Abstract
The fallopian tube plays an important role in the mechanical transport and physiological sustenance of the gametes and early conceptus. Complex and coordinated neuromuscular activity, cilial action and endocrine secretions are required for successful tubal function. Compromised tubal damage can occur after external or internal injury, inhibiting the normal transport of gametes. The overall prognosis for fertility depends principally on the insult and the severity of the tissue damage; hence, assessment of tubal damage plays a major role in predicting occurrence of pregnancy and the likelihood of developing ectopic pregnancy.
Keywords: Assessment, damage, fallopian tube, gametes, transport
INTRODUCTION
The fallopian tube, far from being a passive channel or conduit for gametes and early embryos, plays an important role in many reproductive functions such as sperm transport and capacitation, ova retrieval and transport, fertilization, embryo storage, nourishment and transport.
Tubal stenoocclusive and dilative disease is an important cause of infertility and should be specifically looked for. Tubal disease includes tubal obstruction, narrowing, dilatation, as well as conditions that alter tubal function due to changes in the tubal mucosal lining, muscular wall or any pathology present external to the tube. Tubal disease with blockages can involve the proximal part, the mid part or the distal part. Pelvic adhesions due to infection, inflammation, tuberculosis, endometriosis and previous surgery (tubal or nontubal surgery, appendicitis, others) and ectopic pregnancy (medically or surgically managed) are common factors in tubal subfertility and need to be assessed. Prior abortions, medical termination of pregnancy and myomectomy may predispose to subclinical inflammation or infection with coexistent tubal damage.
Incidence
The tubal factor is reported to account for 25–35% of subfertility in the western medical literature, but the prevalence appears to be higher in India due to the higher rates of unrecognised pelvic inflammatory disease (PID) and tuberculosis.
Proximal (uterotubal) obstruction reportedly occurs in 10–25% of women with tubal disease.[1] However, population-based data from India are lacking.
Proximal tubal obstruction may be due to muscular spasm, stromal edema, amorphous debris, mucosal agglutination and viscous secretions. Other factors include cornual polyps, chronic salpingitis, endometriosis, salpingitis isthmica nodosa, intrauterine synechiae and parasite infection.
Proximal tubal obstruction secondary to tubal spasm or intratubal debris may be a reversible condition. Pelvic inflammatory disease is a major clinically unsuspected reason for tubal subfertility. PID may be responsible for more than 50% of the causes of tubal factor infertility. PID can damage the tube at multiple sites and also predispose to ectopic pregnancy. Frequently, unsuspected mild subclinical, mucosal disease may be present on performing investigations. Fimbrial end involvement may lead to hydrosalpinx [Figure 1] and fimbrial agglutination. Midtubal disease causes stenoocclusions, typically with bulbous termination from scarring and fibrosis.
Figure 1.
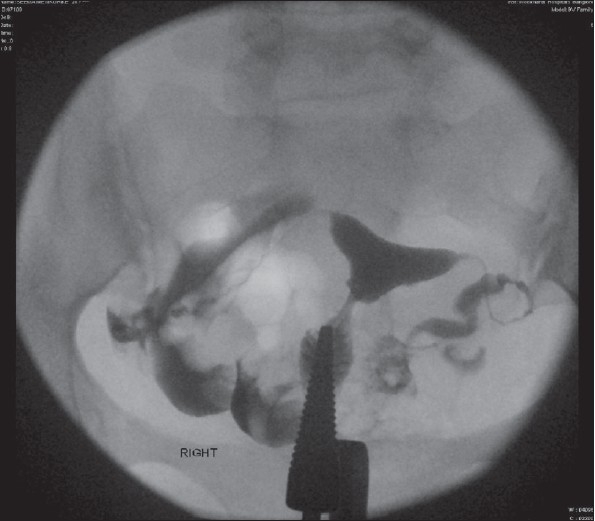
Left hydrosalpinx
Less-severe disease may cause distal tubal stenodilatations, fimbrial adhesions, tubal kinking and fixity from adhesions with preserved tubal patency. Tuberculous involvement of the tube can be mild with damage to the tubal lining or more severe, with tubal scarring, rigidity, fibrosis, stenoocclusions, dilatations, hydrosalpinx, peritubal and pelvic adhesions. Other important causes of tubal damage include endometriosis (7–14%), salpingitis isthmica nodosa and cornual polypoidal lesions (10%).
Assessment of fallopian tubes
Various methods for the evaluation of tubal factor are complementary and not mutually exclusive. Evaluation of tubal patency and tubal integrity is a key component of the diagnostic work-up in infertile couples.
An ideal (or “gold standard”) test for tubal disease would correctly identify all women with tubal disease. The test should be able to predict pregnancy and also improve pregnancy rates and at the same time, be cost-effective.
Fallopian tube assessment tests
Several tests have been described but only few of them are currently in vogue in clinical practice.
Method of assessment
Laparoscopy: Jacobaeus (1910), Palmer (1947).
Hysterosalpingogram: Carey (1914).
-
Rubin's test: Tubal perfusion pressures
Oxygen: Rubin (1920).
Carbon dioxide: Rubin (1952).
Dye injections with culdocentesis: Decker (1952).
Injection of radiolabeled xenon solution with gamma-camera screening: Pertynski et al. (1977).
Selective salpingography and tubal catheterization: Corfman and Taylor (1966).
Salpingoscopy: Brosens et al. (1987).
Falloposcopy: Kerin et al. (1990a).
Hysterocontrast sonography: Deichert (1993).
Fertiloscopy: Watrelot et al. (1999).
Accurate diagnosis of tubal integrity and effective treatment of tubal subfertility often require more than one technique.
Hysterosalpingography (HSG)
Using either a water- or lipid-soluble contrast media is the time-honored method for evaluating tubal patency.
It can document proximal and distal tubal occlusion, demonstrate salpingitis isthmica nodosa, reveal tubal architectural details of potential prognostic value and suggest the presence of fimbrial phimosis or peritubular adhesions when escape of contrast is delayed or becomes loculated, respectively.
Findings suggesting proximal tubal obstruction require further evaluation to exclude transient occlusion resulting from tubal/myometrial contractions.
Tubal cannulation with advanced HSG and tubal assessment categories
(Recent advances in tubal factor - unpublished data, work in progress - Dr. Raghav, Wockhardt Hospitals, Bangalore – personal communication) Improvements in HSG using real time guidance, uterotubal manipulation, tubal cannulation and tubal pressure asessments have yielded the classification of the tubal factor into simple, clinically relevant categories.
The presence or lack of distal tubal disease is an important factor in outcomes in tubal factor subfertility and this classification attempts to address this situation.
By classifying patients to these categories, one can decide the line of treatment and also predict the probability of pregnancy and the likelihood of ectopic pregnancy.
The tubes are categorized into three categories depending on their morphology and patency.
Category 1 - normal [Figure 2]
Figure 2.
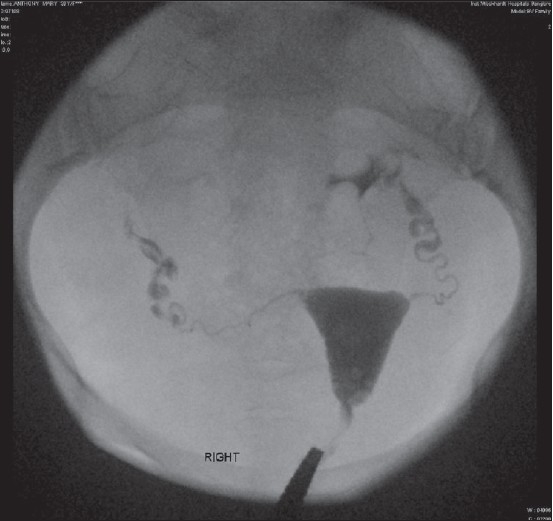
Category 1
Characteristics - patency with free spill, preserved distal tubal folds, normal proximal, mid, distal tubal dimensions and appearance, no fimbrial end clumping, no detected peritubal disease, normal tubal pressures with free flow, lack of sharp pain on forceful flushing.
Outcome - good prognosis and outcome for future fertility, very low incidence of ectopic pregnancy on follow-up.
Category 2 - patent tube with tubal disease [Figure 3]
Figure 3.
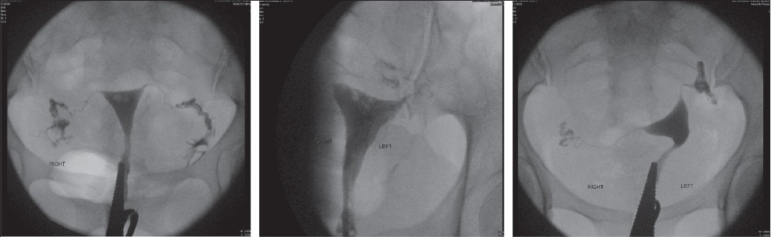
Category 2
2a - mild tubal disease
2b - moderate tubal disease.
Characteristics - patency with good spill, partly or fully preserved distal tubal folds, normal or slightly altered proximal, mid, distal tubal dimensions and appearance, fimbrial end clumping may or may not be present, peritubal disease may or may not be seen, normal or elevated tubal pressures.
Outcome - moderate prognosis and outcome for future fertility, highest group incidence of ectopic pregnancy on follow-up.
Category 3 - patent or blocked tubes, severe tubal disease [Figure 4].
Figure 4 (a and b).
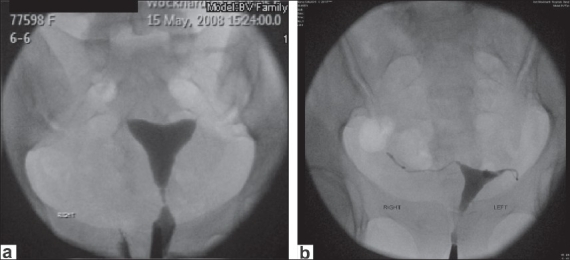
Category 3 bilateral tubal block
Characteristics - patent or blocked tubes, loss of distal tubal folds, altered proximal, mid, distal tubal dimensions and appearance with dilatation/narrowing/scarring/tubal rigidity, fimbrial end dilatation/narrowing with clumping present, peritubal disease may or may not be seen, usually elevated tubal pressures but can be normal.
Outcome - poor prognosis and outcome for nonassisted future fertility, very low group incidence of ectopic pregnancy. In vitro fertilization (IVF) is to be considered.
Further long-term study is required to assess whether tubal cannulation [Figure-5] with advanced HSG can be used as a single primary test for tubal factor integrity assessment in the future.
Figure 5.
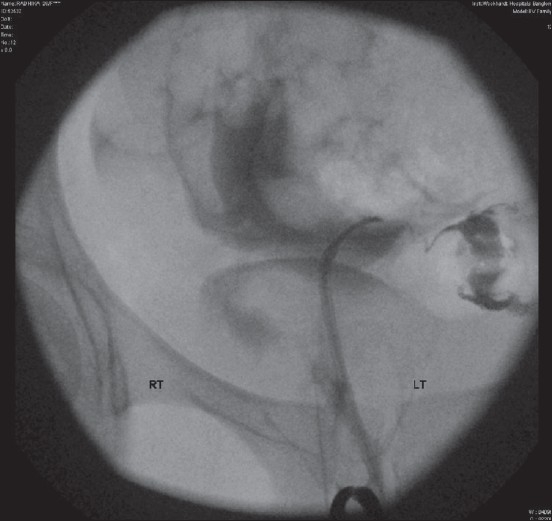
Cannulation
In the presence of patent tubes, intravasation of dye has no significance [Figure 6].
Figure 6.
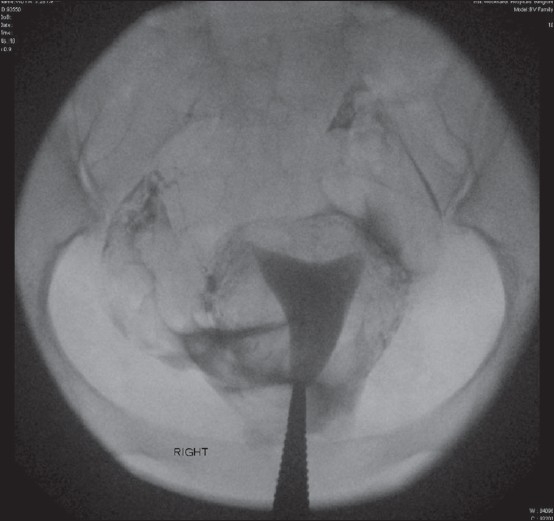
Intravasation of dye
Management implications
Because of its low sensitivity (0.65), simple HSG is of limited use in the assessment of adhesions or endometriosis.[2] Confirmation by laparoscopy is not necessary if HSG shows no abnormality or tubal obstruction.[2] (Category 1 by above classification.)
In patients who are going to be treated with intrauterine insemination (IUI), laparoscopy may be performed if HSG shows abnormal results. (Category 2, especially 2b, with more severe disease by above classification.)
In the era of IVF, it is cost-effective to omit laparoscopy, especially in the cases of bilateral tubal abnormalities detected at HSG. (Category 3 by the above classification.) However, some authors report that the predictive value of HSG occurrence of pregnancy is poor and its routine use in the fertility work-up should be reconsidered.[3,4]
Laparoscopy is of additional value with respect to diagnosis, further treatment decisions after abnormal findings with HSG and before IUI. Laparoscopy provides an external view of the tubes, while HSG provides an internal view and is often complementary.
Tubal cannulation and selective salpingography
The advent of tubal cannulation [Figure 5] and selective salpingography– fluoroscopic and hysteroscopic – has allowed restoration of patency in cases of isolated proximal tubal occlusion due to spasm, tubal plugs and synechiae.[5] Tubal cannulation enables an indirect assessment of tubal function via measurement of the intratubal pressure as high, suggesting decreased tubal wall compliance.[6]
Papaioannou et al.[7] found that persistence of elevated perfusion pressure after cannulation using guide wire was an important determinant in the success rate. The pregnancy rate was significantly lower at 12–14% versus 35% in the low perfusion pressure group. Moreover, in the elevated pressure group, there was a higher ectopic pregnancy rate (25–50% versus 8%).
Several authors have reported case series of radiographic selective salpingography and tubal catheterization. The reported pregnancy rates are between 12 and 44%, with ectopic pregnancy rates ranging from 1.6 to 50%.[7–10] The pregnancy rates do not differ from those achieved after the hysteroscopic technique.
There is only one meta analysis comparing tubal cannulation radiographic versus hysteroscopic techniques versus microsurgical anastomosis for proximal tubal blockage, which was based on data from cohort and observational studies.[11] The total pregnancy rate per patient after hysteroscopic tubal cannulation was 48.9% (65/133 patients). The analysis of data showed that microsurgical anastomosis yields an ongoing pregnancy rate of 47.4%, which is higher than that obtained with radiographic cannulation (25.9%).
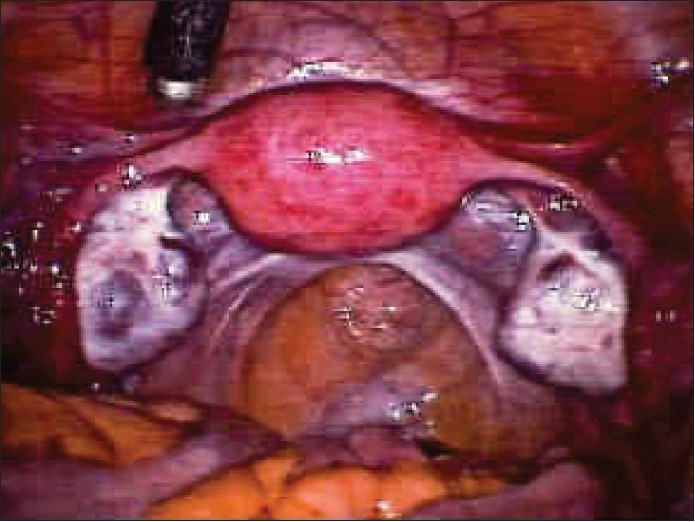
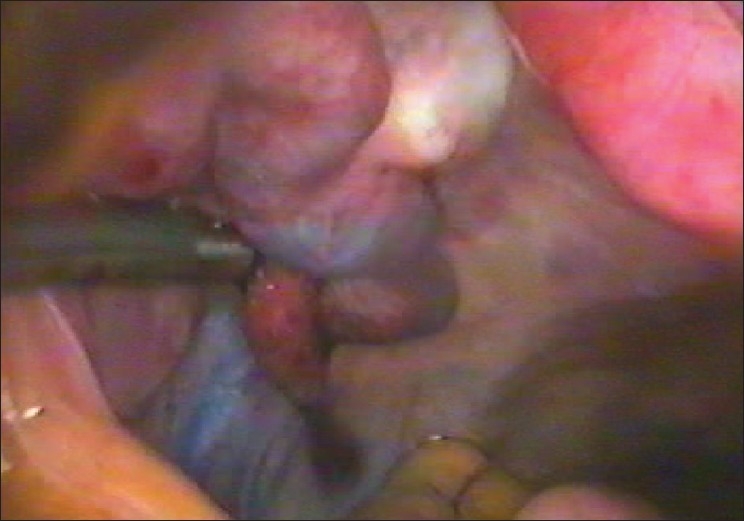
Laparoscopy and “chromotubation” with a dilute solution of methylene blue introduced via the cervix can demonstrate normal pelvic anatomy [Figure 7] and tubal patency or document proximal or distal tubal occlusive disease. Laparoscopy can also identify subtle tubal factors such as hydrosalpinx [Figure 8], fimbrial phimosis [Figure 9] or peritubular adhesions [Figure 10], endometriosis [Figure 11] that may escape detection with less-invasive methods. It is also beneficial because when abnormalities are encountered during laparoscopy, treatment [Figures 12 and 13] is possible at the same time. Thus, laparoscopy will either confirm or exclude a suspected preoperative diagnosis.
Figure 7.
Normal tubes at laparoscopy
Figure 8.
Laparoscopy hydrosalpinx
Figure 9 (a and b).
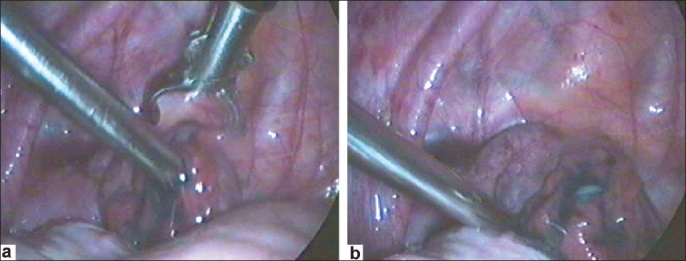
Deagglutination of fimbria or broadening of the phimotic tubal opening
Figure 10 (a–d).
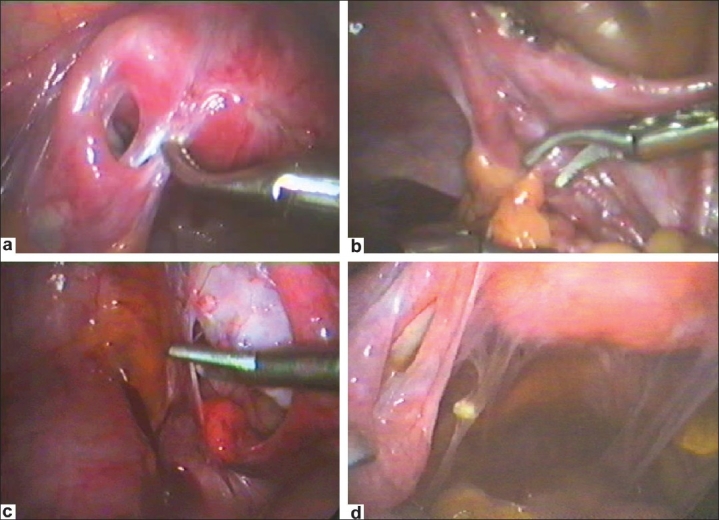
Peritubal adhesions
Figure 11 (a and b).
Endometriosis
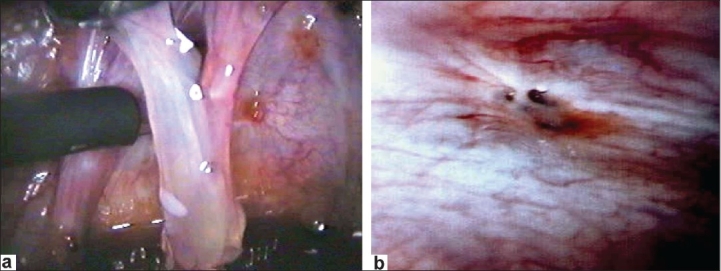
Figure 12.
Microscissor cutting adhesions to tube
Figure 13 (a–d).
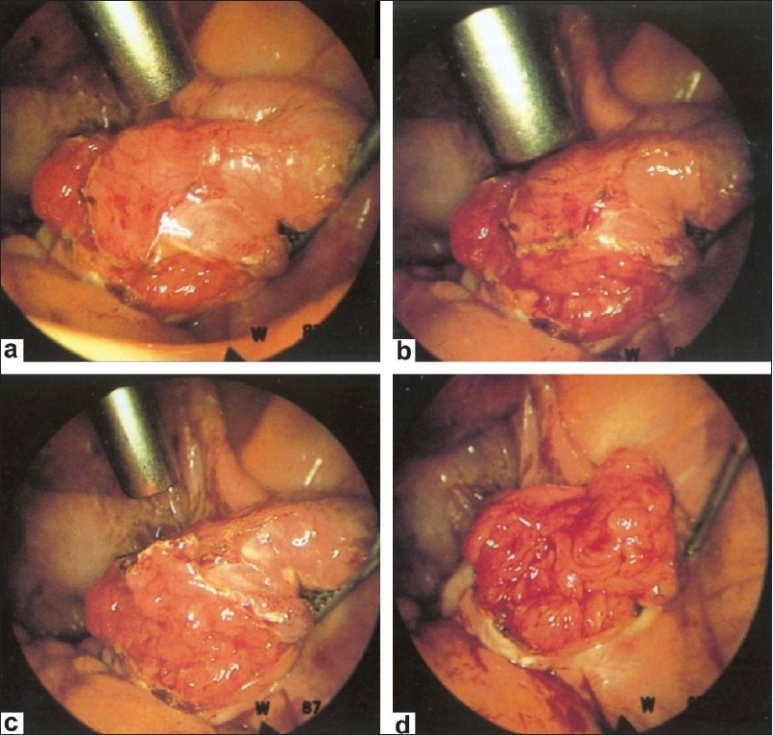
Salpingostomy
Fluoroscopic/hysteroscopic selective tubal cannulation will confirm or exclude any proximal tubal occlusion suggested by HSG or laparoscopy with chromotubation and provide the means for possible correction via recanalization using specialized catheter systems. Selective salpingography performs well regarding safety and the only complication that can occur is tubal perforation, reported in about 5% of the cases. An ectopic pregnancy has been reported in 5% of the cases and infection is very rare if screening or routine prophylaxis for infection is implemented.
Selective salpingography and tubal catheterization[12] provides the examiner with the opportunity to measure tubal perfusion pressures, which have been found by life table analysis to be prognostic of future spontaneous fertility.
Hysterosalpingo-contrast-sonography
It is a transvaginal ultrasound technique in which a solution of galactose and 1% palmitic acid (Echovist -Schering-AG, Germany) or a mixture of air and saline is infused into the uterine cavity and observed to flow along the fallopian tubes to assess tubal patency. The bright echoes generated by the Hysterosalpingo-contrast-sonography (HyCoSy) solution make tubal visualization possible. Results can be further improved by the use of color Doppler imaging or 3D technology.
HyCoSy is a safe outpatient procedure with a relatively low cost and its accuracy has been assessed in a metaanalysis, which compared the results of HyCoSy and laparoscopy and dye tests in 428 infertile women. Sensitivity was 93.3% and specificity was 89.7%.[13]
Salpingoscopy
Salpingoscopy was originally performed during laparotomy for reconstructive tubal surgery to assess the mucosa of the infundibulum and ampulla. Prediction of fertility outcome by laparoscopy can be improved by the concomitant performance of salpingoscopy.[14] The two tests probably complement rather than substitute one another. There is no information about accuracy, reliability, prognosis and effectiveness. Special equipment and expertise are required, making salpingoscopy an expensive proposition. It can clearly demonstrate the presence or absence of anatomical distortions, especially adhesions between and destruction of mucosal folds on a microendoscopic, i.e. mucosal level. Salpingoscopy could direct the infertility investigation and treatment, either toward reconstructive (micro)surgery or toward assisted reproduction technologies. Therefore, not enough is known about these lesions and salpingoscopy remains a research tool. Salpingoscopy can also be performed during transvaginal hydrolaparoscopy as an office procedure.
Falloposcopy
Falloposcopy [Figure 14] is microendoscopy of the oviductal lumen from the uterotubal ostium to the fimbriae by a transcervical approach.[15] Initially, the technique involved the passage into the tubal lumen, under hysteroscopic vision, of a flexible cannula into which the falloposcope was introduced with the help of continuous fluid irrigation through the flexible cannula (coaxial delivery system). Today, a miniature tubular balloon system has been used that is rolled out, along the fallopian tube lumen, by the use of hydraulic pressure. This carries the falloposcope forward with it at the same time (linear eversion system).
Figure 14 (a–c).
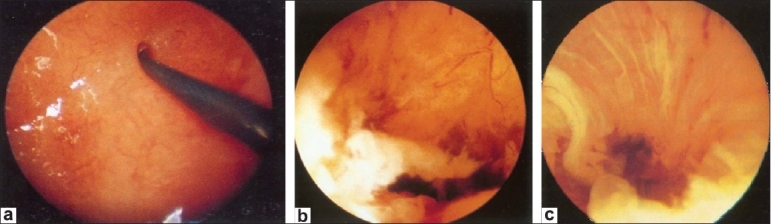
Falloposcopy
Transvaginal hydrolaparoscopy (fertiloscopy)
Fertiloscopy, performed under local anesthesia or sedation, is an outpatient assessment of the female reproductive system.
Microsalpingoscopy is routinely performed, which allows the cells of the tubal mucosa to be examined following the dye test. Staining of the tubal cell nuclei provides a means of assessing the functional capacity of the fallopian tubes: the more colored the nuclei, the less functional is the mucosa.[16] Although some operative procedures are possible, they are limited due to the absence of a panoramic view.[17] The sensitivity of fertiloscopy was 86%. In terms of reliability, the interobserver agreement for tuboovarian adhesions at transvaginal hydrolaparoscopy has been reported at 95%, comparable with that of standard laparoscopy.[17] Complications occurred in 2% of the cases.
Comparing the various modalities of tubal assessment Royal College of Obstetrics and Gynecology[18]
HSG compared with laparoscopy and dye:
HSG and laparoscopy with dye are the two most widely used methods to test for tubal pathology.[19]
For tubal evaluation, laparoscopy remains the gold standard. In an era where cost-effectiveness becomes more important, it is debatable whether laparoscopy should always be a mandatory step in the subfertility work-up after HSG.
HSG and laparoscopy are both invasive procedures but HSG is much less. Among women whose tubes were found to be patent using HSG, 18% were found to have tubal obstruction or peritubal adhesions using laparoscopy and a further 34% were found to have endometriosis and/or fibroids.[20] However, the detection and treatment of pathology missed by HSG did not increase live birth rates. (Evidence level 2b.)
The diagnostic accuracy of HSG has been compared with that of laparoscopy and dye in a systematic review of 20 studies that distinguished between tubal obstruction and peritubal adhesions.[21] However, only three studies involved judgement of laparoscopy without knowledge of HSG results. Metaanalysis based on these three studies gave pooled estimates of sensitivity and specificity for HSG as a test for tubal obstruction of 0.65 (95% [confidence interval] CI 0.50-0.78) and 0.83 (95% CI 0.77-0.88), respectively.[21] (Evidence level 2b.) Because of its low sensitivity (0.65), HSG is of limited use for detecting tubal patency and is hardly reliable in the assessment of adhesions or endometriosis. It is estimated that tubal damage accounts for 14% of fertility problems, which suggests that when HSG suggests the presence of tubal obstruction, this will be confirmed by laparoscopy in only 38% of the women.
When HSG suggests that the tubes are patent, this will be confirmed at laparoscopy in 94% of the women and, thus, HSG is a reliable indicator of tubal patency. These findings imply that after abnormal HSG, even in cases of bilateral pathology such as obstruction, it is still worth performing laparoscopy because, in a considerable number of these patients, the laparoscopic diagnosis allows IUI treatment to remain an option.[22]
Tubal pathology detected at laparoscopy has a stronger effect on future fertility than that detected at HSG.
Bilateral obstruction or hydrosalpinx found by laparoscopy was seen on HSG in 78% of the cases. However, of all bilateral obstructions and hydrosalpinx found by HSG, only 41% was confirmed by laparoscopy and was scheduled for IVF. Thus, if in these cases laparoscopy was omitted, about 60% were incorrectly treated with IVF. A possible explanation for this discrepancy is that tubal spasm could play a role in some of these cases.[23] Furthermore, especially in cases of bilateral hydrosalpinx diagnosed by laparoscopy, bilateral salpingectomy before IVF is indicated in order to optimize IVF outcome. In several patients, laparoscopy after abnormal HSG reveals normal findings or abnormalities not requiring IVF treatment, even when the results of HSG suggest bilateral pathology. Thus, laparoscopy is indicated in all patients with abnormal HSG to determine the exact treatment that should be offered to these patients.
HyCoSy compared with laparoscopy and dye or HSG
Evaluative studies of HyCoSy showed good statistical comparability and concordance with HSG and laparoscopy combined with dye.[24] (Evidence level 1b.) HyCoSy is well tolerated and can be a suitable alternative outpatient procedure.[25] (Evidence level 1b.) HyCoSy using contrast agent Infoson® appears to be more efficient than saline solution in detecting tubal obstruction.[26] (Evidence level 1b).
Fertiloscopy and falloposcopy
Diagnostic fertiloscopy has also been used to identify tubal pathology as an alternative to laparoscopy. (Evidence level 3.) However, the procedure is not without risk and bowel and rectal injuries following fertiloscopy have been reported. (Evidence level 3.) The diagnostic accuracy of fertiloscopy in comparison with HSG and laparoscopy needs further evaluation.
Falloposcopy may be a more discriminatory test of tubal pathology because women with normal fallopian tubes at falloposcopy achieve higher spontaneous pregnancy rates (27.6%) than those with mild or severe endotubal lesions (11.5-0%). In another study, the management plan was changed in 90% of the women following falloposcopy and 24% conceived naturally.[27] (Evidence level 3.) Technical problems with falloposcopy limit the use of the procedure in routine clinical practice.[28,29]
RCOG RECOMMENDATIONS FOR TUBAL ASSESSMENT
Women who are not known to have comorbidities (such as pelvic inflammatory disease, previous ectopic pregnancy or endometriosis) should be offered HSG to screen for tubal occlusion because this is a reliable test for ruling out tubal occlusion and it is less invasive and makes more efficient use of resources than laparoscopy.
Where appropriate expertise is available, screening for tubal occlusion using HyCoSy should be considered because it is an effective alternative to HSG for women who are not known to have comorbidities.
Women who are thought to have comorbidities should be offered laparoscopy and dye so that tubal and other pelvic pathology can be assessed at the same time.
RESEARCH RECOMMENDATIONS BY RCOG
Further research is needed to ascertain the value of fertiloscopy and falloposcopy in the investigation of couples who experience problems with fertility.
Further randomized controlled trials are needed to evaluate the potentially therapeutic effects of tubal flushing with water-soluble media.
Good clinical treatment in assisted reproduction: An ESHRE [European Society of Human Reproduction and Embryology] guideline[30]
Couples who have not conceived after 1 year of regular unprotected sexual intercourse should be offered further clinical investigation, including semen analysis and assessment of ovulation. ESHRE Capri workshop in 2000 published three categories of test: tests that have an established correlation with pregnancy - semen analysis, tubal patency tests by hysterography or laparoscopy and tests to detect ovulation.
Guidelines published in 2008 advocate semen analysis and ovulation assessment before a test of tubal patency is performed. Women thought to have comorbidities should be offered laparoscopy so that any tubal and other pelvic pathology can be investigated and treated at the same time.
Thus, the ESHRE guidelines concluded that if there are no concerns about pelvic or tubal health, it may be appropriate to perform three cycles of ovulation induction before checking tubal patency.
Recommendations by ASRM [American Society of Reproductive Medicine] for tubal factor evaluation[31]
Evaluation of tubal patency is a key component of the diagnostic work-up in infertile couples. All available methods for evaluation of tubal factors have technical limitations that must be considered when any one technique yields abnormal results. Further evaluation with a second, complementary method is prudent whenever specific diagnosis or the best treatment strategy is uncertain.
CONCLUSION
In assessing the tube by the various tests available, the complexities of tubal function are not completely looked at by a single test. Looking for patency by flushing liquids through the tubes should not be taken into account. Just a simple patency of the tube might give false reassurance for women with tubes that might be open as simple pipes that conduct fluid, but not necessarily functional as far as eggs and sperm are concerned. The tubes may be thickened, which affects the contractility or there may be loss of cilia, which is required for normal transport of the gametes.
There is no single test available today that is an “ideal test” in terms of safety, accuracy, effectiveness and prognostic ability. However, today, the evidence base is good for the older tests, i.e. HSG and laparoscopy with dye test.
Acknowledgments
Dr. Raghav, Wockhardt Hospital, Bangalore.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Serafini P, Batzofin J. Diagnosis of female infertility: A comprehensive approach. J Reprod Med. 1989;34:29–40. [PubMed] [Google Scholar]
- 2.Swart P, Mol BW, van der Veen F, van Beurden M, Redekop WK, Bossuyt PM. The accuracy of ysterosalpingography in the diagnosis of tubal pathology: A meta-analysis. Fertil Steril. 1995;64:486–91. doi: 10.1016/s0015-0282(16)57781-4. [DOI] [PubMed] [Google Scholar]
- 3.Perquin DA, Beersma MF, de Craen AJ, Helmerhorst FM. The value of Chlamydia trachomatis-specific IgG antibody testing and hysterosalpingography for predicting tubal pathology and occurrence of pregnancy. Fertil Steril. 2007;88:224–6. doi: 10.1016/j.fertnstert.2006.11.078. [DOI] [PubMed] [Google Scholar]
- 4.Perquin DA, Dörr PJ, de Craen AJ, Helmerhorst FM. Routine use of hysterosalpingography prior to laparoscopy in the fertility workup: A multicentre randomized controlled trial. Hum Reprod. 2006;21:1227–31. doi: 10.1093/humrep/dei478. [DOI] [PubMed] [Google Scholar]
- 5.Novy MJ. Transhysteroscopic techniques for tubal catheterization. Ref Gynecol Obstet. 1995;3:67–71. [Google Scholar]
- 6.Gleicher N, Karande V. The diagnosis and treatment of proximal tubal disease. Hum Reprod. 1996;11:1823–8. doi: 10.1093/oxfordjournals.humrep.a019497. [DOI] [PubMed] [Google Scholar]
- 7.Papaioannou S, Afnan M, Girling AJ, Coomarasamy A, Ola B, Olufowobi O, et al. The effect on pregnancy rates of tubal perfusion pressure reductions achieved by guide-wire tubal catheterization. Hum Reprod. 2002;17:2174–9. doi: 10.1093/humrep/17.8.2174. [DOI] [PubMed] [Google Scholar]
- 8.Confino E, Tur-Kaspa I, DeCherney A, Corfman R, Coulam C, Robinson E, et al. Transcervical balloon tuboplasty. A multicentre study. JAMA. 1990;264:2079–82. [PubMed] [Google Scholar]
- 9.Lang EK, Dunaway HH. Recanalization of obstructed fallopian tube by selective salpingography and transvaginal bougie dilatation: Outcome and cost analysis. Fertil Steril. 1996;66:210–5. doi: 10.1016/s0015-0282(16)58440-4. [DOI] [PubMed] [Google Scholar]
- 10.Woolcott R, Petchpud A, O'Donnell P, Stanger J. Differential impact on pregnancy rate of selective salpingography, tubal catheterization and wire-guide recanalisation in treatment of proximal Fallopian tube obstruction. Hum Reprod. 1995;10:1423–6. doi: 10.1093/humrep/10.6.1423. [DOI] [PubMed] [Google Scholar]
- 11.Honoré GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71:785–95. doi: 10.1016/s0015-0282(99)00014-x. [DOI] [PubMed] [Google Scholar]
- 12.Papaioannou S, Afnan M, Girling AJ, Ola B, Hammadieh N, Coomarasamy A, et al. The learning curve of selective salpingography and tubal catheterization. Fertil Steril. 2002;77:1049–52. doi: 10.1016/s0015-0282(02)03061-3. [DOI] [PubMed] [Google Scholar]
- 13.Holz K, Becker R, Schürmann R. Ultrasound in the investigation of tubal patency: A meta-analysis of three comparative studies of Echovist-200 including 1007 women. Zentralbl Gynakol. 1997;119:366–73. [PubMed] [Google Scholar]
- 14.Marchino GL, Gigante V, Gennarelli G, Mazza O, Mencaglia L. Salpingoscopic and laparoscopic investigations in relation to fertility outcome. J Am Assoc Gynecol Laparosc. 2001;8:218–21. doi: 10.1016/s1074-3804(05)60581-6. [DOI] [PubMed] [Google Scholar]
- 15.Kerin J, Daykhovsky L, Segalowitz J, Surrey E, Anderson R, Stein A, et al. Falloposcopy: A microendoscopic technique for visual exploration of the human fallopian tube from the uterotubal ostium to the fimbria using a transvaginal approach. Fertil Steril. 1990;54:390–400. doi: 10.1016/s0015-0282(16)53750-9. [DOI] [PubMed] [Google Scholar]
- 16.Watrelot A, Dreyfus JM, Cohen M. Systematic salpingoscopy and microsalpingoscopy during fertiloscopy. J Am Assoc Gynecol Laparosc. 2002;9:453–9. doi: 10.1016/s1074-3804(05)60518-x. [DOI] [PubMed] [Google Scholar]
- 17.Gordts S, Puttemans P, Gordts S, Brosens I, Campo R. Transvaginal laparoscopy. Best Pract Res Clin Obstet Gynaecol. 2005;19:757–67. doi: 10.1016/j.bpobgyn.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Clinical Excellence (NICE) Fertility: Assessment and treatment of people with fertility problems. London: RCOG Press; 2004. [PubMed] [Google Scholar]
- 19.Watrelot A, Nisolle M, Chelli H, Hocke C, Rongières C, Racinet C, et al. Is laparoscopy still the gold standard in infertility assessment? A comparison of fertiloscopy versus laparoscopy in infertility: Results of an international multicentre. doi: 10.1093/humrep/deg180. [DOI] [PubMed] [Google Scholar]
- 20.prospective trial: the ‘FLY’ (Fertiloscopy-Laparoscopy) study. Hum Reprod. 2003;18:834–9. doi: 10.1093/humrep/deg180. [DOI] [PubMed] [Google Scholar]
- 21.Belisle S, Collins JA, Burrows EA, Willan AR. The value of laparoscopy among infertile women with tubal patency. J Soc Obstet Gynaecol Can. 1996;18:326–36. [Google Scholar]
- 22.Watrelot A, Dreyfus JM, Andine JP. Evaluation of the performance of fertiloscopy in 160 consecutive infertile patients with no obvious pathology. Hum Reprod. 1999;14:707–11. doi: 10.1093/humrep/14.3.707. [DOI] [PubMed] [Google Scholar]
- 23.Mol BW, Collins JA, Burrows EA, van der Veen F, Bossuyt PM. Comparison of hysterosalpingography and laparoscopy in predicting fertility outcome. Hum Reprod. 1999;14:1237–42. doi: 10.1093/humrep/14.5.1237. [DOI] [PubMed] [Google Scholar]
- 24.Tanahatoe S, Hompes PG, Lambalk CB. Accuracy of diagnostic laparoscopy in the infertility work-up before intrauterine insemination. Fertil Steril. 2003;79:361–6. doi: 10.1016/s0015-0282(02)04686-1. [DOI] [PubMed] [Google Scholar]
- 25.Dijkman AB, Mol BW, van der Veen F, Bossuyt PM, Hogerzeil HV. Can hysterosalpingocontrast-sonography replace hysterosalpingography in the assessment of tubal subfertility? Eur J Radiol. 2000;35:44–8. doi: 10.1016/s0720-048x(99)00127-8. [DOI] [PubMed] [Google Scholar]
- 26.Ayida G, Kennedy S, Barlow D, Chamberlain P. A comparison of patient tolerance of hysterosalpingo-contrast sonography (HyCoSy) with Echovist-200 and X-ray hysterosalpingography for outpatient investigation of infertile women. Ultrasound Obstet Gynecol. 1996;7:201–4. doi: 10.1046/j.1469-0705.1996.07030201.x. [DOI] [PubMed] [Google Scholar]
- 27.Boudghène FP, Bazot M, Robert Y, Perrot N, Rocourt N, Antoine JM, et al. Assessment of fallopian tube patency by HyCoSy: Comparison of a positive contrast agent with saline solution. Ultrasound Obstet Gynecol. 2001;18:525–30. doi: 10.1046/j.0960-7692.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- 28.Downing BG, Wood C. Predictive value of falloscopy: 200 case study. Ref Gynecol Obstet. 1995;3:156–62,394. [Google Scholar]
- 29.Lundberg S, Rasmussen C, Berg AA, Lindblom B. Falloposcopy in conjunction with laparoscopy: Possibilities and limitations. Hum Reprod. 1998;13:1490–2. doi: 10.1093/humrep/13.6.1490. [DOI] [PubMed] [Google Scholar]
- 30.Rimbach S, Bastert G, Wallwiener D. Technical results of falloposcopy for infertility diagnosis in a large multicentre study. Hum Reprod. 2001;16:925–30. doi: 10.1093/humrep/16.5.925. [DOI] [PubMed] [Google Scholar]
- 31.Good Clinical Treatment in Assisted Reproduction. An ESHRE position paper The Practice Committee of the American Society for Reproductive Medicine - Optimal evaluation of the infertile female.
- 32.The Practice Committee of the American Society for Reproductive Medicine - Optimal evaluation of the infertile female.


