Abstract
Currently, a high carbohydrate/low fat diet is recommended for patients with hypertension; however, the potentially important role that the composition of dietary fat and carbohydrate plays in hypertension and the development of pathological left ventricular hypertrophy (LVH) has not been well characterized. Recent studies demonstrate that LVH can also be triggered by activation of insulin signaling pathways, altered adipokine levels, or the activity of peroxisome proliferator-activated receptors (PPARs), suggesting that metabolic alterations play a role in the pathophysiology of LVH. Hypertensive patients with high plasma insulin or metabolic syndrome have a greater occurrence of LVH, which could be due to insulin activation of the serine-threonine kinase Akt and its downstream targets in the heart, resulting in cellular hypertrophy. PPARs also activate cardiac gene expression and growth and are stimulated by fatty acids and consumption of a high fat diet. Dietary intake of fats and carbohydrate and the resultant effects of plasma insulin, adipokine, and lipid concentrations may affect cardiomyocyte size and function, particularly in the setting of chronic hypertension. This review discusses potential mechanisms by which dietary carbohydrates and fats ca affect cardiac growth, metabolism, and function, mainly in the context of pressure overload-induced LVH.
Keywords: diet, fat, glucose, hypertension, hypertrophy, metabolism
1. Introduction
Hypertension is a major cause of left ventricular hypertrophy (LVH), which frequently leads to heart failure[1-5]. Neurohormonal stimulation in hypertension activates signaling pathways in cardiomyocytes that lead to the development of LVH [6-11]. Recent studies demonstrate that cardiomyocyte hypertrophy can also be triggered by activation of insulin signaling pathways [12-14], altered adipokine levels [15-17,17,18] or the activity of peroxisome proliferator-activated receptors (PPARs)[19-22], suggesting that metabolic alterations can play a role in the pathophysiology of LVH. Hypertensive patients with high plasma insulin or metabolic syndrome have a greater occurrence of LVH[23-30], which could be due to insulin activation of the serine-threonine kinase Akt and its downstream targets, resulting in cellular hypertrophy [12,13,31,32]. PPARs also activate cardiac gene expression and growth, and are stimulated by fatty acids[33,34] and consumption of a high fat diet[35-37]. Taken together, these findings suggest that dietary intake of fats and carbohydrate, particularly the intake of simple sugars and the resultant effects of plasma insulin, adipokine, and lipid concentrations, may affect cardiomyocyte size and function, especially with chronic hypertension.
Consuming a high saturated fat and/or high cholesterol diet is atherogenic in humans[38,39], thus current dietary guidelines recommend a high carbohydrate/low saturated fat/low cholesterol diet to prevent heart disease[40]. Less consideration, however, has been given to the effects of dietary lipid and carbohydrate on activation of hypertrophic signaling pathways and the development of LVH. In addition, recent studies show that the adipokines leptin and adiponectin may play a role in the development and progression of LVH [15-17,17,41-43] and may be altered by composition of dietary lipids and carbohydrates [35,44-47]. Recent studies found that dietary composition of fat and carbohydrate can effect the development of LVH and cardiac pathology[48-50]. The aim of this review is to present the potential mechanisms by which dietary macronutrients can affect cardiomyocyte growth and cardiac dysfunction in response to pressure overload. It is important to note that diet and LVH effect myocardial substrate metabolism, however this topic is outside the scope of the present discussion, and the reader is referred to several recent reviews on this topic [34,51-53].
2. Chronic hypertension and LVH
Despite aggressive diagnosis and treatment, hypertension remains a major clinical problem[54] and predictor of pathological LVH and heart failure[1,2,55,56]. Classically, LVH is viewed as an initial positive adaptation to normalize wall stress by increasing wall thickness[57]. However, these same compensatory mechanisms frequently lead to LV dysfunction and remodeling that eventually culminates in overt heart failure[58,59]. Recent data from transgenic mice with attenuated LVH in response to pressure overload show better cardiac function and better maintenance of LV end diastolic volume than wild type mice despite increased end systolic wall stress [60,61]. Additionally, long-term treatment of hypertensive patients with angiotensin converting enzyme (ACE) inhibitors leads to a reduction in LVH and subsequent improvement in cardiovascular risk outcomes[62]. Taken together, LVH results in poor long-term outcome. Thus, it is important to prevent LVH in hypertension.
Several lines of evidence link dietary factors as a cause of hypertension and subsequent LVH[63,64]. The link between increased sodium intake and elevated blood pressure is well documented[65,66], as is the link between dietary intake of saturated fatty acids, elevated low density lipoprotein cholesterol [39,67] and occurrence of coronary artery disease. However, these topics are outside the focus of the present review. Nevertheless, there is a paucity of knowledge regarding the role of macronutrients in the development of LVH in the setting of hypertension. Recent guidelines issued by the American Heart Association (AHA) emphasize the consumption of low fat/high carbohydrate diets in order to reduce cardiovascular disease (CVD) risk factors [40]. The AHA statement on sugar consumption emphasizes the lack of information on the relationship between sugar intake and CVD [68]. Early studies from Yudkin in the 1960s and 1970s demonstrated a potential link between simple sugar consumption in ischemic heart disease and atherosclerosis[69,70], but these intriguing studies did not evaluate the impact of simple sugars on LVH.
3. Insulin, lipids, and cardiac growth
Cardiomyocyte hypertrophy occurs when protein synthesis exceeds protein breakdown, resulting in a net accumulation of protein and expansion in cell size. On the other hand, the molecular mechanisms that cause LVH are extremely complex and redundant, and despite significant progress over the last 20 years, the precise signaling pathways remain only partially understood[6-8,57,71-75]. Insulin stimulates protein synthesis and inhibits protein breakdown in the heart[76-78], and clinical studies have found that elevated plasma insulin is associated with LVH [23-30]. The dietary intake of carbohydrates (particularly simple sugars) and lipids largely determines the exposure of the heart to insulin and various long chain fatty acid ligands for PPARs, and may play a role in the regulation of cardiomyocyte size under pathological conditions. While a great deal of attention has been placed on the effects of activation of the signaling pathways for angiotensin, endothelin, and adrenergic receptors in pathologic cardiomyocyte hypertrophy, the potential role of metabolic hormones and lipid ligands in stimulation of hypertrophic growth has been less studied.
3.1.1. Insulin-Akt-mTOR signaling in LVH
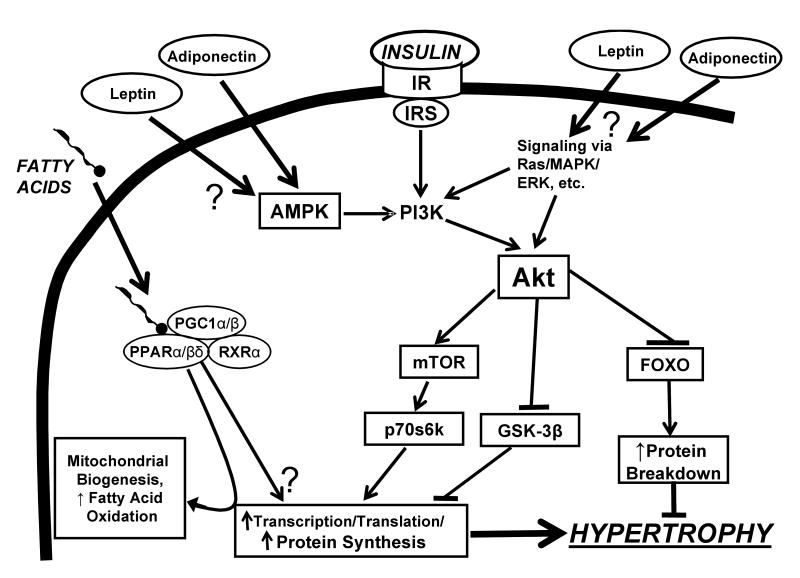
Insulin receptor stimulation activates phosphoinositol-3 kinase (PI3K), and subsequently phosphorylates and activates of Akt[79] (Figure 1). PI3K and Akt can also be activated by G-protein-coupled receptor stimulation [80-82]. Common to these hypertrophic pathways is the phosphorylation and activation of the downstream mammalian target of rapamycin (mTOR)[71,79,82]. Recent studies suggest that mTOR controls the translational machinery via activation of ribosomal p70 s6 kinase (p70s6k), which is critical for protein synthesis and hypertrophy[12,31,73]. Akt activation also leads to phosphorylation and inhibition of glycogen synthase kinase-3β (GSK-3β), which inhibits cyclin D and gene transcription and can result in greater transcription and cell growth[82] (Figure 1). In addition to promoting protein synthesis, Akt activation can suppress protein degradation by inactivation of forkhead dependent transcription factors (FOXO), which promote proteolysis[72,83]. Thus, insulin stimulation of Akt and downstream targets (mTOR, FOXO) link insulin receptor stimulation and downstream growth responses.
Figure 1.
Proposed schematic depiction of activation of LV hypertrophy by insulin- and fatty acid-mediated signaling pathways. In addition to the “classical” mediators of LVH (adrenergic signalling, endothelin, renin-angiotensin system, etc.) dietary macronutrients can be involved in the hypertrophic response. Insulin enhances Akt-stimulated protein synthesis by activating mTOR and p70s6k., and inhibiting GSK-3β. Akt inhibits protein breakdown through inhibition of FOXO. Upstream of Akt, PI3K can also be activated by signaling mechanisms linked to G-protein coupled receptor stimulation (e.g. Ras, MAPK, ERK, etc) as well as AMP kinase (AMPK). The adiponkines leptin and adiponectin can affect hypertrophy through their actions on ERK and AMPK. PPAR/RXR/PGC1 activates expression of genes involved in cardiac fatty acid oxidation and mitochondrial biogenesis, and can stimulate hypertrophy under some conditions (transgenic over-expression or pharmacologic stimulation with high doses of agonist).
Strong evidence for a key role for insulin signaling in LVH comes from studies in transgenic animals. Insulin-like growth factor (IGF) receptor signaling can contribute to progressive cardiac dysfunction in aging as recently shown in fruit flies[84]. Age-related heart dysfunction and heart failure were minimized with a reduction of systemic levels of insulin-like peptides, inactivating mutations in the insulin receptor, or over-expression of FOXO[84]. Cardiac insulin receptor knockout mice have smaller hearts[13] and reduced activation of Akt and p70s6k, which are critical for protein synthesis and hypertrophy[12]. Conversely, over-expression of Akt in isolated cardiomyocytes increases insulin-stimulated protein synthesis[12], and mice with cardiac over-expression of Akt have greater p70s6k activity and extreme LVH (2.3-fold increase above non-over-expressing mice)[85].
Activation of mTOR stimulates LVH in response to hypertension[86,87] or aortic banding[31,32,88,89] in rats and mice. Treatment with rapamycin prevents activation of p70s6k and LVH in mice subjected to aortic banding, and rapamycin administration following the establishment of LVH reduces p70s6k activation and blunts the increase in LV mass[32]. These findings illustrate the key role of mTOR activation in the development of LVH in response to various forms of pressure overload. While a role for mTOR activation in regulating pressure overload-induced LVH is clearly established, the interaction between insulin- and pressure overload-induced activation of mTOR is unclear. Nutritional status is a major regulator of Akt activity in the heart, as evidenced by a ∼3 fold higher Akt and p70s6k in the fed state (with standard high carbohydrate rodent chow) compared to overnight fasted conditions[12]. Thus, when insulin levels are elevated by eating high carbohydrate food, there is greater activation of Akt, mTOR, and p70s6k, suggesting a higher rate of protein synthesis and/or less protein breakdown. As expected, these feeding effects are lost in the cardiac insulin receptor knockout (CIRKO) mouse due to the absence of normal insulin signaling[12]. This suggests the possibility that the increase in insulin exposure to the heart after consuming a high carbohydrate meal activates the Akt-mTOR-p70s6k pathway and increases protein synthesis.
3.1.2. Akt isoforms have various roles in cardiac physiology and growth
There are three isoforms of Akt in mammals: Akt1, Akt2, and Akt3. Akt1 and Akt2 are expressed in the heart[90-92], while Akt3 is expressed primarily in brain where it regulates growth[92,93]. Studies in Akt1-/- mice[90,94-96] and mice with cardiac over-expression of Akt1[96] suggest that this isoform mainly functions to regulate growth[90,94,95]. Akt1-/- mice have impaired fetal and postnatal growth[90] but normal glucose tolerance and insulin responses[90]. Mice with constitutively active cardiac Akt1 develop LVH[96], while Akt1-/- mice are resistant to swim training-induced cardiac hypertrophy[14]. Additionally, isolated myocytes from Akt1-/- mice are resistant to IGF-1-stimulated protein synthesis, suggesting that PI3K-Akt pathways is key for normal heart growth[97]. Akt2-/- mice also exhibit growth retardation but also have severe diabetic symptoms such as insulin resistance, hyperglycemia, hyperinsulemia, and glucose intolerance[91,98]. On a systemic basis, the Akt2-/- mouse has a more severe phenotype, with both retarded growth and severe symptoms of diabetes[91,98], while that of the Akt1-/- is mainly isolated to growth[90].
3.2.1. Lipid activation of PPAR signaling
Cardiac gene expression and myocyte growth can also be activated by ligand binding to PPARs, specifically PPARα and PPARβ/δ[33,34,99-102]. These transcription factors control gene expression by forming a heterodimer with the retinoid X receptors (RXR) and then binding to specific PPAR response elements (PPRE) located within promoter regions of many genes encoding metabolic enzymes[33]. In addition, the PPAR/RXR complex requires the cofactor PPARγ coactivator-1α (PGC-1α)[100]. Once bound to the PPRE, the PPAR/RXR/PGC-1 complex increases the rate of transcription of fatty acid oxidation genes [99,101,103,104]. The activity of PPAR/RXR heterodimers is increased by fatty acids and eicosanoids. Thus, PPAR/RXR heterodimers act as lipid sensors in the cell, increasing the capacity for fatty acid catabolism in response to a greater cell exposure to lipid (Figure 1)[100]. While the expression of PPARα and PPARβ/δ[99] are high in the heart, PPARγ mRNA is very low and does not appear to play a direct role in regulating fatty acid oxidation [99,105].
3.2.2. PPAR regulation and response to LVH
The mRNA for PPARα-regulated genes are downregulated in severe LVH and heart failure in humans and animal models[34,106-110]. Changes in the mRNA expression of PPAR-regulated genes do not consistently result in a similar change in protein expression or enzyme activity [19,107,111], thus one must use caution when inferring changes in protein expression from mRNA data. Several studies found decreased expression of PPARα mRNA or protein in response to hypertrophic growth in cell culture or with LVH following aortic banding[34,101,109,110,112]. On the other hand, in the rat infarct and canine pacing models of heart failure (both which result in LVH), there is no decrease in PPARα protein expression despite reduced mRNA levels for PPARα-regulated genes[19,107,111,113,114]. Mice with a cardiac-specific deletion of PPARβ/δ have LVH, myocardial lipid accumulation, and reduced survival[115], but PPARα-/- mice have a normal heart mass/body mass ratio[22]. Since PPARα and PPARβ/δ appear to perform redundant functions, it is difficult to draw conclusions from studies in knock out mice about the role of either receptor in the regulation of LVH in response to pressure overload. At present, one can conclude that there is a consistent decrease in the transcript levels of PPARα/PPARβ/δ regulated genes in advanced LVH, but it does not require a decrease in PPARα protein expression.
Data on the effects of activation of the PPAR pathway on LVH are conflicting. Cardiac-specific over-expression of PPARα in transgenic mice results in LVH and LV dysfunction[21], particularly with high fat feeding[22]. Conversely, the PPARα ligands fenofibrate and Wy-14,463 inhibited cardiomyocyte hypertrophy in response to endothelin in cell culture[116]. A similar anti-hypertrophic action has been observed for other nuclear ligands, including 1,25 dihydroxyvitamin D and retinoic acid[117]. On the other hand, treatment of Fischer 344 rats with the PPARα agonists Wy-14,643 for 26 weeks resulted in a 23% increase in cardiomyocyte diameter and a greater heart mass/body mass ratio [20]. Activation of PPARα with fenofibrate for 12 weeks in rats with infarct-induced heart failure increased mRNA levels for PPARα-regulated genes and LV mass but had no effect on cardiac systolic function or LV end diastolic volume[19]. Treatment of rats subjected to either sham surgery or proximal aortic banding with Wy-14,643 did not increase LV mass in either group despite increasing the mRNA level of PPARα regulated genes[110]. Activating RXRα by feeding retinoic acid to normotensive rats for 90 days resulted in a 10% increase in LV mass/body mass ratio[118]. Taken together, it appears the pharmacological activation of the PPAR/RXR system can result in a modest degree of LVH, though this is not a uniform finding.
The effects of a high fat diet on PPAR activity and LVH are more complex and not well studied. Feeding a high fat diet (60% of total energy from fat) to normotensive rats for 8 weeks increased the mRNA expression for PPAR regulated genes, but did not result in LVH compared to a high carbohydrate/low fat diet[35]. It is important to note that the high fat diet reduced plasma insulin in the fed state, which may act to reduce insulin-stimulated growth in the face of PPAR activation. Another study found that feeding a high fat diet (45% of energy from fat) to rats with infarct-induced heart failure had no effect on LV mass or function, or the mRNA and protein levels of PPAR-regulated genes[19]. On the other hand, feeding a high fat diet to hypertensive Dahl salt-sensitive rats increased the expression of the mRNA expression for medium chain acyl-CoA dehydrgoentase, a PPARα regulated gene, and attenuated hypertension-induced LVH[49], which is consistant with cell culture data showing an antihypertrophic effect with pharmacological activation of PPARα[116,117].
In summary, there appears to be decreased activity of the PPARα/PPARβ/δ pathways in advanced LVH, which results in a reduced capacity for fatty acid oxidation. It remains unclear if ligand activation of PPARα and/or PPARβ/δ by fatty acids (as might occur with some high fat diets, insulin resistance or diabetes) or PPAR agonists has a direct effect on the development of LVH under conditions of pressure overload. In addition, it is not clear if stimulation of PPAR activity under conditions of pressure overload prevents the down-regulation of proteins involved in fatty acid metabolism[33,34].
4. Insulin resistance and LVH
Cardiac contractile function, gene expression, LV chamber volume, and LV mass are affected by circulating hormones and substrates, including the concentrations of fatty acids and insulin in the plasma[34]. Plasma fatty acid concentration is largely a function of net fatty acid output from adipocytes, which is inhibited by plasma insulin and increased by adrenergic stimulation. Plasma insulin levels reflect the rate of insulin secretion from pancreatic beta cells, which secrete insulin in response to the plasma glucose concentration. Impaired insulin signaling in adipose tissue and skeletal muscle is the hallmark of the whole body insulin resistance observed with metabolic syndrome or type 2 diabetes, and is largely responsible for the increase in plasma insulin and free fatty acids found in these patients[119]. Thus, impaired insulin stimulation of glucose uptake in skeletal muscle and adipose tissue will drive the system to a higher insulin concentration to stimulate the same rate of whole body glucose uptake[119].
Clinically, patients with essential hypertension often display both LVH and insulin resistance [120-123]
Hypertensive patients with either high plasma insulin or metabolic syndrome have a greater occurrence of LVH[23-29]. Echocardiographic assessment of 1,388 nondiabetic American Indians showed that greater LV size (adjusted for blood pressure and body mass) is positively related to fasting insulin level[27]. Approximately two-thirds of patients with essential hypertension have abnormal glucose metabolism[124], and there is a positive relationship between LVH and plasma insulin concentration [23,24], suggesting that elevated insulin contributes to cardiac growth when the heart is subjected to chronic pressure overload. There have been few measurements of the effects of hyperinsulinemia on the human heart; however, it appears that insulin resistance in the myocardium in type 2 diabetic patients is relatively minor[125,126], particularly if plasma fatty acid concentrations are matched[127]. This is in stark contrast to the high levels of resistance observed in adipose tissue and skeletal muscle [119]. Thus, if there is insulin resistance in adipose and skeletal muscle, the plasma insulin and fatty acid levels will increase, which may activate insulin signaling and the PPAR pathway in the heart.
5. Effects of adipokines on the heart
Since the discovery of leptin over ten years ago[128], there has been a great deal of interest in the role of adipose tissue as an endocrine organ, and the effects of adipokines (e.g. leptin, adiponectin, resistin, ghrelin, visfatin) on eating behavior, substrate metabolism, and cardiac growth and function. At present, relatively little is known about how diet effects the secretion of these peptides or about their sites of production (visceral vs. subcutaneous adipose) and the effects on the heart and vascular system [129-132]. The most studied adipokines are leptin and adiponectin, which both have effects on cardiac growth and metabolism.
5.1. Leptin
Leptin has been implicated as a potential mediator of LVH, primarily due to initial observations that it may increase sympathetic vasoconstrictor tone and increase arterial blood pressure [133,134]. However, the direct role that leptin plays in vivo in triggering LVH remains unclear. Leptin normally acts to trigger satiety and reduce food intake[128,135], and is increased ∼4-fold in obese normotensive people compared to lean individuals[136]. Treatment of isolated perfused hearts with leptin increases fatty acid oxidation and reduces cardiac triglyceride stores[137]. In skeletal muscle, leptin stimulates AMP activated protein kinase (AMPK) and inhibits acetyl-CoA carboxylase (ACC), which increases fatty acid oxidation, presumably due to lower malonyl-CoA levels [138,139]. However, this effect was not observed in the isolated heart[137]. Interestingly, leptin activation of fatty acid oxidation is greater in innervated skeletal muscle than in denervated muscle, suggesting the possibility that the hypothalamic effects of leptin are mediated through the discharge of peripheral nerves[138,139].
A strong positive correlation has been observed between fasting plasma leptin levels and increased LV wall thickness independent of blood pressure in a study comparing hypertensive and normotensive male patients[140]. The concept that leptin acts as a direct stimulant for cardiomyocyte growth is supported by studies showing an increase in cell size and protein synthesis in neonatal cardiomyocytes[18,141,142]. The mechanism and in vivo implications for these effects are unclear. Studies in isolated rat myocytes have demonstrated that leptin treatment results in increased protein synthesis and hypertrophy, and that pretreatment of these cells with a leptin receptor antibody attenuates these hypertrophic effects[141-145]. Additionally, these studies have demonstrated that the leptin-induced ERK1/2 activation was attenuated by Rho protein signaling inhibition, suggesting a possible role for these proteins in leptin-mediated hypertrophy.
On the other hand, it has been suggested that diet-induced hyperleptinemia resulting from overnutrition confers differential metabolic effects than obesity-induced hyperleptinemia, which may provide an explanation for hyperleptinemia preventing lipid-induced cardiac dysfunction[146]. The cardiac hypertrophy and increase in cardiomyocyte apoptosis that is observed in the obese leptin deficient (ob/ob) mouse is reversed by long-term leptin infusion [17,41]. Recent interest has focused on the ciliary neurotrophic factor (CNTF), which has receptors on cardiomyocytes that closely resemble those for leptin[147]. Activation of the CNTF signaling pathway regresses LVH in leptin-deficient mice[148], however the mechanism for this effect is not clear. Studies demonstrated that treatment with CNTF activates AMPK and inhibits ACC, increases fatty acid oxidation, and lowers tissue triglyceride and ceramide content in skeletal muscle, presumably due lower malonyl-CoA levels[149,150]. These observations are similar to the effects attributed to leptin treatment in skeletal muscle[138], however this mechanism has not been demonstrated in the heart. Little is known about the effects of diet on plasma leptin in the absence of obesity. We observed that feeding rats a high saturated fat diet for 12 weeks reduced plasma leptin concentrations by 50% compared to normal chow or a high unsaturated fat chow, however there were no effects on body mass, blood pressure, LV mass or cardiac function[35]. Clearly additional work is required before the role of leptin in LVH is understood.
5.2. Adiponectin
Low circulating levels of adiponectin are observed in healthy obese people[136] and are an independent risk factor for hypertension [151-153]. Adiponectin knockout mice with sodium-induced hypertension demonstrated normalized blood pressure when treated with adiponectin, suggesting that adiponectin exerts a hypotensive action in response to sodium overload [15]. Aortic banding of adiponectin knockout mice results in enhanced concentric LVH and mortality compared to wild-type animals and is associated with increased activation of extracellular signal-regulated kinase (ERK) and reduced AMPK activation in the heart[16,154]. Restoration of cardiac adiponectin levels with adenovirus-mediated supplementation partially prevented LVH in response to pressure overload both in adiponectin knockout and wild-type mice and reduced mortality [16]. In addition, adiponectin knockout mice have a larger infarct following ischemia/reperfusion. Treatment with adiponectin reduced infarct size in knockout and wild-type mice. Studies in endothelial cells have shown that adiponectin promotes cell growth and angiogenesis by promoting cross-talk between AMPK and Akt signaling pathways[155]. Taken together, these findings suggest that adiponectin is cardioprotective and anti-hypertrophic and may act through activation of AMPK signaling (as also shown in skeletal muscle[156]). In addition, low levels of adiponectin, such as those present in obesity, may put the heart at risk for LVH and greater injury when subjected to ischemia.
6. Macronutrient influences on LVH
The effect of dietary composition on the development of LVH has not been well studied. Our laboratory recently observed that feeding a high fat diet had no effect on cardiac mass or function in rats with established infarct-induced heart failure[19]. On the other hand, we observed that a high fat diet (60% of energy from fat) fed to hypertensive Dahl salt sensitive rats for six weeks prevented the development of LVH and improved systolic function compared to a standard high carbohydrate diet despite similar blood pressures [48]. In a subsequent study we assessed the long term effects of diet on hypertension-induced LVH, remodeling, contractile dysfunction, and induction of molecular markers of hypertrophy (i.e. expression of mRNA for atrial natriuretic factor and myosin heavy chain β)[49]. Similar levels of hypertension were achieved with high salt feeding in both diet groups (systolic pressure of ∼190 mmHg), however hypertensive rats fed low fat/high carbohydrate chow demonstrated increased LV mass and myocyte cross sectional area, while end diastolic volume were increased and ejection fraction was decreased. Thus, increased dietary lipid intake can reduce cardiac growth, left ventricular remodeling, contractile dysfunction, and alterations in gene expression in response to hypertension. The mechanism of this effect is unclear, but could be due to greater ligand activation of PPARs and reduced insulin activation of growth pathyways (Figures 1 and 2). We have demonstrated that feeding Wistar rats a high fat diet (∼60% calories) for 8 weeks leads to a significant reduction in plasma insulin levels[35]. These findings suggest that there is a decrease in insulin signaling with chronic administration of various high fat diets. This may provide a mechanism for reduced hypertrophy due to reduced stimulation of the insulin signaling pathways. However, the effect of a high fat/low carbohydrate diet on insulin signaling in the heart has not been elucidated.
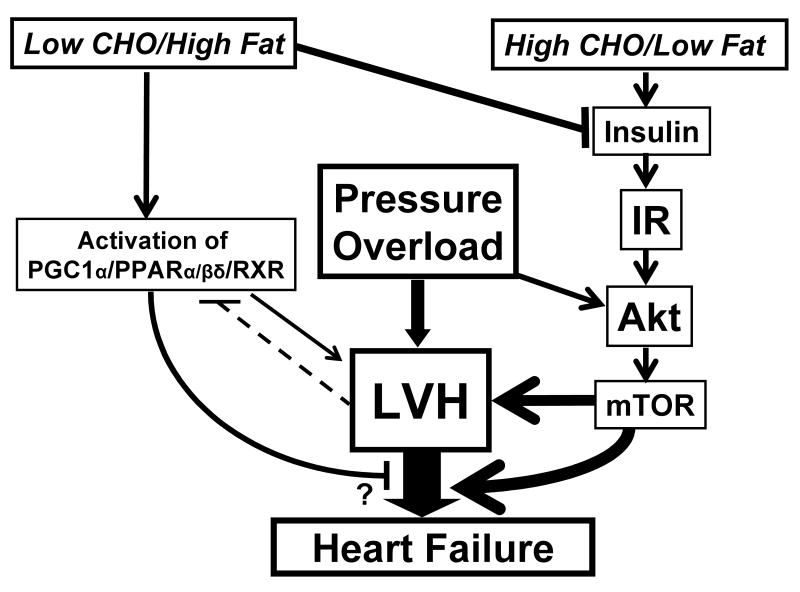
Figure 2.
Schematic speculation of how macronutrient intake might influence LV hypertrophy and subsequent dysfunction during pressure overload. Pressure overload stimulates LVH via Akt/mTOR dependent and independent mechanisms, and chronic LVH progresses to LV remodeling and subsequent heart failure. vvEating a diet that is high in carbohydrate (particular simple sugar) and low in fat will increase plasma insulin and activate hypertrophic growth pathways through stimulation of insulin receptors (IR), and activation of Akt and mTOR. On the other hand, a low carbohydrate/high fat diet will reduce result in low plasma insulin concentration and less activation stimulation of insulin mediate growth, and will elevate plasma fatty acid, resulting in greater activation of the PPAR pathway.
In a subsequent study, we investigated the effects of consuming either a high complex carbohydrate diet, a simple sugar diet (fructose), a high fat diet, or a mixed high fat/high sugar diet (“western diet”) on cardiac hypertrophy and mortality in the same model[50]. Despite similar levels of hypertension, there was 18% in the western diet group, 30% mortality in the complex carbohydrate group, 85% mortality in the high sugar group, but no mortality in the high fat fed animals after 90 days of treatment. Thus, a high simple sugar diet consumed during hypertension increases mortality compared to either a high fat, high starch, or a “western” diet. A high sugar diet should result in a higher insulin exposure of the heart, which should trigger greater insulin-induced cardiomyocyte growth via Akt mediated mechanisms (Figures 1 and 2) [12].
Foods differ greatly in their ability to elevate blood glucose and insulin levels. The concept of glycemic index (GI) is an established measure to quantify the increase in blood glucose attributed to a food following a meal[157]. The increased consumption of low GI foods (i.e. composed of high levels of simple sugars) has been implicated as a potential cause for the profound increase in type II diabetes, obesity, and cardiovascular disease in developed countries[158-160]. These metabolic disturbances appear to underlie the induction of insulin resistance and hyperinsulinemia commonly observed with high simple sugar feeding in both humans and animal models[161-164] and are the basis for recommending a diet low in simple sugars in patient with insulin resistance and metabolic syndrome[157,165-167]. Despite what is known about the hyperinsulinemic response to simple sugars, it is not clear whether diets high in simple sugars contribute to greater LVH in patients with hypertension.
There is extensive clinical evidence suggesting that high fat diets, especially those high in saturated fats, are closely associated with increased risk of CVD[67,168,169]. Recently, a low carbohydrate/high fat diet has been advocated as a weight loss/weight maintenance strategy[170-172]. However, the impact of such a diet on cardiac function, hypertrophy, and LV remodeling in patients with hypertension or with established LVH or heart failure is not known. While it is possible that one can change cardiac phenotype by altering their dietary carbohydrate and fat intake, there is a paucity of long term data on the effect of low carbohydrate/high fat diets on plasma hormones and lipid concentrations, and cardiac function and mass. Almost all clinical investigations on low carbohydrate/high fat diets have focused on weight loss, not weight maintenance or prevention of obesity. Nevertheless, there is a clear possibility that the composition of carbohydrate and lipid in the diet has a major effect in the development of LVH and cardiac dysfunction (Figure 2).
6.1. Polyunsaturated fats and fish oils
Polyunsaturated fats (PUFAs) and fish oils (which contain omega-3 fatty acids (n-3)) can contribute significantly to caloric intake, and have been shown to reduce the risk of arrhythmias and cardiovascular disease [173-178]. The mechanisms behind the beneficial effects of PUFAs and fish oils are unclear[179,180], but might be partially due to greater PUFA incorporation into phospholipids in mitochondrial membranes and increased Ca2+ activation of matrix dehydrogenased, and improved mitochondrial function, as suggested by PUFA feeding studies in aged rats[181-183]. There are few studies that have investigated the role of PUFAs and fish oils in hypertension and the progression of LVH. Diets high in fish oils prevent hyperinsulinemia, hypertriglyceridemia, and hypertension in fructose- and dexamethasone-induced hypertensive rat models[184-186]. Increased PUFA intake is associated with a reduction of reactive oxygen species and an increase in antioxidants during hypertension[187-189]. Dietary fish oils can prevent LVH in transgenic mice with a carnitine transporter mutation, which was attributed to modified diacylglycerol composition and resultant inhibition of PKC activation[190]. Siddiqui et al. demonstrated that n-3 polyunsaturated lipid docosahexaenoic acid (DHA) prevents cardiac hypertrophy by inhibition of the Ras-Raf1-Erk1/2-p90rsk signaling pathway in a phenylephrine-induced hypertrophy model[191]. The role of PUFAs on lipid signaling via the PPAR system in cardiomyocytes remains to be clarified.
Ingestion of fish oils has recently been shown to increase the expression of adiponectin in adipose tissue, and increase plasma adiponectin concentration in mice and rats [44,45,47,192]. Neschen et al. observed a dose-dependent increase in adiponectin up to maximum of 3-fold above normal values after 15 days of treatment [44]. This effect was also seen in PPARα-/- mice, but was absent in PPARγ-/- mice, thus fish oil activation of adiponectin synthesis is regulated by PPARγ. Rossi et al show that feeding cod liver oil to rats that were made insulin resistant by sucrose feeding also significantly elevated plasma adiponectin concentration [192]. An increase in adiponectin levels with increase fish oil consumption may explain the improved cardiovascular health observed in clinical studies [173-177].
7. Summary
The role that dietary fat and carbohydrate composition play in the development and progression of LVH has not been well characterized. At present, the dietary recommendations for hypertensive individuals regarding energy macronutrients have not been determined. Animal studies demonstrate that enhanced stimulation of insulin-mediated growth pathways can trigger cardiomyocyte hypertrophy. Recent work suggests that the composition of dietary carbohydrate and fat can effect the development of LVH, ventricular remodeling, systolic function and survival in hypertensive rats. The role that dietary lipids - specifically PUFAs and fish oils - play in the regulation of cardiac size and function during chronic cardiac stress is unclear. Interaction between dietary lipids and heart size and function may be mediated through direct effects (e.g. PPAR stimulation in cardiomyocytes), or perhaps by indirect effects mediated by adipokines. It remains be determined whether manipulation of dietary macronutrient in human hypertensive patients can prevent LVH. Additional work is needed before the optimal diet for hypertension can be prescribed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kannel WB. Hypertension and other risk factors in coronary heart disease. Am Heart J. 1987;114:918–925. doi: 10.1016/0002-8703(87)90588-6. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Epidemiology and prevention of cardiac failure: Framingham Study insights. Eur Heart J. 1987;8 F:23–26. doi: 10.1093/eurheartj/8.suppl_f.23. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Angeli F, Gattobigio R, Guerrieri M, Benemio G, Porcellati C. Does the reduction in systolic blood pressure alone explain the regression of left ventricular hypertrophy? J Hum Hypertens. 2004;18 2:S23–S28. doi: 10.1038/sj.jhh.1001797. [DOI] [PubMed] [Google Scholar]
- 4.Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006;48:326–341. doi: 10.1016/j.pcad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 6.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 7.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 8.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 9.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol. 2003;35:871–886. doi: 10.1016/s0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 10.Sugden PH. Signalling pathways in cardiac myocyte hypertrophy. Ann Med. 2001;33:611–622. [PubMed] [Google Scholar]
- 11.Schluter KD, Wollert KC. Synchronization and integration of multiple hypertrophic pathways in the heart. Cardiovasc Res. 2004;63:367–372. doi: 10.1016/j.cardiores.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, et al. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 13.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, et al. Akt1 Is Required for Physiological Cardiac Growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin Replenishment Ameliorates Obesity-Related Hypertension. Hypertension. 2006:01. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of Leptin Signaling Contributes to Cardiac Hypertrophy Independently of Body Weight in Mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 18.Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, et al. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004;110:1269–1275. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- 19.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, et al. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 20.Hamano T, Kobayashi K, Sakairi T, Hayashi M, Mutai M. Peroxisome proliferator-activated receptor alpha (PPAR alpha) agonist, WY-14,643, increased transcription of myosin light chain-2 in cardiomyocytes. J Toxicol Sci. 2001;26:275–284. doi: 10.2131/jts.26.275. [DOI] [PubMed] [Google Scholar]
- 21.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shigematsu Y, Hara Y, Ohtsuka T, Ohgimoto A, Inoue K, Higaki J. Relation of genetic predisposition and insulin resistance to left ventricular hypertrophy in hypertension. Am J Hypertens. 2005;18:457–463. doi: 10.1016/j.amjhyper.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Karason K, Sjostrom L, Wallentin I, Peltonen M. Impact of blood pressure and insulin on the relationship between body fat and left ventricular structure. Eur Heart J. 2003;24:1500–1505. doi: 10.1016/s0195-668x(03)00312-9. [DOI] [PubMed] [Google Scholar]
- 25.Stiefel P, Miranda ML, Rodriguez-Puras MJ, Garcia-Morillo S, Carneado J, Pamies E, et al. Glucose effectiveness is strongly related to left ventricular mass in subjects with stage I hypertension or high-normal blood pressure. Am J Hypertens. 2004;17:146–153. doi: 10.1016/j.amjhyper.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 27.Ilercil A, Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, et al. Associations of insulin levels with left ventricular structure and function in American Indians: the strong heart study. Diabetes. 2002;51:1543–1547. doi: 10.2337/diabetes.51.5.1543. [DOI] [PubMed] [Google Scholar]
- 28.Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, et al. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:1991–1998. doi: 10.1097/00004872-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Mule G, Nardi E, Cottone S, Cusimano P, Volpe V, Piazza G, et al. Influence of metabolic syndrome on hypertension-related target organ damage. J Intern Med. 2005;257:503–513. doi: 10.1111/j.1365-2796.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 30.Ceravolo R, Maio R, Cuda G, Scozzafava A, Sciacqua A, Vatrano M, et al. Relation of fasting insulin related to insertion/deletion polymorphism of angiotensin-converting enzyme-gene and cardiac mass in never-treated patients with systemic hypertension. Am J Cardiol. 2003;92:1234–1237. doi: 10.1016/j.amjcard.2003.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 33.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 34.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 35.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 36.Young ME, Patil S, Ying J, Depre C, Ahuja HS, Shipley GL, et al. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J. 2001;15:833–845. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 37.Stavinoha MA, RaySpellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol Endocrinol Metab. 2004;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 38.McNamara DJ. Dietary cholesterol and atherosclerosis. Biochim Biophys Acta. 2000;1529:310–320. doi: 10.1016/s1388-1981(00)00156-6. [DOI] [PubMed] [Google Scholar]
- 39.German JB, Dillard CJ. Saturated fats: what dietary intake? Am J Clin Nutr. 2004;80:550–559. doi: 10.1093/ajcn/80.3.550. [DOI] [PubMed] [Google Scholar]
- 40.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 41.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 42.Minhas KM, Khan SA, Raju SV, Phan AC, Gonzalez DR, Skaf MW, et al. Leptin repletion restores depressed {beta}-adrenergic contractility in ob/ob mice independently of cardiac hypertrophy. J Physiol. 2005;565:463–474. doi: 10.1113/jphysiol.2005.084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 44.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 45.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 46.Huang BW, Chiang MT, Yao HT, Chiang W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes Metab. 2004;6:120–126. doi: 10.1111/j.1462-8902.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- 47.Ide T. Interaction of fish oil and conjugated linoleic acid in affecting hepatic activity of lipogenic enzymes and gene expression in liver and adipose tissue. Diabetes. 2005;54:412–423. doi: 10.2337/diabetes.54.2.412. [DOI] [PubMed] [Google Scholar]
- 48.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, et al. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 49.Okere IC, Young ME, McElfresh TE, Chess DJ, Sharov VG, Sabbah VG, et al. Low carbohydrate/high fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006 doi: 10.1161/01.HYP.0000248430.26229.0f. in press. [DOI] [PubMed] [Google Scholar]
- 50.Sharma N, Okere IC, Duda MK, Johnson J, Yuan C, Chandler MP, et al. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2006 doi: 10.1016/j.amjhyper.2006.09.022. in press. [DOI] [PubMed] [Google Scholar]
- 51.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 52.Ventura-Clapier RF, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2003 doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allard MF. Energy substrate metabolism in cardiac hypertrophy. Curr Hypertens Rep. 2004;6:430–435. doi: 10.1007/s11906-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651–1662. doi: 10.1161/CIRCULATIONAHA.104.490599. [DOI] [PubMed] [Google Scholar]
- 55.Panidis IP, Kotler MN, Ren JF, Mintz GS, Ross J, Kalman P. Development and regression of left ventricular hypertrophy. J Am Coll Cardiol. 1984;3:1309–1320. doi: 10.1016/s0735-1097(84)80192-8. [DOI] [PubMed] [Google Scholar]
- 56.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic Significance of Left Ventricular Mass Change During Treatment of Hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 57.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 58.Izzo JL, Jr, Gradman AH. Mechanisms and management of hypertensive heart disease: from left ventricular hypertrophy to heart failure. Med Clin North Am. 2004;88:1257–1271. doi: 10.1016/j.mcna.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Benjamin EJ, Levy D. Why is left ventricular hypertrophy so predictive of morbidity and mortality? Am J Med Sci. 1999;317:168–175. doi: 10.1097/00000441-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Sano M, Schneider MD. Still stressed out but doing fine: normalization of wall stress is superfluous to maintaining cardiac function in chronic pressure overload. Circulation. 2002;105:8–10. [PubMed] [Google Scholar]
- 61.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 62.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 63.de Lorgeril M, Salen P. Diet as preventive medicine in cardiology. Curr Opin Cardiol. 2000;15:364–370. doi: 10.1097/00001573-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113 9B:13S–24S. doi: 10.1016/s0002-9343(01)00987-1. [DOI] [PubMed] [Google Scholar]
- 65.Frohlich ED. Risk Mechanisms in Hypertensive Heart Disease. Hypertension. 1999;34:782–789. doi: 10.1161/01.hyp.34.4.782. [DOI] [PubMed] [Google Scholar]
- 66.Meneton P, Jeunemaitre X, de Wardener HE, Macgregor GA. Links Between Dietary Salt Intake, Renal Salt Handling, Blood Pressure, and Cardiovascular Diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 67.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 68.Howard BV, Wylie-Rosett J. Sugar and cardiovascular disease: A statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2002;106:523–527. doi: 10.1161/01.cir.0000019552.77778.04. [DOI] [PubMed] [Google Scholar]
- 69.Yudkin J. Sugar and ischaemic heart disease. Practitioner. 1967;198:680–683. [PubMed] [Google Scholar]
- 70.Yudkin J. Dietary factors in arteriosclerosis: sucrose. Lipids. 1978;13:370–372. doi: 10.1007/BF02533732. [DOI] [PubMed] [Google Scholar]
- 71.Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63:500–509. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 73.Proud CG. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63:403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- 75.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 76.Brownsey RW, Boone AN, Allard MF. Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc Res. 1997;34:3–24. doi: 10.1016/s0008-6363(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 77.McNulty PH, Jacob R, Deckelbaum LI, Young LH. Effect of hyperinsulinemia on myocardial amino acid uptake in patients with coronary artery disease. Metabolism. 2000;49:1365–1369. doi: 10.1053/meta.2000.9510. [DOI] [PubMed] [Google Scholar]
- 78.Young LH, Dahl DM, Rauner D, Barrett EJ. Physiological hyperinsulinemia inhibits myocardial protein degradation in vivo in the canine heart. Circ Res. 1992;71:393–400. doi: 10.1161/01.res.71.2.393. [DOI] [PubMed] [Google Scholar]
- 79.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 80.Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Condorelli G, et al. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res. 2005;96:180–188. doi: 10.1161/01.RES.0000152968.71868.c3. [DOI] [PubMed] [Google Scholar]
- 81.Ceci M, Ross J, Jr, Condorelli G. Molecular determinants of the physiological adaptation to stress in the cardiomyocyte: a focus on AKT. J Mol Cell Cardiol. 2004;37:905–912. doi: 10.1016/j.yjmcc.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 82.Latronico MV, Costinean S, Lavitrano ML, Peschle C, Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann N Y Acad Sci. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 83.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 85.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 86.Sanada S, Node K, Minamino T, Takashima S, Ogai A, Asanuma H, et al. Long-acting Ca2+ blockers prevent myocardial remodeling induced by chronic NO inhibition in rats. Hypertension. 2003;41:963–967. doi: 10.1161/01.HYP.0000062881.36813.7A. [DOI] [PubMed] [Google Scholar]
- 87.Sanada S, Kitakaze M, Node K, Takashima S, Ogai A, Asanuma H, et al. Differential subcellular actions of ACE inhibitors and AT(1) receptor antagonists on cardiac remodeling induced by chronic inhibition of NO synthesis in rats. Hypertension. 2001;38:404–411. doi: 10.1161/01.hyp.38.3.404. [DOI] [PubMed] [Google Scholar]
- 88.Boluyt MO, Li ZB, Loyd AM, Scalia AF, Cirrincione GM, Jackson RR. The mTOR/p70S6K signal transduction pathway plays a role in cardiac hypertrophy and influences expression of myosin heavy chain genes in vivo. Cardiovasc Drugs Ther. 2004;18:257–267. doi: 10.1023/B:CARD.0000041245.61136.56. [DOI] [PubMed] [Google Scholar]
- 89.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 90.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 91.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, III, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 92.Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- 93.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh K. Akt Signaling and Growth of the Heart. Circulation. 2006;113:2032–2034. doi: 10.1161/CIRCULATIONAHA.106.615138. [DOI] [PubMed] [Google Scholar]
- 98.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilde AJ, Van Der Lee KA, Willemsen PH, Chinetti G, Van Der Leij FR, van der Vusse GJ, et al. Peroxisome Proliferator-Activated Receptor (PPAR) {alpha} and PPAR{beta}/{delta}, but not PPAR{gamma}, Modulate the Expression of Genes Involved in Cardiac Lipid Metabolism. Circ Res. 2003 doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 100.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 101.Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–185. doi: 10.1023/a:1015332726303. [DOI] [PubMed] [Google Scholar]
- 102.van Bilsen M, van der Vusse GJ, Gilde AJ, Lindhout M, Van Der Lee KA. Peroxisome proliferator-activated receptors: lipid binding proteins controling gene expression. Mol Cell Biochem. 2002;239:131–138. [PubMed] [Google Scholar]
- 103.Harris RA, Huang B, Wu P. Control of pyruvate dehydrogenase kinase gene expression. Adv Enzyme Regul. 2001;41:269–288. doi: 10.1016/s0065-2571(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 104.Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes. 2002;51:276–283. doi: 10.2337/diabetes.51.2.276. [DOI] [PubMed] [Google Scholar]
- 105.Kelly DP. PPARs of the heart: three is a crowd. Circ Res. 2003;92:482–484. doi: 10.1161/01.RES.0000064382.46274.95. [DOI] [PubMed] [Google Scholar]
- 106.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 107.Lei B, Lionetti V, Young ME, Chandler MP, D' Agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, et al. Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am J Physiol Heart Circ Physiol. 2002;283:H1750–H1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 109.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 111.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, et al. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Kanda H, Nohara R, Hasegawa K, Kishimoto C, Sasayama S. A nuclear complex containing PPARalpha/RXRalpha is markedly downregulated in the hypertrophied rat left ventricular myocardium with normal systolic function. Heart Vessels. 2000;15:191–196. doi: 10.1007/s003800070022. [DOI] [PubMed] [Google Scholar]
- 113.Morgan EE, Chandler MP, Young ME, McElfresh TA, Kung TA, Rennison J, et al. Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail. 2006 doi: 10.1016/j.ejheart.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 115.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 116.Liang F, Wang F, Zhang S, Gardner DG. Peroxisome proliferator activated receptor (PPAR)alpha agonists inhibit hypertrophy of neonatal rat cardiac myocytes. Endocrinology. 2003;144:4187–4194. doi: 10.1210/en.2002-0217. [DOI] [PubMed] [Google Scholar]
- 117.Wu J, Garami M, Cheng T, Gardner DG. 1,25 (OH)2 Vitamin D3 and Retinoic Acid Antagonize Endothelin-stimulated Hypertrophy of Neonatal Rat Cardiac Myocytes. J Clin Invest. 1996;97:1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Paiva SA, Zornoff LA, Okoshi MP, Okoshi K, Matsubara LS, Matsubara BB, et al. Ventricular remodeling induced by retinoic acid supplementation in adult rats. Am J Physiol Heart Circ Physiol. 2003;284:H2242–H2246. doi: 10.1152/ajpheart.00646.2002. [DOI] [PubMed] [Google Scholar]
- 119.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 120.Jeng JR. Carotid wall thickening, left ventricular hypertrophy, and insulin resistance in patients with hypertension. Am J Hypertens. 2002;15:422–425. doi: 10.1016/s0895-7061(02)02259-8. [DOI] [PubMed] [Google Scholar]
- 121.Evrengul H, Dursunoglu D, Kaftan A, Kilicaslan F, Tanriverdi H, Kilic M. Relation of insulin resistance and left ventricular function and structure in nondiabetic patients with essential hypertension. Acta Cardiol. 2005;60:191–198. doi: 10.2143/AC.60.2.2005031. [DOI] [PubMed] [Google Scholar]
- 122.Miyazato J, Horio T, Takishita S, Kawano Y. Fasting plasma glucose is an independent determinant of left ventricular diastolic dysfunction in nondiabetic patients with treated essential hypertension. Hypertens Res. 2002;25:403–409. doi: 10.1291/hypres.25.403. [DOI] [PubMed] [Google Scholar]
- 123.Tomiyama H, Kimura Y, Okazaki R, Kushiro T, Abe M, Kuwabara Y, et al. Close relationship of abnormal glucose tolerance with endothelial dysfunction in hypertension. Hypertension. 2000;36:245–249. doi: 10.1161/01.hyp.36.2.245. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Puig J, Ruilope LM, Luque M, Fernandez J, Ortega R, Dal Re R. Glucose metabolism in patients with essential hypertension. Am J Med. 2006;119:318–326. doi: 10.1016/j.amjmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Paternostro G, Camici PG, Lammerstma AA, Marinho N, Baliga RR, Kooner JS, et al. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest. 1996;98:2094–2099. doi: 10.1172/JCI119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:219–226. doi: 10.2174/1568008054064869. [DOI] [PubMed] [Google Scholar]
- 127.Jagasia D, Whiting JM, Concato J, Pfau S, McNulty PH. Effect of non-insulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation. 2001;103:1734–1739. doi: 10.1161/01.cir.103.13.1734. [DOI] [PubMed] [Google Scholar]
- 128.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 129.Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006 doi: 10.1016/j.febslet.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 130.Matsuzawa Y. Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 131.Kobayashi K. Adipokines: therapeutic targets for metabolic syndrome. Curr Drug Targets. 2005;6:525–529. doi: 10.2174/1389450054021972. [DOI] [PubMed] [Google Scholar]
- 132.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 133.Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, et al. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997;10:1171–1174. doi: 10.1016/s0895-7061(97)00310-5. [DOI] [PubMed] [Google Scholar]
- 134.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 135.Friedman JM. Leptin and the regulation of body weight. Harvey Lect. 1999;95:107–136. [PubMed] [Google Scholar]
- 136.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem. 2002;277:29424–29430. doi: 10.1074/jbc.M203813200. [DOI] [PubMed] [Google Scholar]
- 138.Minokoshi Y, Kahn BB. Role of AMP-activated protein kinase in leptin-induced fatty acid oxidation in muscle. Biochem Soc Trans. 2003;31:196–201. doi: 10.1042/bst0310196. [DOI] [PubMed] [Google Scholar]
- 139.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 140.Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, et al. Plasma Leptin Level Is Associated With Myocardial Wall Thickness in Hypertensive Insulin-Resistant Men. Hypertension. 1999;34:1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 141.Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA, Karmazyn M. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006;41:265–274. doi: 10.1016/j.yjmcc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 142.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 143.Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol. 2004;287:H2877–H2884. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- 144.Zeidan A, Javadov S, Karmazyn M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc Res. 2006;72:101–111. doi: 10.1016/j.cardiores.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 145.Zeidan A, Purdham DM, Rajapurohitam V, Javadov S, Chakrabarti S, Karmazyn M. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J Pharmacol Exp Ther. 2005;315:1075–1084. doi: 10.1124/jpet.105.091561. [DOI] [PubMed] [Google Scholar]
- 146.Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension. 2005;45:1031–1034. doi: 10.1161/01.HYP.0000165683.09053.02. [DOI] [PubMed] [Google Scholar]
- 147.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 148.Raju SVY, Zheng M, Schuleri KH, Phan AC, Bedja D, Saraiva RM, et al. Activation of the cardiac ciliary neurotrophic factor receptor reverses left ventricular hypertrophy in leptin-deficient and leptin-resistant obesity. PNAS. 2006;103:4222–4227. doi: 10.1073/pnas.0510460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 150.Watt MJ, Hevener A, Lancaster GI, Febbraio MA. Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of c-Jun N-terminal kinase in peripheral tissues. Endocrinology. 2006;147:2077–2085. doi: 10.1210/en.2005-1074. [DOI] [PubMed] [Google Scholar]
- 151.Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–75. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- 152.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia Is an Independent Risk Factor for Hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 153.Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 154.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 155.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 158.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. doi: 10.1093/ajcn/78.4.873S. [DOI] [PubMed] [Google Scholar]
- 159.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 161.Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens. 2003;16:973–978. doi: 10.1016/s0895-7061(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 162.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 163.Daly M. Sugars, insulin sensitivity, and the postprandial state. Am J Clin Nutr. 2003;78:865S–872S. doi: 10.1093/ajcn/78.4.865S. [DOI] [PubMed] [Google Scholar]
- 164.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A Low-Carbohydrate as Compared with a Low-Fat Diet in Severe Obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 165.Laaksonen DE, Toppinen LK, Juntunen KS, Autio K, Liukkonen KH, Poutanen KS, et al. Dietary carbohydrate modification enhances insulin secretion in persons with the metabolic syndrome. Am J Clin Nutr. 2005;82:1218–1227. doi: 10.1093/ajcn/82.6.1218. [DOI] [PubMed] [Google Scholar]
- 166.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 167.Wilson PWF, Grundy SM. The Metabolic Syndrome: A Practical Guide to Origins and Treatment: Part II. Circulation. 2003;108:1537–1540. doi: 10.1161/01.CIR.0000089506.12223.F1. [DOI] [PubMed] [Google Scholar]
- 168.Roche HM. Fatty acids and the metabolic syndrome. Proc Nutr Soc. 2005;64:23–29. doi: 10.1079/pns2004405. [DOI] [PubMed] [Google Scholar]
- 169.Kuller LH. Nutrition, lipids, and cardiovascular disease. Nutr Rev. 2006;64:S15–S26. doi: 10.1111/j.1753-4887.2006.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 170.Astrup A, Meinert LT, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- 171.Ornish D. Was Dr Atkins right? J Am Diet Assoc. 2004;104:537–542. doi: 10.1016/j.jada.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 172.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 173.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 174.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 175.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary {alpha}-Linolenic Acid Reduces Inflammatory and Lipid Cardiovascular Risk Factors in Hypercholesterolemic Men and Women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 176.Harper CR, Jacobson TA. Usefulness of omega-3 fatty acids and the prevention of coronary heart disease. Am J Cardiol. 2005;96:1521–1529. doi: 10.1016/j.amjcard.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 177.Segal-Isaacson CJ, Wylie-Rosett J. The cardiovascular effects of fish oils and omega-3 fatty acids. Heart Dis. 1999;1:149–154. [PubMed] [Google Scholar]
- 178.Albert C. Fish oil--an appetising alternative to anti-arrhythmic drugs? Lancet. 2004;363:1412–1413. doi: 10.1016/S0140-6736(04)16135-7. [DOI] [PubMed] [Google Scholar]
- 179.Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- 180.Stanley WC, Recchia FA, Okere IC. Metabolic therapies for heart disease: fish for prevention and treatment of cardiac failure? Cardiovasc Res. 2005;68:175–177. doi: 10.1016/j.cardiores.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 181.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 182.Pepe S. Effect of dietary polyunsaturated fatty acids on age-related changes in cardiac mitochondrial membranes. Exp Gerontol. 2005;40:369–376. doi: 10.1016/j.exger.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 183.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 184.Huang YJ, Fang VS, Juan CC, Chou YC, Kwok CF, Ho LT. Amelioration of insulin resistance and hypertension in a fructose-fed rat model with fish oil supplementation. Metabolism. 1997;46:1252–1258. doi: 10.1016/s0026-0495(97)90226-2. [DOI] [PubMed] [Google Scholar]
- 185.Rousseau D, Helies-Toussaint C, Moreau D, Raederstorff D, Grynberg A. Dietary n-3 PUFAs affect the blood pressure rise and cardiac impairments in a hyperinsulinemia rat model in vivo. Am J Physiol Heart Circ Physiol. 2003;285:H1294–H1302. doi: 10.1152/ajpheart.00651.2002. [DOI] [PubMed] [Google Scholar]
- 186.Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of Programmed Hyperleptinemia and Hypertension by Postnatal Dietary {omega}-3 Fatty Acids. Endocrinology. 2006;147:599–606. doi: 10.1210/en.2005-0748. [DOI] [PubMed] [Google Scholar]
- 187.Frenoux JM, Prost ED, Belleville JL, Prost JL. A Polyunsaturated Fatty Acid Diet Lowers Blood Pressure and Improves Antioxidant Status in Spontaneously Hypertensive Rats. J Nutr. 2001;131:39–45. doi: 10.1093/jn/131.1.39. [DOI] [PubMed] [Google Scholar]
- 188.Nyby MD, Matsumoto K, Yamamoto K, Abedi K, Eslami P, Hernandez G, et al. Dietary fish oil prevents vascular dysfunction and oxidative stress in hyperinsulinemic rats. Am J Hypertens. 2005;18:213–219. doi: 10.1016/j.amjhyper.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 189.Wang HH, Hung TM, Wei J, Chiang AN. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc Res. 2004;61:169–176. doi: 10.1016/j.cardiores.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 190.Takahashi R, Okumura K, Asai T, Hirai T, Murakami H, Murakami R, et al. Dietary fish oil attenuates cardiac hypertrophy in lipotoxic cardiomyopathy due to systemic carnitine deficiency. Cardiovasc Res. 2005;68:213–223. doi: 10.1016/j.cardiores.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 191.Siddiqui RA, Shaikh SR, Kovacs R, Stillwell W, Zaloga G. Inhibition of phenylephrine-induced cardiac hypertrophy by docosahexaenoic acid. J Cell Biochem. 2004;92:1141–1159. doi: 10.1002/jcb.20135. [DOI] [PubMed] [Google Scholar]
- 192.Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G, et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R486–R494. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]