Abstract
Opening and closing of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel is regulated by the interaction of ATP with its two cytoplasmic nucleotide-binding domains (NBD). Although ATP hydrolysis by the NBDs is required for normal gating, the influence of ATP binding versus hydrolysis on specific steps in the gating cycle remains uncertain. Earlier work showed that the absence of Mg2+ prevents hydrolysis. We found that even in the absence of Mg2+, ATP could support channel activity, albeit at a reduced level compared with the presence of Mg2+. Application of ATP with a divalent cation, including the poorly hydrolyzed CaATP complex, increased the rate of opening. Moreover, in CFTR variants with mutations that disrupt hydrolysis, ATP alone opened the channel and Mg2+ further enhanced ATP-dependent opening. These data suggest that ATP alone can open the channel and that divalent cations increase ATP binding. Consistent with this conclusion, when we mutated an aspartate thought to bind Mg2+, divalent cations failed to increase activity compared with ATP alone. Two observations suggested that divalent cations also stabilize the open state. In wild-type CFTR, CaATP generated a long duration open state, whereas ATP alone did not. With a CFTR variant in which hydrolysis was disrupted, MgATP, but not ATP alone, produced long openings. These results suggest a gating cycle for CFTR in which ATP binding opens the channel and either hydrolysis or dissociation leads to channel closure. In addition, the data suggest that ATP binding and hydrolysis by either NBD can gate the channel.
The presence of two cytoplasmic nucleotide-binding domains (NBDs) is the conserved feature of ATP-binding cassette (ABC) transporters (1, 2). In most ABC transporters, the NBDs bind and hydrolyze ATP to actively pump a substrate across membranes. However in the cystic fibrosis transmembrane conductance regulator (CFTR), the NBDs open and close a transmembrane pore through which Cl− flows passively down its electrochemical gradient (3, 4). Understanding how the interaction between ATP and the CFTR NBDs gates the channel remains an important goal.
Previous work demonstrated that CFTR and individual NBD proteins bind and hydrolyze ATP (5–8). In addition, studies of the functional effect of ATP concentration, nucleoside analogs, site-directed mutations, and chemical modifications have established the central role of the NBDs and ATP in controlling channel gating (3, 4). However, the molecular mechanisms of gating and the function of the two NBDs remain uncertain. Consequently, several models have been proposed to describe the ATP-dependent gating cycle (3, 4, 9–14). There is general agreement that cAMP-dependent phosphorylation of the regulatory domain (R domain) is a prerequisite for ATP-dependent activity. In addition, all of the proposed models emphasize a role for ATP interactions with both NBD1 and NBD2. However, there is less agreement about the role of ATP binding versus ATP hydrolysis in controlling specific steps in the gating cycle.
Several previous models, including our own, proposed that channel opening requires ATP hydrolysis (3, 4, 9–11, 13, 14). This conclusion rests heavily on the observation that the hydrolysis-resistant nucleoside triphosphate 5′-adenylyl imidodiphosphate (AMP-PNP) generates little channel activity on its own (9, 13, 15–17). Even though it does not open the channel, three observations suggest that AMP-PNP binds to CFTR: (i) AMP-PNP inhibits ATP binding as measured by photoaffinity labeling methods (18); (ii) adding AMP-PNP in the presence of ATP can cause occasional bursts of long duration, although only a small fraction of bursts are affected (9, 10, 16, 17); and (iii) AMP-PNP can compete with ATP binding under some conditions (13, 17). However the inability of AMP-PNP to support channel activity may not, on its own, be sufficient to conclude that hydrolysis is absolutely required to open the channel. That conclusion requires the assumption that ATP and AMP-PNP are equivalent in their binding and interaction with the NBDs. In some other systems this is not always the case. For example, subtle structural differences were found in ATP and AMP-PNP interactions with the sarcoplasmic reticulum Ca2+-ATPase (19). In a 70-kDa heat-shock protein, AMP-PNP bound 2–3 orders of magnitude more weakly than ATP (20). Moreover in the chaperonin GroEL, the AMP-PNP-bound structure is similar to the ADP-bound structure in the upper ring and to the ATP-bound structure in the lower ring (21, 22). Thus AMP-PNP is not a perfect ATP analogue (22).
On the other hand, several observations suggest that hydrolysis may not be required to open the channel. For example, mutation of the conserved lysine in the NBD Walker A motif abolishes ATPase activity in many ABC transporters (23). In CFTR, the NBD1 mutation K464A reduces ATPase activity to ≈15%, and the NBD2 mutation K1250A eliminates ATPase activity (24). Yet despite this, gating continues, albeit at a reduced level (10, 11, 14, 24). This led Ramjeesingh and colleagues (24) to propose a loose relationship between catalytic activity and channel gating. Strikingly, a variant with both NBDs mutated (K464A/K1250A) showed significant activity and gating not different from CFTR-K1250A (10). The requirement for hydrolysis has also been assessed by reducing the Mg2+ concentration. Mg2+ coordinates the γ phosphate of ATP and is required for hydrolysis by most enzymes (25). In CFTR, Mg2+ chelation abolished ATP hydrolysis (6). Despite this, some studies showed that gating persisted in the absence of Mg2+ (26, 27).
Thus, although it is clear that the NBDs bind and hydrolyze ATP to influence gating, questions remain about whether hydrolysis is required to open the channel. In this study, we examined further the role of ATP binding and hydrolysis in gating by the two NBDs.
Materials and Methods
Wild-type and mutant CFTR were transiently transfected into HeLa cells by using the vaccinia virus expression plasmid pTM-CFTR4, as previously described (28). We studied excised, inside-out membrane patches by using the patch-clamp technique. The pipette (external) solution contained (in mM): 140 N-methyl-d-glucamine, 2 MgCl2, 5 CaCl2, 100 l-aspartic acid, and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes), pH 7.3 with HCl (Cl− concentration, 50 mM). The bath (internal) solution contained (in mM): 140 N-methyl-d-glucamine, 4 MgCl2 (except when indicated), 1 Cs ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (CsEGTA), and 10 N-[(tris(hydroxymethyl)methyl]glycine (Tricine), pH 7.3 with HCl (Cl− concentration, 140 mM). In all cases, CFTR was first phosphorylated with 75 nM of the catalytic subunit of cAMP-dependent protein kinase (PKA; Promega) and MgATP. Patches were continuously perfused with a multichannel rapid change perfusion system.
For nominally Mg2+- and Ca2+-free conditions, divalent cation concentrations were measured by atomic-emission spectroscopy (Environmental Protection Agency, Iowa State Hygienic Laboratory, Des Moines, IA). Values were below detectable limits; Mg2+ < 4 μM and Ca2+ < 25 μM. We also added 1 mM EDTA disodium salt (EDTA) to chelate Mg2+. Thus, if the total Mg2+ concentration was <4 μM, we calculate that free Mg2+ concentration was <6 nM and the MgATP concentration (with 1 mM ATP) was <22 nM (29). In some studies, we used trans-1,2-cyclohexanediamine-N,N,N,N-tetraacetic acid (CDTA) to chelate Mg2+.
The methods for patch-clamp recording were similar to those previously described (28). Experiments were performed at room temperature (22–24°C) and membrane voltage was held at −40 mV unless otherwise indicated. Pipette resistance was 3–10 MΩ, and seal resistance was 3–30 GΩ. An Axopatch 200-B amplifier and pClamp software were used for data acquisition and analysis (Axon Instruments, Foster City, CA). For macroscopic current measurements, interventions were bracketed by the control conditions. For single-channel analysis, replayed data were filtered at 1 kHz with a variable eight-pole Bessel filter, digitized at 5 kHz, and digitally filtered at 500 Hz. Single-channel analyses were performed as previously described; a burst delimiter of >20 ms was used (10, 30).
Data are shown as mean ± SEM. A Student's t test or one-way analysis of variance (ANOVA) was used to test statistical significance (gb-stat, Dynamic Microsystems, Silver Spring, MD). When ANOVA was used, post hoc least significance difference test and Scheffé comparison test were used to identify significantly different conditions.
Results
CFTR Channel Activity in the Absence of Mg2+ and Presence of Ca2+.
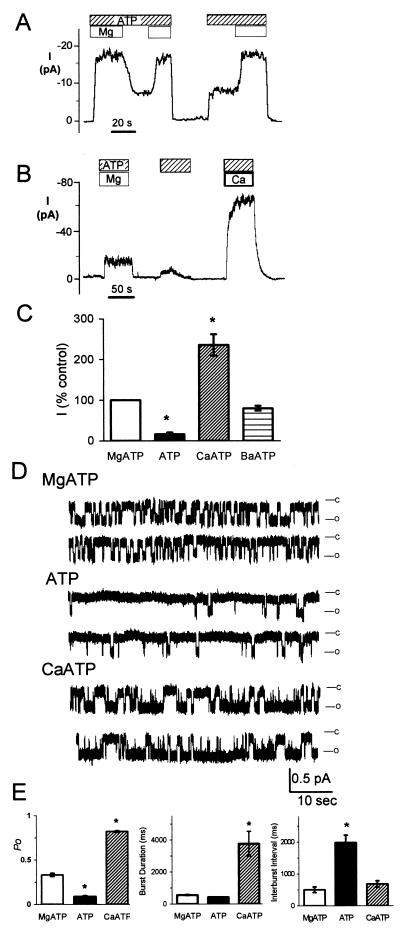
We asked whether Mg2+ was required for channel activity. Fig. 1A shows macroscopic Cl− current in an excised, inside-out membrane patch containing many phosphorylated CFTR Cl− channels. Removing the Mg2+ from the cytosolic (bath) solution reduced but did not abolish activity. Re-adding Mg2+ increased current. In contrast, without ATP, activity fell to near zero. Reintroducing ATP, even in the absence of Mg2+, rapidly increased current. As described in Materials and Methods, the Mg2+-free solution contained 1 mM EDTA to chelate residual Mg2+. We obtained similar results with 1 mM CDTA, although it occasionally irreversibly abolished activity (3 of 7 experiments). These results are consistent with earlier reports indicating gating in the absence of Mg2+ (26, 27). Importantly, Li and colleagues (6) have shown that chelating Mg2+ abolishes CFTR ATPase activity. These data suggest that ATP stimulates gating even in the absence of hydrolysis.
Figure 1.
Effect of divalent cations on macroscopic (A–C) and single-channel (D and E) CFTR Cl− channel current. Data are from excised, inside-out membrane patches of HeLa cells expressing wild-type CFTR. (A and B) ATP (1 mM) and Ca2+ or Mg2+ (4 mM) were present on cytosolic surface during times indicated by bars. Protein kinase A (75 nM) was removed before data collection. (C) Effect of divalent ion–ATP complexes on current; current with MgATP is shown as 100%, n = 4–6. *, P < 0.05. (D) Single-channel traces obtained with 1 mM ATP and either 0 or 4 mM Mg2+ or Ca2+ as indicated. Membrane potential was at −80 mV. (E) Open state probability (Po), burst duration, and interburst interval; n = 5. *, P < 0.01 compared with MgATP.
To further investigate the requirement for hydrolysis, we replaced Mg2+ with Ca2+. Other ABC transporters show little or no hydrolysis of CaATP (31–33). The same is true of other ATPases (25, 34). CaATP increased current to levels greater than those obtained with ATP alone or with MgATP (Fig. 1 B and C), as previously reported (35). Ca2+ applied in the absence of ATP did not stimulate current (not shown). BaATP also stimulated current compared with ATP alone (Fig. 1C). It is possible that differential effects of the various divalent cations depend upon the extent of electrostatic interaction with ATP. The channel required phosphorylation for CaATP to stimulate current (not shown). CaATP also stimulated CFTR-ΔR/S660A channels, which lack the R domain and show no response to phosphorylation (36) (data not shown), indicating that stimulation was largely independent of the R domain. These data suggest that ATP hydrolysis is not required for the channel to open. However, the complex of ATP with divalent cations stimulated channel activity more than did ATP alone.
Effect of Divalent Cations on Single-Channel Gating.
We investigated the effect of divalent cations on single-channel gating. When ATP was applied in the absence of divalent cations, the open-state probability (Po) decreased, primarily because the closed interval between bursts increased (Fig. 1 D–F). The burst duration showed minimal change. Probability density histograms suggest these burst durations and interburst intervals are fit by single exponential functions (not shown). Earlier data showed that reducing the MgATP concentration prolonged the interburst interval (6, 14, 37). The observation that eliminating Mg2+ also prolonged the interburst interval is consistent with reduced ATP binding and hence reduced opening without the divalent cation. These results also suggest that closing can occur in the absence of hydrolysis, presumably by dissociation of ATP.
CaATP increased Po by markedly prolonging the burst duration (Fig. 1 D–F). The interburst interval was not significantly different from that with MgATP. Compared with MgATP, CaATP had no effect on the single-channel conductance (6.2 ± 0.2 pS with MgATP and 6.6 ± 0.4 pS with CaATP, n = 4, P > 0.1), the current–voltage relationship (data not shown), or the anion selectivity (PI/PCl: 0.69 ± 0.4 with MgATP and 0.71 ± 0.3 with CaATP, n = 4, P > 0.1). These data suggest that CaATP binds to CFTR and facilitates channel opening. However, because CaATP is not a good substrate for hydrolysis, after binding CaATP, the channel may not progress through the hydrolysis cycle. As a result, the channel would be locked open until ATP dissociates. Alternatively, rare hydrolysis of CaATP might terminate a burst. In either case, burst duration would increase. Prolongation of the burst duration is similar to the result of two other interventions that allow nucleotide binding but not hydrolysis: binding of the nonhydrolyzable AMP-PNP (9, 10, 16, 17, 38) and an NBD2 mutation that prevents hydrolysis, K1250A (10, 11, 14, 24).
Effect of Divalent Cations on CFTR Bearing a Walker B Mutation.
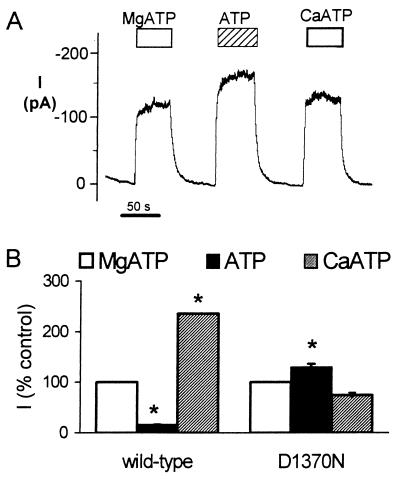
Because these data suggested that Mg2+ and Ca2+ facilitate ATP binding and that binding opens the channel, we studied CFTR with a mutation of the conserved aspartate in the Walker B motif that is thought to bind divalent cations. Its mutation inhibits ATP hydrolysis in other ABC transporters (23). We studied the CFTR-D1370N mutant; this channel has reduced activity (11, 39). We did not study the analogous mutation in NBD1, D572N, because it shows defective biosynthesis and is not processed to the cell surface. We predicted that CFTR-D1370N would not discriminate between ATP, MgATP, and CaATP. Fig. 2 shows that neither MgATP nor CaATP increased current compared with ATP alone, and MgATP and CaATP had currents of similar size. These data are consistent with the conclusion that Mg2+ and Ca2+ facilitate ATP binding. Because the presence or absence of a divalent cation did not influence this NBD2 mutant, the data are consistent with the idea that NBD2 may be responsible for most of the channel activity of CFTR.
Figure 2.
Effect of MgATP, ATP alone, and CaATP on Cl− current of CFTR-D1370N. (A) Example of macroscopic current. Measurements were made in the presence of either 0 or 4 mM Mg2+ or Ca2+ and 1 mM ATP as indicated by bars. (B) Effect of divalent ion–ATP complexes on current: MgATP is shown as 100%; n = 4. *, P < 0.05 compared with MgATP.
Effect of MgATP and ATP on CFTR Bearing Walker A Mutations.
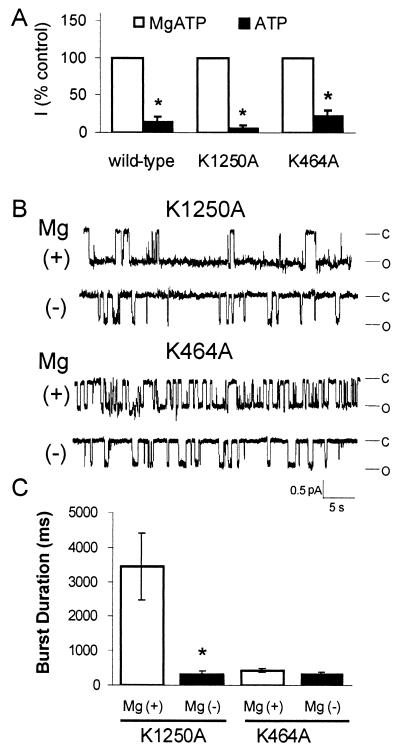
To learn more about how nucleotide binding—in contrast to hydrolysis—controls activity of the two NBDs, we examined the effect of MgATP and ATP alone on CFTR variants that do not hydrolyze ATP. In this way, we could eliminate the consequences of hydrolysis after ATP binding. We tested variants with mutations in the Walker A lysine, CFTR-K464A and -K1250A. These mutations reduce hydrolysis to ≈15% and zero, respectively (24). In both mutants, removing Mg2+ decreased current (Fig. 3A): this observation is consistent with reduced ATP binding.
Figure 3.
Effect of MgATP and ATP alone on CFTR-K1250A and -K464A. (A) Macroscopic current measurements were made in the presence of either 0 or 4 mM Mg2+, membrane potential was −80 mV, n = 4. Current with MgATP is shown as 100%. *, P < 0.05 compared with MgATP. (B) Example of single-channel traces. (C) Burst duration, n = 4. *, P < 0.01 compared with MgATP.
Although removing Mg2+ reduced macroscopic current in both Walker A mutants, single-channel analysis revealed different aspects to the response. With MgATP, CFTR-K1250A showed prolonged bursts (Fig. 3 B and C), as previously reported (10, 11, 14, 24). However in the absence of Mg2+, burst duration (552 ± 40 ms) fell to a value in the range of that obtained in wild-type CFTR with ATP alone (418 ± 30 ms). These data suggest that Mg2+ reduces the dissociation of ATP from this hydrolysis-resistant mutant; or in other words Mg2+ stabilizes ATP binding, thereby prolonging the open state. In contrast, with CFTR-K464A, the burst duration was the same with MgATP and ATP alone (Fig. 3 B and C). There are two potential explanations for the difference between K464A and K1250A. Dissociation of ATP may be different at NBD1 and NBD2. Alternatively, in K464A, most of the gating may be due to ATP interactions with NBD2.
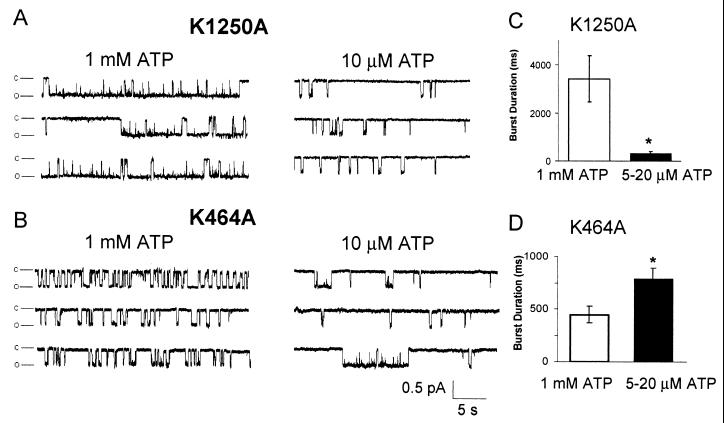
The conclusion that the two NBDs have significantly different effects on gating is consistent with earlier results, including evidence that ADP has differential effects on variants with NBD1 and NBD2 mutations (39), that covalent modification of the two NBDs has different effects (30), and that increasing MgATP concentrations have a complex effect on channel activity (6, 14, 17, 37, 39). Moreover, the two NBDs of ABC transporters, including CFTR, can show different ATP affinities (8, 40). Therefore, we studied CFTR-K1250A and CFTR-K464A at two different ATP concentrations. With 1 mM ATP and 4 mM Mg2+, CFTR-K1250A showed prolonged bursts, but with a low MgATP concentration (5–20 μM ATP and 4 mM Mg2+), burst duration decreased (Fig. 4 A and C). This result is similar to a previous report (14). One interpretation of this finding is that either NBD can gate the channel, and a higher ATP affinity at NBD1 than NBD2 would shift the majority of gating to NBD1 at low ATP concentrations. This might prevent the channel from entering the prolonged bursts that result from NBD2 gating in K1250A.
Figure 4.
Effect of ATP concentration on gating of CFTR-K1250A (A and C) and K464A (B and D) channels. (A and B) Single-channel traces with 1 mM or 10 μM ATP (4 mM Mg2+). (C and D) Effect of high and low ATP concentrations (4 mM Mg2+) on burst duration. Data are mean ± SEM (n = 4–6). *, P < 0.01 compared with 1 mM ATP.
If NBD1 can bind and hydrolyze MgATP to gate the channel, then inhibiting NBD1 hydrolysis might be expected to prolong those bursts that originate at NBD1. Yet, prolonged burst durations have not been observed with K464A (10, 11, 14, 24). Our results provide a potential explanation for this paradox. If NBD2 were responsible for the majority of channel gating, it would obscure most NBD1-dependent gating. However, if NBD1 has a higher ATP affinity than NBD2, this premise predicts that K464A would have long bursts at low MgATP concentrations. Fig. 4 B and D shows this was the case. With 1 mM ATP and 4 mM Mg2+, CFTR-K464A showed durations approximately the same as observed with wild-type CFTR. However with 5–20 μM ATP and 4 mM Mg2+, burst duration increased, and some bursts of long duration were observed.
Discussion
In most ABC transporters, ATP hydrolysis is absolutely required for active pumping of substrate across the cell membrane (23). Our data and earlier studies suggest this is not the case with CFTR; ATP binding alone may open a pore through which Cl− flows passively. Here we suggest a model for ATP-dependent gating (Fig. 5). This model incorporates features of several earlier models (3, 4, 10, 11, 13, 14), although there are differences. The most important differences are that ATP binding can open the channel, and that gating can arise from ATP interactions with either NBD.
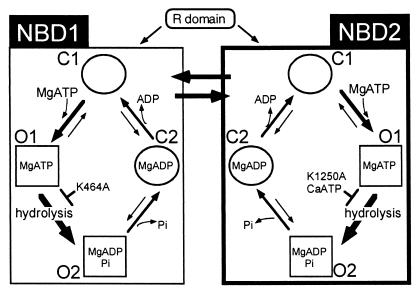
Figure 5.
Model of the interaction of the CFTR NBDs with ATP and effect on gating. See text for details.
A Model of ATP-Dependent Gating.
CFTR has at least four discernible states. Two open conformations, “O1” and “O2,” occur during a burst of activity and are distinguished by flickery Mops-dependent block (11, 38). The O1 state is ATP bound and hydrolysis moves the channel to the O2 state. As indicated by Gadsby and Nairn (4), there are also at least two closed states, which we designate C1 and C2. At NBD2, binding of ATP without Mg2+ alters protein structure, opening the channel from the C1 to the O1 state. The presence of Mg2+ increases the rate of ATP binding, shortening the interburst closed state. Mg2+ also increases the affinity of the O1 state for ATP, reducing ATP dissociation and stabilizing the O1 state. From O1, hydrolysis of MgATP shifts the conformation to O2. When the channel leaves the O2 conformation it closes.
Our current data support several aspects of this model; we first consider NBD2. In the absence of divalent cations, ATP binding and dissociation generate C1 → O1 and O1 → C1 transitions, respectively (Fig. 5). The presence of Mg2+ or Ca2+ facilitates the C1 → O1 transition, shortening the interburst interval compared with ATP alone (Fig. 1). The divalent cation also stabilizes the O1 state. However, because CaATP is not a good substrate for hydrolysis, once it binds and carries the channel into the O1 state, progress to O2 is blocked. As a result, the burst duration is prolonged (Fig. 1). The K1250A variant has a similar effect, blocking the O1 → O2 transition and thereby prolonging bursts with MgATP (Fig. 3). In contrast, with ATP alone, the O1 state of K1250A is unstable and ATP dissociates more quickly (Fig. 3). The K1250 mutation has an additional effect, decreasing the rate of channel opening into bursts, i.e., prolonging the interburst interval. This result suggests that the K1250A mutation reduces ATP binding to NBD2. Previous studies of other ABC transporters have shown that mutation of the Walker A lysine reduces ATP binding (41). It is also noteworthy that MgATP did not stimulate current to a greater extent than ATP alone when CFTR had a mutation in NBD2 (D1370N) that disrupts divalent cation binding (Fig. 2).
In Fig. 5, we model the consequences of ATP binding and hydrolysis at NBD1 in the same way as at NBD2. However, our own data and that of others speak less to NBD1 than NBD2. We suggest that ATP binding and hydrolysis at NBD1 can also gate the channel. Our finding that the K464A mutation can generate bursts with a prolonged duration (Fig. 4) supports this conclusion. Why we see this at low ATP concentrations is not certain; however, it may be that the two NBDs have significantly different ATP affinities: NBD1 may have a high affinity with a low cycling rate, and NBD2 a low affinity and a high cycling rate (42). As indicated below, this is the case in other ABC transporters.
Previous work has indicated that the two NBDs interact. This is evidenced by the cooperativity shown in the relationship between ATP concentration and activity (6, 14, 39) and by the ability of a mutation in one NBD to inhibit by greater than 50% the rate of ATP hydrolysis (24). The extent of interaction and the contribution to gating of the two NBDs may be influenced by phosphorylation (9, 36, 43). It is also possible that ATP concentration or other factors may play a role.
The data also suggest that NBD2 activity may generate the majority of gating in the presence millimolar ATP concentrations. This conclusion is supported by the finding that mutations in NBD2 markedly reduce the opening rate, whereas equivalent mutations in NBD1 have smaller effects on channel opening (10, 11, 14, 24). In addition, ADP competitively inhibits the majority of channel activity (39). This inhibition appears to occur through NBD2 because mutations in NBD2 prevent the effect of ADP (39), and ADP slows the rate of N-ethylmaleimide modification of NBD2 but not NBD1 (30).
Relationship to Earlier Models of CFTR Gating.
The mechanism of gating in Fig. 5 has several features that are consistent with earlier models. First, the model in Fig. 5 proposes that an ATP interaction with either NBD can open the channel. Hwang et al. (9) also speculated that both NBDs might gate the channel. Second, most models suggest that ATP hydrolysis is required for the channel to open (3, 4). However, several studies suggest that the channel can open in the absence of hydrolysis (26, 27, 44, 45). Gunderson and Kopito (11) proposed a model in which ATP binding to NBD2 opens the channel, but only after hydrolysis at NBD1. As in their model, we propose that ATP binding alone is sufficient to open CFTR, but we suggest that binding at either NBD can open the channel. Third, as with earlier models, we emphasize interactions between the two NBDs. Whether ATP binding alone or hydrolysis is necessary for interactions is unknown.
The observation that would seem to make the greatest challenge to the model in Fig. 5 is the inability of nonhydrolyzable nucleoside triphosphates to support substantial activity. This observation has suggested that hydrolysis is required for opening. We discussed the limitations of this finding in the Introduction. However, at present we do not know how to resolve this issue with confidence.
There are additional observations that bear on the model in Fig. 5. First, concentration-dependent effects of ATP on burst duration could be consistent with the model. Some reports suggest that increasing ATP concentrations increase burst duration (6, 14), and some suggest no effect (37). If the two NBDs have distinct ATP affinities and openings with different kinetics, then ATP-dependent burst durations might be observed under some conditions. Second, the closed-time distribution of CFTR contains a negative exponential component (ref. 14 and J. F. Cotten and M.J.W., unpublished). This observation indicates that there is an irreversible step in the ATP-dependent gating cycle; such a step could occur either before or after ATP binding (46). On the basis of our data and that of others, we suggest that such a step occurs at some point after ATP binding. Third, the channel opening rate saturates at high ATP concentrations (6, 14, 37); this suggests the presence of a rate-limiting step. Such an ATP-independent step could occur at multiple sites in a gating cycle, either before or after ATP binding.
Relationship to the Function of Other ABC Transporters.
Several aspects of the function of other ABC transporters are consistent with features of the model we propose for CFTR. First, we propose that ATP binding causes a conformational change that opens the CFTR channel. Intrinsic fluorescence studies of MalK demonstrate that ATP binding induces a conformational change (47). Although elimination of Mg2+ abolished hydrolysis, it did not prevent the fluorescence changes, suggesting that ATP binding alone altered conformation. Limited proteolysis also revealed conformational changes when MgATP was applied; these changes did not require hydrolysis, as they occurred in a MalK hydrolysis-defective variant. CaATP generated similar although less marked effects, even though CaATP did not substitute for MgATP in supporting hydrolysis (32). Limited proteolysis has shown that MgATP binding also induced conformational changes in the hemolysin exporter HlyB and in P-glycoprotein (31, 48).
Second, we propose that activity of either NBD can gate the channel, although NBD2 may be responsible for most of the gating at millimolar ATP concentrations. Recent studies of HisP (49) showed that in a membrane complex, both HisP subunits hydrolyze ATP. Importantly, Nikaido and Ames found that a complex composed of one hydrolysis-defective subunit and one wild-type subunit still hydrolyzed ATP and translocated ligand, albeit at half the rate observed with two wild-type subunits. These results suggested that a single subunit is capable of supporting a catalytic cycle that actively transports substrate.
Third, we suggest that the two NBDs may have different ATP-binding properties and that one of the NBDs may be responsible for most of the gating. In SUR1, NBD1 has a high affinity for nucleotide binding and does so in the absence of Mg2+, whereas NBD2 has a low ATP affinity and requires Mg2+ (40). Moreover in SUR1, NBD2 showed ATPase activity, whereas NBD1 had little or none (40). Interestingly, in the Escherichia coli SecA subunit of the preprotein translocase, ATP binding and hydrolysis at one NBD drives SecA membrane insertion and deinsertion, whereas ATP binding alone at the other NBD couples SecA cycling to preprotein translocation (50).
In summary, the model of CFTR gating supported by our data incorporates features of several earlier models. However, the hypothesis that ATP binding at either NBD is sufficient to cause a conformational change that opens the channel diverges from earlier models. More importantly, this hypothesis may provide a framework for future investigations designed to understand the gating of this complex channel. Such knowledge may also be of value in understanding mechanisms in other ABC transporters and in the diseases with which they are associated.
Acknowledgments
We thank Pary Weber, Phil Karp, Tamara Nesselhauf, and Theresa Mayhew for excellent assistance and our laboratory colleagues for helpful discussions. We especially appreciate the thoughtful comments and suggestions of Dr. David Sheppard. This work was supported by the National Heart, Lung and Blood Institute and the Howard Hughes Medical Institute. M.I. is an Associate and M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- NBD
nucleotide-binding domain
- ABC transporter
ATP-binding cassette transporter
- AMP-PNP
5′-adenylyl imidodiphosphate
- CDTA
trans-1,2-cyclohexanediamine-N,N,N,N-tetraacetic acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140220597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140220597
References
- 1.Ames G F-L, Mimura C S, Shyamala V. FEMS Microbiol Rev. 1990;75:429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 2.Hyde S C, Emsley P, Hartshorn M J, Mimmack M M, Gileadi U, Pearce S R, Gallagher M P, Gill D R, Hubbard R E, Higgins C F. Nature (London) 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard D N, Welsh M J. Physiol Rev. 1999;79:S43–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 4.Gadsby D C, Nairn A C. Physiol Rev. 1999;79:s77–s107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- 5.Ko Y H, Pedersen P L. J Biol Chem. 1995;270:22093–22096. doi: 10.1074/jbc.270.38.22093. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens J M, Galley K, Bear C E. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 7.Randak C, Neth P, Auerswald E A, Eckerskorn C, Assfalg-Machleidt I, Machleidt W. FEBS Lett. 1997;410:180–186. doi: 10.1016/s0014-5793(97)00574-7. [DOI] [PubMed] [Google Scholar]
- 8.Logan J, Hiestand D, Daram P, Huang Z, Muccio D D, Hartman J, Haley B, Cook W J, Sorscher E J. J Clin Invest. 1994;94:228–236. doi: 10.1172/JCI117311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang T-C, Nagel G, Nairn A C, Gadsby D C. Proc Natl Acad Sci USA. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson M R, Travis S M, Welsh M J. J Biol Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- 11.Gunderson K L, Kopito R R. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- 12.Schultz B D, Venglarik C J, Bridges R J, Frizzell R A. J Gen Physiol. 1995;105:329–361. doi: 10.1085/jgp.105.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinreich F, Riordan J R, Nagel G. J Gen Physiol. 1999;114:55–70. doi: 10.1085/jgp.114.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeltwanger S, Wang F, Wang G T, Gillis K D, Hwang T C. J Gen Physiol. 1999;113:541–554. doi: 10.1085/jgp.113.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson M R, Welsh M J. Am J Physiol. 1993;265:L27–L32. doi: 10.1152/ajplung.1993.265.1.L27. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson K L, Kopito R R. J Biol Chem. 1994;269:19349–19353. [PubMed] [Google Scholar]
- 17.Mathews C J, Tabcharani J A, Hanrahan J W. J Membr Biol. 1998;163:55–66. doi: 10.1007/s002329900370. [DOI] [PubMed] [Google Scholar]
- 18.Travis S M, Carson M R, Ries D R, Welsh M J. J Biol Chem. 1993;268:15336–15339. [PubMed] [Google Scholar]
- 19.von Germar F, Barth A, Mantele W. Biophys J. 2000;78:1531–1540. doi: 10.1016/S0006-3495(00)76705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao B, Greene L, Eisenberg E. Biochemistry. 1994;33:2048–2054. doi: 10.1021/bi00174a010. [DOI] [PubMed] [Google Scholar]
- 21.Roseman A M, Chen S, White H, Braig K, Saibil H R. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- 22.Rye H S, Burston S G, Fenton W A, Beechem J M, Xu Z, Sigler P B, Horwich A L. Nature (London) 1997;388:792–798. doi: 10.1038/42047. [DOI] [PubMed] [Google Scholar]
- 23.Holland I B, Blight M A. J Mol Biol. 1999;293:381–399. doi: 10.1006/jmbi.1999.2993. [DOI] [PubMed] [Google Scholar]
- 24.Ramjeesingh M, Li C, Garami E, Huan L-J, Galley K, Wang Y, Bear C E. Biochemistry. 1999;38:1463–1468. doi: 10.1021/bi982243y. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke B. Biochem Pharmacol. 1993;46:1103–1112. doi: 10.1016/0006-2952(93)90456-7. [DOI] [PubMed] [Google Scholar]
- 26.Reddy M M, Quinton P M. Am J Physiol. 1996;271:C35–C42. doi: 10.1152/ajpcell.1996.271.1.C35. [DOI] [PubMed] [Google Scholar]
- 27.Schultz B D, Bridges R J, Frizell R A. J Membr Biol. 1996;151:63–75. doi: 10.1007/s002329900058. [DOI] [PubMed] [Google Scholar]
- 28.Anderson M P, Berger H A, Rich D P, Gregory R J, Smith A E, Welsh M J. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 29.Brooks S P J, Storey K B. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 30.Cotten J F, Welsh M J. J Biol Chem. 1997;272:25617–25622. doi: 10.1074/jbc.272.41.25617. [DOI] [PubMed] [Google Scholar]
- 31.Koronakis V, Hughes C, Koronakis E. Mol Microbiol. 1993;8:1163–1175. doi: 10.1111/j.1365-2958.1993.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 32.Morbach S, Tebbe S, Schneider E. J Biol Chem. 1993;268:18617–18621. [PubMed] [Google Scholar]
- 33.Urbatsch I L, al-Shawi M K, Senior A E. Biochemistry. 1994;33:7069–7076. doi: 10.1021/bi00189a008. [DOI] [PubMed] [Google Scholar]
- 34.Mendlein J, Sachs G. J Biol Chem. 1989;264:18512–18519. [PubMed] [Google Scholar]
- 35.Harrington M A, Gunderson K L, Kopito R R. J Biol Chem. 1999;274:27536–27544. doi: 10.1074/jbc.274.39.27536. [DOI] [PubMed] [Google Scholar]
- 36.Winter M C, Welsh M J. Nature (London) 1997;389:294–296. doi: 10.1038/38514. [DOI] [PubMed] [Google Scholar]
- 37.Winter M C, Sheppard D N, Carson M R, Welsh M J. Biophys J. 1994;66:1398–1403. doi: 10.1016/S0006-3495(94)80930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishihara H, Welsh M J. Am J Physiol. 1997;273:C1278–C1289. doi: 10.1152/ajpcell.1997.273.4.C1278. [DOI] [PubMed] [Google Scholar]
- 39.Anderson M P, Welsh M J. Science. 1992;257:1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- 40.Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Proc Natl Acad Sci USA. 1999;96:1268–1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo M, Kioka N, Amachi T, Ueda K. J Biol Chem. 1999;274:37479–37482. doi: 10.1074/jbc.274.52.37479. [DOI] [PubMed] [Google Scholar]
- 42.Szabo K, Szakacs G, Hegeds T, Sarkadi B. J Biol Chem. 1999;274:12209–12212. doi: 10.1074/jbc.274.18.12209. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson D J, Mansoura M K, Watson P Y, Smit L S, Collins F S, Dawson D C. J Gen Physiol. 1996;107:103–119. doi: 10.1085/jgp.107.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinton P M, Reddy M M. Nature (London) 1992;360:79–81. doi: 10.1038/360079a0. [DOI] [PubMed] [Google Scholar]
- 45.Aleksandrov A A, Riordan J R. FEBS Lett. 1998;431:97–101. doi: 10.1016/s0014-5793(98)00713-3. [DOI] [PubMed] [Google Scholar]
- 46.Colquhoun D, Hawkes A G. In: Single-Channel Recording. 2nd Ed. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 397–482. [Google Scholar]
- 47.Schneider E, Wilken S, Schmid R. J Biol Chem. 1994;269:20456–20461. [PubMed] [Google Scholar]
- 48.Wang G, Pincheira R, Zhang M, Zhang J T. Biochem J. 1997;328:897–904. doi: 10.1042/bj3280897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikaido K, Ames G F-L. J Biol Chem. 1999;274:26727–26735. doi: 10.1074/jbc.274.38.26727. [DOI] [PubMed] [Google Scholar]
- 50.Economou A, Pogliano J A, Beckwith J, Oliver D B, Wickner W. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]