Abstract
Argentine hemorrhagic fever (AHF), a systemic infectious disease caused by infection with Junin virus, affects several organs, and patients can show hematologic, cardiovascular, renal, or neurologic symptoms. We compared the virulence of two Junin virus strains in inbred and outbred guinea pigs with the aim of characterizing this animal model better for future vaccine/antiviral efficacy studies. Our data indicate that this passage of the XJ strain is attenuated in guinea pigs. In contrast, the Romero strain is highly virulent in Strain 13 as well as in Hartley guinea pigs, resulting in systemic infection, thrombocytopenia, elevated apartate aminotransferase levels, and ultimately, uniformly lethal disease. We detected viral antigen in formalin-fixed, paraffin-embedded tissues. Thus, both guinea pig strains are useful animal models for lethal Junin virus (Romero strain) infection and potentially can be used for preclinical trials in vaccine or antiviral drug development.
INTRODUCTION
Junin virus, a member of the family Arenaviridae, is the etiologic agent of Argentine hemorrhagic fever (AHF), a human disease first described in 1955.1 Infection with Junin virus typically manifests as a non-specific febrile illness, which then may progress to the severe form, AHF, characterized by multiorgan symptoms with gastrointestinal involvement, hemorrhage, shock, and in some cases, coma. Ultimately, death may result from AHF; the case-fatality rate is estimated to be 15–30% if untreated. Laboratory findings include leukopenia, thrombocytopenia, proteinuria,2 and slightly elevated aspartate aminotransferase (AST) levels.
Arenaviruses are enveloped and contain two segments of ambisense single-stranded RNA, from which an RNA-dependent RNA polymerase (protein L) and four structural proteins are produced: nucleocapsid associated protein, Z protein, and two enveloped glycoproteins (GP1 and GP2) that arise from proteolytic cleavage of the cell-associated precursor.3 The geographic distribution of particular arenaviruses is generally determined by the presence of the natural reservoir, with Tacaribe serocomplex (New World group) viruses found in North and South America, whereas the Lassa-lymphocytic choriomeningitis serocomplex (Old World group) is found in Africa, although one member, lymphocytic choriomeningitis virus, is distributed worldwide. Classification in the respective serocomplex is in accordance with antigenic properties (antibody to glycoprotein, detected by virus neutralization assay) as well as phylogenetic analysis.4
Junin virus is transmitted by direct contact with the rodent reservoir, mainly Calomys musculinis, or by aerosol from the rodent excreta, and is predominantly associated with agricultural activities.5 Junin virus was first isolated in 1958 from a human case6,7 and subsequently, has been isolated from rodents as well as from humans.8–12 No Junin virus–specific antiviral drug exists, although studies suggest that ribavirin may be an effective therapy13,14 and convalescent-phase plasma is used for treatment; despite the ability of convalescent-phase plasma to reduce the fatality ratio to less than 1%, approximately 10% of treated patients develop a transient cerebellar–cranial nerve syndrome.15,16 In addition, a safe, efficacious Junin virus vaccine, Candid 1, has been developed and is in use in risk groups to reduce disease in Argentina.5,17
Several experimental animal models exist for study of Junin virus–mediated pathogenesis, as multiple rodent species (C3H and athymic mice,18–21 guinea pigs,22 and rats23), as well as New and Old World primates (Cebus paella, Callithrix jacchus, and Macaca mulatta)24–28 are susceptible to infection with Junin virus. However, the pathogenesis, e.g., 50% lethal dose, timing of death, and the viral distribution in organs, varies widely, depending upon the animal species, virus strain, and/or passage history,22,25,29 and is age-dependent.23,30,31 Newborn athymic (nude) mice develop persistent, relatively avirulent infection after intracranial inoculation of Junin virus, whereas nude mice in which bone marrow reconstitution is performed (AT × BM mice) develop fatal encephalitis similarly to age-matched immunocompetent mice.20,32,33 However, guinea pigs and rhesus macaques, when infected by the intraperitoneal or aerosol route, respectively, most closely manifest a disease syndrome similar to AHF22,25,34–37 and have been used for the evaluation of antiviral drugs as well as Junin virus vaccine candidates.38,39 Yet the cost, space, and safety concerns have limited the use of primates in high-level biocontainment.
In an effort to establish an accessible and consistent model for the testing of antiviral drugs in a Biosafety Level 4 (BSL 4) facility, we have evaluated the pathogenesis of Junin virus in inbred (Strain 13) and outbred (Hartley) guinea pigs by using Junin XJ and Romero strains. The Junin XJ strain was isolated from human blood and underwent 37 suckling mouse brain passages prior to cell culture inoculation to create a virus stock in our laboratory. The Romero strain was isolated from a patient and was passed twice in fetal rhesus lung cells and once in Vero cells.25 The primary aim of our investigation was to characterize the differences between XJ and Romero strains of Junin virus in two alternative guinea pig strains.
MATERIALS AND METHODS
Cells
Vero cells (American Tissue Culture Collection, Manassas, VA) were maintained at 37°C in minimum essential medium (MEM) supplemented with 10% fetal bovine serum and vitamins.
Viruses
The Junin Romero strain (GenBank accession nos. AY619640 and AY619641) was obtained from Dr. Thomas G. Ksiazek (Centers for Disease Control and Prevention, Atlanta, GA). The Junin XJ strain (GenBank accession nos. AY358022 and AY358023), which was isolated from human blood in 1958 and passaged 37 times in suckling mouse brain, was provided by Dr. Robert Tesh (University of Texas Medical Branch, Galveston, TX). Virus stock for animal infection studies was obtained by amplification in Vero E6 cells and stored at −80°C in 1-mL aliquots until use. All work with Junin virus was performed in the University of Texas Medical Branch (UTMB) BSL 4 facility in accordance with institutional health and safety guidelines.
Viral replication in cell culture
Cells were seeded at a concentration of 5 × 105/35-mm diameter dish. After a 4-hour incubation at 37°C, monolayers were infected at a multiplicity of infection of 0.1, incubated for 1 hour at 37°C, and the inoculum was then replaced with MEM. At selected times after infection, supernatants were harvested, and virus titers in the harvested samples were determined by a plaque assay on Vero cells.
Plaque assay
The level of infectious virus in supernatants of Junin virus–infected cells or in tissue homogenates was assessed by plaque assay on Vero cells. Ten-fold specimen dilutions were added to cell monolayers in six-well culture plates for 1 hour at the 37°C in an atmosphere of 5% CO2, and then overlaid with MEM containing 8% fetal bovine serum, 1% penicillin–streptomycin solution, and 0.5% agarose. Plates were incubated for five days, and a second agarose overlay was added (0.5% agarose/0.05% neutral red in MEM). Plates were then incubated overnight at 37°C and plaques were counted the next day.
Virus sequencing
To confirm virus identity, RNA was extracted from the supernatant obtained from Romero virus– or XJ virus–infected cells, reverse transcription–polymerase chain reaction (RT-PCR) was performed, and the amplicons were sequenced. The RNA was isolated from the cell culture supernatants with Trizol reagent (Invitrogen, Carlsbad, CA), followed by chloroform extraction and isopropanol precipitation, as recommended by the manufacturer. The RNA was resuspended in 15 µL of sterile, nuclease-free water and frozen at −80°C. Five microliters of the RNA extract was used in each RT reaction (Superscript III; Invitrogen) using random primers to generate cDNA for the PCR. The PCR primers were designed on the basis of conserved sequence of glycoprotein precursor sequences of XJ and Romero strains of Junin virus (GenBank accession nos. U70800 and AY619641, respectively): 305d, 5′-TGTCCTTCTCAATGGTGGGTC-3′ and 1196r 5′-CCTGTTGTGAGTAATGGTTCC-3′. The PCR products were submitted to the UTMB Core Facility for sequencing. Sequence confirmed virus stock was used for growth curves and for animal experiments as described below.
Animals
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and were carried out according to National Institutes of Health (Bethesda, MD) guidelines. Guinea pigs were anesthetized using an isoflurane precision variable bypass vaporizer prior to virus inoculation by the intraperitoneal route. Standardized recording of death and disease symptoms was performed using the following definitions: encephalitis; development of discoordination, ataxia or transient seizures with retention of the ability to drink and feed; paralysis; and hind limb (hemiplegic) or quadriplegic paralysis with the inability to reach the feeder or water bottle.
Infection with the Junin virus XJ strain
Eight- to twenty-week-old female Strain 13 (United States Army Medical Research Institute for Infectious Disease [USAMRIID], Fort Detrick, MD) and 5- to 10-week-old female Hartley guinea pigs (Charles River, Wilmington, MA) were infected with Junin XJ strain using doses in the range of 1.5 × 103 to 5 × 105 PFU/animal (Strain 13) or 1.0–1.5 × 103 PFU/animal (Hartley) and monitored for 16–19 days. Daily telemetric monitoring of body temperature was performed for 14 days.
Infection with the Junin virus Romero strain
Eight- to twenty-week-old female Strain 13 (USAMRIID) and one-year-old female Hartley guinea pigs (Charles River) were infected with Junin Romero strain using doses in the range of 1.5 × 103 to 6 × 105 PFU/animal (Strain 13) or 7.5 × 103 PFU/animal (Hartley). Animals were monitored as described for Junin XJ virus studies.
Hematologic and clinical chemical analysis
Blood was collected from guinea pigs into tubes containing EDTA and standard hematologic analysis was performed using the HEMAVET® 1700; Drew Scientific, Inc. (Waterbury, CT) on whole blood specimens to determine platelet and differential counts, in accordance with the manufacturer’s recommendation. Clinical chemical analysis was performed using the ACE Alera™ Clinical Chemistry System (Alfa Wassermann, West Caldwell, NJ) according to the manufacturer’s instructions.
Telemetry
For measurement of body temperature, animals were anesthetized and implanted subcutaneously with BMDS IPTT-300 transponders (chips) obtained from Bio Medic Data Systems, Inc. (Seaford, DE), using a trocar needle assembly. Animals were monitored for signs of infection or migration of transponder for two days prior to transfer of animals into the BSL-4 facility. Chips were scanned daily using a DAS-6007 transponder reader (Bio Medic Data Systems, Inc.). Downloading of digital temperature data was performed in accordance with the manufacturer’s protocol.
Infectious virus titration in organs
For quantitation of Junin virus in tissues, specimens were dissected at necropsy, and homogenized in MEM containing 1% penicillin–streptomycin solution. Suspensions were clarified by centrifugation, and the supernatants were harvested and frozen at −80°C until analysis was performed. The titer of infectious virus was determined using a plaque assay.
Histopathologic analysis
Brain, liver, and spleen sections were fixed in 10% buffered formalin for a minimum of 7 days and stored in 70% ethanol for 12 hours. The samples were then embedded in paraffin, sectioned (4 µm), mounted on slides, and standard staining with hematoxylin and eosin was performed. Histopathologic analysis of tissue sections was performed by a pathologist blinded to the sample identification and grouping. Semi-quantitative scoring was determined as indicated in Table 1.40
TABLE 1.
Semi-quantitative histopathologic analysis of spleen and liver from Strain 13 guinea pigs 9 days after infection with Junin virus
| Histopathology description/score† | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental description* | Spleen | Liver | |||||||
| Junin virus | Dose (PFU/animal) | Guinea pig no. | FN | AI | WPN | PI | LI | N | S |
| Romero | 5.0 × 103 | GP1 | 0 | 2 | 1 | 0 | 2 | 0 | 1 |
| GP2 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | ||
| GP3 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | ||
| 0 | 2 | 1 | 1 | 1 | 0 | 1 | |||
| XJ | 1.5 × 103 | GP12 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| GP13 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| GP14 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | ||
| 0 | 1 | 0 | 0 | 1 | 0 | 0 | |||
| – | – | GP15 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| GP16 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| 0 | 0.5 | 0 | 0.5 | 1.5 | 0 | 0 | |||
Eight- to twenty-week-old female Strain 13 guinea pigs were infected with the indicatedstrain and dose (in plaque-forming units [PFU]) of Junin virus by the intraperitoneal route,or as a control, age- and sex-matched Strain 13 guinea pigs were mock-infected (indicated by−) by the intraperitoneal route. Randomly pre-selected animals were humanely killed at9-days post-infection, and the spleen and liver were harvested for histopathologic analysis,performed as described in the Materials and Methods.
FN = fibrinoid necrosis; AI = acute inflammation; WPN = white pulp necrosis; PI =portal inflammation; LI = lobular inflammation; N = necrosis; S = steatosis; 0 = none; 1= mild, 2 = moderate; 3 = severe. Tabulated score is indicated on the bottom row.
Immunohistochemical analysis
Tissue sections were deparaffinized and rehydrated through xylene and graded ethanol solutions. To block endogenous peroxidase activity, slides were then treated with a solution of Tris-buffered saline containing 0.1% Tween-20, 3% hydrogen peroxide, and 0.03% sodium azide for 15 minutes, followed by heat antigen retrieval in a water bath at 95°C for 40 min in DAKO Target Retrieval Solution, pH 6.1 (DAKO Corporation, Carpinteria, CA). To block endogenous biotin reactivity, sequential 15-minute incubation with avidin D and biotin solutions (Vector Laboratories, Inc; Burlingame, CA) was performed. Subsequently, to prevent nonspecific protein binding, sections were incubated in blocking solution (Histomouse™-SP Kit, catalog no. 95-9541; Zymed, South San Francisco, CA), according to manufacturer’s instructions. For viral antigen staining, mouse-specific hyperimmune ascitic fluid (HIS) raised against Junin XJ virus, which cross-reacts with the Junin Romero virus, was used (provided by Dr. Robert Tesh), as follows. Tissue sections were incubated for 60 minutes with HIS at a 1:500 dilution in antibody diluent solution (BD Pharmingen, San Jose, CA). Tissue sections from uninfected guinea pigs were used as a negative control for immunostaining. As an additional negative control, tissue sections from Junin virus–infected guinea pigs were incubated with diluent alone. To detect HIS bound to Junin antigen in guinea pig tissue, the Histomouse™-SP Kit (catalog no. 95-9541; Zymed) and biotinylated secondary antibody was used, followed by streptavidin-peroxidase. Color development was achieved using the chromogenic substrate, according to the manufacturer’s instructions. Slides were counter-stained with Mayer’s modified hematoxylin for microscopy.
RESULTS
Replication of Junin virus Romero and XJ strains in vitro
To determine the kinetics of Junin virus XJ and Romero replication, we infected Vero cells at a multiplicity of infection of 0.1 PFU/cell and collected virus supernatants over a period of 6 days at 24-hour time points for virus titration (Figure 1A). The peak in virus replication for both strains occurred at 72-hours post-infection for both Junin virus strains. However, the level for the Romero strain was higher then the XJ strain after 72-hours post-infection.
FIGURE 1. In vitro and in vivo studies of Junin virus.
A, In vitro replication of Junin virus. Vero cell monolayers were infected with Junin virus (XJ or Romero strain) per cell at multiplicity of infection of 0.1 and supernatant was harvested at 24-hour time points. Production of infectious virus was measured by plaque assay on Vero cells. After a 5-day incubation, cells were overlaid with agarose and stained with neutral red solution. The number of plaques per milliliter of supernatant is indicated for each virus strain. The limit of detection was 40 plaque-forming units (PFU)/mL. B, Survival of guinea pigs infected with Junin Romero strain. Eight- to twenty-week-old female inbred (Strain 13) or one-year old female outbred (Hartley strain) guinea pigs were infected by the intraperitoneal route with the indicated dose of Junin Romero strain virus. Animals were monitored up to 19-days post-infection for disease development or death. Animals were humanely killed upon development of severe disease, e.g., paralysis or encephalitis. Histopathologic examination of spleens and livers from three inbred animals that developed severe disease is shown in Table 3. Survival of inbred (panel i) and outbred (panel ii) guinea pigs is shown. C, Level of infectious virus in brain and peripheral organs after infection with Junin Romero strain. Eight- to twenty-week-old inbred (Strain 13) guinea pigs were infected by the intraperitoneal route with Junin Romero strain in the range of 1.5 × 103 to 6 × 105 PFU/animal or were inoculated with minimal essential medium alone (mock). Necropsy was performed on animals that were pre-scheduled for humane killing at 9 days post-infection (n = 3) or that developed severe disease, e.g., paralysis or encephalitis, between 9 and 13 days post-infection (n = 4). Organs were collected for analysis of virus levels by plaque assay. The total number of animals tested is indicated for each organ. The limit of detection of 50 PFU/g tissue is indicated by the dashed line.
Attenuation of Junin virus XJ strain in both guinea pig strains
Neither Hartley (n = 9, two independent replicates) nor Strain 13 (n = 17, two independent replicates) guinea pigs developed severe disease after infection with the XJ strain of Junin virus at doses in the range of 1 × 103 to 5 × 105 PFU/animal. With the exception of development of fever at a low frequency on 17 days post-infection (dpi) (2 of 3 and 1 of 4 guinea pigs infected with a dose of 2.5 × 104 and 5 × 105 PFU, respectively), there were no signs of disease development in Junin XJ–infected Strain 13 guinea pigs. There was little or no weight loss over the study period. None of the Junin XJ–infected animals developed severe clinical disease symptoms or fever at any time prior to or on the day of scheduled humane killing (day 9). Histopathologic examination of spleen sections from Strain 13 guinea pigs nine days after infection with Junis virus XJ at a dose of 1.5 × 103 PFU (Table 1) indicated that three of three infected animals had a mild level of acute inflammation in contrast to 1 of 2 with mild inflammation for the mock-infected controls; one of three had a mild level of white pulp necrosis, whereas no white pulp necrosis was observed in the two control animals. Liver examination indicated that one of three infected animals had a moderate level of portal inflammation in contrast to mild portal inflammation in one of two mock-infected controls. Three of three infected animals showed mild-to-moderate level of lobular inflammation (LI); similarly, two of two mock-infected controls showed mild-to-moderate lobular inflammation.
Pathogenicity of Junin virus Romero strain in inbred and outbred guinea pigs
In contrast to the limited pathogenicity of the XJ strain in either guinea pig strain (described above), infection with the Romero strain produced severe disease symptoms, e.g., clinical encephalitis or paralysis, in Strain 13 guinea pigs, and was uniformly lethal by 19 dpi (Figure 1B, panel i). To further examine guinea pig strain differences, we infected one-year-old female, Hartley guinea pigs (n = 4) with a dose of 7.5 × 103 PFU/animal of Romero strain. In Hartley guinea pigs, the survival pattern was similar to that for Strain 13; infection with the Romero strain was 100% lethal by 17 dpi (Figure 1B, panel ii). A steady decrease in body weight occurred after 7 dpi. All animals that maintained their monitoring chip became febrile at 10 dpi (three of three animals with a body temperature ≥ 39.5°C) and at 14 dpi, body temperature rapidly decreased, with a 15–24% decrease between 12 and 14 dpi. Clinical signs initially were observed at 12–13 dpi. Fifty percent (two of four) of the guinea pigs developed clinical encephalitis and 25% (one of four) developed paralysis and subsequently died at 17 dpi. Infectious virus levels were highest in the spleen, liver, and adrenal glands with log10 PFU/mL in the range of 4.5–7.7, 5.3–7.7, and 7.4–7.7, respectively (Figure 1C); levels were also relatively high in the brain and kidney, with values in the range of 2.4–4.7 and 4.4–5.4 log10 PFU/g, respectively.
In a second Romero strain infection study, Strain 13 guinea pigs (n = 7) were infected with a dose of 5 × 103 PFU/animal and monitored for disease development and survival up to nine days. At 9 dpi, all animals were humanely killed and the level of infectious virus in the organs was determined (Figure 1C). Histopathologic analysis was performed on three of these animals (Table 1). None of these animals showed signs of severe clinical disease at any time during the monitoring period; however, fever occurred between day 5 and 6 (3 of 3, 100% febrile on day 6). As in the first study (survival shown in Figure 1B, panel i), virus was detectable in the spleen, liver, and brain on 9 dpi in Romero strain-infected animals, indicating that virus spread to and replicated in all organs tested. In addition, blood was collected at 9 dpi from three of the infected animals and from the two uninfected animals for analysis of the hematologic profile, clinical biochemical patterns, and serum antibody titer (Table 2). At 9 dpi, all animals tested (three of three) were positive by plaque reduction neutralization test (PRNT) on day 9, with 50% PRNT titers in the range of 25–50 (Table 2).
TABLE 2.
Indices of severe disease development by 9-days post-infection in Junin Romero virus–infected Strain 13 guinea pigs
| Hematology, clinical chemistry and neutralizing antibody at indicated time point† | ||||||
|---|---|---|---|---|---|---|
| Experimental description* | PLT (K/µL) | AST (U/L) | ||||
| Junin virus |
Animal ID |
No. febrile days |
Pre- infection (day 0) |
Post- infection (day 9) |
Post- infection (day 9) |
50% PRNT (day 9)‡ |
| Romero | GP-01 | 3 | 610 | 224 | 157 | 1:50 |
| GP-02 | 4 | 774 | 246 | 111 | 1:25 | |
| GP-03 | 3 | 474 | 188 | 94 | 1:25 | |
| Uninfected | GP-15 | 0 | 369 | 584 | 55 | < 1:25 |
| GP-16 | 0 | 541 | 634 | NT | NT | |
Eight- to twenty-week-old Strain 13 guinea pigs were infected with 5 × 103 plaque-forming units/animal of Junin Romero virus by the intraperitoneal route. Disease development, body temperature, and survival were monitored for 19 days. Fever development (body temperature > 39.5°C) is summarized by individual animal.
Blood was drawn at the indicated time points pre-infection or post-infection for hematologic and clinical analysis. Representative parameters, e.g., platelet count (PLT) and aspartate aminotransferase (AST) levels, respectively, are shown. The normal ranges for guinea pigs are PLT = 380–800 K/µL (Mascot™ Hematology Profile, HEMAVET® 1700; Drew Scientific, Inc., Waterbury, CT) and AST = 26.5–67.5 IU/L (ACE Alera™ Clinical Chemistry System, Alfa Wassermann, West Caldwell, NJ). NT = not tested.
Seroconversion was assessed by 50% plaque reduction neutralization test (PRNT). Samples that were below the limit of detection are indicated as < 1:25.
Among the hematologic parameters evaluated (leukocyte, neutrophil, lymphocyte, monocyte, and eosinophil counts and thrombocyte and erythrocyte panels) for Romero virus–infected and uninfected control guinea pigs, platelet (PLT) count differences were the most notable among parameters tested (Table 2); The PLT was reduced at 9 dpi in three of three guinea pigs tested in comparison to the corresponding pre-infection (day 0) baseline values obtained for each the indicated animal, and the two uninfected guinea pigs tested in parallel, for which no reduction occurred.
The following clinical biochemistry parameters were analyzed for samples obtained at 9 dpi: amylase, alanine aminotransferase, aspartate aminotransferase, calcium, cholesterol, albumin, creatinine, glucose, and phosphorous. However, paired pre-infection baseline (day 0) samples were not analyzed. Most of the parameters tested did not show notable differences between infected animals and uninfected control animals, and values were sufficiently variable among all animals (irrespective of whether infected or uninfected) to preclude any statement on trends related to infection. However, among these parameters, AST levels for infected animals (n = 3) were higher than those in the two uninfected controls tested (Table 2).
Histopathologic changes in liver and spleen of Romero virus–infected inbred and outbred guinea pigs
Histologic examinations were performed on Strain 13 guinea pigs infected with Romero virus (n = 6), XJ virus (n = 3) or, as controls, mock-infected (n = 2) or uninfected (n = 1) Strain 13 guinea pigs. Representative hematoxylin and eosin–stained sections of liver and spleen from Romero and XJ virus–infected animals are shown in Figure 2A and B). Sections were examined by a pathologist blinded to the grouping of the animals. A numerical score for liver and spleen pathology was assigned on the basis of modification of a scoring system previously reported.40 Scores for tissues of Junin virus–infected guinea pigs collected 9 dpi are summarized in Table 1. No clinical disease (with the exception of fever) was observed in these three animals.
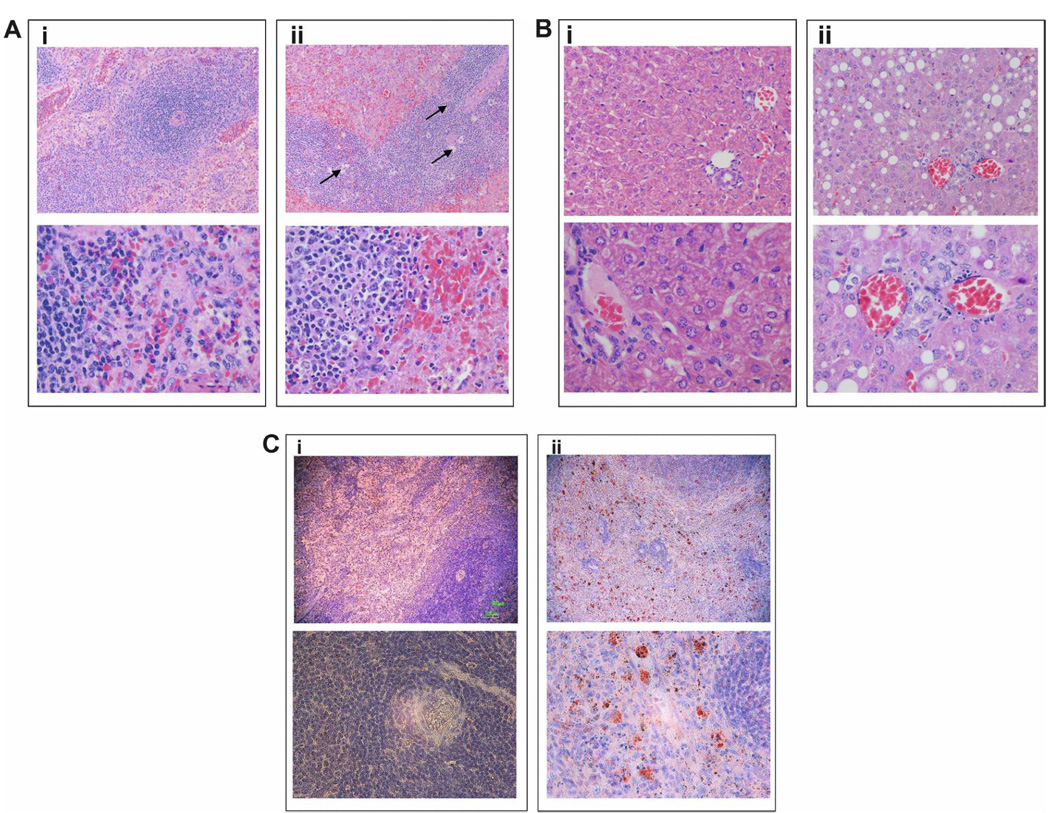
FIGURE 2. Comparative histopathology and tissue viral antigen distribution of Junin Romero virus-infected inbred guinea pigs.
A and B, Histopathologic analysis of spleen and liver. Eight- to twenty-week-old Strain 13 guinea pigs were infected with 5 × 103 plaque-forming units per animal of Junin Romero virus by the intraperitoneal route. Animals were humanely killed at the time of development of severe neurologic symptoms. The spleen and liver were harvested at the indicated day post-infection for histopathologic analysis (see scoring in Table 1), performed as described in the Materials and Methods. Representative spleen (A) and liver (B) sections from mock-infected (panel i) and Junin Romero-infected (panel ii) guinea pigs at 12-days post-infection are shown. Upper panels shown low power (10×) magnification and bottom panels show high power (40×) magnification. Arrows indicate apoptotic cells and tingible body macrophages. C, Viral antigen in spleen. Eight- to twenty-week-old female Strain 13 guinea pigs were infected with a dose of 5.0 × 103 Junin Romero virus by the intraperitoneal route. Animals were humanely killed at 9-days post-infection, and the spleen and liver were harvested for histopathologic analysis (see scoring in Table 1), performed as described in the Materials and Methods. Uninfected sex- and age-matched Strain 13 guinea pigs were used as controls. Viral antigen localization was determined by incubation of spleen sections with Junin hyperimmune serum. Anti-Junin binding in tissue was detected using a secondary biotinylated antibody and color development performed using streptavidin-peroxidase, followed by addition of a chromogenic substrate. Slides were counterstained with Mayer’s modified hematoxylin prior to mounting and microscopy. Representative spleen sections from uninfected-(panel i) and Junin Romero-infected (panel ii) guinea pigs at 12-days post-infection are shown. Upper panels shown low power (10×) magnification and bottom panels show high power (40×) magnification. Viral antigen (brown-red) was detected in association with macrophage-like cells in both red pulp and white pulp.
Scores for animals in a second study are shown in Table 3. Severe disease (paralysis, encephalitis, and/or seizure) occurred on the day of humane killing for the three animals evaluated. Noteworthy pathologic changes were seen in spleen (Figure 2A, panel ii) and liver (Figure 2B, panel ii) of Romero-infected guinea pigs at 12 dpi in comparison to the mock-infected controls (Figure 2A and B, panel i) and lesions were consistent among infected animals. Spleen showed a “moth-eaten” appearance of the white pulp with numerous tingible body macrophages and large, reactive pale cells suggestive of macrophages (Figure 2A, panel ii). This change involved up to 50% of the white pulp in all animals examined. The red pulp contained clusters of neutrophils, particularly in the region of the marginal zones. Among the severely affected animals humanely killed at later time points of 12–13 dpi, the red pulp showed generalized cellular depletion, with smudgy or fibrinoid necrosis and scattered nuclear debris. Liver pathology in animals examined at day 9 of infection with Romero strain was characterized by diffuse microvesicular steatosis, mild portal inflammation, and mild lobular inflammation. Kupffer cell hyperplasia, acidophil bodies, or nuclear inclusions were not appreciable. In animals examined at later time points (day 12–13), steatosis was more pronounced, predominantly macrovesicular, without zonal predeliction (Figure 2B, panel ii). Scattered foci of nuclear debris were present in portal triads and in lobular foci, suggesting leukocyte degeneration. Patchy coagulative necrosis was seen in the Romero virus–infected animal examined at 9 dpi. In contrast, guinea pigs infected with the XJ strain (n = 3) at a similar dose (1.5 × 103 PFU/animal) showed minimal splenic and hepatic pathology. In random sections of cerebrum, cerebellum, and hippocampus, no significant inflammation or necrosis was identified in brains of any (none of three, each virus strain) of the Junin Romero– or XJ–infected guinea pigs using routine histologic stains. Immunohistochemical staining with Junin-specific antiserum indicated that viral antigen is distributed in association with large cells with abundant cytoplasm, consistent with macrophages, in both red pulp and white pulp (Figure 2C).
TABLE 3.
Semi-quantitative histopathologic analysis of spleen and liver from Junin Romero virus strain–infected Strain 13 guinea pigs at the time of development of severe neurologic symptoms
| Histopathologic description/score† | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental description* | Spleen | Liver | |||||||
| Dose (PFU/animal) | Organ harvest (day of study) | Guinea pig no. | FN | AI | WPN | PI | LI | N | S |
| 1.50 × 103 | 9 dpi | GP15 | 0 | 1 | 2 | 1 | 1 | 0 | 2 |
| 1.50 × 103 | 12 dpi | GP17 | 1 | 0 | 2 | 1 | 1 | 0 | 2 |
| 2.50 × 103 | 13 dpi | GP20 | NA | NA | NA | 0 | 1 | 0 | 2 |
Eight- to twenty-week-old female Strain 13 guinea pigs were infected with the indicated dose (in plaque-forming units [PFU]) of Junin Romero strain virus by the intraperitoneal route. Animals were humanely killed at the time of development of severe neurologic symptoms. The spleen and liver were harvested at the indicated day post-infection (dpi) for histopathologic analysis, performed as described in the Materials and Methods. The survival curves for animals that were followed-up throughout the study period of 19 days are shown in Figure 1B, panel i.
FN = fibrinoid necrosis; AI = acute inflammation; WPN = white pulp necrosis; PI = portal inflammation; LI = lobular inflammation; N = necrosis; S = steatosis; 0 = none; 1 = mild, 2 = moderate; NA = not available.
DISCUSSION
To better characterize the animal model for future studies aimed at testing efficacy of antiviral drug or vaccine candidates, we have performed additional pathogenesis studies with Junin viruses in two guinea pig strains. One of the requirements for the successful application of animal models in preclinical trials is the availability of well-characterized animal models that reproduces human disease as closely as possible. Although some non-human primates such as Rhesus monkeys are excellent animal models,25,35,36 small animals, especially rodents, are advantageous for the work in the BSL-4 laboratory and more easily accessible for experimental work.
It was previously shown that guinea pigs infected with virulent Junin virus develop (dose-dependent) severe and often fatal disease, which is similar to 10–30% of human clinical cases.2 In general, two forms of the disease can be described: the visceral form and the neurologic form. Some viral strains are more viscerotropic and others may potentially have increased neurotropism.22 However, both disease forms can be observed in Junin Romero virus–infected guinea pigs. In our study, Romero virus–infected guinea pigs developed a systemic disease associated with clinical symptoms that are also associated with human AHF and are summarized in Table 4. Similar to results reported for human patients, infected guinea pigs developed thrombocytopenia, although our sample size was small (n = 2–3 per group). In this animal model, AST levels may provide a useful measure of drug or vaccine efficacy, although increased sample size is needed, along with the paired pre-infection baseline values. Interestingly, elevated AST levels in Junin virus Romero–infected guinea pigs would be consistent with the findings in humans. Further examination of clinical biochemical and hematologic parameters is warranted because abnormal values may be indicative of liver and/or spleen pathology. However, current accurate interpretation of clinical biochemical and hematologic results is limited by the lack of compelling information in the literature on guinea pig normal ranges for age-, weight-and/or strain-matched animals. Studies are ongoing to address these important limitations and to validate the utility of this and other parameters for establishment of the correlation between this animal model and human disease, which would facilitate monitoring of antiviral drug/vaccine efficacy.
TABLE 4.
Comparative clinical presentation in humans versus experimentally infected guinea pigs*
| Characteristic | Symptom | Human† | Guinea pig |
|---|---|---|---|
| Disease phase | |||
| Acute | |||
| Fever | + | + | |
| Myalgia | + | Unknown | |
| Malaise | + | Unknown | |
| Dizziness | + | Unknown | |
| Tremors | + | + | |
| Petechiae | + | − | |
| Severe | |||
| Shock | + | + | |
| Mucosal hemorrhage | + | + | |
| Coma | + | + | |
| Convulsion | + | + | |
| Death | + | + | |
| Hematology | |||
| Leukopenia | + | Unknown | |
| Thrombocytopenia | + | + | |
| Clinical biochemistry | |||
| Proteinuria | + | Unknown | |
| Elevated AST | + | + | |
| Hypercholesteremia | Unknown | Unknown |
AST = aspartate aminotransferase.
Harrison and others2
Disease development and survival of both guinea pig strains (Hartley and Strain 13) after infection with Junin virus Romero were similar. This is an important advantage for the future antiviral drug testing because it provides the opportunity to use outbred guinea pigs raised under specific pathogen-free conditions that can be obtained from commercial vendors. We also found that guinea pig histopathology is comparable to those of lethal human cases, with involvement of visceral organs, such as the spleen and liver. In this study, we have also shown that the viral antigen can be detected in the spleen by using immunohistochemical techniques, which may be useful diagnostic procedure in cases where only formalin-fixed and paraffin-embedded tissue is available. In contrast to the Romero strain, the XJ isolate we used was highly attenuated in guinea pigs and caused only mild disease with all animals surviving the infection, although little difference in numerical scoring was seen between XJ- and Romero-infected guinea pigs at the tissue level to explain the dissimilar clinical disease/survival outcomes (albeit from a small sample size). This virus was obtained after 37 additional suckling mouse brain passages and has obviously lost its virulence for both guinea pig strains. However, this virus was capable of inducing immune response in guinea pigs and minor pathologic manifestations in visceral organs and can potentially be used to produce immune serum in guinea pigs, as well as to study attenuation of Junin viruses.
Acknowledgments
We thank Dr. Robert Tesh (UTMB) for providing the Junin XJ strain and anti-XJ mouse ascitic fluid; Dr. Tom Ksiazek (Centers for Disease Control and Prevention, Atlanta, GA) for providing the Junin Romero strain; Drs. Dennis Hruby, Tove’ Bolken, Sean Amberg (SIGA Technologies, Inc., Corvallis, OR), and Dr. Mary C. Guttieri (United States Army Medical Research Institute for Infectious Disease, Fort Detrick, MD) for helpful scientific and technical advice; Melinda J. Kelley for helpful technical assistance; and Jenna Linde and Nicolette Ward for assistance with preparation of the manuscript.
Financial support: The study was supported by National Institutes of Health (NIH) contract no. 5R44A1056525, NIH K08 award no. AI059491-01, and faculty start-up funding provided by the Institute for Human Infections and Immunity at UTMB.
REFERENCES
- 1.Arribalzaga RA. New epidemic disease due to unidentified germ: nephrotoxic, leukopenic and enanthematous hyperthermia. Dia Med. 1955;27:1204–1210. [PubMed] [Google Scholar]
- 2.Harrison LH, Halsey NA, McKee KT, Jr, Peters CJ, Barrera Oro JG, Briggiler AM, Feuillade MR, Maiztegui JI. Clinical case definitions for Argentine hemorrhagic fever. Clin Infect Dis. 1999;28:1091–1094. doi: 10.1086/514749. [DOI] [PubMed] [Google Scholar]
- 3.Buchmeier MJ, de la Torre J-C, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DM, editor. Fields’ Virology. Philadelphia: Lippincott, Williams and Wilkins; 2007. pp. 1635–1668. 51. [Google Scholar]
- 4.Bowen MD, Peters CJ, Nichol ST. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol. 1997;8:301–316. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 5.Enria DA, Barrera Oro JG. Junin virus vaccines. Curr Top Microbiol Immunol. 2002;263:239–261. doi: 10.1007/978-3-642-56055-2_12. [DOI] [PubMed] [Google Scholar]
- 6.Parodi AS, Greenway DJ, Rugiero HR, Frigerio M, De La Barrera JM, Mettler N, Garzon F, Boxaca M, Guerrero L, Nota N. Concerning the epidemic outbreak in Junin. Dia Med. 1958;30:2300–2301. [PubMed] [Google Scholar]
- 7.Pirosky I, Zuccarini J, Molinelli EA, Di Pietro A, Barrera Oro JG, Martini P, Capello AR. Virosis Hemorrágica del Noroeste Bonaerense (Endemo Epidémica, Febril, Enantémática y Leucopenica) Buenos Aires: Instituto Nacional de Microbiologia, Minesterio de Asistencia Social y Salud Publica; 1959. [Google Scholar]
- 8.Salazar-Bravo J, Ruedas LA, Yates TL. Mammalian reservoirs of arenaviruses. Curr Top Microbiol Immunol. 2002;262:25–63. doi: 10.1007/978-3-642-56029-3_2. [DOI] [PubMed] [Google Scholar]
- 9.Parodi AS, Coto CE, Boxaca M, Lajmanovich S, Gonzalez S. Characteristics of Junin virus. Etiological agent of Argentine hemorrhagic fever. Arch Gesamte Virusforsch. 1966;19:393–402. doi: 10.1007/BF01250608. [DOI] [PubMed] [Google Scholar]
- 10.Parodi AS, Rugiero HR, Greenway DJ, Mettler N, Boxaca M. Isolation of the Junin virus from rodents of non-epidemic areas. Prensa Med Argent. 1961;48:2321–2322. [PubMed] [Google Scholar]
- 11.Parodi AS, Rugiero HR, Greenway DJ, Mettler N, Martinez A, Boxaca M, De La Barrera JM. Isolation of the Junin virus (epidemic hemorrhagic fever) from the mites of the epidemic area (Echinolaelaps echidninus, Barlese) Prensa Med Argent. 1959;46:2242–2244. [PubMed] [Google Scholar]
- 12.Mills JN, Ellis BA, McKee KT, Jr, Ksiazek TG, Oro JG, Maiztegui JI, Calderon GE, Peters CJ, Childs JE. Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. Am J Trop Med Hyg. 1991;44:589–597. doi: 10.4269/ajtmh.1991.44.589. [DOI] [PubMed] [Google Scholar]
- 13.Enria DA, Briggiler AM, Levis S, Vallejos D, Maiztegui JI, Canonico PG. Tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res. 1987;7:353–359. doi: 10.1016/0166-3542(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 14.Enria DA, Maiztegui JI. Antiviral treatment of Argentine hemorrhagic fever. Antiviral Res. 1994;23:23–31. doi: 10.1016/0166-3542(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 15.Enria DA, Briggiler AM, Fernandez NJ, Levis SC, Maiztegui JI. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984;2:255–256. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]
- 16.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 17.Maiztegui JI, McKee KT, Jr, Barrera Oro JG, Harrison LH, Gibbs PH, Feuillade MR, Enria DA, Briggiler AM, Levis SC, Ambrosio AM, Halsey NA, Peters CJ, AHF Study Group Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J Infect Dis. 1998;177:277–283. doi: 10.1086/514211. [DOI] [PubMed] [Google Scholar]
- 18.Weissenbacher MC, de Guerrero LB, Boxaca MC. Experimental biology and pathogenesis of Junin virus infection in animals and man. Bull World Health Organ. 1975;52:507–515. [PMC free article] [PubMed] [Google Scholar]
- 19.Barrios HA, Rondinone SN, Blejer JL, Giovanniello OA, Nota NR. Development of specific immune response in mice infected with Junin virus. Acta Virol. 1982;26:156–164. [PubMed] [Google Scholar]
- 20.Weissenbacher MC, Calello MA, Quintans CJ, Panisse H, Woyskowsky NM, Zannoli VH. Junin virus infection in genetically athymic mice. Intervirology. 1983;19:1–5. doi: 10.1159/000149330. [DOI] [PubMed] [Google Scholar]
- 21.Campetella OE, Galassi NV, Sanjuan N, Barrios HA. Susceptible adult murine model for Junin virus. J Med Virol. 1988;26:443–451. doi: 10.1002/jmv.1890260412. [DOI] [PubMed] [Google Scholar]
- 22.Kenyon RH, Green DE, Maiztegui JI, Peters CJ. Viral strain dependent differences in experimental Argentine hemorrhagic fever. Intervirology. 1988;29:133–143. doi: 10.1159/000150039. [DOI] [PubMed] [Google Scholar]
- 23.Remesar MC, Blejer JL, Lerman GD, Dejean C, Nejamkis MR. Protection against encephalitis in rats caused by a pathogenic strain of the Junin virus, using peripheral inoculation of an attenuated strain. Rev Argent Microbiol. 1989;21:120–126. [PubMed] [Google Scholar]
- 24.Weissenbacher MC, Calello MA, Colillas OJ, Rondinone SN, Frigerio MJ. Argentine hemorrhagic fever: a primate model. Intervirology. 1979;11:363–365. doi: 10.1159/000149059. [DOI] [PubMed] [Google Scholar]
- 25.McKee KT, Jr, Mahlandt BG, Maiztegui JI, Eddy GA, Peters CJ. Experimental Argentine hemorrhagic fever in rhesus macaques: viral strain-dependent clinical response. J Infect Dis. 1985;152:218–221. doi: 10.1093/infdis/152.1.218. [DOI] [PubMed] [Google Scholar]
- 26.McKee KT, Jr, Green DE, Mahlandt BG, Bagley LR, Lyerly WH, Jr, Peters CJ, Eddy GA. Infection of Cebus monkeys with Junin virus. Medicina (B Aires) 1985;45:144–152. [PubMed] [Google Scholar]
- 27.Avila MM, Samoilovich SR, Laguens RP, Merani MS, Weissenbacher MC. Protection of Junin virus-infected marmosets by passive administration of immune serum: association with late neurologic signs. J Med Virol. 1987;21:67–74. doi: 10.1002/jmv.1890210109. [DOI] [PubMed] [Google Scholar]
- 28.Carballal G, Oubina JR, Molinas FC, Nagle C, de la Vega MT, Videla C, Elsner B. Intracerebral infection of Cebus apella with the XJ-Clone 3 strain of Junin virus. J Med Virol. 1987;21:257–268. doi: 10.1002/jmv.1890210309. [DOI] [PubMed] [Google Scholar]
- 29.Kenyon RH, McKee KT, Jr, Maiztegui JI, Green DE, Peters CJ. Heterogeneity of Junin virus strains. Med Microbiol Immunol (Berl) 1986;175:169–172. doi: 10.1007/BF02122442. [DOI] [PubMed] [Google Scholar]
- 30.Campetella OE, Barrios HA, Galassi NV. Junin virusinduced delayed-type hypersensitivity suppression in adult mice. J Med Virol. 1988;25:227–235. doi: 10.1002/jmv.1890250213. [DOI] [PubMed] [Google Scholar]
- 31.Kenyon RH, Peters CJ. Cytolysis of Junin infected target cells by immune guinea pig spleen cells. Microb Pathog. 1986;1:453–464. doi: 10.1016/0882-4010(86)90007-0. [DOI] [PubMed] [Google Scholar]
- 32.Calello MA, Rabinovich RD, Boxaca MC, Weissenbacher MC. Relationship between Junin virus infection of thymus and the establishment of persistence in rodents. Med Microbiol Immunol (Berl) 1986;175:109–112. doi: 10.1007/BF02122427. [DOI] [PubMed] [Google Scholar]
- 33.Weissenbacher MC, Calello MA, Merani S, Oubina JR, Laguens RP, Montoro L, Carballal G. Induction of Junin virus persistence in adult athymic mice. Intervirology. 1986;25:210–215. doi: 10.1159/000149677. [DOI] [PubMed] [Google Scholar]
- 34.Kenyon RH, Green DE, Eddy GA, Peters CJ. Treatment of Junin virus-infected guinea pigs with immune serum: development of late neurological disease. J Med Virol. 1986;20:207–218. doi: 10.1002/jmv.1890200303. [DOI] [PubMed] [Google Scholar]
- 35.McKee KT, Jr, Mahlandt BG, Maiztegui JI, Green DE, Peters CJ. Virus-specific factors in experimental Argentine hemorrhagic fever in rhesus macaques. J Med Virol. 1987;22:99–111. doi: 10.1002/jmv.1890220202. [DOI] [PubMed] [Google Scholar]
- 36.Kenyon RH, McKee KT, Jr, Zack PM, Rippy MK, Vogel AP, York C, Meegan J, Crabbs C, Peters CJ. Aerosol infection of rhesus macaques with Junin virus. Intervirology. 1992;33:23–31. doi: 10.1159/000150227. [DOI] [PubMed] [Google Scholar]
- 37.Elsner B, Boxaca MC, Weissenbacher M, De Guerrero LB. Experimental Junin virus infection of guinea pigs. I. Pathological anatomy. Medicina (B Aires) 1976;36:197–206. [PubMed] [Google Scholar]
- 38.Lopez N, Scolaro L, Rossi C, Jacamo R, Candurra N, Pujol C, Damonte EB, Franze-Fernandez MT. Homologous and heterologous glycoproteins induce protection against Junin virus challenge in guinea pigs. J Gen Virol. 2000;81:1273–1281. doi: 10.1099/0022-1317-81-5-1273. [DOI] [PubMed] [Google Scholar]
- 39.Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT, Jr, Barrera Oro JG. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 40.Aronson JF, Herzog NK, Jerrells TR. Pathological and virological features of arenavirus disease in guinea pigs. Comparison of two Pichinde virus strains. Am J Pathol. 1994;145:228–235. [PMC free article] [PubMed] [Google Scholar]