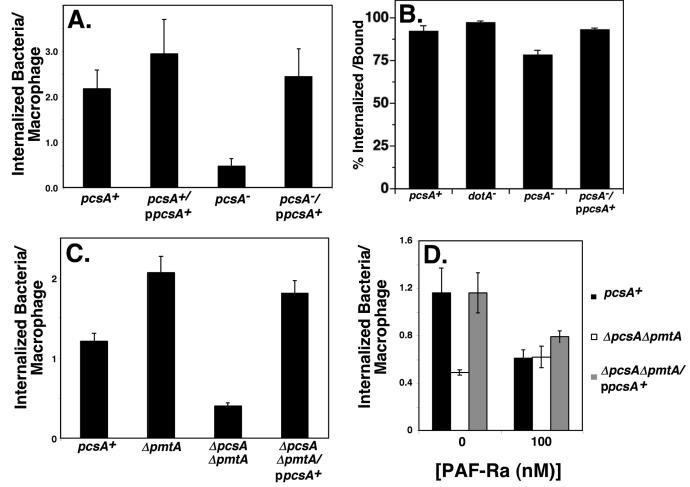
From the growth curves (Fig. 2A) and examination of replication vacuoles (Figs. 2C and 3A), it was clear that there were two levels of defect caused by the lack of PC. First there was a depressed yield of bacteria after three days incubation with macrophages. Secondly, the number of bacteria internalized by macrophages appeared to be lower for the PC mutants. To compare internalization levels by target cells, L. pneumophila strains were incubated with macrophages for 1 hour, fixed and assayed for internalization by microscopy, using an antibody probing strategy (Experimental Procedures). Internalization of a pcsA− mutant was approximately 3-5 fold less efficient than strains expressing either chromosomal and/or plasmid-encoded copies of pcsA+ (Fig. 4A; pcsA−). Uptake was fully restored by introducing a wild type copy of pcsA+ into the mutant background (Fig. 4A; pcsA−/ppcsA+), and the identical results were obtained using an alternative assay for internalization involving gentamicin protection (data not shown; (Lobo and Mandell, 1973)). The defect in uptake was due to reduced association of the mutant with macrophages rather than disrupted internalization of bound bacteria. Uptake of the macrophage-associated pcsA− mutant was indistinguishable from a number of other L. pneumophila strains (Fig. 4B). We also found that the kinetics of cell association differed for the wild type and pcsA− mutant. The maximal association of the mutant occurred immediately after its centrifugation onto macrophages, whereas the wild type showed increasing association over a one hour time period of incubation with macrophages (data not shown).
Figure 4. Absence of PC results in defective association with target macrophages.
(A). Efficient cell association requires the activity of PcsA. Macrophages were infected at MOI ≈ 10, after synchronizing uptake with a 5 min centrifugation at room temperature followed by 10 min incubation at 37°C. The number of internalized bacteria per macrophage was scored by double indirect immunofluorescence microscopy. (B). Uptake efficiency of bound bacteria. Uptake efficiency was determined by antibody probing as in panel A. This measurement is defined as the percentage of cell associated bacteria that are internalized. (C). Bacterial association with macrophages is altered by the absence of PmtA. Internalization was determined as above. (D). PAF-Ra inhibits PC-dependent uptake. Macrophages were pretreated with PAF-R antagonist (1-O-hexadecyl-2-acetyl-sn-glycerol-3-phospho-(N,N,N-trimethyl)-hexanolamine; PAF-Ra) for 30 min., bacteria were allowed to bind for 10 minutes at 37°C, and attachment was assayed as above. Data shown are the mean ± standard deviations of triplicate determinations. Experiment was performed 4 times, and displayed is a typical experiment. Strains used were: pcsA+, GL233; pcsA+/ppcsA+, CCL29; pcsA−, GL263; pcsA−/ppcsA+, GL266; dotA−, GL83; ΔpmtA, CCL25; ΔpmtA ΔpcsA, CCL26; ΔpmtA ΔpcsA/ppcsA, CCL39.
The absence of the pmtA gene caused an unexpected effect. Although the ΔpcsAΔpmtA double mutant was defective for uptake by macrophages, the simple loss of pmtA from L. pneumophila resulted in a modest stimulation of bacterial association with macrophages (Fig. 4C). This was observed either by analyzing a deletion (Fig. 4C; ΔpmtA) or the double mutant complemented in trans with pcsA+ (Fig. 4C; ΔpcsAΔpmtA/ ppcsA+). It is possible that the mono- and dimethyl intermediates in the pathway promoted by the PmtA enzyme may interfere with L. pneumophila adhesion, and their absence in the pmtA− mutant is stimulatory for uptake. Alternatively, since we observed enhanced PC content in both the pmtA mutant and the complemented clone relative to wild type (Fig. 1E), the increased efficiency of cell association may be directly related to the increased PC content of these strains.
The possibility that lipid derivatives could interfere with PC-dependent uptake was further pursued. Absence of bacterial PC could indirectly reduce uptake by interfering with the ability of the bacterium to export a ligand that is bound by a cellular receptor, or directly, by preventing the synthesis of a PC-derived molecule that is recognized by the macrophage. There is precedent for respiratory pathogens using choline-derivatives as ligands for recognition by cellular receptors, in which case adhesion is inhibited by small molecule PAF receptor antagonists (PAF-Ra) (Swords et al., 2000; Cundell et al., 1995). When a PAF-Ra was added during a short incubation of bacteria with macrophages, it specifically interfered with uptake of the pcsA+ strains, and had no effect on association of the ΔpcsAΔpmtA mutant (Fig. 4D). We saw similar levels of inhibition when, instead of PAF-Ra, PC vesicles were added to the infections (data not shown). These results are consistent with the model that some bacterial PC derivative plays a direct role in microbial association with macrophages.
The absence of PC interferes with selected functions associated with bacterial transmission to macrophages
A number of L. pneumophila virulence-associated functions are preferentially induced during the post-exponential phase of growth, and the induction of these factors is correlated with efficient establishment of intracellular growth (Hammer et al., 2002; Byrne and Swanson, 1998). These properties include the Dot/Icm-dependent induction of macrophage cell death after high multiplicity infection, enhanced bacterial motility, and the induction of expression of translocated substrates of the Dot/Icm system (Luo and Isberg, 2004). Each of these properties was analyzed.
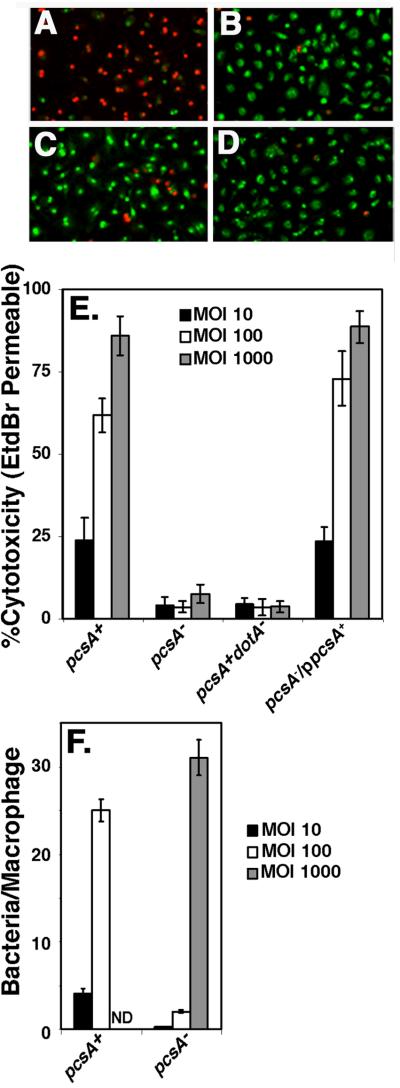
Rapid cell death to macrophages resulting from bacterial contact-dependent high multiplicity infection has been attributed to the insertion of a bacterium-derived pore into host macrophages by the Dot/Icm system (Zink et al., 2002; Kirby et al., 1998). To assay for this function, macrophages were allowed to come into contact with bacteria for one hour (Fig. 5A-D). A pcsA− mutant was defective for contact-dependent cytoxicity (Fig. 5A-D). This result was observed at all MOI tested, with the levels of cytotoxicity only slightly higher than a dot/icm− strain. An intact pcsA+ gene provided in trans completely restored cytotoxicity (Fig. 5E). To determine if the defect in association of bacteria with the macrophage (Fig. 4) was the cause of the defect in cytotoxicity, bacteria were introduced at various MOI in the cytotoxicity assay and then analyzed for efficiency of bacterial binding to macrophages. At an MOI = 1000, there was little cytotoxicity (Fig. 5E), although the pcsA− mutant had large numbers of bacteria bound to macrophages (Fig. 5F). In contrast, when a similar number of pcsA+ bacteria were bound to cells (using a 10 fold lower MOI; Fig. 5F), there was large-scale cytoxicity (Fig. 5E). Therefore, the defect in cytotoxicity is not due to a defect in bacterial association with host cells.
Figure 5. The defect in Dot/Icm-dependent cytotoxicity caused by the absence of PC is not the result of lack of adhesiveness.
Bacteria were allowed to incubate with macrophages for 55 min at 37°C at the indicated series of MOI with the following strains: pcsA+, GL84; pcsA−, GL263; pcsA+dotA−, GL83; pcsA−/ppcsA+, GL266. Merged images of 10X magnifications of incubations with bone marrow macrophages incubated at an MOI = 100 are displayed for: (A) dotA+pcsA+, (B) dotA− pcsA+, (C) pcsA− dotA+, and (D) lidC dotA+ (Conover et al., 2003). Green cells are those staining with acridine orange, while red cells are those that are dead and permeable to EtdBr. (E). Defective cytotoxicity in a pcsA− mutant is independent of bacterial dose. Cytotoxicity was measured by identifying cells permeable to ethidium bromide (EtdBr), which was added after removing the medium immediately after the infection period. % cytotoxicity was determined as the percentage of cells observed to be EtdBr-permeable in the rhodamine fluorescence channel relative to the total number of acridine orange stained cells observed in the FITC channel, using grabbed images (Experimental Procedures). (F) Incubation of the pcsA− mutant at high multiplicity allows adhesion of bacteria to macrophages. In parallel to the cytotoxicity assay, incubations were fixed, bacteria were identified with an anti-L. pneumophil antibody and the number of bacteria bound per macrophage was determined at the noted MOI. Strains are same as in panel (E). Quantitation of binding at MOI = 1000 for the pcsA+ strain was not determined (ND). All experiments were repeated twice.
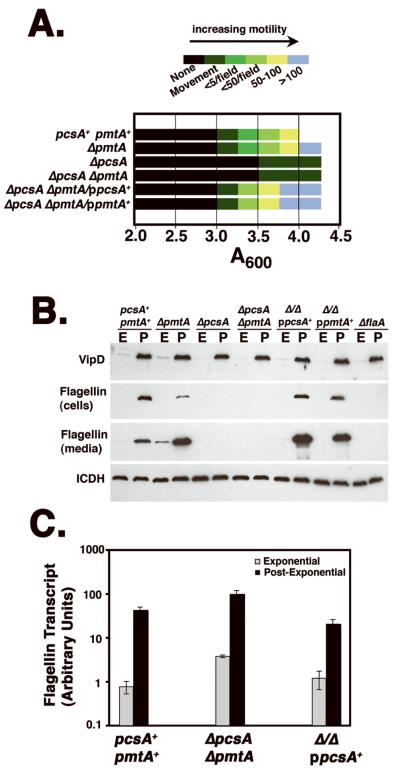
The defective cytotoxicity is attributed to either defective Dot/Icm assembly (Kirby et al., 1998) or an inability of the bacterium to assume a state of heightened competence for intracellular growth that has been closely correlated with entry into postexponential phase of broth culture (Molofsky and Swanson, 2004). This latter property has been penned the “Transmission Phenotype” to denote that the postexponential phase allows the bacteria to be transmitted from an extracellular locale to an intracellular niche and initiate replication efficiently (Molofsky and Swanson, 2004). To test for properties that are similarly associated with the Transmission Phenotype, the PC-defective mutants were assayed for motility and growth phase-dependent expression of translocated substrates. Mutants lacking PcsA were highly defective for establishing a motile state in postexponential phase (Fig. 6A), with only a few bacteria showing some movement in very dense bacterial cultures. In contrast, strains competent for PC synthesis, including the ΔpmtA strain, and PC mutants expressing either pcsA+ or pmtA+ on a plasmid, were able to establish a high degree of motility (Fig. 6A).
Figure 6. Selective defects in L. pneumophila factors associated with high efficiency growth in host cells.
(A). A deficit in PC results in a block in induction of motility in postexponential phase. Bacteria were grown to in culture in AYE broth and at noted A600 readings, a drop of culture was placed on a coverslip and several fields using a 40X lens were analyzed for the presence of motile bacteria. The degree of motility was noted using the color-coded scale in the figure, with each color representing the number of bacteria observed moving per field. If no bacteria were showing overt motility, but there was occasional movement of tethered bacteria, these cultures were scored as “movement,” which was the first sign that some degree of motility was beginning to occur in a culture. Experiment was repeated three times. (B) The absence of PC causes a reduction in steady state flagellin production. The noted bacterial strains were grown in AYE medium (Experimental Procedures) to either exponential (A600= 1.4-1.8;“E”) or postexponential phase (A600= 3.5-4.0; “P”). The cells were adjusted to the identical densities, pelleted, subjected to SDS-PAGE on a 10% gel, and either cell pellet (cells) or culture supernatant (media) were immunoprobed with antibody to VipD, L. pneumophila flagellin or to isocitrate dehydrogenase (ICDH), to control for identical loading conditions. Strains: pcsA+pmtA+, GL233; ΔpmtA, CCL25; ΔpcsA, CCL24; ΔpmtAΔpcsA, CCL26; Δ Δ /ppcsA+, CCL39; Δ Δ /ppmtA+, CCL41. (C). The defect in flagellin production occurs at the post-transcriptional level. Bacteria from exponential (E) or post-exponential (P) phase were prepared and subjected to qPCR, using flagellin structural gene (flaA)-specific primers. Amount transcript was then determined as described (Experimental Procedures). Experiment was preformed twice.
Although two phenotypes (motility and cytotoxicity) associated with the Transmission Phenotype were defective in mutants lacking PC, the mutants were still able to respond to postexponential phase signals based on the levels of translocated Dot/Icm substrates associated with the bacteria. In postexponential phase cultures, bacteria accumulate the translocated substrate VipD (Fig. 6B, “P;”), with little VipD expression during logarithmic growth (Fig. 6B, “E;” (VanRheenen et al., 2006; Shohdy et al., 2005)). In all mutants tested, including the ΔpcsAΔpmtA double mutant, this growth phase response appeared identical to wild type (Fig. 6B; pcsA+pmtA+). Similar results were observed with the substrate SidC (data not shown). Therefore, it is unlikely that the absence of PC prevents detection of nutrient conditions associated with entry into the Transmissive phase.
As the lack of motility was unlikely to be due to general misregulation of post-exponential phase transcription, we looked directly for a loss in the steady state levels of flagellin protein due the absence of PC (Fig. 6B). Mutants with defects in PC biosynthesis (either ΔpcsA or ΔpcsAΔpmtA) were devoid of either cell-associated or culture-associated flagellin after growth to post-exponential phase, a defect that could be complemented by either plasmid borne pcsA+ or pmtA+(Fig. 6B). Consistent with the lack of effects of these deletion mutations on postexponential phase expression, transcription of the flagellin (flaA) structural gene was highly induced when cultures approached stationary phase (Fig. 6C). Therefore, the lack of motility observed in PC-defective mutants can be attributed to a post-transcriptional defect in accumulation of flagellin protein. Furthermore, as both cell association and cytotoxicity of L. pneumophila have been previously reported to be highly reduced in flaA− mutants (Molofsky et al., 2005), the loss of assembled flagellin is at least part of the reason that defects in host cell interaction were observed in bacteria lacking PC.
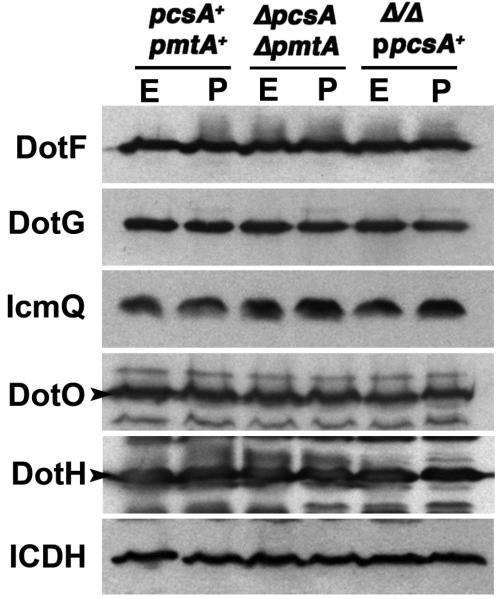
In contrast to the lowered flagellin levels observed in the absence of PC, the expression and stability of individual components of the Dot/Icm complex appeared unaffected. Immunoprobing with antibody directed against inner membrane components DotF, DotG and DotO, the outer membrane protein DotH and the cytoplasmic IcmQ showed that steady state levels for each of these proteins were identical in wild type, ΔpcsAΔpmtA, and ΔpcsAΔpmtA/ppcsA+ strains (Fig. 7). The lack of effect was observed in both exponential and post-exponential phase, and was identical to what was observed for the control cytoplasmic protein isocitrate dehydrogenase (Fig. 7). Therefore, although translocation rates of Dot/Icm substrates were reduced in the absence of PC, the cause of this defect was not due to global reduction of steady state levels of Dot/Icm components in mutants having lowered PC content.
Figure 7. Absence of PC does not affect the steady state levels of a subset of Dot/Icm proteins.
The noted bacterial strains were grown in AYE medium (Experimental Procedures) to either exponential (A600= 1.4-1.8; “E”) or post-exponential phase (A600=3.5-4.0; “P”). The cells were adjusted to identical densities, pelleted, lysed in sample buffer and resolved by SDS-PAGE. Proteins were blotted to PVDF membranes and immunoprobed with rabbit antibodies to DotF, DotG, DotO, DotH, or IcmQ. Samples were also immunoprobed with antibodies to isocitrate dehydrogenase (ICDH) to control for identical loading conditions. Strains: pcsA+ pmtA+, GL233; ΔpcsA ΔpmtA, CCL26; ΔpcsA ΔpmtA/ ppcsA+, CCL41.
DISCUSSION
As most bacterial species do not have the capacity to synthesize phosphatidylcholine (PC), the reason for the presence of PC in some bacterial species has remained mysterious. It was originally proposed that bacteria with large amounts of internal membranes, such as photosynthetic bacteria, have a requirement for this lipid (Hagen et al., 1966). Interestingly, several species that interact intimately with host cells have significant PC content in their membranes, so this lipid may play an important function in allowing these microorganisms to interface with their hosts (Lopez-Lara and Geiger, 2001; Goldfine, 1984). There had been little information supporting this hypothesis, and each species having PC may synthesize the lipid for a different reason. The original observation supporting its importance was the demonstration that mutations depressing PC synthesis in the nitrogen fixing Bradyrhizobium japonicum cause reduced root nodule occupancy (Minder et al., 2001). Similarly, Brucella abortus mutants defective for synthesis of PC show unexplained defects in cell interactions and virulence (Comerci et al., 2006; Conde-Alvarez et al., 2006). The clearest connection between a molecular defect and a phenotype is the case of A. tumefaciens, in which loss of virulence in PC-defective mutants results from lowered transcription of the genes encoding the T DNA transfer apparatus, a result very different from what we observed with the Dot/Icm complex (Fig. 7). There is no mechanism, however, that links membrane composition to transcription of this locus (Wessel et al., 2006). The experiments described here are consistent with an important role for PC in the virulence of an intracellular pathogen. Many of the defects observed could be ascribed to loss of the flagellin protein, which unlike the A. tumefaciens example, occurs at the post-transcriptional level.
The low yield of bacteria after infection of macrophages by PC- mutants appears to be the consequence of two related defects: poor initiation of intracellular replication and a defect in the ability of the bacteria to associate with macrophages. Once initiation of replication occurs, however, the rate of replication of these mutants did not appear strongly altered by the absence of PC. This small effect on replication rate is somewhat surprising given the increased association with LAMP-containing compartments for mutants relative to wild type. Having a lowered initial level of infection, however, could be the result of both an uptake defect as well as loss of viability of the improperly targeted bacteria. Therefore, the viable PC- mutants seen at early timepoints may be primarily those that have targeted properly and which will eventually replicate. Furthermore, different initial doses of infected macrophages results in nonidentical monolayer conditions that can greatly affect the rate of bacterial intracellular replication. For instance, we have observed that at low MOI, there is a detectable cytokine response that is a function of bacterial infectivity levels (M. Liu and R. Isberg, data not shown). This response, in which monolayers having lower numbers of viable bacteria result in less restriction of L. pneumophila growth, makes it difficult to directly correlate replication rates to targeting defects.
There is a large body of work indicating that L. pneumophila entry into post-exponential phase allows expression of functions required for productive growth in macrophages (Byrne and Swanson, 1998). We found that the loss of PC interfered with proper function of a subset of activities associated with this state, such as cytotoxicity, motility, cell association and intracellular growth (Molofsky and Swanson, 2004). These defects did not appear to be a result of an inability to respond to signals resulting from nutrient depletion, as cultures of wild type strains and PC- mutants showed identical induction of both translocated substrates and the flagellin structural gene after entry into post-exponential phase. The absence of PC, however, did result in the loss of flagellin protein even though the flaA structural gene transcript was strongly induced in post-exponential phase (Fig. 6). As lack of flagella on the bacterial cell surface reduces both bacterial binding to macrophages as well as cytotoxicity (Molofsky et al., 2005), the aberrant cellular interactions observed in PC- mutants may largely result from an inability to maintain high steady state levels of the flagellin protein. The molecular basis for the absence of this protein is unclear. Presumably, lack of PC prevents post-exponential phase assembly of the flagellar complex, with the result that unassembled subunits are degraded. PC may be required for assembly of this complex in L. pneumophila because the events involved in production of flagella all occur in nondividing bacteria, potentially raising problems not observed during exponential phase assembly. PC has many characteristics that may alter protein interactions. For instance, it has been shown to antagonize folding of the LacY protein, indicating it may control folding rates of inner membrane proteins (Bogdanov et al., 1999; Goldfine, 1984).
The second explanation for the role of PC is that the choline head group directly interacts with target cells. In particular, some mucosal pathogens are believed to use bacterial surface molecules that have been modified by phosphorylcholine as ligands for host cell receptors (Swords et al., 2000; Cundell et al., 1995). The PC head group could act as a source for such modifications. The primary evidence that bacterial phosphorylcholine is recognized by mammalian receptors are studies indicating that an antagonist of platelet activating factor receptor (PAF-Ra) interferes specifically with adhesion of phosphorylcholine-modified bacterial ligand (Swords et al., 2000; Cundell et al., 1995). As PAF has a phosphorylcholine group, it is attractive to speculate that mammalian receptors that recognize the PAF-Ra antagonist also recognize phosphorylcholine-modified bacterial cell surfaces. Similar to results in Streptococcus pneumoniae and Haemophilus influenzae, we found that the PC-dependent cell adhesion of L. pneumophila could be blocked by the addition of a PAF-Ra, with adhesion of a mutant lacking PC being totally resistant to the action of the antagonist (Fig. 4). Mammalian cells may directly recognize an L. pneumophila ligand that contains either the phosphocholine headgroup or a derivative of PC. Consistent with this model, we found that the efficiency of bacterial association with macrophages was a direct function of the PC concentration, as derivatives that had higher PC levels than wild type also associated with macrophages more efficiently than wild type, as if a PC derivative were limiting for cell association.
The clear preference of L. pneumophila for using PcsA, rather than the PmtA pathway must be explained. One possibility is that the products of the PmtA pathway negatively impact either bacterial viability or the ability of the organism to interact with the host. Unlike PcsA, which only produces PC as a product, the PmtA pathway results in low-level synthesis of the monomethylated and dimethylated derivatives of phosphatidylethanolamine, which have uncertain roles in membrane biology. These lipids could interfere with either adhesion of the cells or assembly of membrane proteins. A second explanation for the predominance of the PcsA pathway is that the synthase is a direct sensor of environmental conditions, using choline availability as an indicator of the status of the locale in which the bacterium is found. Limiting choline concentrations may be a signal for the bacterium to either slow its growth or make compensatory changes in lipid side chain composition to accommodate environments in which choline is limiting. The presence of large pools of choline could be a signal for the bacterium that host cells are available for intracellular replication, allowing the proper assembly of envelope components critical for host cell interaction.
Although it may be counterintuitive that the bacterium relies on an extracellular source of precursor rather than utilize its own biosynthetic pools, the fact that interaction with hosts is a central aspect of the biology of L. pneumophila must be an important determinant of how this preference for PcsA arose. PC synthesis appears to be a critical sensor of the nature of the environment surrounding the microorganism. Biosynthetic precursors found in lung tissue, therefore, can modulate virulence of this organism by controlling the ability of the organism to incorporate PC into its cell envelope. Investigating how the environment controls the PC content in cells and how this phospholipid modulates host cell interaction are of central importance for understanding the pathogenesis of L. pneumophila.
EXPERIMENTAL PROCEDURES
Bacterial strains, plasmids, cell culture and media
The bacterial strains and plasmids used in this study are listed with their relevant characteristics in Table 1. The L. pneumophila strains used are derivatives of the L. pneumophila Philadelphia 1 strain Lp02 (thyA Δ[hsdR-lvh] rpsL) (Berger and Isberg, 1993), and were grown on casamino acids-yeast extract (CYE) solid medium or ACES-yeast extract (AYE) broth (Berger and Isberg, 1993). For L. pneumophila, antibiotics were used at the following concentrations: chloramphenicol, 5 μg/ml; kanamycin, 40 μg/ml; streptomycin, 50 μg/ml; and gentamicin, 50μg/ml. The growth media were supplemented with thymidine at 100 μg/ml or 5% sucrose when appropriate. The L. pneumophila strains used in all assays were grown to post-exponential phase (OD600 ≈ 3.2 – 3.5) unless stated otherwise. Antibiotics were used at the following concentrations with E.coli strains: kanamycin, 40 μg/ml; ampicillin 100 μg/ ml and chloramphenicol, 30 μg /ml.
Table 1.
Bacterial strains and plasmids used in this work.
| Plasmid / Strain |
Genotype/ Relevant Characteristics | Reference |
|---|---|---|
| PLASMIDS | ||
| pDOT1 | pMMB66EH tdΔi bla+ dotA+ | (Berger and Isberg, 1993) |
| pSR47s | KanRsacB suicide vector | (Merriam et al., 1997) |
| pGC36 | pKB5 dotA+ | (Conover et al., 2003) |
| pGC41 | pGC36 pcsA+ | This study |
| pTC5 | pGC36 pmtA+ | This study |
| pTC6 | pSR47s ΔpcsA::GmR | This study |
| pGC51 | pSR47s ΔpmtA | This study |
| pKB5 | pMMB66EH ori RSF1010 tdΔi bla+ | (Berger and Isberg, 1993) |
| STRAINS 1 | ||
| LP02 | Philadelphia-1 rpslL Δ[hsdR-lvh] thyA- | (Berger and Isberg, 1993) |
| LP03 | LP02 dotA03- thyA- | (Berger and Isberg, 1993) |
| JR32 | Philadelphia-1 hsdR | (Sadosky et al., 1993) |
| GL83 | LP02dotA / pKB5 | (Conover et al., 2003) |
| GL84 | LP02dotA / pDOT1 | (Conover et al., 2003) |
| GL233 | LP02dotA / pGC36 | (Conover et al., 2003) |
| GL263 | LP02 pcsA::miniTn10kan dotA/pGC36 | This study, NT |
| GL266 | LP02 pcsA::miniTn10kan dot /pGC41 | This study, NT |
| GL301 | LP02 ΔpmtA dotA- | This study |
| GL302 | LP02 ΔpmtA dotA / pGC36 | This study |
| GL303 | LP02 ΔpmtA thyA- | This study |
| GC197 | LP02 pcsA::miniTn10kan dotA | (Conover et al., 2003) |
| CCL1 | LP02 ΔpmtA dotA thyA- | This study |
| CCL17 | LP02 ΔpcsA::GmR dotA-/ pGC41 | This study |
| CCL21 | LP02 ΔpmtA ΔpcsA::GmR dotA- | This study |
| CCL24 | LP02 ΔpcsA::GmR dotA-/ pGC36 | This study |
| CCL25 | LP02 ΔpmtA dotA-/ pGC36 | This study |
| CCL26 | LP02 ΔpmtA ΔpcsA::GmR dotA- / pGC36 |
This study |
| CCL29 | LP02/pGC41 | This study |
| CCL39 | LP02 ΔpmtA ΔpcsA::GmR dotA-/ pGC41(dotA+ pcsA+) |
This study |
| CCL41 | LP02 ΔpmtA ΔpcsA::GmR dotA-/ pTC5(dotA+ pmt+) |
This study |
All strains are derivatives of L. pneumophila Philadelphia 1
All DNA manipulations were as described previously (Conover et al., 2003).
Primary bone marrow-derived macrophages were isolated from the femurs of female A/J mice and maintained as described (Swanson and Isberg, 1995). Typically, 1×105 macrophages were plated per well for immunofluorescence studies, and 4-5 × 105 macrophages per well were used to monitor bacterial growth.
Construction of the pcsA and pmtA deletion mutants
The pcsA(lidk)::miniTn10 insertion was isolated previously (Conover et al., 2003). The ΔpmtA deletion on the L. pneumophila chromosome was isolated by first constructing an internal deletion in pmtA using PCR generated fragments, leaving 6 amino acids at the amino terminus and 3 amino acids at the carboxyl terminus (Supplemental Data). These were then cloned on the suicide plasmid pSR47s (kanRsacB) to generate the plasmid pGD51. The plasmid was introduced into appropriate L. pneumophila strains as described (Conover et al., 2003). After selecting for integration by plating on kanamycin-containing medium, transconjugants were purified and plated onto 5% sucrose CYET plates to select for sucrose resistant recombinants that had lost the plasmid. Construction of the ΔpcsA::GmR mutant used the same strategy, with a GmR fragment inserted into a total deletion of the pcsA (lidK) gene in the suicide plasmid pSR47s (Supplemental Data). Construction of the ΔpcsA::GmR ΔpmtA double mutation in a strain harboring the plasmid pGC41td+pcsA+ (Thy+) is described in the text (Fig. 2). The double mutant was isolated in the LP03 strain background, which has a chromosomal thyA− mutation. This allowed a selection for curing (loss) of the pGC41 plasmid, which has the phage T4 td gene enabling growth in the absence of thymidine (Berger and Isberg, 1993), but also rendering the strain trimethoprim sensitive. In the presence of medium containing trimethoprim and thymidine, bacteria harboring pGC41 are unable to grow, while strains that have lost pGC41 are resistant to trimethoprim, as described (Berger and Isberg, 1993).
Construction of complementation plasmids
The two complementing plasmids encoding the complete ORFs of pcsA (pGC41) and pmtA (pTC5) were constructed in the plasmid pGC36 (Conover et al., 2003) by placing each gene under the control of Ptac in an operon with td?i. The complementing genes containing sufficient upstream DNA to allow efficient translation were amplified by PCR from chromosomal DNA using primers with the indicated engineered restriction sites.
Lipid profiles of L. pneumophila PC mutants
L. pneumophila cultures (1 ml) were grown in AYE at 37°C to an optical density of A600=3.5, labeled for 40 min with 5 μl of [1-14C]acetate (60 mCi/mmol; 200 μCi/ml) and subsequently harvested. Lipids were then extracted (Bligh and Dyer, 1959) and analyzed by 2-D thin layer chromatography (TLC) (de Rudder et al., 1997). The TLC plates were exposed to Kodak X-Omat film, lipid spots were scraped, and quantified by liquid scintillation counter. In addition to the results reported, strains growing in exponential phase were also analyzed, using an identical approach.
LAMP-1 targeting assay
L. pneumophila strains were grown and tested in the LAMP-1 targeting assay exactly as previously described (Conover et al., 2003). The number of LAMP-1-positive phagosomes per bacterial strain was quantified by counting cell-associated bacteria per strain (N = 312). The average and standard deviation reported here were calculated from three independent infections per strain.
Intracellular growth assays
Intracellular growth curves were performed in bone marrow-derived macrophages from the A/J mouse as described, incubating macrophages with bacteria at an MOI ≈ 0.05 or as indicated (Conover et al., 2003). Each experiment was performed in triplicate.
To examine microscopically the intracellular growth of L. pneumophila inside macrophages, mammalian cells were plated at density of 1×105 per coverslip and infected with the indicated strains at MOI ≈ 1, fixed in in PLP-sucrose (McLean and Nakane, 1974) and analyzed as described previously (Conover et al., 2003). The number of intracellular bacteria in each phagosome in separate macrophages was recorded by visual inspection. Data shown represent the distribution of phagosomes containing 1 to 3, 4 to 9 and more than 10 bacteria. These data were calculated by averaging triplicate samples of 104 scored phagosomes per coverslip.
Cytotoxicity assay
Contact dependent cytotoxicity assays were performed in macrophages plated on coverslips 24 hours prior to infection, as described performing 1 hour infections at 37°C in 5% CO2 (Kirby et al., 1998). Pore-forming activity was measured as the percentage of total cells that stained positive for ethidium bromide relative to acridine orange stained cells.
Immunofluoresence assay of bacterial uptake and SidC translocation
Mammalian cells were plated one day prior to infection with the indicated post-exponentially grown motile L. pneumophila. Unless otherwise indicated, the infected macrophages were spun for 5 minutes and the infection proceeded at 37°C for the times indicated, until stopped by three washes with RPMI medium. Samples were fixed in PLP- sucrose and stained for extracellular bacteria with rabbit anti-L. pneumophila serum and Texas Red-anti rabbit IgG. The monolayers were then methanol permeabilized (Conover et al., 2003) and stained with rabbit anti-L. pneumophila serum and FITC-anti rabbit IgG to detect total bacteria. Percentage of internalized/bound bacteria was scored as fraction of bacteria resisting staining with Texas Red-IgG but which stain with FITC-IgG. The data reported in each experiment is the average of triplicate infections scoring 104 macrophages per coverslip.
Translocation of the Dot/Icm substrate SidC was performed as described (Luo and Isberg, 2004; Conover et al., 2003). Macrophages were incubated with noted bacterial strains for one hour, and fixed samples were then probed with affinity purified rabbit anti-SidC and rat anti-L. pneumophila, followed by detection with fluorescent secondary antibodies. 104 phagosomes per coverslip were scored, and data were expressed as mean percentage of phagosomes showing staining for SidC ± S.D.
Assay for bacterial uptake in platelet activating factor receptor antagonist (PAF-Ra) treated macrophages
Platelet Activating Factor-16 Antagonist (PAF-Ra, Calbiochem, La Jolla, CA, catalog # 511082) was dissolved in EtOH following manufacturer specifications. The compound was serially diluted in RPMI to obtain the appropriate concentration. Macrophages were pretreated at 37°C with 100 nM PAF-Ra for 30 minutes prior to infection as described (Swords et al., 2000). Once the drug was added to the mammalian cells, they were immediately infected with post-exponential motile L. pneumophila at MOI ≈ 10 for 10 minutes. The infection was stopped by extensive washes and the samples were processed for immunofluorescence as described above.
Immunoprobing of L. pneumophila lysates for post-exponential phase expressed proteins
For analysis of growth-stage specific expression of proteins, L. pneumophila strains were grown at 37° C in AYE broth. The equivalent of 0.5 OD units of each strain were harvested at both exponential (A600 = 1.0-1.5) and post-exponential (A600 = 3.5-4.0) phases of growth, centrifuged at 16,000 × g to separate bacterial cells from culture supernatants and the resulting supernatants were frozen at −80° C for later analysis. Bacterial cell pellets were then resuspended in 150 μl SDS-PAGE sample buffer and boiled for 5 minutes. 5 μl of each sample was resolved by SDS-PAGE and analyzed by Western blotting using various antisera. For the analysis of flagellin in culture supernatants, frozen supernatants were thawed on ice and trichloroacetic acid was added to a final concentration of 10%. Samples were vortexed for 1 min and incubated on ice for 20 min. Samples were pelleted at 16,000 × g for 10 min and resulting precipitates were washed twice with ice-cold ethanol and air dried for 15 min at 37° C. Dried pellets were then resuspended with 30 μl SDS-PAGE sample buffer and boiled for 5 min. 10 μl of each sample was resolved by SDS-PAGE and analyzed by Western blotting using rabbit antisera against the noted L. pneumophila proteins. Serum specific for B. subtilus isocitrate dehydrogenase (ICDH) was provided by A. L. Sonenshein (Tufts University School of Medicine, Boston MA). Serum specific for flagellin was provided by W. F. Dietrich (Harvard Medical School, Boston MA), antisera directed against L. pneumophila proteins were raised previously in our laboratory. Affinity purified serum directed against VipD has been previously described (VanRheenen et al., 2006).
RNA extraction, reverse transcription, and real-time PCR
To determine the amounts of flaA transcript in L. pneumophila strains by using realt time quantitative PCR (qPCR), L. pneumophila was grown in AYE medium at 37°C and samples were removed in either late exponential phase (A600= 1.0) or postexponential phase (A600= 3.5) to prepare RNA. Total RNA was then isolated using RNeasy (QIAGEN). Reverse transcription was performed on the samples using 1 μg of RNA with Superscript II reverse transcriptase (Invitrogen) and random decamers (Ambion). qPCR was performed in real time in a Clontech quantitative thermocycling spectrophotometer using the SYBR green PCR master mix (Applied Biosystems) with primers specific for flaA (flaA forward: 5′ CCATCAGCAGCAGTGAGTGT 3′, flaA reverse: 5′TCAATGGCGTCAGTAACCAA 3′) and 16S rRNA (16S forward: 5′ CTAAGGAGACTGCCGGTGGTGAC, 16S reverse: 5′CGTAAGGGCCATGATGACTT 3′). The transcript levels of flaA were normalized to 16s rRNA values for each condition and displayed in graphical format.
Supplementary Material
Acknowledgments
We thank Matthias Machner, Tamara O'Connor, Marion Dorer and Zhiru Li for review of the manuscript. This work was supported by Training Grant T32AI1007422, NIDDK Center Grant P30 DK34928, a fellowship from the Life Sciences Foundation to Z-Q. Luo, an NIH postdoctoral fellowship to M.H. and grants from the Consejo Nacional de Ciencia y Tecnología de México (CONACyT 42578/A-1), as well as the Howard Hughes Medical Institute (HHMI 55003675) to O. Geiger. R. I. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- Andrews HL, Vogel JP, Isberg RR. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arondel V, Benning C, Somerville CR. Isolation and functional expression in Escherichia coli of a gene encoding phosphatidylethanolamine methyltransferase (EC 2.1.1.17) from Rhodobacter sphaeroides. J Biol Chem. 1993;268:16002–16008. [PubMed] [Google Scholar]
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Umeda M, Dowhan W. Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J Biol Chem. 1999;274:12339–12345. doi: 10.1074/jbc.274.18.12339. [DOI] [PubMed] [Google Scholar]
- Buscher BA, Conover GM, Miller JL, Vogel SA, Meyers SN, Isberg RR, Vogel JP. The DotL protein, a member of the TraG-coupling protein family, is essential for viability of Legionella pneumophila strain Lp02. J Bacteriol. 2005;187:2927–2938. doi: 10.1128/JB.187.9.2927-2938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JW, Yu VL. Legionella: a major opportunistic pathogen in transplant recipients. Semin Respir Infect. 1998;13:132–139. [PubMed] [Google Scholar]
- Comerci DJ, Altabe S, de Mendoza D, Ugalde RA. Brucella abortus Synthesizes Phosphatidylcholine from Choline Provided by the Host. J Bacteriol. 2006;188:1929–1934. doi: 10.1128/JB.188.5.1929-1934.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Alvarez R, Grillo MJ, Salcedo SP, de Miguel MJ, Fugier E, Gorvel JP. Synthesis of phosphatidylcholine, a typical eukaryotic phospholipid, is necessary for full virulence of the intracellular bacterial parasite Brucella abortus. Cell Microbiol. 2006;8:1322–1335. doi: 10.1111/j.1462-5822.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- Conover GM, Derre I, Vogel JP, Isberg RR. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol. 2003;48:305–321. doi: 10.1046/j.1365-2958.2003.03400.x. [DOI] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- de Rudder KE, Lopez-Lara IM, Geiger O. Inactivation of the gene for phospholipid N-methyltransferase in Sinorhizobium meliloti: phosphatidylcholine is required for normal growth. Mol Microbiol. 2000;37:763–772. doi: 10.1046/j.1365-2958.2000.02032.x. [DOI] [PubMed] [Google Scholar]
- de Rudder KE, Sohlenkamp C, Geiger O. Plant-exuded choline is used for rhizobial membrane lipid biosynthesis by phosphatidylcholine synthase. J Biol Chem. 1999;274:20011–20016. doi: 10.1074/jbc.274.28.20011. [DOI] [PubMed] [Google Scholar]
- de Rudder KE, Thomas-Oates JE, Geiger O. Rhizobium meliloti mutants deficient in phospholipid N-methyltransferase still contain phosphatidylcholine. J Bacteriol. 1997;179:6921–6928. doi: 10.1128/jb.179.22.6921-6928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG, Harris J, Mallison GF, Martin SM, McDade JE, et al. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Gao LY, Abu Kwaik Y. Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect Immun. 1999;67:4886–4894. doi: 10.1128/iai.67.9.4886-4894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine H. Bacterial membranes and lipid packing theory. J Lipid Res. 1984;25:1501–1507. [PubMed] [Google Scholar]
- Hagen PO, Goldfine H, Williams PJ. Phospholipids of bacteria with extensive intracytoplasmic membranes. Science. 1966;151:1543–1544. doi: 10.1126/science.151.3717.1543. [DOI] [PubMed] [Google Scholar]
- Hammer BK, Tateda ES, Swanson MS. A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol Microbiol. 2002;44:107–118. doi: 10.1046/j.1365-2958.2002.02884.x. [DOI] [PubMed] [Google Scholar]
- Hindahl MS, Iglewski BH. Isolation and characterization of the Legionella pneumophila outer membrane. J Bacteriol. 1984;159:107–113. doi: 10.1128/jb.159.1.107-113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JE, Vogel JP, Andrews HL, Isberg RR. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- Lobo MC, Mandell GL. The effect of antibiotics on Escherichia coli ingested by macrophages. Proc Soc Exp Biol Med. 1973;142:1048–1050. doi: 10.3181/00379727-142-37173. [DOI] [PubMed] [Google Scholar]
- Lopez-Lara IM, Geiger O. Novel pathway for phosphatidylcholine biosynthesis in bacteria associated with eukaryotes. J Biotechnol. 2001;91:211–221. doi: 10.1016/s0168-1656(01)00331-5. [DOI] [PubMed] [Google Scholar]
- Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko E, Richards JC, Cox AD, Stewart A, Martin A, Kapoor M, Weiser JN. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales F, Schobert M, Lopez-Lara IM, Geiger O. Pathways for phosphatidylcholine biosynthesis in bacteria. Microbiology. 2003;149:3461–3471. doi: 10.1099/mic.0.26522-0. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Merriam JJ, Mathur R, Maxfield-Boumil R, Isberg RR. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun. 1997;65:2497–2501. doi: 10.1128/iai.65.6.2497-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minder AC, de Rudder KE, Narberhaus F, Fischer HM, Hennecke H, Geiger O. Phosphatidylcholine levels in Bradyrhizobium japonicum membranes are critical for an efficient symbiosis with the soybean host plant. Mol Microbiol. 2001;39:1186–1198. [PubMed] [Google Scholar]
- Molofsky AB, Swanson MS. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol Microbiol. 2004;53:29–40. doi: 10.1111/j.1365-2958.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Shetron-Rama LM, Swanson MS. Components of the Legionella pneumophila flagellar regulon contribute to multiple virulence traits, including lysosome avoidance and macrophage death. Infect Immun. 2005;73:5720–5734. doi: 10.1128/IAI.73.9.5720-5734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash TW, Libby DM, Horwitz MA. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl Environ Microbiol. 1994;60:1585–1592. doi: 10.1128/aem.60.5.1585-1592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham TJ. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadosky AB, Wiater LA, Shuman HA. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G, Shuman HA. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JA, Vogel JP. Type IVB secretion by intracellular pathogens. Traffic. 2002;3:178–185. doi: 10.1034/j.1600-0854.2002.030303.x. [DOI] [PubMed] [Google Scholar]
- Shohdy N, Efe JA, Emr SD, Shuman HA. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc Natl Acad Sci USA. 2005;102:4866–4871. doi: 10.1073/pnas.0501315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlenkamp C, de Rudder KE, Rohrs V, Lopez-Lara IM, Geiger O. Cloning and characterization of the gene for phosphatidylcholine synthase. J Biol Chem. 2000;275:18919–18925. doi: 10.1074/jbc.M000844200. [DOI] [PubMed] [Google Scholar]
- Sohlenkamp C, Lopez-Lara IM, Geiger O. Biosynthesis of phosphatidylcholine in bacteria. Prog Lipid Res. 2003;42:115–162. doi: 10.1016/s0163-7827(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Stewart AG, Grigoriadis G. Structure-activity relationships for platelet-activating factor (PAF) and analogues reveal differences between PAF receptors on platelets and macrophages. J Lipid Mediat. 1991;4:299–308. [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE, Buscher BA, Ver Steeg Ii K, Preston A, Nichols WA, Weiser JN, Gibson BW, Apicella MA. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- VanRheenen SM, Luo Z-Q, O'Connor T, Isberg RR. Members of a Legionella pneumophila family of proteins with ExoU/phospholipase A active sites are translocated to target cells. Infect Immun. 2006;74:3597–3606. doi: 10.1128/IAI.02060-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- Wessel M, Klusener S, Godeke J, Fritz C, Hacker S, Narberhaus F. Virulence of Agrobacterium tumefaciens requires phosphatidylcholine in the bacterial membrane. Mol Microbiol. 2006;62:906–915. doi: 10.1111/j.1365-2958.2006.05425.x. [DOI] [PubMed] [Google Scholar]
- Wilderman PJ, Vasil AI, Martin WE, Murphy RC, Vasil ML. Pseudomonas aeruginosa synthesizes phosphatidylcholine by use of the phosphatidylcholine synthase pathway. J Bacteriol. 2002;184:4792–4799. doi: 10.1128/JB.184.17.4792-4799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink SD, Pedersen L, Cianciotto NP, Abu-Kwaik Y. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect Immun. 2002;70:1657–1663. doi: 10.1128/IAI.70.3.1657-1663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.