Abstract
Damage to rat perirhinal cortex (PR) profoundly impairs fear conditioning to 22 kHz ultrasonic vocalizations (USVs), but has no effect on fear conditioning to continuous tones. The most obvious difference between these two sounds is that continuous tones have no internal temporal structure, whereas USVs consist of strings of discrete calls separated by temporal discontinuities. PR was hypothesized to support the fusion or integration of discontinuous auditory segments into unitary representations or “auditory objects”. This transform was suggested to be necessary for normal fear conditioning to occur. These ideas naturally assume that the effect of PR damage on auditory fear conditioning is not peculiar to 22 kHz USVs. The present study directly tested these ideas by using a different set of continuous and discontinuous auditory cues. Control and PR-damaged rats were fear-conditioned to a 53 kHz USV, a 53 kHz continuous tone, or a 53 kHz discontinuous tone. The continuous and discontinuous tones matched the 53 kHz USV in terms of duration, loudness, and principle frequency. The on/off pattern of the discontinuous tone matched the pattern of the individual calls of the 53 kHz USV. The on/off pattern of the 50 kHz USV was very different from the patterns in the 22 kHz USVs that have been comparably examined. Rats with PR damage were profoundly impaired in fear conditioning to both discontinuous cues, but they were unimpaired in conditioning to the continuous cue. The implications of this temporal discontinuity effect are explored in terms of contemporary ideas about PR function.
Keywords: medial temporal lobe, rat social signals, fear conditioning
Introduction
Perirhinal cortex (PR) plays an essential role in fear conditioning to some but not other stimuli. Several studies have shown that damage to rat PR profoundly impairs fear conditioning to contexts (Bucci, Phillips, and Burwell, 2000; Burwell, Bucci, Sanborn, and Jutras, 2004; Corodimas, and LeDoux, 1995; Kholodar-Smith, Allen, and Brown, 2008a; Kholodar-Smith, Boguszewski, and Brown, 2008b; Lindquist, Jarrard, and Brown, 2004). PR damage also profoundly impairs fear conditioning to 22 kHz ultrasonic vocalizations (USVs) recorded from conspecifics (Kholodar-Smith et al., 2008a; Lindquist et al., 2004). Rodent USVs are believed to serve as ethologically-important social signals (Borta, Wöhr, and Schwarting, 2006; Blanchard, Blanchard, Agullana, and Weiss, 1991; Brudzynski, 2005; 2007; Knutson, Burgdorf, and Panksepp, 2002; Litvin, Blanchard, and Blanchard, 2007; Panksepp, 2007). Interestingly, PR damage has no detectable effect on fear conditioning to continuous tones at ~22 kHz or at audible frequencies (Bucci et al., 2000; Campeau and Davis, 1995; Kholodar-Smith et al., 2008a; Lindquist et al., 2004; Romanski and LeDoux, 1992).
One prominent hypothesis proposes that rat PR is required for fear conditioning to “complex” stimuli (USVs and contexts) but not “simple” ones (continuous tones; see Bucci et al., 2000; Bucci, Saddoris, and Burwell, 2002; Burwell et al., 2004; Lindquist et al., 2004; Yaniv, Desmedt, Jaffard, and Richter-Levin, 2004). Instead of focusing on the concept and measurement of stimulus complexity, a different approach has been attempting to deconstruct USVs into sub-features that might control the requirement for PR function (see Allen, Furtak, and Brown, 2007; Bang, Allen, Jones, Boguszewski, and Brown, 2008; Furtak, Allen, and Brown, 2007a; Kholodar-Smith et al., 2008a; 2008b). Obvious candidates have included the principle frequency, which is centered near 22 kHz or 50 kHz; the characteristic frequency and amplitude modulations; and the frank temporal discontinuities.
The contributions of these sub-features were explored by measuring fear conditioning in PR-damaged rats and sham-operated control animals to one of three cues: a 19 kHz USV, which was pre-recorded from a conspecific; a 19 kHz continuous tone; and a 19 kHz discontinuous tone, whose on-off pattern mimicked the calls of the USV (Kholodar-Smith et al., 2008a). By convention, this USV is classified as and termed a “22 kHz USV”. The discontinuous tone matched the USV in terms of principle frequency, loudness, overall duration, and on/off pattern. However, it lacked the frequency and amplitude modulations that characterize all USVs. The continuous tone matched the frequency, loudness, and duration of the discontinuous tone, but it lacked the temporal discontinuities. PR damage caused profound and comparable impairments in fear conditioning to both discontinuous auditory cues, but had no effect on conditioning to the continuous auditory cue.
Based on these and other findings, PR was suggested to support the fusion or binding of discontinuous auditory segments into unitary representations (Kholodar-Smith et al., 2008a), which were conceived as “auditory objects” (see Cusack, Carlyon, and Robertson, 2000; Dyson and Ishfaq, 2008; Gentner and Margoliash, 2003; Griffiths and Warren, 2004; Kubovy and Van Valkenburg, 2001; Laufer and Pratt, 2003; Tian, Reser, Durham, Kustov, and Rauschecker, 2001). The creation of auditory objects was argued to be necessary for efficient fear conditioning to occur (Kholodar-Smith et al., 2008a). Related ideas have been advanced to describe the role of PR in the recognition of “non-auditory objects” (Barense, Bussey, Lee, Rogers, Davies, Saksida, Murray, and Graham, 2005; Bartko, Winters, Cowell, Saksida, and Bussey, 2007a; Buffalo, Bellgowan, and Martin, 2006; Brown and Aggleton, 2001; Brown and Eldridge, 2009; Haskins, Yonelinas, Quamme, and Ranganath, 2008; Murray and Bussey, 1999; Murray, Bussey, and Saksida, 2007; Norman and Eacott, 2004; Petrulis and Eichenbaum, 2003; Taylor, Moss, Stamatakis, and Tyler, 2006; Winters, Saksida, and Bussey, 2006; Tankiwale and Brown, in press). The latter are conventionally referred to more simply as “objects”.
The hypothesis that PR supports auditory objects assumes that the lesion-produced conditioning deficits are not peculiar to 22 kHz USVs, but extend to other natural sounds. The present study directly tested this assumption by quantifying the effect of PR damage on fear conditioning to a “50 kHz USV” whose principle frequency was 53 kHz. This stimulus differed markedly from all previous ones that have been comparably investigated. Obvious spectrotemporal differences included the principle frequency, the amplitude and frequency modulations, and the pattern of temporal discontinuity. The latter differed in terms of call durations, inter-call intervals, and overall duty cycle (for recent examples of USV spectrograms, see Allen et al., 2007; Bang et al., 2008; Burgdorf, Wood, Kroes, Moskal, and Panksepp, 2007; Furtak et al., 2007a; Kholodar-Smith et al., 2008a; Wöhr, Borta, and Schwarting, 2005).
Materials and Methods
Subjects
A total of fifty-eight male Sprague-Dawley rats were used (240 – 310 g; Charles River Laboratories, Kingston, NY). Rats were individually housed, maintained on a 12 hr light/dark cycle, and given ad libitum access to food and water. Subjects were handled for 3 to 5 days prior to surgery. Experiments were in strict compliance with Yale University’s Institutional Animal Care and Use Committee guidelines.
Surgery
The surgical methods are described elsewhere in detail (Kholodar-Smith et al., 2008a). Rats were anesthetized with a mixture of ketamine (100 mg/kg, i.p.) and xylazine (20 mg/kg, i.p.). Throughout the surgery, subjects were secured in a stereotaxic instrument. Temperature was maintained by a heating pad (37°C; Braintree Scientific, Braintree, MA). The skin was cut along the two lateral ridges and the mediolateral line above lambda. The dorsal skull and temporal muscle were exposed. Two holes were drilled on the dorsal skull and filled with stainless-steel screws (Small Parts, Inc; Miami Lakes, FL). The lateral surfaces of the skull were exposed bilaterally by pulling the temporal muscle away from the skull with a tissue spreader. PR cortex was exposed in the region near the intersection of the zygomatic arch and the temporal bone.
A micromanipulator (Sutter Instrument Company, Novato, CA) drove the tip of a 10 μL hypodermic microsyringe (26 gauge, non-coring; Hamilton Co., Reno, NV) 1–2 mm into PR at a 45° angle from the horizontal plane. Each subject received either 0.01 M phosphate-buffered 0.9% saline (PBS) or 0.34 M N-methyl-D-aspartate (NMDA; 50 mg/mL; Sigma, St. Louis, MO) injections into PR. Seven to eight injections were made bilaterally along the most of the rostro-caudal extent of PR (from 2.8 to 7.6 mm posterior to Bregma). Each injection (0.07 μL each) was made at a rate of 0.05 μL/min using a motorized infusing apparatus (Stoelting, Wood Dale, IL). When the injections were completed, the exposed tissue was covered with bone wax and the incisions were sutured. Animals were given 10% acetaminophen in their drinking water (v/v; Silarx Pharmaceuticals, Spring Valley, NY) and allowed 7 to 10 days to recover.
Conditioning and testing apparatus
Two rectangular chambers were used for conditioning and testing. A miniature infrared CCD camera (CB-21; Circuit Specialists, Mesa, AZ), mounted on the top inner surface of each chamber, was used for observing and making video recordings of behavior. Both chambers were housed in a larger sound attenuating enclosure (Med Associates Inc, St. Albans, VT). One chamber was used exclusively for conditioning and context testing. The inside dimensions were 25.4 cm wide × 29.4 cm deep × 53.3 cm high. This chamber had a standard grid floor made of stainless steel rods. The shock US was delivered to the grid by a shock generator (ENV-410, MED Associates) and grid scrambler (ENV-412, MED Associates). The floor and inner walls were sprayed with white vinegar/water (1:3) solution before the experiments. During conditioning and context testing, both the inner chamber and the experimental room were illuminated. The second chamber was used for testing the cue, after conditioning, in a “shifted” context. The inside dimensions were 25.5 cm wide × 29 cm deep × 32 cm high. The flooring was hard plastic. Prior to cue testing, the chamber was sprayed with Windex®. Both the chamber and the room were kept dark during cue testing.
Auditory Stimuli
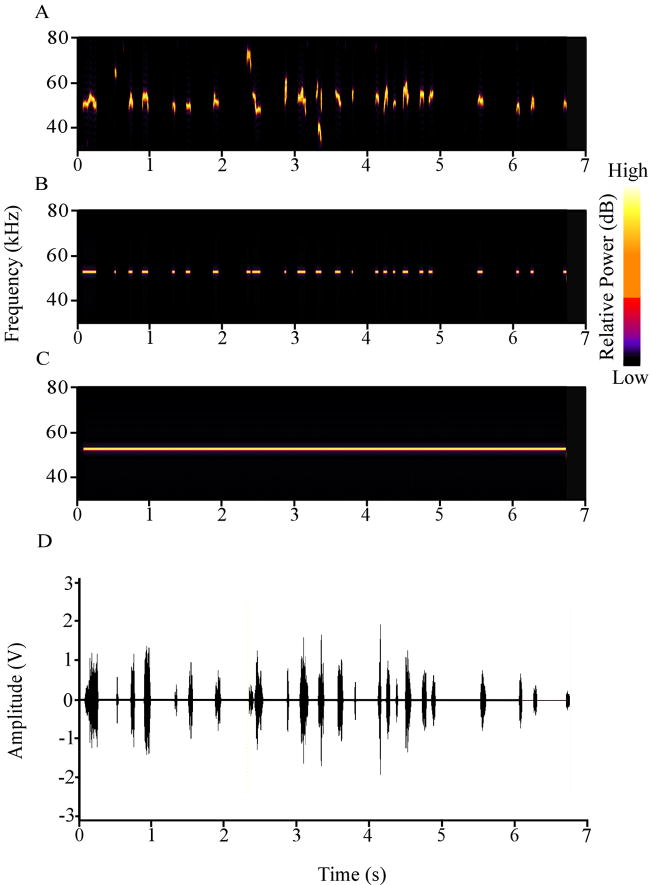
Three stimuli were used as auditory CSs: a 53 kHz USV (Fig. 1A), a 53 kHz discontinuous tone (Fig. 1B), and a 53 kHz continuous tone (Fig. 1C). The USV was recorded from experimentally naïve rats during rough-and-tumble play. The sound was transduced by a high-frequency microphone (Model 7012, ACO Pacific, Belmont, CA) and amplified by a Tucker-Davis Technologies (TDT, Gainesville, FL) MA2 preamplifier. The amplified USV was digitized at 200 kHz by an RP2.1 digital signal processor (TDT), band-pass filtered between 30 and 80 kHz, and then processed off-line using standard auditory editing software (Raven 1.1, Cornell Bioacoustics Lab; Adobe Audition 2.0, Adobe, San Jose, CA). Note that adult USVs are classified and identified based on whether the principle frequency is closer to 22 kHz or 50 kHz. The 53 kHz USV is therefore also termed a “50 kHz USV”. The other two cues were continuous and discontinuous 53 kHz tones (Figs. 1C & B, respectively), whose loudness (65 dBSLP; measured using an Ultraprobe 9000, UE Systems) and duration (6.74 s) matched the USV. The rise/fall times of the tones were 10 ms. The rise/fall times of the calls within the USV were variable and more difficult to quantify. The on-off pattern of the discontinuous tone (Fig. 1B) mimicked the on-off pattern of the individual calls that comprised the 53 kHz USV (Figs. 1A & D). Playback of the USV and tones was delivered free-field via the RP2.1 and a high-frequency electrostatic loudspeaker (ES1, TDT). A heterodyne bat detector (Mini-3, Noldus Technology), which transformed ultrasound into audible frequencies, enabled the experimenter to monitor cue presentations.
Figure 1.
Features of the three auditory cues that were used as conditional stimuli. (A) Spectrogram of the 53 kHz USV. (B) Spectrogram of the 53 kHz discontinuous tone. (C) Spectrogram of the 53 kHz continuous tone. (D) Amplitude plot of the 53 kHz USV.
Behavioral procedures
Seven to 10 days after surgery, subjects were fear-conditioned to one of the three auditory cues (Fig. 1A – C). Conditioning consisted of 10 trials in which the cue or conditional stimulus (CS) co-terminated with the unconditional stimulus (US; a 0.8 mA footshock lasting 0.4 s). The inter-trial interval (ITI) was 180 ± 60 s. One minute after the last CS-US presentation, subjects were returned to their home cages. Conditional freezing responses (CRs) to the auditory cue and the conditioning context were measured, on consecutive days, in a counterbalanced order. Auditory cue conditioning was tested in a novel context. Freezing behavior was measured in this shifted context for 2 minutes before the CS and for 6 min after the CS presentation. Context conditioning was assessed by placing the subjects in the original conditioning chamber. Freezing was measured for the first 8 min after the animal was placed into the chamber. Testing sessions were recorded for off-line video analysis of freezing.
Histology
At the end of the experiment, rats were deeply anesthetized from an overdose of sodium pentobarbital (100 mg/kg, i.p.). Transcardial perfusion with 0.01 M PBS was followed by perfusion with 4 % paraformaldehyde. The brain was removed, placed in 4 % paraformaldehyde for 24 hr, and cryoprotected in 30 % sucrose for at least 2 days. Each brain was serially-sectioned in the coronal plane (75 μm) using a freezing microtome. Next the brain sections were submerged in an antifreeze cryoprotectant, where they were stored at −20° C. Finally, the sections were stained using Nissl, myelin, or NeuN protocols, described elsewhere (see Kholodar-Smith et al., 2008a).
Lesion Reconstructions
The volume of NMDA-produced brain damage was quantified in NeuN-stained sections using a Neurolucida computer-microscope system (MicroBrightField, Williston, VT). The volume of damage was quantified (based on Paxinos and Watson, 2005; Burwell, 2001) in the following regions: PR (from −3.00 mm to −7.56 mm relative to Bregma); the caudal end of the posterior insular cortex (pIC; from −1.72 mm to −3.00 mm relative to Bregma), the amygdala (AM; from −1.72 mm to −3.36 mm relative to Bregma), the entorhinal cortex (EC; from −3.12 mm to −7.56 mm relative to Bregma), area TE of the temporal cortex (TE; from −4.56 mm to −7.56 mm relative to Bregma), and the ventral hippocampus (vHC; from −4.36 mm to −6.60 mm relative to Bregma). For comparisons among brain regions, results are reported as percentages of total volume within these restricted anterior-posterior limits.
Behavioral analysis
Freezing behavior was measured using video-analysis software (see Kholodar-Smith et al., 2008b) and defined as immobilization that lasts more than 3 seconds, excluding minor movements associated with breathing or the elicitation of USVs. A separate experiment (see Supplemental Figure) found a nearly-perfect correlation between human and machine analysis of freezing. For the cue test, the 6 min of CS presentation was further divided into three 2-min bins to see the extinction pattern. For the context test, freezing to the conditioning context was further analyzed in four 2-min bins. The results were analyzed using analysis of variance (ANOVA) and t-tests (SPSS 14.0, Chicago, IL).
The lesion effect size was calculated using Cohen’s d statistic (Cohen, 1988):
| (1) |
where the numerator is the difference in the mean percentage of freezing between the control and lesioned animals and the denominator is a pooled estimate of the standard deviation in freezing levels. Cohen’s rule of thumb (Cohen, 1988) for the size of d is that small, medium, and large effect sizes respectively correspond to d = 0.2, 0.5, and 0.8. Note that the value of d is calculated separately for each cue type.
The effect size for the interaction between surgical condition and cue type was quantified based on eta-squared (η2):
| (2) |
where the numerator is the sum of squares due to the interaction between the surgical condition (S) and type of cue (C) and the denominator is the total sum of squares. Cohen’s rule of thumb for the size of η2 is that “small”, “medium”, and “large” effect sizes correspond, respectively, to η2 = 0.01, 0.06, and 0.14 (Cohen, 1988).
Results
Histological reconstructions of neurotoxic damage
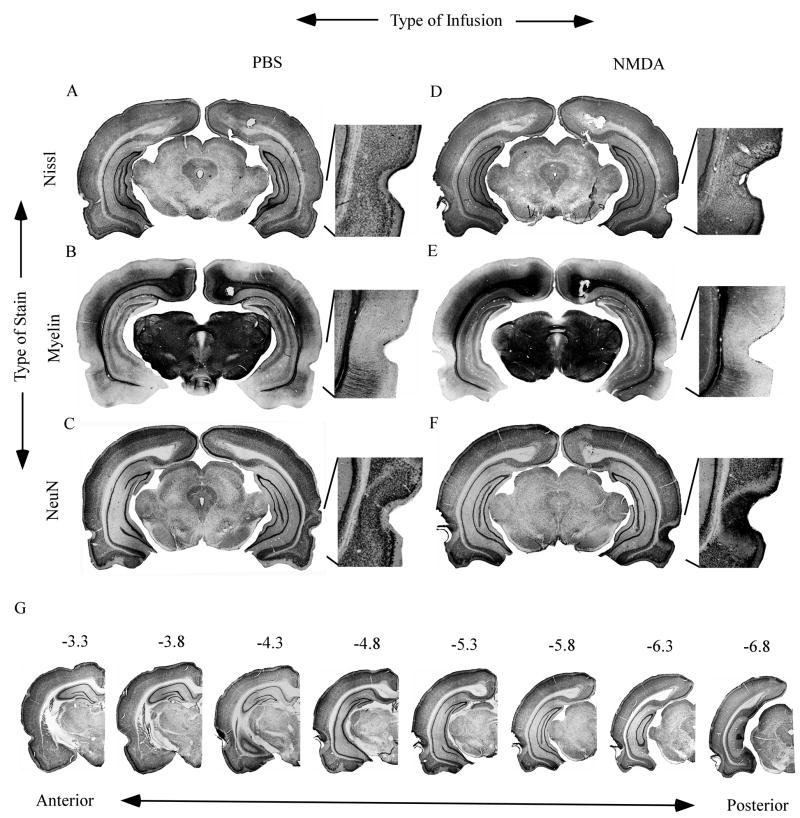
Figure 2 shows coronal sections through the brain of a sham-operated control rat that received saline infusions (Fig. 2A through 2C) and the brain of an experimental rat that received NMDA infusions (Fig. 2D through 2F). Nissl-, myelin-, and NeuN-stained sections are illustrated in the first, second, and third rows of the panel, respectively. As indicated in Fig. 2C, the needle track caused minimal PR damage in the brain of the sham-operated control rat (see enlarged image of the boxed region). In sharp contrast, the brain of the NMDA-infused rat incurred extensive and bilateral PR damage (Fig. 2D through 2F).
Figure 2.
Coronal sections from an animal in the sham-operated control group (PBS; Panels A through C) and an animal in the neurotoxic-lesioned group (NMDA; Panels D through G). Panels A and D are Nissl-stained sections; panels B and E are myelin-stained sections; and panels C and F are NeuN-stained sections. Panel G shows unilateral NeuN-stained sections taken at eight different anterior-posterior locations (from −3.3 to −6.8 mm relative to Bregma).
The percentage of damage to PR and surrounding regions was quantified (see Methods) using the NeuN-stained sections (Fig. 2F). Unlike the Nissl stain, the NeuN stain is specific for neurons. This property enables more precise quantification of the extent of neuronal damage. In Nissl-stained sections, gliosis is evidenced by lighter staining around the rhinal fissure (see enlarged images in Fig. 2A and 2D). The myelin stain (Figs. 2B and 2E) highlights the borders between PR and adjacent cortex (Brown and Furtak, 2006; Burwell, 2001; Furtak, Wei, Agster, and Burwell; 2007b) and it can reveal breaching of the external capsule. The conspicuously low myelin in PR (Figs. 2B and 2E) reflects the fact that there are relatively fewer fibers of passage. As shown in the enlarged image in Fig. 2E, the external capsule was well-preserved in the NMDA-infused brain. None of the NMDA-infused rats was excluded based on the amount or extent of PR damage. Averaging across NMDA-infused rats (n = 28), the mean percentage of damage to PR (± the standard error) on the left and right sides was, respectively, 76.8 ± 5.1 % and 75.4 ± 4.6 %. Averaged across hemispheres, the mean percentage of PR damage was 76.08 ± 4.3 %. The minor damage in the saline-infused rats (n = 30; see Fig. 2A – C) was not quantified. In a comparable study (Kholodar-Smith et al., 2008a) the mean percentage of damage to PR was < 1% in the saline-infused group.
Neuronal loss was evident throughout the anterior-posterior (AP) axis of PR (see Fig. 2G). In come cases, the damage also included the most caudal portion of the posterior insular cortex (pIC), the amygdala (AM), the entorhinal cortex (EC), the temporal cortex (TE), and the ventral hippocampus (vHC). The mean (± the standard error) bilateral damage to these neighboring regions was as follows: pIC, 18.0 ± 4.5 %; AM, 3.6 ± 1.3 %; EC, 33.5 ± 4.5 %; TE, 17.1 ± 2.2 %; and vHC, 6.0 ± 1.2 %. Analysis of variance revealed no significant differences among cue groups in the volume of damage to PR (F(2, 25) = 0.54, p > 0.05), pIC (F(2, 25) = 0.83, p > 0.05), AM (F(2, 25) = 0.23, p > 0.05), EC (F(2, 25) = 0.21, p > 0.05), TE (F(2, 25) = 0.39, p > 0.05), or vHC (F(2, 25) = 1.15, p > 0.05). Paired-samples t-test revealed no significant differences between the left and right hemispheres in the amount of damage to PR (t(27) = 0.34, p > 0.05), AM (t(27) = −0.48, p > 0.05), EC (t(27) = 0.94, p > 0.05), TE (t(27) = 1.49, p > 0.05), and vHC (t(27) = −0.89, p > 0.05; see Fig. 3). However, there was a significant difference in bilateral damage to pIC (t(27) = −2.81, p < 0.01), reflecting greater anterior damage to the right hemisphere (Fig. 3). ANOVA revealed no significant differences among cue groups in the mean differences in damage to the right and left pIC (F(2, 25) = 0.78, p > 0.05).
Figure 3.
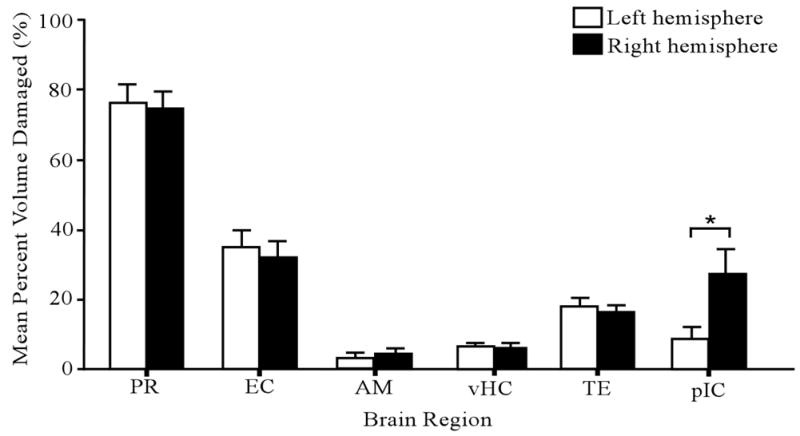
Amount of neurotoxic damage in six different brain regions. The open and filled bars represent the left and right hemispheres, respectively. The asterisk denotes a significant difference between left and right hemispheres in the mean amount of damage. Error bars represent ± 1 SE. Abbreviations: PR, perirhinal cortex; EC, entorhinal cortex; AM, amygdala; vHC, ventral hippocampus; TE, temporal cortex; and pIC; posterior insular cortex.
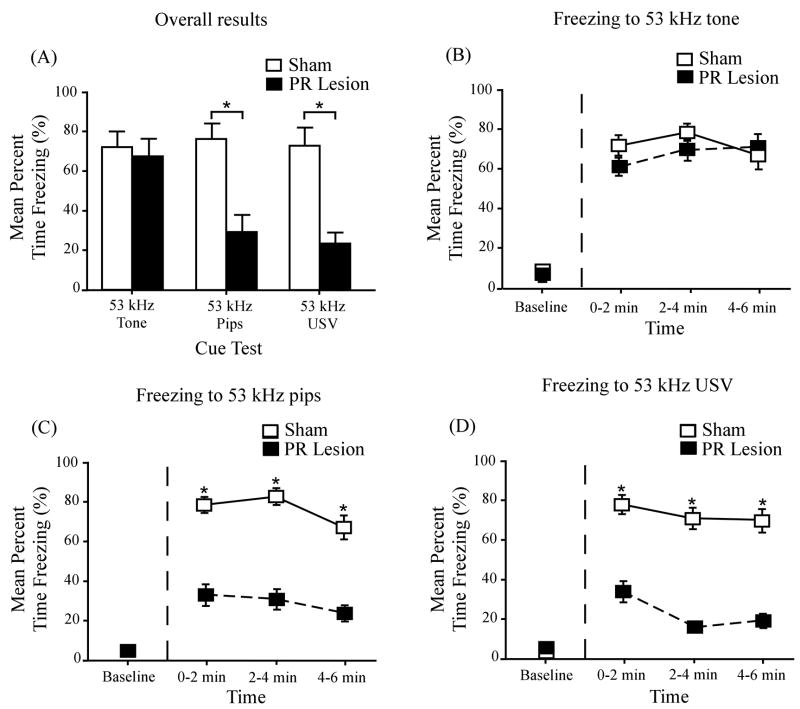
PR lesion effects on cue conditioning
Figure 4A shows CS-elicited conditional freezing in sham-operated control animals (open bars) and NMDA-infused animals (filled bars). The asterisks denote statistically significant differences (p < 0.05) between the sham and lesioned animals. ANOVA revealed a significant main effect of cue type (F(2, 52) = 4.14, p < 0.05) and surgical condition (F(1, 52) = 25.70, p < 0.001) as well as a significant interaction between cue type and surgical condition (F(2, 52) = 4.35, p < 0.05; η2 = 0.09). Subsequent t tests revealed significant differences between the sham and lesioned groups in the amount of freezing to the 53 kHz USV (t(18) = 4.73, p < 0.001) and the 53 kHz discontinuous tone (t(17) = 4.11, p < 0.05), but not to the 53 kHz continuous tone (t(17) = 0.42, p > 0.05).
Figure 4.
Effects of perirhinal lesions on conditional freezing in response to each of the three auditory cues. (A) Mean percent freezing to the 53 kHz tone, the 53 kHz discontinuous tone (tone pips), and the 53 kHz USV during cue testing in a shifted context. The open and filled bars represent, respectively, mean freezing levels in the sham-operated control group and the neurotoxic-lesioned group. Asterisks denote significant group differences. (B) Time course of freezing, in response to the continuous tone, in the sham-operated control group (open squares) and the lesioned group (filled squares). There were no significant group differences before or during the presentation of the cue. (C) Time course of freezing, in response to the discontinuous tone (pips), in the sham-operated control group (open squares) and the lesioned group (filled squares). There were significant group differences in all three time periods after the cue presentation, but no significant differences during the baseline period. (D) Time course of freezing, in response to the USV, in the sham-operated group (open squares) and the lesioned group (filled squares). There were significant differences in all three time periods after the cue presentation, but no significant differences during the baseline period. Error bars represent ± 1 SE.
Parts B – D of Figure 4 show the time course of freezing in the three cue groups. In all three groups, the level of freezing was negligible during the two-min baseline period (to the left of the vertical dashed line). In response to the 53 kHz continuous tone, both the control and lesioned animals displayed robust and comparable levels of freezing throughout the 6-min CS presentation (Fig. 4B). By contrast, in response to the 53 kHz discontinuous tone (pips), the lesioned animals showed significantly less freezing than the sham-operated animals in all three 2-min bins: first bin, t(17) = 3.61, p < 0.01; second bin, t(17) = 4.32, p < 0.01; and third bin, t(17) = 3.27, p < 0.01. Similarly, in all three time bins, the amount of freezing to the 53 kHz USV (see Fig. 4D) was significantly lower in the lesioned group than in the control group: first bin, t(18) = 3.42, p < 0.01; second bin, t(18) = 4.92, p < 0.01; and third bin, t(18) = 3.88, p < 0.01.
Lesion effect sizes on conditional freezing (from Eq. 1) were as follows: 53 kHz USV, d = 2.12; 53 kHz pips, d = 1.90; and 53 kHz tone, d = 0.19. In summary, PR damage profoundly impaired conditioning to both of the discontinuous cues but had no effect on conditioning to the continuous cue. In a related study (Kholodar-Smith et al., 2008a), PR lesion effect sizes on delay cue conditioning were as follows: 22 kH USV, d = 1.4; 22 kHz pips, d = 1.7; and 22 kHz tone, d = 0.1). The mean percentage of PR damage (60%) was smaller in that study than in the present one (76%).
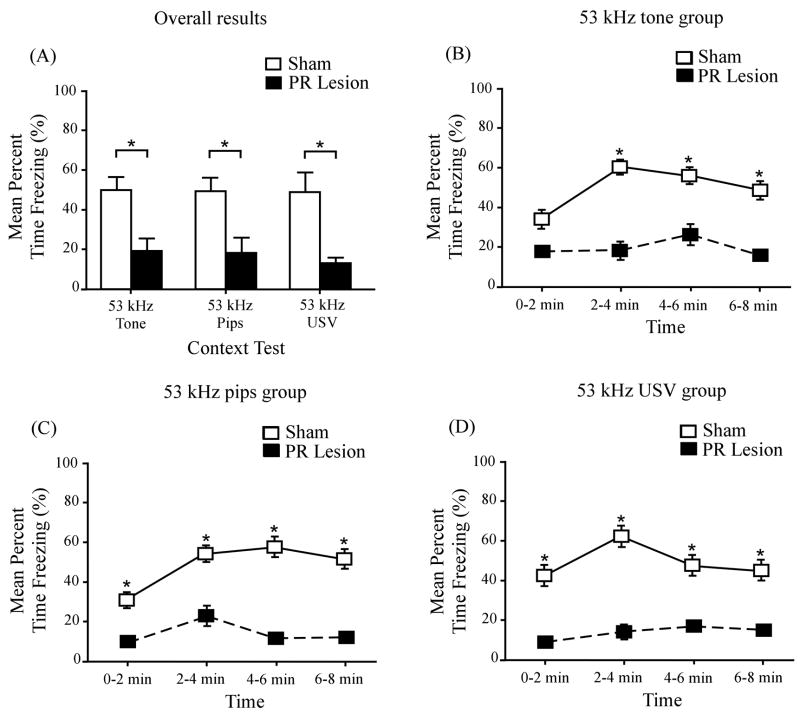
PR lesion effects on context conditioning
Figure 5A depicts contextual freezing in sham-operated control animals (open bars) and NMDA-infused animals (filled bars). As indicated, PR damage profoundly depressed freezing to the training context in all three cue groups. Asterisks identify significant differences between the sham and lesioned groups. ANOVA revealed a significant main effect of surgery (F(1, 52) = 38.11, p < 0.01), indicating a significant PR-lesion effect on context conditioning. There was no significant effect of the type of cue (F(2, 52) = 0.19, p > 0.05) and no significant interaction between the surgical condition and type of cue (F(2, 52) = 0.84, p > 0.05).
Figure 5.
Effects of PR lesions on conditional freezing to the training context. (A) Mean percent freezing to the training context in all three cue groups. Freezing was significantly depressed in the lesioned group (filled bars) relative to the sham-operated control group (open bars). Group differences in mean freezing levels are indicated by an asterisk. (B) Time course of freezing to the conditioning context among animals that were conditioned to the continuous tone. There were significant differences between the lesioned animals (filled squares) and control animals (open squares) in three of the four 2-min time periods. (C) Time course of freezing to the conditioning context among animals that were conditioned to the discontinuous tone. There were significant differences between the lesioned animals (filled squares) and control animals (open squares) in all four time periods. (D) Time course of freezing to the conditioning context among animals that were conditioning to the USV. There were significant differences between the lesioned animals (filled squares) and control animals (open squares) in all four time periods. Error bars represent ± 1 SE.
Parts B – D of Figure 5 show the time course of context-elicited freezing in all three cue groups. In the control group (open squares), freezing levels always peaked after the first time bin (0 – 2 min). At every time point, freezing was significantly lower in the lesioned group (filled squares). Significant group differences are denoted by asterisks. The lesion effect sizes (Eq. 1) on context conditioning were as follows: 53 kHz tone group, d = 1.54; 53 kHz discontinuous tone group, d = 1.34; and 53 kHz USV group, d = 1.58. Pooling the cue groups, d̄ = 1.5. A similar study (Kholodar-Smith et al, 2008a) recently reported d̄ = 1.8.
Discussion
Brief summary
The present study examined the effect of PR damage on fear conditioning to a 53 kHz USV, a 53 kHz continuous tone, and a 53 kHz discontinuous tone (Fig. 1). Using a lateral surgical approach, PR damage was produced through multiple injections of NMDA along the length of PR (Fig. 2). Subsequent 3D histological reconstructions revealed well-localized damage along the full anterior-posterior extent of PR (Figs. 2 – 3). PR damage profoundly impaired fear conditioning to the 53 kHz USV and the matched 53 kHz tone pips (d > 1.8; Fig. 4), but had no significant effect on conditioning to the 53 kHz continuous tone (Fig. 4). PR damage also profoundly impaired context conditioning (d > 1.3 across cue groups; Fig. 5), as anticipated from several previous studies (Bucci et al., 2000; Corodimas and LeDoux, 1995; Kholodar-Smith et al., 2008a; 2008b; Lindquist et al., 2004). These and previous results are discussed in the context of contemporary ideas and established facts that are central to understanding the role of PR in fear conditioning.
Evaluation of the lateral surgical approach
The lateral surgical approach to PR was first used to make aspirative lesions (Lindquist et al., 2004) and later extended to include neurotoxic lesions (Kholodar-Smith et al., 2008a; 2008b). Massive destruction of PR was a primary surgical goal. Burwell and coworkers have suggested that the effects of PR damage critically depend on the amount of PR damage (Burwell et al., 2004). In using the lateral approach, PR is directly visualized during the surgical procedure. In the present study, 76% of PR was damaged (see Figs. 2 and 3). An equally-important surgical goal was to spare AM, since damage to this region is well-known to cause a general impairment in fear conditioning (for a review, see Fanselow and Gale, 2003; LeDoux, 2003; Sigurdsson, Doyère, Cain, and LeDoux, 2007). In fact, only 3.6% of AM incurred damage (see Fig. 3).
Thus the lateral surgical approach was remarkably successful in terms of the two most important goals. However, there was substantial damage to structures of lesser interest to this study. The greatest unintended damage was to EC (33 %). Currently, there is no evidence that pre-training EC damage can impair fear conditioning to either continuous or discontinuous tones. In one previous study, 60% damage to PR was accompanied by 18% damage to EC (Kholodar-Smith., 2008a). Another study reported 62 % damage to PR and 18 % damage to EC (Kholodar-Smith et al., 2008b). If minimizing EC damage is a goal, then smaller NMDA injections are recommended. The second greatest unintended damage was to caudal pIC (18%; see Fig. 3). There is no evidence that pre-training lesions of pIC can produce the pattern of deficits reported here and elsewhere (Kholodar-Smith et al., 2008a). Damage to pIC can be minimized, if desired, by eliminating the most anterior PR injections.
Four essential sets of facts about delay fear conditioning to auditory cues
Four sets of facts form the basis of our current understanding of the role of PR in auditory fear conditioning in delay paradigms. First, PR damage has no detectable effect on conditioning to continuous tones. This generalization is based on studies of continuous tone cues at 0.8 kHz (Romanski and LeDoux, 1992), 2 kHz (Campeau and Davis, 1995), 4 kHz (Lindquist et al., 2004), 10 kHz (Bucci et al., 2000), 22 kHz (Kholodar-Smith et al., 2008a; Lindquist et al., 2004), and 53 kHz (Fig. 4).
Second, PR damage profoundly impairs fear conditioning to both 22 kHz USVs and 50 kHz USVs (Lindquist et al., 2004; Kholodar-Smith et al., 2008a; Fig. 4). These two types of vocalizations differ markedly from each other in terms of spectrotemporal structure (also see Bang et al, 2008; Brudzynski, 2005; 2007). More specifically, they differ in terms of principle frequency, frequency modulations, amplitude modulations, and the overall pattern of temporal discontinuity.
Third, the use of deconstructed USVs as cues revealed that temporal discontinuity is the essential auditory sub-feature that determines the effect of PR damage (Kholodar-Smith et al., 2008a; Figs 1 and 4). By contrast, neither the principle frequency of a cue nor its intrinsic modulations predict the effect of PR damage. Elsewhere we have shown that rats fail to discriminate between USVs and matched pips in a differential delay fear-conditioning paradigm (Bang et al., 2008). Notably, the presence of temporal discontinuities is not a unique feature of USVs. Instead, discontinuities are characteristic of natural sounds. The discovery of this temporal discontinuity effect illustrates how the use of “arbitrary” instead of “ecological” stimuli (Domjan, Cusato, and Krause, 2004) can fail to reveal normal brain function.
Fourth, the lesion-produced deficits in fear conditioning to the two types of USVs appear to be unrelated to the affective states that give rise to their production. We have now shown that PR damage profoundly impairs conditioning to both 22 kHz USVs (Kholodar-Smith et al., 2008a; Lindquist et al., 2004) and 50 USVs (Fig. 4). The latter are emitted in association with presumptively positive affective states. They occur during sexual behavior, rough and tumble play, tickling, and the anticipation of food, play, or rewarding drugs (Brudzynski, 2005; 2007; Knutson et al., 2002). On the other hand, 22 kHz USVs are produced in association with presumptively negative affective states. They occur during confrontation by a predator, defeat by a conspecific, or the presence of other threatening or aversive stimuli (Blanchard et al., 1991; Brudzynski, 2005; 2007; Knutson et al., 2002; Litvin et al., 2007; but see Choi and Brown, 2003). Among singly-caged Sprague-Dawley rats, no differences have been detected, among USVs and continuous tones, in either unconditional or conditional cue-elicited freezing (Bang et al., 2008; Tankhiwale, Bang, Allen, Boguszewski, and Brown, 2008). Freezing in response to all three types of auditory cues depends on experience.
Based on these four sets of facts, we suggest that temporal discontinuity is an essential variable that determines the requirement for PR function in auditory fear conditioning. The discovery of this temporal discontinuity effect has changed how we think about the role of PR in auditory fear conditioning, as discussed below.
Temporal discontinuity, auditory objects, and perceptual learning
Our interpretation of the temporal discontinuity effect is that PR plays a critical role in integrating or unitizing sound segments into larger entities, which we have termed auditory objects. One way to conceptualize an auditory object, in the present context, is to consider a different representation of the discontinuous cues—one that does not depend on stimulus unitization. In the absence of stimulus unitization, each of the individual sound segments could theoretically be processed and conditioned as 24 statistically-independent and temporally-unrelated events.
In this case, the conditional probability of the US, given that an auditory segment occurred, would be close to zero (p = 0.042). Based on what is known about CS-US contingency effects (Rescorla, 1968; 2000), one would expect individual sound segments to be ineffective in supporting fear conditioning because they convey little predictive information regarding the occurrence of the US. Furthermore, recent empirical evidence suggests that single calls cannot support delay fear conditioning (Kholodar-Smith et al., 2008a), possibly because of the short interval between the CS and US onsets. In stark contrast to this “elementistic” form of stimulus representation, if the whole USV is treated as a single entity, then the conditional probability of the US, given that the USV occurred, is unity (p = 1.000).
Stimulus unitization thus enables learning about causal relationships and other statistical regularities that would otherwise go undetected. Temporal integration of successive auditory stimulus segments is recognized to be an essential feature of auditory perception (see Rosen, 1992; Sussman, 2005; Sussman, Winkler, Ritter, Alho, and Näätänen, 1999; Wang, 2000; Zaehle, Wüstenberg, Meyer, and Jäncke, 2004). Our suggestion that rat PR plays a special role in auditory perception accords well with the more general contention that PR supports perceptual aspects of learning and memory (Bartko, Winters, Cowell, Saksida, and Bussey, 2007b; Buckley and Gaffan, 2006; Bussey, Saksida, and Murray, 2002; 2003; 2005; Murray and Bussey, 1999; Murray, Bussey, and Saksida, 2007; Murray, Graham, and Gaffan, 2005). Several different measures of object recognition memory have been shown to depend on PR function (reviewed in Tankhiwale and Brown, in press).
General theory of the role of perirhinal cortex in acquired fear
The present study focused primarily on the dramatic effect of auditory discontinuity on the requirement for PR function (Fig. 4). We also noted that PR damage profoundly impaired context conditioning (Fig. 5), a finding that has been repeatedly reported (Bucci et al., 2000; Corodimas and LeDoux, 1995; Kholodar-Smith et al., 2008a; 2008b; Lindquist et al., 2004). Both of these deficits could reflect the loss of a common PR function; one that might be loosely described in terms of unitization of the conditional stimulus. However, there is a third major effect of neurotoxic PR damage, which has not been mentioned thus far, that cannot be comparably explained. The third effect is a profound impairment in trace fear conditioning that does not depend on the temporal structure of the cue (Kholodar-Smith et al., 2008b). Before attempting to create a general theory of the role of PR in acquired fear, we need to know whether or how these three major deficits might be isolated from one another.
Supplementary Material
Acknowledgments
This research was supported by NIH grant MH58405 and Yale University. We thank Dianna Kholodar-Smith for useful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TA, Furtak SC, Brown TH. Single-unit responses to 22 kHz ultrasonic vocalizations in rat perirhinal cortex. Behavioural Brain Research. 2007;182(2):327–336. doi: 10.1016/j.bbr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Allen TA, Jones LK, Boguszewski P, Brown TH. Asymmetrical stimulus generalization following differential fear conditioning. Neurobiology of Learning and Memory. 2008;90(1):200–216. doi: 10.1016/j.nlm.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee ACH, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. Journal of Neuroscience. 2005;25(44):10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learning & Memory. 2007a;14(12):821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. Journal of Neuroscience. 2007b;27(10):2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiology & Behavior. 1991;50(5):967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Borta A, Wöhr M, Schwarting RKW. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behavioural Brain Research. 2006;166(2):271–280. doi: 10.1016/j.bbr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Eldridge M. Perirhinal Cortex: Neural Representations. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 7. Oxford: Academic Press; 2009. pp. 565–577. [Google Scholar]
- Brown TH, Furtak SC. Low myelin staining in rat perirhinal cortex and parts of the amygdala. Society for Neuroscience Abstracts. 2006;32 Program no. 638.17. [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior Genetics. 2005;35(1):85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine-dopamine interaction and acoustic coding. Behavioural Brain Research. 2007;182(2):261–273. doi: 10.1016/j.bbr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phllips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behavavioral Neuroscience. 2002;116(3):479–488. [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortical contributions to object perception. Trends in cognitive sciences. 2006;10(3):100–107. doi: 10.1016/j.tics.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & Memory. 2006;13(5):638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behavioural Brain Research. 2007;182(2):274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. Journal of Comparative Neurology. 2001;437(1):17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. Journal of Neuroscience. 2004;24(49):11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The role of perirhinal cortex in memory and perception: conjunctive representations for object identification. In: Witter MP, Wouterlood FG, editors. The Parahippocampal Region: Organization and Role in Cognitive Functions. Oxford University Press; Oxford: 2002. pp. 239–254. [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. European Journal of Neuroscience. 2003;17(3):649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray FA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Quarterly journal of experimental psychology B, Comparative and physiological psychology. 2005;58(3–4):269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Brown TH. Central amygdale lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. Journal of Neuroscience. 2003;23(25):8713–8721. doi: 10.1523/JNEUROSCI.23-25-08713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Corodimas KP, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behavioral Neuroscience. 1995;109(4):613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- Cusack R, Carlyon RP, Robertson IH. Neglect between but not within auditory objects. Journal of Cognitive Neuroscience. 2000;12(6):1056–1065. doi: 10.1162/089892900563867. [DOI] [PubMed] [Google Scholar]
- Domjan M, Cusato B, Krause M. Learning with arbitrary versus ecological conditioned stimuli: Evidence from sexual conditioning. Psychonomic Bulletin & Review. 2004;11(2):232–246. doi: 10.3758/bf03196565. [DOI] [PubMed] [Google Scholar]
- Dyson BJ, Ishfaq F. Auditory memory can be object based. Psychonomic Bulletin & Review. 2008;15(2):409–412. doi: 10.3758/pbr.15.2.409. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Allen TA, Brown TH. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. Journal of Neuroscience. 2007a;27(45):12277–12291. doi: 10.1523/JNEUROSCI.1653-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007b;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424(6949):669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. What is an auditory object? Nature Reviews Neuroscience. 2004;5(11):887–892. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59(4):554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Allen TA, Brown TH. Fear Conditioning to Discontinuous Auditory Cues Requires Perirhinal Cortical Function. Behavioral Neuroscience. 2008a;122(5):1178–1185. doi: 10.1037/a0012902. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Boguszewski P, Brown TH. Auditory trace fear conditioning requires perirhinal cortex. Neurobiology of Learning and Memory. 2008b;90(3):537–543. doi: 10.1016/j.nlm.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychological Bulletin. 2002;128(6):961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kubovy M, Van Valkenburg D. Auditory and visual objects. Cognition. 2001;80(1–2):97–126. doi: 10.1016/s0010-0277(00)00155-4. [DOI] [PubMed] [Google Scholar]
- Laufer I, Pratt H. The electrophysiological net response (‘F-complex’) to spatial fusion of speech elements forming an auditory object. Clinical Neurophysiology. 2003;114(5):818–834. doi: 10.1016/s1388-2457(03)00029-4. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist DH, Jarrard LE, Brown TH. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. Journal of Neuroscience. 2004;24(14):3610–3617. doi: 10.1523/JNEUROSCI.4839-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Blanchard RJ. Rat 22 kHz ultrasonic vocalizations as alarm cries. Behavioural Brain Research. 2007;182(2):166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annual review of neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Murray EA, Graham KS, Gaffan D. Perirhinal cortex and its neighbours in the medial temporal lobe: contributions to memory and perception. Quarterly journal of experimental psychology B, Comparative and physiological psychology. 2005;58(3–4):378–396. doi: 10.1080/02724990544000077. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural brain research. 2004;148(1–2):79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroevolutionary sources of laughter and social joy: Modeling primal human laughter in laboratory rats. Behavioural Brain Research. 2007;182(2):231–244. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates — the new coronal set. 5. Academic Press; Sydney: 2005. [Google Scholar]
- Petrulis A, Eichenbaum H. The perirhinal-entorhinal cortex, but not the hippocampus, is critical for expression of individual recognition in the context of the Coolidge effect. Neuroscience. 2003;122(3):599–607. doi: 10.1016/j.neuroscience.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of Comparative and Physiological Psychology. 1968;66(1):1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Associative changes with a random CS-US relationship. Quarterly Journal of Experimental Psychology: B Comparative and Physiological Psychology. 2000;53(4):325–340. doi: 10.1080/713932736. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Bilateral destruction of neocortical and perirhinal projection targets of the acoustic thalamus does not disrupt auditory fear conditioning. Neuroscience Letters. 1992;142(2):228–232. doi: 10.1016/0304-3940(92)90379-l. [DOI] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1992;336(1278):367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neurophmacology. 2007;52(1):215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Sussman ES. Integration and segregation in auditory scene analysis. Journal of the Acoustical Society of America. 2005;117:1285–1298. doi: 10.1121/1.1854312. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Ritter W, Alho K, Näätänen R. Temporal integration of auditory stimulus deviance as reflected by the mismatch negativity. Neuroscience Letters. 1999;264(1–3):161–164. doi: 10.1016/s0304-3940(99)00214-1. [DOI] [PubMed] [Google Scholar]
- Tankhiwale AA, Brown TH. Temporal lobe and object recognition. In: Koob G, Thompson RF, Michel Le Moal M, editors. Encyclopedia of Behavioral Neuroscience. Oxford University Press; Oxford: (in press) [Google Scholar]
- Tankhiwale AA, Bang S, Allen TA, Boguszewski P, Brown TH. Role of experience in eliciting fear responses to rat auditory social signals. Society for Neuroscience Abstracts. 2008:34. Program no. 93.4. [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292(5515):290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Wang X. On cortical coding of vocal communication sounds in primates. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):11843–11849. doi: 10.1073/pnas.97.22.11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Paradoxial facilitation of object recognition memory after infusion of scopolamine into perirhinal cortex: implications for cholinergic system function. Journal of Neuroscience. 2006;26(37):9520–9529. doi: 10.1523/JNEUROSCI.2319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Borta A, Schwarting RKW. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: a dose-response study in the rat. Neurobiology of Learning and Memory. 2005;84(3):228–240. doi: 10.1016/j.nlm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Yaniv D, Desmedt A, Jaffard R, Richter-Levin G. The amygdala and appraisal processes: stimulus and response complexity as an organizing factor. Brain Research Brain Research Reviews. 2004;44(2–3):179–186. doi: 10.1016/j.brainresrev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Wüstenberg T, Meyer M, Jäncke L. Evidence for rapid auditory perception as the foundation of speech processing: a sparse temporal sampling fMRI study. European Journal of Neuroscience. 2004;20(9):2447–2456. doi: 10.1111/j.1460-9568.2004.03687.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.