Abstract
Little is known about normative variation in stress response over the adolescent transition. This study examined neuroendocrine and cardiovascular responses to performance and peer rejection stressors over the adolescent transition in a normative sample. Participants were 82 healthy children (ages 7-12 years, n=39, 22 females) and adolescents (ages 13-17, n=43, 20 females) recruited through community postings. Following a habituation session, participants completed a performance (public speaking, mental arithmetic, mirror tracing) or peer rejection (exclusion challenges) stress session. Salivary cortisol, alpha amylase (sAA), systolic and diastolic blood pressure (SBP, DBP), and heart rate (HR) were measured throughout. Adolescents showed significantly greater cortisol, sAA, SBP and DBP stress response relative to children. Developmental differences were most pronounced in the performance stress session for cortisol and DBP, and in the peer rejection session for sAA and SBP. Heightened physiological stress responses in typical adolescents may facilitate adaptation to new challenges of adolescence and adulthood. In high-risk adolescents, this normative shift may tip the balance toward stress response dysregulation associated with depression and other psychopathology. Specificity of physiological response by stressor type highlights the importance of a multi-system approach to the psychobiology of stress and may also have implications for understanding trajectories to psychopathology.
Keywords: adolescent, child, stress, cortisol, cardiovascular, amylase, depression
Although the notion of adolescence as a time of “storm and stress” has been questioned (Arnett, 1999), it is clear that the transition between middle childhood and adolescence represents a confluence of social, academic, cognitive, physiological and physical changes. Pubertal maturation brings morphological changes heralding reproductive maturity as well as increases in gonadal hormones which influence central and peripheral stress response systems throughout the body and brain (Chrousos, Torpy, & Gold, 1998; Spear, 2000). Emerging animal research has also revealed that puberty represents a period of rapid growth of brain pathways followed by pruning, suggesting that it may be a critical period of brain plasticity and sensitivity to environmental stimuli (Dahl, 2004a; Steinberg et al., 2004). On a macro-level, transitions to middle school or junior high bring new academic challenges as well as social pressures. Adolescents increasingly become focused on peer and romantic relationships, and show decreased reliance and focus on parents and family (Eccles et al., 1993; Steinberg & Morris, 2001). They are also confronted with increased numbers of stressors relative to children and show increased negative mood and mood variability (Colten & Gore, 1991; Compas, Hinden, & Gerhardt, 1995). Finally, the transition to adolescence is associated with dramatic increases in psychopathology, particularly depressive symptoms and syndromes, and represents a critical time for the emergence of gender differences in depression and other psychopathology (Hayward, 2003).
Many of the systems and features showing most marked changes over the transition to adolescence (gonadal hormones/puberty, increased stressors, increases in psychopathology) have also been associated with alterations in physiological stress response systems. Peripheral physiological responses to stressors involve two key systems: the hypothalamic pituitary adrenocortical (HPA) axis and the autonomic nervous system (ANS). Activation of the HPA axis results in a cascade of neural events eventuating in the release of glucocorticoids (principally cortisol in humans and corticosterone in animals) from the adrenal cortex. The ANS is composed of two coordinated and often-opposing systems: sympathetic (mediates activating processes) and parasympathetic (mediates vegetative processes), which interact to produce various degrees of arousal. Increases in sympathetic tone lead to increased release of epinephrine (E) and norepinephrine (NE) from the adrenal medulla. Changes in cardiovascular measures (e.g., heart rate, blood pressure) are the result of complex interactions between sympathetic and parasympathetic input as well as other factors (Porth, 2006). Although appropriate activation of physiological stress responses are necessary for survival, repeated exaggerated or prolonged physiological reactivity to stressors may result in persistent dysregulation of stress systems (e.g., HPA, ANS) leading to a variety of physical and psychiatric disorders (Charmandari, Tsigos, & Chrousos, 2005; McEwen, 1998).
Dysregulation of HPA, ANS, and cardiovascular stress response systems (including alterations in central and peripheral tonic activity, sensitivity to negative feedback inhibition, and response to biological and social challenge) is a risk process implicated in the pathophysiology of depression in adults (Guinjoan, Bernabo, & Cardinali, 1995; Matthews, Nelesen, & Dimsdale, 2005; Plotsky, Owens, & Nemeroff, 1998). Dysregulation of stress response systems has also been demonstrated in adolescent depression and other psychopathology (Shea, Walsh, Macmillan, & Steiner, 2005; Susman, 2006; van Goozen, Fairchild, Snoek, & Harold, 2007). However, direction and significance of findings has varied based on participant characteristics and study design (e.g., severity of disorder, Dahl et al., 1992), type of stress response dysregulation (i.e., basal versus reactivity, ANS versus HPA, van Goozen et al., 2007), and whether stress systems were examined in relation to concurrent disorder or as a predictor of future disorder (Dahl et al., 1991; Halligan, Herbert, Goodyer, & Murray, 2007)). Numerous studies have also demonstrated reciprocal interactions between HPA/ANS stress response (dys)regulation and activity of the hypothalamic pituitary gonadal (HPG) axis, highlighting the possibility of pubertal influence on stress response systems (Chrousos et al., 1998; Spear, 2000). Given associations with risk for depression and other psychopathology and evidence for potent reciprocal regulation by the HPG axis, stress response (dys)regulation may represent an important candidate risk process in adolescent depression. As such, a developmental psychopathology approach would point to the need to characterize both atypical and normative developmental variation in physiological stress response across the pubertal/adolescent transition (Cicchetti & Rogosch, 2002). The present study tests the hypothesis that adolescents relative to children will show increased physiological responses to ecologically valid laboratory stressors.
Despite important implications for understanding development of depression, only a small number of studies in animals and humans have examined developmental influences on stress response systems over the adolescent transition. Studies in rodents reveal increased behavioral responses (e.g., increased immobility, social inhibition) to a variety of stressors over adolescence (Primus & Kellogg, 1989; Walker, Trottier, Rochford, & Lavallee, 1995). Alterations in central and peripheral autonomic activity in the direction of increased sympathetic activity and parasympathetic withdrawal across adolescence have also been demonstrated (Choi & Kellogg, 1996; Kurtz & Campbell, 1994). Animal models have also revealed relatively consistent increases in HPA activity at baseline and in response to stressors over puberty/adolescence—particularly in females (Critchlow, Liebelt, Bar-Sela, Mountcastle, & Lipscomb, 1963; Romeo, Lee, & McEwen, 2005; Viau, Bingham, Davis, Lee, & Wong, 2005).
In humans, seminal work by Allen and Matthews (1997) demonstrated a developmental increase in cardiovascular responses to stressors between children (ages 8-10/no signs of pubertal development) and adolescents (15-17/late to post puberty). Adolescents showed greater blood pressure and cardiac output, suggesting increased beta-adrenergic activity, in response to laboratory challenges--including a stress interview, mirror tracing and reaction time tasks, and a cold stressor--compared to children. However, under baseline conditions, they found decreased sympathetic input to cardiac regulation. Greater ambulatory blood pressure response to daytime activities has also been demonstrated in adolescents relative to younger children (Modesti et al., 1994). In addition, a number of cross-sectional and longitudinal studies in humans have shown higher basal cortisol levels in older adolescents or adolescents in later stages of puberty (Adam, 2006; Elmlinger, Kuhnel, & Ranke, 2002; Jonetz-Mentzel & Wiedemann, 1993; Kenny, Gancayco, Heald, & Hung, 1966; Kiess et al., 1995; Legro, Lin, Demers, & Lloyd, 2003; Netherton, Goodyer, Tamplin, & Herbert, 2004; Walker, Walder, & Reynolds, 2001), although inconsistent findings have been demonstrated and exact timing of effects is not clear (Knutsson et al., 1997; Matchock, Dorn, & Susman, 2007). Effects appear most consistent for girls, and for morning cortisol levels — potentially indicating a maturation of the circadian rhythm over the pubertal/adolescent transition (Gunnar & Vazquez, 2006). Adam (Adam, 2006) also demonstrated increased cortisol response to the momentary experience of worry (as assessed by ecological momentary assessment) with increasing age in a community sample of healthy adolescents. Finally, our group demonstrated increased cortisol response to biological (corticotropin-releasing hormone, CRH) challenge with increasing pubertal stage in girls from a sample of physically health children and adolescents with no history of psychopathology (Stroud, Papandonatos, Williamson, & Dahl, 2004).
Thus, preliminary evidence from animal models and humans suggests increased stress responses over or across the adolescent transition (including both longitudinal and cross-sectional studies). However, although both depression and the transition to adolescence are associated with increases in exposure to psychological stressors, human studies of HPA and ANS responses to psychological stressors over the adolescent transition have been limited. As described above, Allen and Matthews (1997) demonstrated increased cardiovascular reactivity to performance-oriented stressors. Walker et al. showed cross-sectional and longitudinal associations of age with initial laboratory cortisol sampling—presumed to indicate response to novelty (Walker et al., 2001; Weinstein, Diforio, Schiffman, Walker, & Bonsall, 1999). Adam (2006) revealed increased cortisol response to momentary negative affect (worry) in naturalistic settings with increasing age in adolescents aged 13 and above. Although not explicitly designed to examine developmental differences, Klimes-Dougan et al. examined cortisol response to a family conflict (conflictual discussion with mother) and social performance (interaction with shy confederate and public speaking task) in a community sample of girls and boys ages 11-13 and 14-16. In response to the social performance task only, older boys showed increased cortisol reactivity relative to older girls, younger boys and younger girls (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001).
In addition, despite calls for measurement across multiple systems in studies investigating the developmental psychobiology of stress (Cicchetti & Blender, 2006), besides Gunnar et al. (this volume) we know of no studies designed to examine developmental influences on HPA, SNS, and cardiovascular responses to stressors over the adolescent transition. Finally, most previous laboratory studies of stress response in adolescents have utilized performance/academic-oriented stressors including public speaking and mental arithmetic tasks such as the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., 1997). Yet, there is a need for investigations of individual differences in responses to additional developmentally salient and ecologically valid psychological stressors. Although adolescence brings increased emphasis on achievement and performance, it is also a time of increased focus and distress around peer and romantic relationships (Graber, Brooks-Gunn, & Petersen, 1996; Steinberg & Morris, 2001). Further, greater links between interpersonal stressors and psychopathology emerge over adolescence, with emotional maladjustment associated with family dysfunction in early adolescence, but more strongly with difficulties with peers and romantic relationships by late adolescence (Nelson, Leibenluft, McClure, & Pine, 2005). Peer rejection, in particular, has been demonstrated as a risk factor for depressive and internalizing symptoms over the adolescent transition (Ladd, 2006; Prinstein, Borelli, Cheah, Simon, & Aikins, 2005).
In the present study, we fill a critical gap in understanding of stress response patterns across the adolescent transition through examination of HPA, SNS, and cardiovascular responses to two developmentally-relevant domains of psychological stressors: performance and peer rejection. Specifically, we examined developmental differences in responses to typical laboratory performance stressors adapted from the TSST-C (public speaking, mental arithmetic, and mirror star tracing) versus responses to a novel peer rejection stressor adapted from a paradigm developed and validated in young adults (Yale Interpersonal Stressor-Child version; YIPS-C; Stroud, Tanofsky-Kraff, Wilfley, & Salovey, 2000). HPA (saliva cortisol), cardiovascular (systolic and diastolic blood pressure and heart rate), and affective responses to the stressors were examined. In addition, we included measures of saliva α-amylase (sAA), an enzyme secreted by the salivary glands, that has been identified as a non-invasive surrogate marker of SNS stress response with demonstrated validity in children and adolescents (Granger et al., 2006; Nater et al., 2006). In the present study, our aims are: a) to test the hypothesis that adolescents relative to children in a normative sample will show increased physiological stress response and b) to explore whether developmental differences in stress response differ by stressor domain (performance versus peer rejection) and physiological stress system (HPA (cortisol), versus SNS (sAA), versus cardiovascular (heart rate, blood pressure)).
Methods
Participants
Participants were 82 children and adolescents ages 7 to17 (M = 12.5, SD = 2.5) recruited through community and online postings. Participants included 43 adolescents (ages 13-17 years, 20 females), and 39 children (7-12 years, 22 females). Racial/ethnic breakdown of participants was 75.5% Caucasian, 4% African American, 14.5% Hispanic, 6% mixed race/ethnicity, and 6% other or “don't know”. Interested participants and parents were screened by telephone to determine study eligibility. Exclusion criteria were based on factors known to influence cortisol reactivity, including the use of oral contraceptives, thyroid medications, steroids, and psychotropic medications (Hibel, Granger, Cicchetti, & Rogosch, 2007). Participants with a previous diagnosis of autism or mental retardation, a history of psychological or behavioral problems, or current physical illness were also excluded from the study. All participants denied smoking and regular drug or alcohol use. Lending support to the normative nature of this sample, mean scores on a) the Child Depression Inventory—Short Form = 1.33 (SD = 1.64, Med = 1, n=81), b) Revised Child Manifest Anxiety Inventory = 7.25 (SD = 5.06, Med = 6, n=79), and c) Child Behavior Checklist (CBCL) Internalizing Problems = 5.15 (SD= 5.24, Med=4, n=62), Externalizing Problems = 5.13 (SD= 5.42, Med= 4, n=62) well below suggested clinical screening cutoffs (Achenbach, 1991; Achenbach & Rescorla, 2001; Kovacs, 1992; Reynolds & Richmond, 2000). Average family income was $60,000 to $80,000 (Range $5000-9,999 to >$100,000). Maternal education ranged from some high school to completion of a graduate degree. Twenty-two percent of mothers had a high school education or less, 37% completed some college or an associate's degree, and 41% graduated college or obtained a graduate degree. Partner education ranged from eighth grade to completion of a graduate degree; 32% had a high school education or less, 21% completed some college, and 46% graduated college or obtained a graduate degree. Eighty-three percent of mothers were married. Eighty-three percent of mothers and ninety-three percent of fathers were employed.
Procedure
Protocols and procedures were reviewed and approved by Lifespan Hospitals Institutional Review Boards. Informed consent was obtained from mothers and assent from children and adolescents. The study included two sessions, each lasting approximately 2 hours each, conducted on separate days. Participants were accompanied by their mothers to the laboratory for both sessions. In the first “rest” session, participants watched G-rated movies and television shows and completed questionnaires. The primary purpose of the rest session was to allow participants to habituate to the laboratory and physiological monitoring prior to undergoing the stress induction. With the influence of laboratory novelty attenuated, differences in reactivity could be attributed to the stress induction. The second (stress) session involved random assignment to the performance or peer rejection stressors. Fifty-one participants (27 adolescents) completed the performance session; 31 participants (16 adolescents) completed the peer rejection session. Both performance and peer rejection stress sessions included a 20-25 minute baseline period where participants were asked to read easy (grade K-2) books or watch G-rated movies and television shows, three stressors, lasting 10, 5, and 5 minutes respectively, and a one-hour recovery period in which participants completed questionnaires and watched G-rated movies and television shows. The performance session included speech (5 minutes preparation, 5-minute speech), mental arithmetic (5 minutes) and mirror tracing (5 minutes) tasks; the social rejection session involved three exclusion challenges (10, 5, and 5 minutes) with gender/age-matched confederates. All mothers were required to observe the stressor portion of the session from an observation room. Seven to nine saliva samples were taken over the baseline, stressor, and recovery periods. Six “matched” time points were selected from the 7-9 samples to optimize timing for cortisol and sAA response assessment, respectively. (See Measures below.) Blood pressure and heart rate recordings were taken at 2-minute intervals during the baseline and stress induction periods, and at 5-minute intervals during the recovery period. Self-reported affect was assessed at baseline, during each stress task, and during the recovery period. All sessions began between 14:00 and 17:00 to control for diurnal variation in cortisol. Participants were asked to refrain from food and drink (besides water) for two hours prior to the stress session, from exercise for 24 hours prior to the session, and from caffeine beginning the evening before the stress session. Following the stress sessions, participants were extensively debriefed. Debriefing included three segments: a) experimenter debriefed child/adolescent, b) mothers joined child/adolescent and experimenter and any further questions were answered, and c) confederates/audience members returned for a positive interaction with child/adolescent. Participants and mothers were then compensated for their time.
Stressors
Performance Challenges
Performance-oriented tasks were based on an adaptation of the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., 1997). The first segment was a public speaking task in which participants were given 5 minutes to prepare, then were asked to speak on academic topics (e.g., English, Science, History) for 5 minutes. Specifically, participants were asked to give a book report on a book of their choice, a science lesson (e.g., the planets), and a history lesson (e.g., describe their favorite president and why). Based on pilot testing, speech topics included minor adjustments for age (e.g., adolescents were asked to “give a plot summary and interpretation” of a book, while children were asked “to tell about [a] book.” Adolescents were asked “to choose an American historic leader and describe his or her significance,” while children were asked “to talk about an important president and to talk about why he was important.” Participants then completed a mental arithmetic task involving serial subtraction under time pressure for 5 minutes. Difficulty was adjusted based on participant age and performance. For example, 15-17 year olds began subtracting by 17's, 10-14 year olds by 11's, and 7-9 year olds by 8's. If an error was made, participants were asked to start again from the beginning. If participant was unable to produce correct responses to a particular serial subtraction request after three tries, the experimenter would go to the next level down; if the participant produced all correct responses 6 times in a row, the experimenter would go up a level. The mirror tracing task also lasted 5 minutes and was adapted from Allen and Matthews (1997). This task involved tracing the figure of a six-sided star while viewing only its mirror image using a mirror star tracing apparatus (Layfayette Instruments, 1987). Mistakes were counted and marked by a sound and a white light. If errors were made, participants were instructed to start tracing the star again from the beginning. All tasks were performed before a two-member audience who remained stern during the procedures and pretended to take notes.
Peer rejection challenges
These challenges involve three social rejection interactions based on an adaptation for children and adolescents of the Yale Interpersonal Stressor (YIPS; (Stroud et al., 2000). The Child-YIPS (YIPS-C) involved interactions with two trained, same-sex, similar-age confederates who subtly excluded the participant by bonding with each other, leaving the participant out of their conversations, and having different interests and activities than the participant. Child confederates were extensively trained child actors. Adolescent confederates were child actors or trained college students. Participants were told that we were studying “how kids get to know one another” and that they were to discuss specified topics while “getting to know one another.” Each participant/confederate was first asked to introduce him/herself. Then, the exclusion interactions focused around three topics: weekend activities, family, and friends. Over the course of the three segments, the two actors gradually excluded the participant. Confederates used a variety of verbal and nonverbal techniques to exclude the participant, while connecting well with each other. Exclusion of the participant began slowly and built gradually so as not to appear planned.
Measures
Salivary Cortisol Measures
Saliva cortisol is considered to be a reliable and valid measure of unbound, or free cortisol levels in plasma (Kirschbaum & Hellhammer, 1989, 1994). It is a particularly useful measure for assessing acute changes in HPA axis activation due to stressors (Kirschbaum & Hellhammer, 1994), and has been utilized in numerous studies with children and adolescents (Gunnar, 1992; Schmidt, 1998) Seven to nine whole saliva samples were collected from each participant by passive drool over the course of each stress session. Participants were asked to fill saliva collection vials to a designated line using a straw or by salivating directly into the tube (Granger et al., In Press). Following collection, samples were frozen at −80 degrees Celsius until shipment overnight delivery on dry ice to Salimetrics Laboratories (State College PA). Cortisol was analyzed in duplicate using a commercially available enzyme immunoassay without modification to the manufacturer's protocol (Salimetrics, PA), range of sensitivity from .007-3.0 μg/dl, and intra and inter-assay coefficients of variation less than 5 and 10% respectively. Salivary cortisol data are expressed in micrograms per deciliter (μg/dl).
Timing and number of saliva samples (7 versus 9) was changed over the course of the study; thus six “matched” samples were selected from the original 7 and 9 time-point sampling frame to optimize timing for cortisol (approximately 20 minutes after onset of each stressor, (Schwartz, Granger, Susman, Gunnar, & Laird, 1998). The first matched saliva sample represented an initial baseline period, and was collected 7-10 minutes prior to the onset of stress. The second matched saliva sample represented a second baseline period, and was collected immediately following the first stressor (speech/interaction 1). The third matched sample represented response to the first stressor, and was collected 17-21 minutes following the onset of the speech/first interaction task. The fourth saliva sample represented response to the second stressor, and was collected 17-20 minutes after the mental arithmetic/second interaction task. The fifth saliva sample represented response to the third stressor and was collected 19-22 minutes following the tracing/third interaction task. The sixth sample represented the recovery period, corresponding to 40-51 minutes following the third stressor. The six “matched” cortisol samples were utilized for all statistical analyses involving cortisol. Two participants were missing one or more cortisol samples.
Saliva Alpha Amylase (sAA)
sAA is an enzyme produced by the salivary gland, and has been shown as a promising surrogate marker for the SNS (Nater et al., 2006; van Stegeren, Rohleder, Everaerd, & Wolf, 2006). sAA was measured using a kinetic reaction assay that employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose (Granger et al., 2006). The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2 min period) in absorbance at 405 nm. Results are computed in U/ml of sAA using the formula: [Absorbance difference per minute × total assay volume (328 ml) × dilution factor (200)]/[millimolar absorptivity of 2-chloro-p-nitrophenol (12.9) × sample volume (.008 ml) × light path (.97)]. Intra-assay variation (CV) computed for the mean of 30 replicate tests was less than 7.5%. Inter-assay variation computed for the mean of average duplicates for 16 separate runs was less than 6%.
Selection of 6 “matched” time points differed for sAA versus cortisol due to differences in optimal timing of sAA versus cortisol sampling following stressor onset (5 versus 20 minutes after onset of each stressor; (Gordis, Granger, Susman, & Trickett, 2006; Granger, Kivlighan, El-Sheikh, Gordis, & Stroud, 2007a). Identical to cortisol, the first matched saliva sample represented an initial baseline period, and was collected 7-10 minutes prior to the onset of stress. The second matched saliva sample represented response to the first stress task, and was collected five minutes following the onset and immediately following the completion of the speech/first interaction task. The third sample represented response to the second stressor, and was collected 5 minutes following the onset and immediately following the completion of the math/second interaction task. The fourth saliva sample represented response to the third stressor, and was collected 5 minutes following the onset and immediately following the completion of the mirror star tracing/third interaction task. The fifth saliva sample represented the first recovery period and was collected 19-22 minutes after the third stressor. The sixth sAA sample represented the second recovery period, corresponding to 49-53 minutes following the third stressor. The six “matched” sAA samples were utilized for all statistical analyses involving sAA. Ten participants were missing one or more sAA samples.
Cardiovascular Measures
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured every two minutes during baseline and stress periods and every five minutes during the recovery period throughout all stress sessions using a Dinamap automatic, oscillometric blood pressure monitor (Critikon Inc., Tampa, FL). Participants were fitted with an appropriately sized cuff on their non-dominant arm prior to the start of the baseline period. The cuff was removed at the end of the recovery period. For all cardiovascular variables (SBP, DBP, HR), stress levels were the average of each measure (SBP, DBP, or HR) during each stress task, and baseline levels, the average of cardiovascular measures during the initial baseline period. Six participants were missing one or more cardiovascular samples.
Self-reported Affect
Self-reported affect was assessed at six points during each stress session: once at baseline, three times following the stress tasks (participants were asked to respond according to how they were feeling during each task), and twice during the recovery period. Affect measures consisted of mood adjectives adapted from the State-Trait Anxiety Inventory for Children (STAI-C; Spielberger, Edwards, Montuori, Lushene, & Platzek, 1973) including “upset,” “nervous,” “sad,” “happy,” “relaxed,” and “scared” rated along 3-point Likert scales. Positive as well as negative affect adjectives were included based on recent research highlighting the mechanistic importance of positive as well as negative affect in determining stress response and as related to depression and other internalizing disorders (Folkman & Moskowitz, 2007; Forbes & Dahl, 2005). Affect measures included emotion faces at the extremes each adjective's scale to assist participants in anchoring high and low levels of each emotion. Based on the results of a principal components analysis, we created two overall affect scales (negative affect (NA: “upset,” “nervous,” “sad,” “scared”) and positive affect (PA: “happy,” “relaxed”)). Both scales showed good internal consistency (Cronbach's alphas were .87 for both NA and PA). Three participants were missing one or more NA samples; four participants were missing one or more PA samples.
Preliminary Analyses/Secondary Measures
Stressor Validation. Task Perceptions Questionnaire (TPQ)
TPQ's were administered to validate conceptualization of the performance versus social nature of the stress sessions. Questions included graded (rated along 3-point Likert scales) and forced choice format questions for each of the three tasks from the performance or peer rejection sessions. When asked to choose, 90-100% of participants who completed the peer rejection session rated the interaction tasks as more “like talking to people” than “like performing something” or “like school or homework”; 75-81% participants who completed the performance tasks rated them as more “like performing something” or “like school or homework” than “like talking to people” (X2 (1) > 25.5, p's < .0001). In the Likert-formatted questions, the performance condition was perceived as more like “times you had to perform,” “you were trying to reach a goal,” “school or homework,” and “doing badly in school” than the peer rejection condition (t's > 2.8, p's <.01). In contrast, the peer rejection condition was perceived as more like “meeting new people,” “activities you do with other kids,” “hanging out,” and “times you have felt left out” than the performance condition (t's > 4.7, p's <.01). These analyses lend credence to our conceptualization of the performance versus social nature of the two stress sessions.
Associations between Age and Pubertal Stage. Tanner Staging
Pubertal stage was assessed on a subset of participants (n=47) using Tanner criterion (breast (B) and pubic hair (PH) for girls, genital (G) and PH for boys). Tanner staging was conducted by physician (pediatric endocrinologist) and/or by participant-report using pictures depicting each Tanner stage (Marshall & Tanner, 1969, 1970). Physician rating was utilized in preference to participant-report for determining staging, when available. As determined by polyserial correlations, high-magnitude associations emerged between continuous age and pubertal stage (r= .84, 95% CI = .75, .92 for Tanner BG); (r = .88, 95% CI = .81, .95 for Tanner PH). Tanner stage was subsequently dichotomized at early-mid vs. late puberty (Tanner I-III vs. IV-V). Next, a sensitivity analysis was conducted to determine the optimal age threshold for minimizing misclassification based on this particular Tanner stage dichotomization. Splitting the sample at ages 7-12 versus 13-17 (based on mean age of 12.5) was the optimal threshold for minimizing misclassification rates, resulting in a 6.4% error rate for Tanner BG and 8.5% error rate for PH. Thus, to the extent that participants with Tanner stage data are representative of the sample as a whole, continuous age may offer a preliminary proxy for Tanner BG or PH, and age dichotomized at 7-12 and 13-17 a proxy for early-mid versus late puberty (Tanner I-III versus IV-V). Given these determinations, although numerically, all data analyses are based on continuous or dichotomous age, we substitute the more integrative term, development, to encompass influences of pubertal stage and age.
Data Analyses
As cortisol values were positively skewed and leptokurtotic, logarithmic transformations of cortisol values were used for all statistical analyses. Similarly, as has been recommended in previous literature (Gordis et al., 2006; Granger, Kivlighan, El-Sheikh, Gordis, & Stroud, 2007b), square root transformations were applied to sAA values to overcome moderate positive skew and leptokurtosis. However, nontransformed cortisol and sAA values are presented in Figures 2 and 3. For cardiovascular measures, reactivity was the difference between the average task and average baseline levels for each stress task (Llabre, Spitzer, Saab, Ironson, & Schneiderman, 1991). Percent change was defined as reactivity divided by average baseline. For figures and follow up analyses, a dichotomous development variable was created based on a 7-12 years (child) versus 13-17 years (adolescent) split. This split was selected as a proxy for Tanner I-III versus IV-V (see above), due to evidence for pronounced increases in depression/psychopathology at age 13, and because this stratification represented a mean split for the sample.
Figure 2.
Salivary cortisol levels (means ± SEM) over performance and peer rejection stress sessions for children (≤ 12 years) and adolescents (13+ years).
Figure 3.
Salivary alpha amylase activity (means ± SEM) over performance and peer rejection stress sessions for children and adolescents. Duration of the stress period is highlighted in grey.
An initial manipulation check involved analyses of self-reported affect over performance and peer rejection stress sessions adjusting for gender and race/ethnicity. Next, we examined the influence of development and stressor type on physiological stress response, also adjusting for gender, and race/ethnicity (dichotomized as Caucasian versus other); cortisol and sAA response outcomes were also adjusted for time of the day. Cortisol, sAA, and cardiovascular (SBP, DBP, and HR) analyses utilized repeated measures analysis of covariance (ANCOVA), within a multiple regression model framework. Within this framework, development was modeled as a continuous variable; interaction effects involving continuous development utilized age centered at its mean. Huynh-Feldt adjustments were made for significant departure from the sphericity assumption (Vasey & Thayer, 1987). Initially, overall development (continuous) X stressor type X time (six time points: baseline, challenge 1, challenge 2, challenge 3, post-stress 1, post-stress 2) repeated measures ANCOVAs were conducted, followed by repeated measures ANCOVAs stratified by session type, all within a multiple regression framework. Stratified analyses by session type were conducted with development examined as a continuous and dichotomous (≤12 years, 13+ years) variable. All repeated measures analyses were conducted with S-Plus statistical software to allow available case analysis for missing data. Significant effects were followed by simple, directional contrasts. For cardiovascular variables (SBP, DBP, HR), follow-up reactivity percent change scores were examined to highlight the influence of development and stressor type on change in cardiovascular responses. Due to small sample size and between-subjects design, effects significant at p < .10 were considered trends. Figures are based on raw means with no adjustment for covariates.
Results
Manipulation Check: Changes in Self-Reported Affect
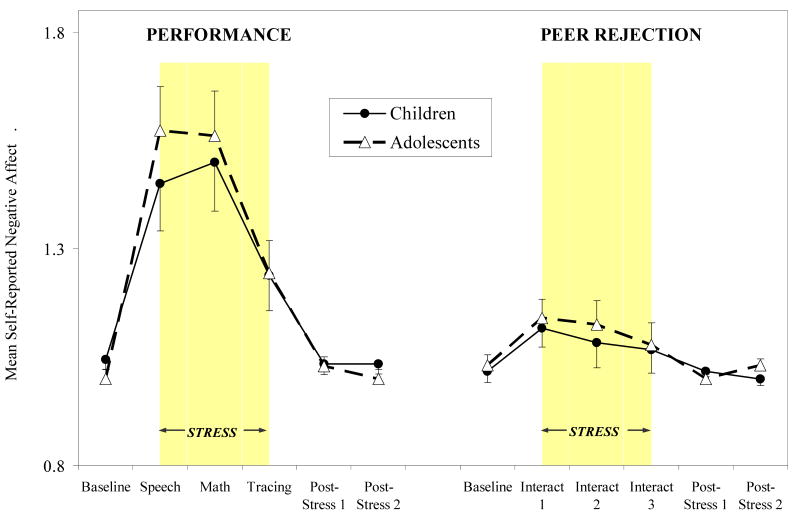
Mean ratings from the NA scale for performance and peer rejection sessions by development group (children versus adolescents) are presented in Figure 1. Positive affect levels followed the same patterns but in reverse. Both stress sessions evoked significant increases in NA and decreases in PA over the six time points (F's > 4.03, p's < .005 for performance session; F's > 2.58, p's < .05 for peer rejection session). Further, we found significant stressor type by time interactions (F's > 9.41, p's < .0001 for NA and PA), with participants in the performance condition showing significantly greater increases in NA and decreases in PA between baseline and stress periods compared to participants in the peer rejection condition. No significant interactions over time emerged for development group for NA or PA.
Figure 1.
Self-reported negative affect ratings (means ± SEM) over performance and peer rejection stress sessions for children (≤ 12 years) and adolescents (13+ years). Stressors included public speaking (speech), mental arithmetic (math), and mirror star tracing (tracing) for the performance session, and three exclusion interaction challenges (interact 1, interact 2, interact 3) for the peer rejection session. Duration of the stress period is highlighted in grey.
Note. Affect values represent levels along a 3-point Likert scale.
Influence of Development and Stressor Type on Physiological Stress Response
Cortisol
We found a significant development (continuous) by stressor type by time interaction for cortisol (F (5, 369) = 3.41, p <.05), such that participants in the performance session showed greater cortisol responses with increasing development, while developmental differences were less pronounced for the peer rejection session. Stratifying by stressor type, we found a significant development by time interaction for the performance condition (F (5, 229) = 5.48, p < .005), but no significant development by time interaction for the peer rejection condition (although between-subjects effect of development trended toward significance, p < .10). Mean cortisol levels for performance and peer rejection sessions by development group (children versus adolescents) are presented in Figure 2. Adolescents showed greater increases in cortisol over time in response to the performance stressors relative to children (F (5, 229) = 3.35, p < .01), with significant developmental differences emerging for each stress task and both recovery periods (p's < .05). Although adolescents also showed greater cortisol responses to the peer rejection session relative to children, differences were less pronounced and not statistically significant.
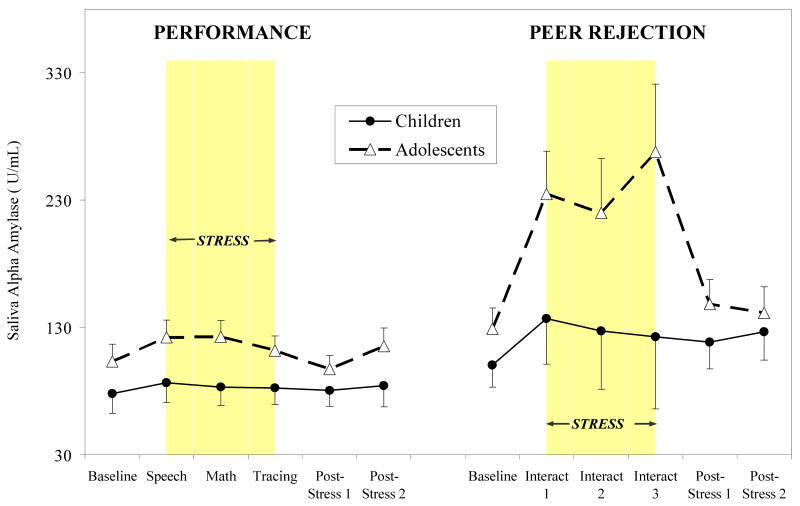
Alpha Amylase (sAA)
We found significant development (continuous) by time (F (5, 336) = 3.26, p < .05) and stressor type by time (F (5, 336) = 4.11, p < .01) interactions and a trend toward a significant development by stressor type by time interaction (p < .10). In contrast to cortisol, for sAA, participants in the peer rejection session showed increasing sAA stress response with increasing development, while little influence of development emerged for the performance session. Similarly, stratifying by stressor type, a significant development by time interaction emerged for the peer rejection condition (F (5, 125) = 3.96, p < .05), but not for the performance session (although a between-subjects effect of development trended toward significance, p < .10). Mean sAA levels for performance and peer rejection sessions by development group are presented in Figure 3. Adolescents showed more pronounced and statistically significant sAA response to the peer rejection session relative to children (F (5, 125) = 4.09, p < .01), with significant developmental differences emerging for the first and third interaction tasks (p's < .05). Although adolescents showed slightly greater sAA responses in to the performance stressors relative to children, differences were not statistically significant.
Systolic Blood Pressure (SBP)
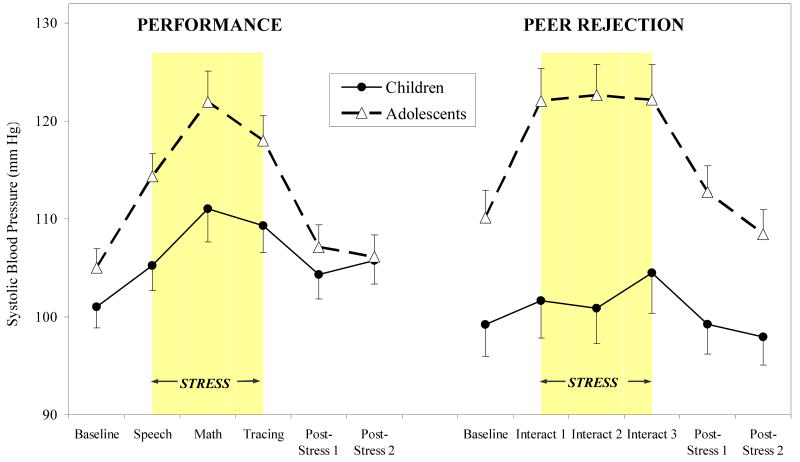
We found significant development (continuous) by time and stressor type by time interactions (F's (5, 374) = 11.27 and 2.96, respectively, p< .05), such that increasing development was associated with increasing SBP responses, with greater SBP responses to the to the peer rejection relative to the performance stress session. Stratifying by session type, increasing development was associated with increased SBP stress response for both performance and peer rejection stressors (development by time interactions: F (5, 232) = 3.82, p < .01 for performance, F (5, 132) = 8.83, p < .001 for peer rejection), with most pronounced effects of development evident for the peer rejection session. As shown in Figure 4, adolescents showed greater SBP responses to the performance and peer rejection stressors relative to children (development group by time interactions: F (5, 232) = 4.11, p < .005 for performance; F (5, 132) = 7.32, p < .0001 for peer rejection stressors), with directional contrasts revealing significant development-group differences for SBP response to speech, math and tracing tasks in the performance session (p's < .05) and at all time points in the peer rejection session (p's < .05). Adolescents showed significantly greater SBP reactivity and percent change in response to all performance stressors (speech, math, tracing; p's < .05) and all peer rejection interactions (p's < .01) relative to children; however development group differences were more pronounced in response to peer rejection stressors (8-10% versus 3-5% change from baseline in SBP following peer rejection relative to performance stressors).
Figure 4.
Systolic blood pressure (means ± SEM) over performance and peer rejection stress sessions for children and adolescents.
Diastolic Blood Pressure (DBP)
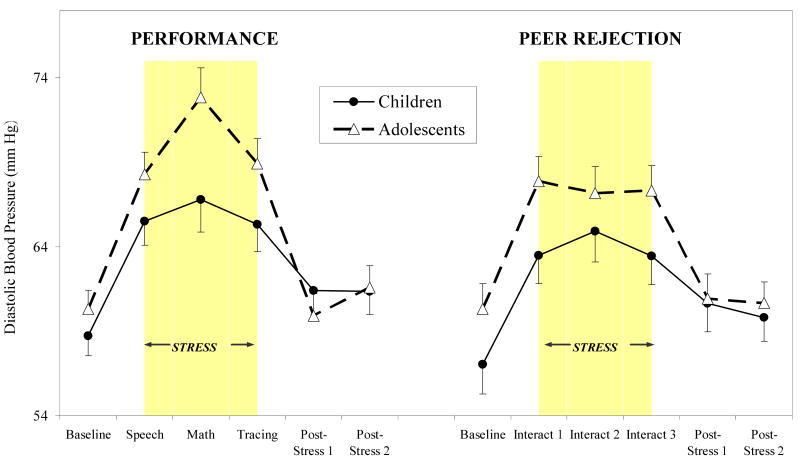
Similar to SBP, we found significant development (continuous) by time interaction for DBP (F (5, 374) = 5.30, p < .001), such that increasing development was associated with increasing DBP responses to stressors, with greater DBP responses to performance relative to peer rejection stressors. Stratifying by session type, increasing development was associated with increased SBP stress response to the performance stress session (development by time interaction: F (5, 232) = 3.94, p < .01). No significant effects of development emerged for peer rejection stressors. As shown in Figure 5, adolescents showed significantly more pronounced DBP responses to performance stressors relative to children (F (5, 232) = 4.36, p < .003), with directional contrasts revealing a significant age difference for DBP response to the mental arithmetic task (p < .05). Although adolescents also showed slightly greater DBP response to the peer rejection session relative to children, effects were not statistically significant. Adolescents showed significantly greater DBP reactivity and percent change in response to the mental arithmetic and mirror tracing stressors (p's < .05) in the performance session, with no significant differences emerging for the peer rejection session.
Figure 5.
Diastolic blood pressure (means ± SEM) over performance and peer rejection stress sessions for children and adolescents.
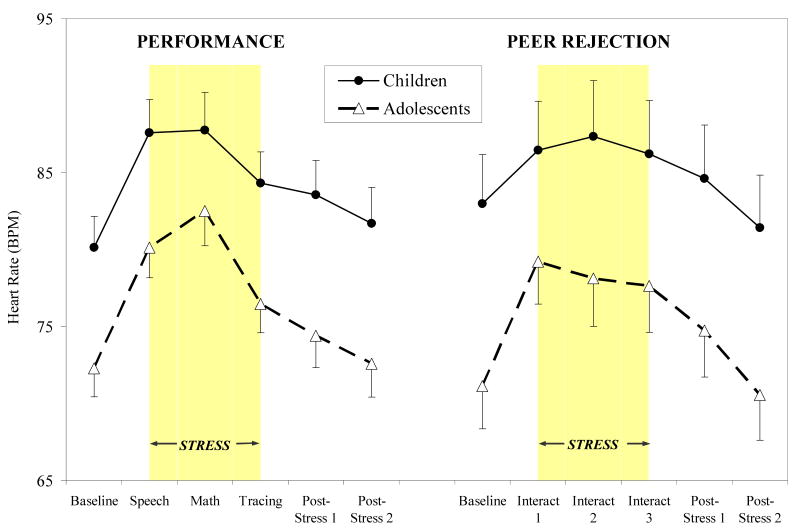
Heart Rate (HR)
For HR, no overall development by time, session by time, or development by session by time interactions emerged. An overall main effect of development (continuous) was shown, such that increasing development was associated with decreasing overall HR response (F (1, 72) = 13.26, p < .001). Stratifying by session type, main effects of development (increasing development associated with attenuated HR) were evident for both performance and peer rejection sessions (F (1, 44) = 12.55, p < .001 and F (1, 24) = 5.58, p < .05, respectively). As shown in Figure 6, children showed significantly greater HR responses across performance and peer rejection sessions relative to adolescents (development group main effects: F (1, 44) = 8.72, p < .005 for performance, (F (1, 24) = 6.23, p < .02 for peer rejection), with directional contrasts revealing a significant developmental difference for HR response to all time points except the math stressor (p's < .01) in the performance session and at all time points except the first interaction task in the peer rejection session (p's < .05). No significant differences in HR reactivity or percent change were evident for the performance session, but adolescents showed increased reactivity and percent change in response to the second interaction task in the peer rejection session (p < .05).
Figure 6.
Heart rate (means ± SEM) over performance and peer rejection stress sessions for children and adolescents.
Discussion
Dysregulation in stress response systems and stress reactivity has been proposed as an important candidate risk process in adolescent depression and other psychopathology. However, little is known about normative patterns of physiological stress reactivity across the adolescent transition, despite the need for this data to inform studies of high-risk and clinical populations (Cicchetti & Rogosch, 2002). The present study investigated developmental influences on physiological stress response across the adolescent transition in a normative sample. Using a cross-sectional approach, we tested the hypothesis of a fundamental (normative) developmental increase in stress response over the adolescent transition. We found consistently increased physiological responses to developmentally-relevant laboratory stressors across adolescence, with increasing development associated with increased HPA (saliva cortisol) and SNS (saliva α-amylase (sAA)) and cardiovascular (SBP, DBP, HR) stress responses. In particular, we found pronounced differences in cortisol and sAA responses in adolescents (ages 13-17, Tanner IV/V) versus children (ages 7-12, Tanner I-III). We also explored differences related to stressor (performance versus peer rejection) and response domain (HPA versus SNS versus cardiovascular) specificity. The present study revealed initial evidence for specificity of developmental influences on stress response by stressor domain and physiological stress system. Developmental influences on cortisol and DBP stress response were most pronounced in the context of the performance stressors, while developmental influences on sAA and SBP were evident primarily in response to the peer rejection stressor.
Although we believe these findings have several noteworthy implications, we acknowledge two important limitations of the study. First, we utilized a cross-sectional approach to development in which we tested stress response of different individuals across a range of ages/pubertal stages. A longitudinal or mixed design where stress response of the same individuals was tested repeatedly over development would provide a more powerful and conclusive investigation of the possibility of a fundamental increase in stress response over the adolescent transition. A longitudinal approach would also allow examination of associations between individual differences in the magnitude of stress response changes over adolescence and change in risk and risk processes relevant to depression/psychopathology during this period. One of the challenges for the field in designing such longitudinal studies will be to create laboratory tasks to which participants are unlikely to habituate over time and/or to consider novel approaches to stress reactivity assessment (e.g., Adam, 2006). A second important limitation of the present study is that pubertal stage data were only available on approximately 60% of the sample. Due to missing pubertal data and between-subjects nature of the design, continuous and dichotomous age measures were utilized as proxies for overall development. Although the use of physician or participant-rated Tanner staging and high-magnitude associations with age lend credence to our use of age as a proxy for development, we were unable to parse the unique influences of pubertal stage versus other developmental processes on stress response.
These important limitations notwithstanding, to our knowledge, the present study and that of Gunnar et al. (this issue) represent the first studies designed explicitly to examine developmental influences on HPA, SNS and cardiovascular responses to psychological stressors over the adolescent transition. Despite evidence for major changes in brain, neuroendocrine, physical, social, contextual, and cognitive function, pronounced increases in psychiatric disorders showing continuity to adulthood, and known links between stress responses and both psychiatric disorders and brain/neuroendocrine/social/cognitive function, the dearth of basic research investigating developmental influences on stress response over the adolescent transition is perplexing. Besides studies of aging, most investigations of developmental influences on the stress response in animals and humans have focused on the fetal period, infancy, and early childhood. In rodent models, the first two weeks of life are characterized by a relative stress hyporesponsive period (SHRP). During this period, the HPA axis is considerably less responsive to external stressors—showing little in the way of an adrenocortical response when stressors are administered (Gunnar & Vazquez, 2001; Vazquez, 1998). The SHRP is believed to protect the maturing infant brain from potential detrimental influence of glucocorticoids and CRH (Gunnar & Vazquez, 2001; Sapolsky & Meaney, 1986). Until recently, it was unclear whether a period analogous to the SHRP existed in humans. In the first months of life, infants show potent response to psychological (e.g., neurobehavioral examinations) and physical stressors (e.g., blood draw); however, by the end of the first year, infants show little response to most stressors suggesting decreased responsiveness to stressors (Armario, Gavalda, & Marti, 1995; Gunnar, 1989). Further, anecdotally, it has been noted that typical laboratory stressors do not evoke a cortisol response in young children (Dahl, 2004b; Gunnar, 2004). Although more research is clearly needed, the present study and the others in this issue provide preliminary evidence that a period of relatively buffered physiological response to many stressors may extend as far as the adolescent/pubertal transition in humans (Gunnar, 2003; Gunnar & Vazquez, 2001).
The present study is consistent with a small number of human studies showing increases in basal cortisol over puberty/the adolescent transition using cross-sectional and longitudinal approaches (Adam, 2006; Elmlinger et al., 2002; Jonetz-Mentzel & Wiedemann, 1993; Kenny et al., 1966; Kiess et al., 1995; Legro et al., 2003; Netherton et al., 2004; Walker et al., 2001), and basal SNS/cardiovascular indicators (Modesti et al., 1994), but extends this literature to examine HPA, SNS, and cardiovascular response to challenge. Results are also consistent with preclinical studies showing increases in central and peripheral stress response over puberty, and a study by our group showing increased cortisol response to biological (CRH) challenge with increasing pubertal stages; with effects evident primarily in females (Stroud et al., 2004). The present study extends this work to investigate cortisol response to two types of psychological challenge. Results also complement those of Allen and Matthews (1997), who found greater cardiovascular response to performance-oriented stressors including mirror star tracing in adolescents versus children in a cross-sectional study and those of Walker et al. (Walker et al., 2001, Weinstein et al., 1999) and Klimes-Dougan et al. (2001), who revealed preliminary evidence for increased cortisol response to psychological stressors over the adolescent transition. Walker et al. showed both cross-sectional and longitudinal influences of development on the initial samples in a series of laboratory saliva cortisol samples. Although the initial saliva sample was described as indicating response to novelty (stress response), because a specific stressor was not imposed, it is possible that results could also indicate a developmental increase in basal cortisol. Similar to the present study, Klimes-Dougan et al. (2001) found evidence for increased cortisol responses to (social) performance tasks, but not a relationship-oriented (family conflict) task across development, but only in boys. Finally, results are consistent with a recent study by Adam (2006), which demonstrated increased cortisol reactivity to negative affect with increasing age using ecological momentary assessment techniques in older (13+) adolescents in a cross-sectional design.
In the present study, the consistent differences between adolescents and children evident for physiological stress responses were not mirrored by differences in affective responses to the stressors. Adolescents showed mildly greater increases in negative affect and decreases in positive affect in response to both stress sessions relative to children; however, developmental differences were not statistically significant. The lack of significant developmental influences on affective responses suggests that developmental influences on physiological stress responses may not be mediated by perceptions of increased stressor intensity, but that at similar levels of perceived stressor intensity, adolescents show increased physiological responses relative to children. However, one possibility that must be ruled out is that adolescents under-report and/or children over-report changes in negative and positive affect in response to laboratory stressors. Future studies involving affective measures less prone to self-reporting bias (e.g., observer coding of facial expression) and allowing for examination of specific emotional states (e.g., fear, disgust) might substantiate the dissociation between developmental influences on affective and physiological responses seen in the present study or, perhaps, reveal closer parallels between developmental influences on affective and physiological responses (Lerner, Dahl, Hariri, & Taylor, 2007).
Evidence for a normative developmental difference in stress response over the adolescent transition has important implications for elucidating mechanisms underlying increases in depression and psychopathology over the adolescent transition. Heightened physiological stress response in typical adolescents may facilitate adaptation to new challenges of adolescence and adulthood (Gunnar et al., this volume). However, in high-risk adolescents, this normative shift may tip the balance toward stress response dysregulation associated with depression and other psychopathology. One possibility is that at-risk or disordered children show alterations in stress response prior to the pubertal/adolescent transition (e.g., do not show evidence for an SHRP), but alterations become exaggerated by a normative maturational increase in stress response during a period of increased biological and social transition. Another possibility is that at-risk/disordered children show few alterations in stress response prior to the adolescent transition, but the normative developmental increase in stress response combined with an increase in stressor exposure during adolescence tips the balance toward even more pronounced increases in stress response and stress response dysregulation. It is also possible that at-risk/disordered children would not show a normative increase in stress response that may be adaptive around this transition (see Gunnar et al., this issue). Future longitudinal research in at-risk children might investigate these possibilities. In addition, although plausible mechanisms have been proposed linking stress-reactivity to the emergence of psychopathology, most human research on stress response and pathology has revealed stress response dysregulation as a concomitant, rather than a predictor of depression and other psychopathology (Charmandari et al., 2005; Holsboer, 2000; McEwen, 1998; Rosen & Schulkin, 1998). Thus, future research is needed to elucidate pathways between stress response alterations and the emergence of disorder.
The present study also revealed specificity of developmental differences in stress response by stressor domain and physiological stress system. In particular, developmental influences on (increased) cortisol and DBP stress responses were most pronounced in response to the performance stressor, while developmental influences on (increased) sAA and SBP stress responses were most striking in response to the peer rejection stressor. Evidence for increased cortisol reactivity to performance versus peer rejection stressors is consistent with a recent meta-analysis of cortisol response to acute laboratory stressors in adults (Dickerson & Kemeny, 2004). In this review, the greatest cortisol responses were elicited by stressors involving both social evaluative threat—potential negative judgment by others--and an uncontrollable outcome. As a prototype of both characteristics, the Trier Social Stress Test (involving public speaking and mental arithmetic before an audience) has been shown to be one of the most potent laboratory challenges for increasing cortisol (Kirschbaum, Pirke, & Hellhammer, 1993). We have previously proposed that the TSST is more performance-oriented than social, and designed the peer rejection task in the current study to be purely social in nature (Stroud, et al., 2000). Closer examination of patterns of cortisol response to peer rejection reveal increased initial baseline relative to the performance session (especially in adolescents), suggesting greater anticipatory response to peer rejection. Thus, it is possible that while adolescents assigned to the performance condition showed greater task reactivity relative to children, adolescents assigned to the peer rejection condition showed greater anticipatory reactivity relative to children. These patterns are similar to those found by Klimes-Dougan et al. (2001) in a study including cortisol response to both interpersonal conflict and performance stressors in a within-subjects design (each participant completed all stress tasks). In this study, elevated baseline followed by decline in cortisol levels were evident for interpersonal conflict task (conflictual mother-child discussion), but a more typical task reaction pattern (increase slight in cortisol in response to the task followed by decline after the task) emerged in response to the performance stressors (interaction with shy confederate and public speaking). Although one possibility suggested by the authors is that the high initial baseline prior to the interpersonal conflict task may have been related to stressful nature of events prior to the task (e.g., experimenter presence in home, consent), a cortisol response in anticipation of the interpersonal conflict is also a possibility.
For DBP, both children and adolescents showed significant response to both challenges, with developmental differences due to increased adolescent response to mental arithmetic and mirror tracing challenges. Increased DBP response and developmental differences in DBP response to performance relative to peer rejection stressors is consistent with Allen and Matthews (1997), who revealed pronounced adolescent-child differences in DBP but not SBP response to a mirror tracing task similar to that utilized in the present study. However, Allen and Matthews found significant developmental differences in DBP response to a social task (interview regarding past stressful interpersonal situation; Social Competence Interview), whereas no developmental differences emerged during the peer rejection situation in the present study. One possibility is that past situations recalled in the Social Competence Interview may have represented a variety of situations evoking both social and performance anxiety as opposed to the exclusive focus on peer rejection in the present study.
For both SBP and sAA, developmental differences in response to peer rejection were driven by more sustained response to peer rejection interactions by adolescents and by a lack of response to peer rejection by children. In response to the performance session, however, neither adolescents nor children showed significant sAA response to the stressors, while for SBP, developmental differences were evident, but smaller magnitude than for the peer rejection session. More pronounced developmental differences in sAA and SBP in response to peer rejection relative to performance stressors are consistent with prior studies in children and adolescents showing associations between interpersonal striving and tasks with increased interpersonal relevance and increased cardiovascular stress responses (Chen, Matthews, Salomon, & Ewart, 2002; Ewart & Jorgensen, 2004; Ewart, Jorgensen, & Kolodner, 1998). Although the performance stressors in the present study were not identical to those in the TSST, the public speaking and mental arithmetic tasks are very similar. Interestingly, the lack of sAA response to performance challenge is not consistent with prior studies of sAA response to the TSST in adolescents (Gordis et al., 2006; Gordis, Granger, Susman, & Trickett, 2008). However, the studies by Gordis et al. were conducted in older adolescents (ages 9-14) in a more diverse sample including maltreated children. Timing of saliva sampling also differed for from the present study. However, the potent sAA response to the peer rejection stressor in the present study using the same saliva sampling timeframe suggests that lack of sAA response is not due to timing of saliva sampling. Additional normative studies are needed to determine further examine sAA response to the TSST and performance stressors over the adolescent transition.
To our knowledge, the present study is the first to show dissociations between developmental differences in HPA versus SNS responses to performance versus peer rejection stressors in children and adolescents. In adults, Henry, Frankenhaeuser and others have proposed that tasks perceived as uncontrollable, and inducing high distress, passive coping, and a “defeat” reaction are associated with increased HPA relative to or in addition to SNS activation, whereas tasks perceived as more controllable leading to increased effort, active coping and “defense” reactions are associated with increased SNS relative to HPA activation (Frankenhaeuser, 1982; Henry, 1992). Thus, it is possible that in older adolescents, the performance task evoked more of a “defeat” response, whereas the social task evoked more of a “defensive” or effortful coping response, while no such dissociations were evident for children. Further research to uncover specific emotions related to cortisol versus sAA response and performance versus social stressors over the adolescent transition is needed.
Although results related to specificity of response to performance versus rejection stressors by physiological stress system were not predicted a priori, they are worthy of replication and future research. Individual differences in response to performance versus interpersonal stressors may be linked with risk for psychopathology. For example, we have proposed that physiological sensitivity to interpersonal stressors may be a risk factor for depressive disorders, particularly among girls (Stroud, Salovey, & Epel, 2002). Exaggerated or dysregulated response to performance stressors may also be associated with specific types of psychopathology/symptoms. Or, individual differences in response across multiple task domains (e.g., exaggerated response to multiple task domains versus a single domain) may be linked to pathology (Klimes-Dougan et al., 2001). Patterns of physiological responses across multiple systems may also have implications for psychopathology (Bauer, Quas, & Boyce, 2002). For example, Gordis et al. (2006, 2008) have shown links between asymmetry of HPA and SNS responses and aggressive behavior as well as maltreatment. Results highlight the importance of including multiple developmentally relevant stressor domains and measuring across multiple physiological stress systems in order to make sense of complex interactions between context and individual differences in the emergence and maintenance of psychopathology.
Although the present study represents an important first step in examining normative, developmental influences on stress response across the adolescent transition, there are a number of additional limitations that point to directions for future research. First, although we included measures of depression, anxiety, and behavioral symptoms in this study, the cross-sectional, between-subjects nature of the design and normative sample precluded conclusive examination of developmental influences on associations between stress response and psychiatric symptoms (depression, anxiety, behavioral problems). However, in prior analyses collapsing across development and session type, we found significant associations between cortisol reactivity and (CBCL) internalizing symptoms, as well as an interaction between cortisol and sAA reactivity in predicting (CBCL) total behavior problems (Handwerger, Allwood, Kivlighan, Granger, & Stroud, In Preparation). Future research is needed to examine differential associations between stress response and psychiatric symptoms/disorders across and over the adolescent transition in normative, at-risk, and clinical populations. Second, fewer participants completed the peer rejection session than the performance session resulting in decreased power for revealing developmental influences on response to social relative to performance stressors. However, that we found statistically significant effects within the peer rejection condition in this small sample highlights the importance of including this stressor domain in future studies. Third, the peer rejection session was perceived as less intense than the performance session (Figure 1), likely due to increased ethical concerns from the investigators and study staff in administering rejection versus performance stressors. Thus, it is possible that differences in developmental influences on and specificity of physiological responses to peer rejection versus performance stressors were due to differences in intensity rather than differences in stressor domain. However, given that SNS and cardiovascular responses are typically associated with stressor intensity, stronger sAA and SBP responses to peer rejection relative to performance stressors suggests that specificity of physiological response and developmental influences were not due to differences in stressor intensity. Fourth, participants were primarily Caucasian and middle to upper-middle class in terms of socio-economic indicators. Given evidence for differences in stress response by race and socio-economic status in children, future studies with more diverse samples are needed (Lupien, King, Meaney, & McEwen, 2001; Murphy, Alpert, Walker, & Willey, 1988). Fifth, although the adolescent transition is a critical period for the emergence of gender differences in psychopathology and previous studies have revealed gender by stressor type interactions in adults (Stroud et al., 2002), the study was not adequately powered to examine interactions of age and stressor type with gender. Finally, although sAA has been validated as a surrogate marker for the SNS, given discrepant results between the present study and Gunnar et al (this issue), future studies involving pure measures of SNS as well as PNS reactivity to ecologically valid stressors are needed.
In sum, paralleling increases in depression and psychopathology over adolescence, we found pronounced increases in stress response across multiple physiological systems over the adolescent transition. Developmental influences differed by stressor domain and physiological stress system, highlighting the importance of assessing responses to multiple developmentally-relevant stressor domains and across multiple physiological stress systems. Synthesizing with prior studies in human infants and a body of preclinical research documenting a rodent stress hyporesponsive period (SHRP), results raise the possibility of a normative period of relatively buffered physiological stress response in humans lasting until the pubertal/adolescent transition. Results from the present study and the possibility of a human SHRP have important implications for elucidating mechanisms underlying increases in depression and other psychiatric disorders over the adolescent transition.
Acknowledgments
This research was supported in part by an NIMH Career Development Award (K23 MH65443), a National Alliance for Research on Schizophrenia and Depression Junior Investigator Award to the first author, and NCI grant P50 CA84719. We are indebted to the mothers and children who participated in this study. We also thank Catherine Solomon, Stephanie Paton, Gia Fiore, and Vincent Capaldi for their assistance in data collection and management, and Mary Curran and Becky Hamilton for biotechnical support. Finally, we thank Megan Gunnar for her comments on earlier versions of this manuscript. Reagents and materials were supported in part by Salimetrics LLC (State College, PA).
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psychology; 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Allen MT, Matthews KA. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology. 1997;34(3):329–339. doi: 10.1111/j.1469-8986.1997.tb02403.x. [DOI] [PubMed] [Google Scholar]
- Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20(8):879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behavior: advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Salomon K, Ewart CK. Cardiovascular reactivity during social and nonsocial stressors: do children's personal goals and expressive skills matter? Health Psychology. 2002;21(1):16–24. [PubMed] [Google Scholar]
- Choi S, Kellogg CK. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Brain Research and Developmental Brain Research. 1996;94(2):144–151. doi: 10.1016/s0165-3806(96)80005-8. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Annals of Internal Medicine. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70(1):6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Colten ME, Gore S. Adolescent Stress: Causes and Consequences. New York: Aldine De Gruyter; 1991. [Google Scholar]
- Compas BE, Hinden BR, Gerhardt CA. Adolescent development: pathways and processes of risk and resilience. Annual Review of Psychology. 1995;46:265–293. doi: 10.1146/annurev.ps.46.020195.001405. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. American Journal of Physiology. 1963;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent development and the regulation of behavior and emotion: introduction to part VIII. Annals of the New York Academy of Sciences. 2004a;1021:294–295. doi: 10.1196/annals.1308.034. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Personal Communication 2004b [Google Scholar]
- Dahl RE, Kaufman J, Ryan ND, Perel J, al-Shabbout M, Birmaher B, et al. The dexamethasone suppression test in children and adolescents: a review and a controlled study. Biological Psychiatry. 1992;32(2):109–126. doi: 10.1016/0006-3223(92)90015-r. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, et al. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biological Psychiatry. 1991;30(1):25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eccles JS, Midgley C, Wigfield A, Buchanan CM, Reuman D, Flanagan C, et al. Development during adolescence. The impact of stage-environment fit on young adolescents' experiences in schools and in families. American Psychologist. 1993;48(2):90–101. doi: 10.1037//0003-066x.48.2.90. [DOI] [PubMed] [Google Scholar]
- Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clinical Chemistry and Laboratory Medicine. 2002;40(11):1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Jorgensen RS. Agonistic interpersonal striving: social-cognitive mechanism of cardiovascular risk in youth? Health Psychology. 2004;23(1):75–85. doi: 10.1037/0278-6133.23.1.75. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Jorgensen RS, Kolodner KB. Sociotropic cognition moderates blood pressure response to interpersonal stress in high-risk adolescent girls. International Journal of Psychophysiology. 1998;28(2):131–142. doi: 10.1016/s0167-8760(97)00091-3. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and meaning-focused coping during significant psychological stress. In: Hewstone M, Schut HAW, De Wit JBF, Van Den Bos K, Stroebe MS, editors. The scope of social psychology: Theory and applications. New York, NY: Psychology Press; 2007. pp. 193–208. [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17(3):827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser M. Challenge-control interaction as reflected in sympathetic-adrenal and pituitary-adrenal activity: Comparison between the sexes. Scandinavian Journal of Psychology. 1982 1:158–164. doi: 10.1111/j.1467-9450.1982.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J, Petersen AC. Transitions Through Adolescence: Interpersonal Domains and Context. Mahwah, N.J: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, et al. Integrating the measurement of salivary alpha amylase into studies of child health, development, and social relationships. Journal of Social and Personal Relationships. 2006;23:267–290. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Assessment of Salivary a-Amylase in Biobehavioral Research. In: Luecken LJ, Gallo L, editors. Handbook of Physiological Research Methods in Health Psychology. New York: Sage; 2007a. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Annals of the New York Academy of Sciences. 2007b;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. doi: 10.1016/j.physbeh.2007.05.004. In Press. [DOI] [PubMed] [Google Scholar]
- Guinjoan SM, Bernabo JL, Cardinali DP. Cardiovascular tests of autonomic function and sympathetic skin responses in patients with major depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;59(3):299–302. doi: 10.1136/jnnp.59.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Studies of the human infant's adrenocortical response to potentially stressful events. New Directions in Child Development. 1989;45:3–18. doi: 10.1002/cd.23219894503. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Reactivity of the hypothalamic-pituitary-adrenocortical system to stressors in normal infants and children. Pediatrics. 1992;90(3 Pt 2):491–497. [PubMed] [Google Scholar]
- Gunnar MR. Integrating neuroscience and psychosocial approaches in the study of early experiences. In: King JA, Ferris CF, Lederhendler II, editors. Roots of Mental Illness in Children. Vol. 1008. New York: New York Academy of Sciences; 2003. pp. 238–247. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Personal Communication 2004 [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress Neurobiology and Developmental Psychopathology. In: Cicchetti D, Cohen, editors. Developmental Psychopathology: Developmental Neuroscience. 2nd. Vol. 2. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62(1):40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Handwerger K, Allwood MA, Kivlighan KT, Granger DA, Stroud LR. Saliva alpha amylase stress reactivity in children and adolescents: Validation, contextual influences, and links to behavioral dysregulation. Hormones and Behavior In Preparation. [Google Scholar]
- Hayward C. Gender differences at puberty. Cambridge, U.K: Cambridge University Press; 2003. [Google Scholar]
- Henry JP. Biological basis of the stress response. Integrative Physiology and Behavioral Sciences. 1992;27(1):66–83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Cicchetti D, Rogosch F. Salivary Biomarker Levels and Diurnal Variation: Associations With Medications Prescribed to Control Children's Problem Behavior. Child Development. 2007;78(3):927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Jonetz-Mentzel L, Wiedemann G. Establishment of reference ranges for cortisol in neonates, infants, children and adolescents. European Journal of Clinical Chemistry and Clinical Biochemistry. 1993;31(8):525–529. [PubMed] [Google Scholar]
- Kenny FM, Gancayco GP, Heald FP, Hung W. Cortisol production rate in adolescent males in different stages of sexual maturation. Journal of Clinical Endocrinology and Metabolism. 1966;26(11):1232–1236. doi: 10.1210/jcem-26-11-1232. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatric Research. 1995;37(4 Pt 1):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(12):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, et al. Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. Journal of Clinical Endocrinology and Metabolism. 1997;82(2):536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Childhood Depression Inventory Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Kurtz MM, Campbell BA. Paradoxical autonomic responses to aversive stimuli in the developing rat. Behavioral Neuroscience. 1994;108(5):962–971. doi: 10.1037//0735-7044.108.5.962. [DOI] [PubMed] [Google Scholar]
- Ladd GW. Peer rejection, aggressive or withdrawn behavior, and psychological maladjustment from ages 5 to 12: an examination of four predictive models. Child Development. 2006;77(4):822–846. doi: 10.1111/j.1467-8624.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Urinary free cortisol increases in adolescent Caucasian females during perimenarche. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):215–219. doi: 10.1210/jc.2002-020256. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Dahl RE, Hariri AR, Taylor SE. Facial expressions of emotion reveal neuroendocrine and cardiovascular stress responses. Biological Psychiatry. 2007;61(2):253–260. doi: 10.1016/j.biopsych.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, Schneiderman N. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28:701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13(3):653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Children. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Children. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International. 2007;24(5):969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Nelesen RA, Dimsdale JE. Depressive symptoms are associated with increased systemic vascular resistance to stress. Psychosomatic Medicine. 2005;67(4):509–513. doi: 10.1097/01.psy.0000160467.78373.d8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Pela I, Cecioni I, Gensini GF, Serneri GG, Bartolozzi G. Changes in blood pressure reactivity and 24-hour blood pressure profile occurring at puberty. Angiology. 1994;45(6):443–450. doi: 10.1177/000331979404500605. [DOI] [PubMed] [Google Scholar]
- Murphy JK, Alpert BS, Walker SS, Willey ES. Race and cardiovascular reactivity. A replication. Hypertension. 1988;11(4):308–311. doi: 10.1161/01.hyp.11.4.308. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, et al. Stress-induced changes in human salivary alpha-amylase activity -- associations with adrenergic activity. Psychoneuroendocrinology. 2006;31(1):49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29(2):125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21(2):293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Porth C. Essentials of Pathophysiology: Concepts of Altered Health States. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Developmental Psychobiology. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Borelli JL, Cheah CS, Simon VA, Aikins JW. Adolescent girls' interpersonal vulnerability to depressive symptoms: a longitudinal examination of reassurance-seeking and peer relationships. Journal of Abnormal Psychology. 2005;114(4):676–688. doi: 10.1037/0021-843X.114.4.676. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. Revised Children's Manifest Anxiety Scale. Los Angeles, CA: Western Psychological Services; 2000. [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential Stress Reactivity in Intact and Ovariectomized Prepubertal and Adult Female Rats. Neuroendocrinology. 2005;80(6):387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research. 1986;396(1):64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schmidt NA. Salivary cortisol testing in children. Issues in Comprehensive Pediatric Nursing. 1998;20(3):183–190. doi: 10.3109/01460869709028262. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69(6):1503–1513. [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30(2):162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Edwards CD, Montuori J, Lushene RE, Platzek D. State-Trait Anxiety Inventory for Children (STAIC) Palo Alto, CA: Mind Garden; 1973. [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine D. The Study of Developmental Psychopathology in Adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, editor. Handbook of Developmental Psychopathology. New York: Wiley; 2004. [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to a corticotropin-releasing hormone challenge: the Pittsburgh psychobiologic studies. Annals of the New York Academy of Sciences. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Tanofsky-Kraff M, Wilfley DE, Salovey P. The Yale Interpersonal Stressor (YIPS): Affective, physiological, and behavioral responses to a novel interpersonal rejection paradigm. Annals of Behavioral Medicine. 2000;22:204–213. doi: 10.1007/BF02895115. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: effect of beta blockade. Psychoneuroendocrinology. 2006;31(1):137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23(7):663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Walker CD, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. Journal of Neuroendocrinology. 1995;7(8):615–622. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13(3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Weinstein DD, Diforio D, Schiffman J, Walker EF, Bonsall R. Minor physical anomalies, dermatoglyphic asymmetries, and cortisol levels in adolescents with schizotypal personality disorder. American Journal of Psychiatry. 1999;156(4):617–623. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]