Abstract
A heightened propensity for risk-taking and poor decision-making underlies the peak morbidity and mortality rates reported during adolescence. Delayed maturation of cortical structures during the adolescent years has been proposed as a possible explanation for this observation. Here, we test the hypothesis of adolescent delayed maturation by using fMRI during a monetary decision-making task that directly examines risk-taking behavior during choice selection. Orbitofrontal/ventrolateral prefrontal cortex (OFC/VLPFC) and dorsal anterior cingulate cortex (ACC) were examined selectively since both have been implicated in reward-related processes, cognitive control, and resolution of conflicting decisions. Group comparisons revealed greater activation in the OFC/VLPFC (BA 47) and dorsal ACC (BA 32) in adults than adolescents when making risky selections. Furthermore, reduced activity in these areas correlated with greater risk-taking performance in adolescents and in the combined group. Consistent with predictions, these results suggest that adolescents engage prefrontal regulatory structures to a lesser extent than adults when making risky economic choices.
Keywords: reward, decision-making, cognitive control, affective regulation, conflict monitoring
Introduction
Adolescence is a major transition period in life, a time when children undergo the physical, psychological, and social changes needed to become adult members of society. Accompanying this period of rapid and necessary change, however, is a heightened propensity for risk-taking, impulsivity, and reckless behavior (Arnett, 1992). Adolescents tend to perceive risks as smaller and more controllable than adults (Benthin, Slovic, Severson, 1993), and they are less adept at setting goals and evaluating their decisions (Byrnes, 2002). Indeed, in a time of generally optimal health, these poor decision-making tendencies confer a high level of morbidity and mortality (Grunbaum, Kann, Kinchen, Ross, Hawkins, Lowry, Harris, McManus, Chyen, Collins, 2004), and may leave adolescents more vulnerable to gambling (Chambers, Potenza, 2003), addiction (Chambers, Taylor, Potenza, 2003), and a number of other psychopathologies (Steinberg, Dahl, Keating, Kupfer, Masten, Pine, 2005).
Parallel to these fundamental behavioral changes, cortical structures also undergo widespread refinement and maturation during the adolescent years (see review, Spear, 2000; Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent, III, Herman, Clasen, Toga, Rapoport, Thompson, 2004). Anatomical neuroimaging studies have shown that the prefrontal areas are some of the last brain regions to mature (Casey, Giedd, Thomas, 2000; Luna, Sweeney, 2004; Giedd, 2004), and that processes such as synaptic pruning, elaboration of dendritic arborization, and increased myelination continue throughout adolescence (see review, Luna, Sweeney, 2001). This is especially true in brain areas associated with higher-order cognition and emotional regulation. For instance, Segalowitz and Davies (2004) showed that error-related potentials continue to develop in the anterior cingulate cortex (ACC) and orbital/ventrolateral prefrontal cortex (OFC/VLPFC) throughout the second decade of life. Additionally, improved performance on tasks of memory and attention (see review, Casey et al., 2000), as well as decision-making (Overman, Frassrand, Ansel, Trawalter, Bies, Redmond, 2004; Ernst, Grant, London, Contoreggi, Kimes, Spurgeon, 2003; Crone, Bunge, Latenstein, van der Molen, 2005; Hooper, Luciana, Conklin, Yarger, 2004) lends indirect support for continued prefrontal maturation during adolescence.
The relationship between improved cognitive performance and age-related changes in patterns of regional brain activation has been examined in a number of studies. Although some studies have revealed weaker neural activation in adults than in children (e.g., Durston et al., 2002), most have reported greater regional activation associated with increasing age (Schapiro, Schmithorst, Wilke, Byars, Strawsburg, Holland, 2004; Marsh, Zhu, Schultz, Quackenbush, Royal, Skudlarski, Peterson, 2006; Rubia, Overmeyer, Taylor, Brammer, Williams, Simmons, Andrew, Bullmore, 2000). For example, Schapiro and colleagues (2004) found positive correlations of activation in motor cortex and language areas with age during motor and language tasks in a sample of 332 healthy individuals aged 5 to 19 years. Similarly, Marsh et al. (2006) compared 20 children (mean age 13.5 years) with 50 adults (mean age 31.9 years), and reported greater activation in adults than in children in brain areas typically activated by the Stroop task (right frontostriatal circuits) and known to subserve self-regulatory function. Finally, using tasks tapping inhibitory processes, Rubia et al. (2000) reported increased prefrontal activation with age (9 adolescents aged 12-19 years compared to 8 adults aged 22-40 years). Taken together, these findings suggest that improved cognitive function is associated with the facilitated recruitment of brain areas and coming on-line of mature neural circuits, which would be reflected as enhanced activation. Additional contributing factors could be an age-related difference in the type of strategies used to perform the tasks, and in learning processes, which depend on both levels of neural maturation and opportunity for practice (i.e., different degrees of expertise).
Recently, knowledge about behavioral changes and the concurrent refinement of cortical structures have been integrated in neuropsychological theories for why adolescents demonstrate poor decision-making. Briefly, a lag in the maturation of cortical regions—particularly those regions involved in the representation of reward values and the control of behavior—may bias adolescents towards taking risks (Ernst, Pine, Hardin, 2006; Steinberg, 2004; Spear, 2000). More specifically, the dorsal ACC and the OFC/VLPFC emerge as the most likely sites for such a lag in control function, based on their consistent and frequently concurrent recruitment during tasks of cognitive flexibility and inhibition, such as go-no go and reversal tasks (Nagahama, Okada, Katsumi, Hayashi, Yamauchi, Oyanagi, Konishi, Fukuyama, Shibasaki, 2001; Casey, Forman, Franzen, Berkowitz, Braver, Nystrom, Thomas, Noll, 2001; Cools, Clark, Owen, Robbins, 2002; O'Doherty, Critchley, Deichmann, Dolan, 2003).
So far, five neuroimaging studies have examined the neural correlates of reward-related processing in adolescence (May, Delgado, Dahl, Stenger, Ryan, Fiez, Carter, 2004; Bjork, Knutson, Fong, Caggiano, Bennett, Hommer, 2004; Ernst, Nelson, Jazbec, McClure, Monk, Leibenluft, Blair, Pine, 2005; Galvan, Hare, Parra, Penn, Voss, Glover and casey, 2006; van Leijenhorst, Crone, Bunge, 2006). Four of these works directly compared adults with youth on various stages of decision-making: selection of options (van Leijenhorst et al., 2006), anticipation of feedback (Bjork et al., 2004), response to outcomes (Ernst et al., 2005), or combined anticipation and response to outcomes (Galvan et al., 2006). Bjork et al. (2004) found that adolescents, relative to adults, had reduced striatal activation in anticipation of high reward when executing a reaction time task. Both Galvan et al. (2006) and Ernst et al. (2005) reported enhanced striatal activation when receiving a reward. In addition, Galvan et al. (2006) showed reduced lateral orbitofrontal activation in adolescents relative to adults. The most recent study (van Leijenhorst et al., 2006) revealed greater activation of the ACC and no differences in medial orbitofrontal cortical activation in children (9-12 years) compared to adults (18-26 years) during the selection of one of two options, based on probability of outcome. Here, we focus (1) on the selection stage in the context of varying probability and magnitude of potential monetary gains, and (2) on the role of ACC and OFC/VLPFC cortex in this stage.
We used rapid event-related fMRI to examine OFC/VLPFC and ACC during performance of a monetary, two-choice decision-making task with varying levels of risk and rewards (Ernst, Nelson, McClure, Monk, Eshel, Zarahn, Leibenluft, Zametkin, Towbin, Charney, Pine, 2004). Performance on this task has been shown to be sensitive to psychopathology, such as children and adolescent with bipolar disorders and history of trauma (Ernst et al, 2004; Guyer et al., in press). In addition, unpublished data collected in a clinic environment, outside the scanner, revealed significantly more risk-taking in psychiatrically healthy adolescents (n=19, mean age 13.5 years) than in psychiatrically healthy adults (n=11, mean age 29.7 years), F(1,28)=46.7, P=0.02. Based on previous neuroimaging literature with this (Ernst et al., 2004) and similar tasks (see review, Krawczyk, 2002), we hypothesized the recruitment, in both adults and adolescents, of regions involved in representing reward values and object/motor features (OFC/VLPFC) and monitoring conflict and error (ACC), as well as visual processing (occipito-parietal cortex), motor preparation (supplementary motor area and premotor cortex), and executive control (prefrontal cortex). More specifically, given the theory that regulatory prefrontal structures continue to develop throughout adolescence and exert more control over behavior with increasing age (Ernst et al., 2006), we predicted that the OFC/VLPFC and ACC would become more active in adults, and that this enhanced activation with age would correlate with reduced risk-taking behavior.
Methods
Sample
Eighteen healthy adolescents and 16 healthy adults participated in the study. This sample is also described in a previous work (Ernst et al. 2005), which examined developmental differences in the processing of feedback, in contrast to the selection stage presented here. Data from four subjects (2 adolescents and 2 adults) were excluded from the analysis because of excessive head movement (greater than 2 mm in any one direction). Subjects were recruited through newspaper advertisements and were financially compensated for their participation. Inclusion criteria were right-handedness, age between 9 and 17 years for adolescents and 20 and 40 years for adults, absence of past and present psychiatric disorders on the basis of a psychiatric diagnostic interview [Schedule for Affective Disorders and Schizophrenia of School-Age Children-Present and Lifetime version for the adolescents (Kaufman, Birmaher, Brent, Ryan, Rao, 2000; Spitzer, Williams, Gibbon, First, 1992), and Structured Clinical Interview for DSM-IV for adults (Segal, Hersen, Van Hasselt, 1994)], and absence of acute or chronic medical illnesses on the basis of medical history and physical examination. All adult participants and parents of adolescent participants signed a consent form after having been explained the study in detail. All adolescents signed an assent form. The study was approved by the NIMH Institutional Review Board.
Task
The Wheel of Fortune (WOF) task is a computerized two-choice decision-making task involving probabilistic monetary outcomes (Ernst et al., 2004; Ernst et al., 2005).
In each trial, a wheel (circle divided into two slices of different size and color, either blue or magenta) was presented to the subjects (see Fig. 1). Four types of monetary wheels, differing on probability (represented by the size of the slices) and magnitude of reward, were presented in random order throughout the task. The present study examined two of these wheels. They included i) a high-risk/reward condition, where subjects had to choose between a 10% chance of winning US$ 4.00 and a 90% chance of winning nothing, versus a 90% chance of winning US$ 0.50 and a 10% chance of winning nothing; and ii) a low-risk/reward condition, where subjects had to choose between a 30% chance of winning US$ 2.00 and a 70% chance of winning nothing, versus a 70% chance of winning US$ 1.00 and a 30% chance of winning nothing.
Figure 1.
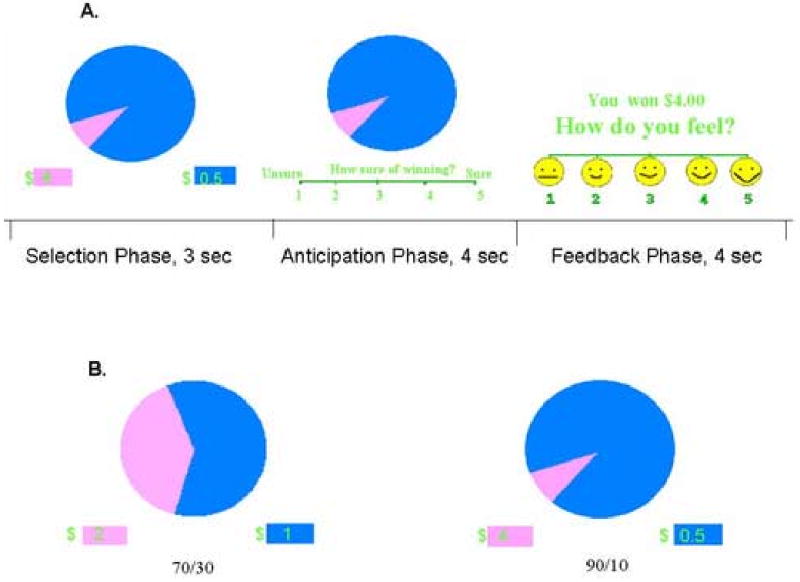
The Wheel of Fortune Task. The top Panel A. represents the three stages of a single trial, beginning with the selection phase, then the anticipation phase and finally the feedback phase. The present work analyzed only the selection phase. The bottom panel B. represents the two wheels used in the analysis.
Subjects were asked to select one of the slices by its color (blue or magenta) using a button press. For example, when the magenta slice was on the left, and subjects opted for this color, they pressed the left button. If the computer randomly selected the same color as the subject, the subject won the designated amount of money (receipt of reward); if the computer randomly selected the other color, the subject won nothing (omission of reward). The smaller slice was always paired with the higher dollar amount (see Fig. 1).
The task comprised three runs of 7.2 min and 43 trials each. Each wheel trial lasted 11 s and included a selection phase, during which subjects selected one of the two slices of the wheel (3 s); an anticipation phase, during which subjected rated how confident they were in their selection (4 s); and a feedback phase, during which the outcome was displayed and subjects rated how they felt about it (4 s). Inter-trial intervals were 1 s long. Only the selection phase was analyzed. We also used a control condition, which included the sensory-motor attributes of the monetary conditions, but lacked the decision-making. In this control condition, participants were presented with a monochromatic wheel (all blue, or all magenta), and instructed to press the button whose color corresponded to the color of the wheel (see Fig. 1). All responses were recorded on a 5-button-box in the fMRI scanner (MRI Devices; Waukesha, WI), and all button presses were done using the right hand. Subjects were instructed that they could take home the money they won and that they should try to win as much money as possible.
fMRI
A General Electric Signa 3 T scanner was employed. Head movement was restricted by the use of foam padding. Visual images were presented via Avotec Silent Vision Glasses (Stuart, FL) located directly above subjects' eyes. All participants were acclimated to the MRI environment with an MRI simulator prior to the study. Gradient echo planar (EPI) images were acquired after sagittal localization and a manual shim procedure. EPI images were acquired in 23 contiguous 5 mm axial slices per brain volume positioned parallel to the AC–PC line. Images were acquired using echo planar single shot gradient echo T2* weighting. The following imaging parameters were used: matrix was 64 × 64 mm; TR = 2000 ms; TE = 40 ms; Field of View = 240 mm; voxels were 3.75 × 3.75 × 5 mm. After EPI acquisition, a high resolution T1-weighted anatomical image was acquired to aid with spatial normalization. A standardized magnetization prepared gradient echo sequence was used (180, 1 mm sagittal slices, FOV = 256, NEX = 1, TR = 11.4 ms, TE = 4.4 ms, matrix = 256 × 256, TI = 300 ms, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels).
Analysis of the Results
Behavioral performance
Performance on the WOF is presented for adults and adolescents as mean (standard deviation) and variability (standard deviation) of the percent of risky (10 and 30) or safe (70 and 90) selections, and mean (standard deviation) of reaction times for the selection of risky and safe options. Group differences were tested using a repeated-measures ANOVA with risk as the within-subjects factor and group (adults vs. adolescents) as the between-subjects factor. In addition, correlational analyses were conducted between percent selection of risky options (option 10 of the 10/90 wheel, and option 30 of the 30/70 wheel) and age.
Imaging Data
For each subject, reconstructed fMRI images were analyzed using Medx software to check for excessive motion. The degree of motion did not differ significantly between groups. The mean of the individual largest displacement values were 1.8 mm (SD = 1.0) in adults and 2.0mm (SD .80) in adolescents (Mann-Whitney U = 67, p=0.2), and in radians 0.02 (SD 0.01) in adults and 0.03 (SD 0.02) in adolescents (Mann-Whitney U = 60, p=0.09). Therefore, we did not include motion parameters as nuisance covariates in the following analyses.
All subsequent analyses were conducted with SPM software (SPM99, Welcome Department of Neurology) and other routines written in Matlab 5.3. Preprocessing of data included, in turn, correction for the sequence of slice acquisition, motion correction, and spatial normalization to the Montreal Neurological Institute (MNI) T1-weighted template image supplied with SPM99. The analysis of the neuroimaging data was based on the assumption that the transform of neural signal to fMRI signal is linear and time-invariant, with a known impulse response function (Zarahn, 2000). This assumption has been shown to be acceptable for events of duration greater than 2 s (Buckner, 1998).
At the individual subject level (i.e., time series), event-related response amplitudes were estimated using the General Linear Model (GLM) for each of the 5 conditions (selection of 90, 10, 70, and 30 slices, and monochromatic control wheel). The waveform used to model each type of event-related response in the GLM was a rectangular pulse of the duration of the event (3 s) convolved with the synthetic hemodynamic response function provided by SPM. Contrast images were generated for each subject using pairwise comparisons of the event-related BOLD changes across event types. Here, the contrast of interest compared BOLD signal changes associated with high-risk selection (10 & 30) to BOLD signal changes associated with low-risk selection (70 & 90). Prior to group-level analysis, each contrast image was divided by the subject-specific voxel time series means, yielding values proportional to percentage fMRI signal change. These normalized contrast images were then smoothed with an isotropic Gaussian kernel (FWHM = 11.4) to mitigate any non-stationarity in spatial autocorrelation structure introduced by the previous step.
For all group-level analyses, a random effects model was employed to permit population-level inferences (Holmes, Friston, 1998). The main analysis was restricted to four a priori defined regions of interest (ROIs): right and left OFC/VLPFC, encompassing Brodmann areas BA 11, 10 and 47 (Kringelbach, Rolls, 2004); and right and left ACC, encompassing BA 24, 25, 32, 33 (Vogt, Nimchinsky, Vogt, Hof, 1995). These regions were ascertained from standard anatomical criteria (Talairach, Tournoux, 1988) on a single MNI template and applied to all normalized brains at the group level. Upon close inspection of individual data, the medial OFC (BA 11) was found to be significantly affected by fMRI artifacts and was mostly absent from the subjects' SPM group mask. Therefore, we could not assess this region. We performed voxel-wise t tests in these anatomically defined volumes of interest. Three-mm spheres were drawn around significant activation peaks of each region of interest, and statistical significance was set at P<0.05 corrected for the 3 mm sphere volume.
Within these ROIs, an analysis was conducted to compare risky selections (10 and 30 options) with safe selections (70 and 90 options). This analysis included three types of tests: (1) combined group activation (N = 30), (2) adolescent vs. adult comparison (i.e., interaction of group by activation), and (3) adolescent group and adult group activation separately. In addition, peak values of ROI activations in the [adolescent vs. adult] comparisons were extracted for each group to facilitate depiction of the interaction. Finally, the percentage of risky selections, used as the measure of individual risk-taking during the task, was entered in a correlation analysis with brain activation within the OFC/VLPFC and ACC ROIs during risky selections relative to safe selections.
As a measure of control for the specificity of our findings, we examined group differences in activation of the dorsolateral prefrontal cortex, an area for which we did not have a priori hypotheses regarding group differences within our sample. Finally, for completeness and the reader's interest, we report results from the subcortical regions of amygdala and ventral striatum since these regions are strongly implicated in reward processes. However, because this manuscript is focused on maturation of cognitive control of decision-making, these subcortical findings are not discussed.
Results
Sample and task performance
Fourteen adults (6 females/8 males; 26.7 ± 5.0 years old) and sixteen adolescents (9 females/7 males; 13.3 ± 2.1 years old) completed the study. Mean IQ (adults 109.1 ± 12.1; adolescents 107.6 ± 12.4) and mean socioeconomic status (adults 69.4 ± 31.2; adolescents 55.3 ± 17.8) measured using the Hollingshead's Four Factor Index of Social Status (Hollingshead, 1975) were similar between the groups.
Percent risky selections and variability of type of selection did not differ significantly between adolescents and adults (see Table 1). The cumulative dollar amount won during the task was also similar between groups (adolescents: $60.3 ± 6.4; adults: $58.9 ± 10.8). However, the percent selection of the risky option (option 30) of the 30/70 wheel was significantly negatively correlated with age (r= -0.41, P=0.02, n=30), indicating that risk-taking decreased with age (see Fig. 2) A similar correlation did not emerge with the 10-90 wheel (r= -0.1). The overall percent selection of risky options (option-10 and option-30) was not significantly correlated with age (r= -.25). The absence of strong group differences in performance removes the possible confound of performance difference in the interpretation of the findings, and the need for controlling for performance in the following fMRI analyses.
Table 1. Performance in Adolescents and Adults.
Task performance in adults and adolescents: mean (standard deviation) and variability (standard deviation) of percent risky selections (number of times 10 and 30 options were selected relative to the total number of presentations of the10/90 and 30/70 wheels); mean (standard deviation) reaction time (ms) for the selection of risky options (10 and 30) and for the selection of safe options (70 and 90).
| Adults (n=14) | Adolescents (n=16) | |
|---|---|---|
| Percent Risky Selections (10 & 30) % | 46 (28) | 51 (27) |
| Variability (SD) in risky vs. safe selections % | 40 (17) | 38 (16) |
| Reaction time for risky selections (10 & 30) ms | 1587.4 (293.8) | 1439.7 (229.1) |
| Reaction time for safe selections (70 & 90) ms | 1587.9 (237.7) | 1463.5 (261.5) |
Figure 2.
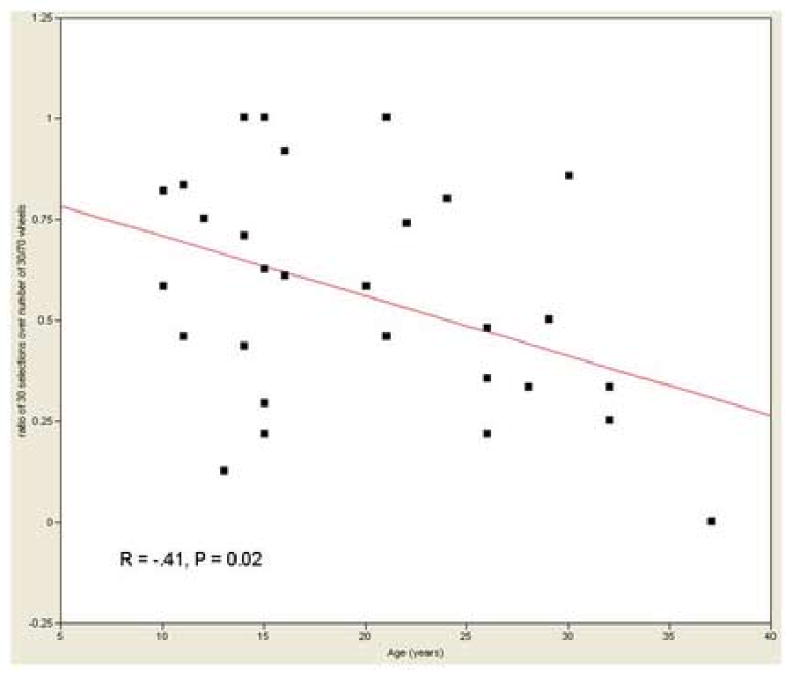
Scatter plot of the negative correlation between percent risky selections in the moderate risk range (option 30 of the 30/70 wheel) and age (years) in the whole group.
fMRI Results
OFC/VLPFC ROI (see Tables 2-3)
Table 2. ROI activation in the combined sample and each group separately.
Region of interest activation in the contrast [Select 1030>7090] for the combined group (n=30) and adolescents (n=16) and adults (n=14) separately. The regions of interest include the left and right orbital/ventrolateral prefrontal cortices (L-OFC/VLPFC, R-OFC/VLPFC) and left and right anterior cingulate cortices (L-ACC, R-ACC). Results show significant activations (P < 0.05 small volume corrected) in small regions of interest (3 mm spheres) centered on peak voxels found to be significantly activated by risky selections relative to safe selections in the combined group, adolescents, and adults respectively. Equivk is the cluster size of voxels, and x y z (mm) are the coordinates of the peak voxel based on the brain template provided by the Montreal Neurological Institute (MNI). No regions of interest showed significantly more activation during safe selections (70-90) than during risky selections (10-30) in any of the groups.
| L-OFC/VLPFC | R-OFC/ VLPFC | |||||||
|---|---|---|---|---|---|---|---|---|
| equivk | P voxel | T | x,y,z | equivk | P voxel | T | x,y,z | |
| Combined Group | 19 | 0.000 | 4.78 | -38 18 2 | 4 | 0.002 | 3.60 | 52 24 -4 |
| Adolescents | 19 | 0.008 | 2.92 | -38 22 4 | - | - | - | - |
| Adults | 13 | 0.000 | 4.73 | -42 14 -2 | 4 | 0.003 | 3.30 | 52 24 -4 |
| L-ACC | R-ACC | |||||||
| Combined Group | 19 | 0.005 | 3.16 | -2 0 30 | 19 | 0.005 | 3.10 | 2 14 46 |
| Adolescents | 19 | 0.007 | 2.97 | -12 56 -2 | 16 | 0.037 | 2.18 | 2 36 20 |
| Adults | 19 | 0.004 | 3.28 | 0 4 26 | 19 | 0.006 | 3.08 | 4 26 32 |
Table 3. Group Differences in ROI Activation.
Group comparison of ROI activation using the contrast: Adult[Select 1030>7090] vs. Adolescent[Select 1030>7090]. The regions of interest include the left and right orbital/ventrolateral prefrontal cortices (L-OFC/ VLPFC, R-OFC/ VLPFC) and left and right anterior cingulate cortices (L-ACC, R-ACC). Results show significant activations (P < 0.05 small volume corrected) in small regions of interest (3 mm spheres) centered on peak voxels found to be significantly activated by risky selections relative to safe selections in the combined group, adolescents, and adults respectively. Equivk is the cluster size of voxels, and x y z (mm) are the coordinates of the peak voxel based on the brain template provided by the Montreal Neurological Institute (MNI)
| Adults > Adolescents | ||||
|---|---|---|---|---|
| equivk | P voxel | T | x,y,z | |
| L-OFC/VLPFC | 13 | 0.011 | 2.80 | -44 14 -4 |
| R-OFC/VLPFC | - | - | - | - |
| L-ACC | 19 | 0.044 | 2.09 | -2 26 30 |
| R-ACC | 19 | 0.044 | 2.09 | 2 26 30 |
| Adolescents > Adults | ||||
| L-OFC/VLPFC | - | - | - | - |
| R-OFC/VLPFC | - | - | - | - |
| L-ventral ACC | 19 | 0.046 | 2.06 | -2 38 2 |
| R-ACC | - | - | - | - |
When subjects chose risky (10 or 30 percent chance of reward) over non-risky (70 or 90 percent chance of reward) selections, robust bilateral activation was found in the lateral OFC/VLPFC (BA 47, prefrontal operculum) (Table 2). When examined separately, adults activated this area bilaterally, while adolescents activated the left side preferentially (Table 2). On direct comparison, adults had significantly greater activation relative to adolescents in this area (see Fig. 3, Table 3).
Figure 3.
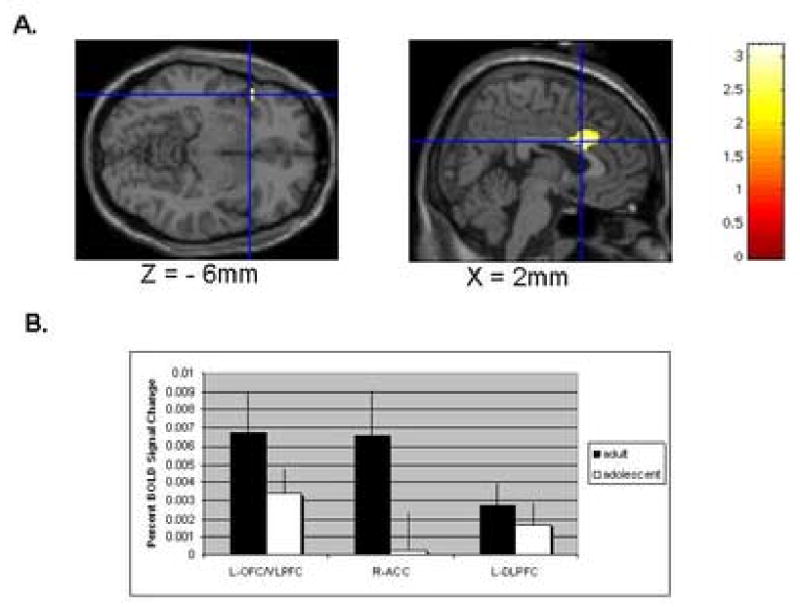
Greater regional activations in adults than in adolescents for the contrast (select 10/30 minus select 70/90) in the lateral orbitofrontal/ventrolateral prefrontal cortex (OFC/VLPFC) and anterior cingulate cortex (ACC) regions of interest. The top panel A. represents regional activation in the left OFC/VLPFC (MNI x, y, z: -44, 14, -6 mm), and right ACC (MNI x, y, z: 2, 26, 30 mm), rendered on a single-subject T1 image provided by SPM99. The bottom panel B. represents the group mean fraction of BOLD signal changes in adults and adolescents at the peak voxels identified on panel A. The left dorsolateral prefrontal cortex (DLPFC, MNI x, y, z: -26, 24, 46) was also included as a control region to inform the regional specificity of group differences.
There was no detectable activation when comparing safe to risky choices. Adolescents showed no greater recruitment than adults in this contrast (safe > risky).
ACC ROI (see Tables 2-3)
Taken as a group, subjects recruited the dorsal ACC (BA 24, extending dorsally into BA 6) when making risky choices (Table 2). Both adolescents and adults recruited this area bilaterally, but the adolescents' activation tended to be stronger on the left side and more anterior and ventral (x y z MNI coordinates in mm: adolescents, -12 56 -2; adults, 0 4 26). A direct comparison showed overall stronger bilateral activation in adults than in adolescents in the dorsal ACC (x y z MNI coordinates in mm: Adults > Adolescents, ACC 2 26 30; BA 32, see Fig. 3, Table 3). Adolescents had stronger activation than adults in the ventral ACC (adolescents > adults, left ACC -2 38 2; BA 24). There was no detectable activation when comparing safe to risky choices. Adolescents showed no greater recruitment than adults in this contrast (safe > risky).
Correlations with performance (see Table 4)
Table 4. Correlations of ROI Activation * Performance.
Peaks of significant negative correlation between ROI activation in [Select 1030>7090] and percent of risky choices for all subjects combined, and adults and adolescents separately. Results show significant activations (P < 0.05 small volume corrected) in small regions of interest (3 mm spheres) centered on peak voxels significantly activated in the negative correlations of the combined group, adolescents, and adults respectively. The regions of interest include the left and right orbital/ventrolateral cortices (L-OFC/VLPFC, R-OFC/VLPFC) and left and right anterior cingulate gyri (L-ACC, R-ACC). Equivk is the cluster size of voxels, and x y z (mm) are the coordinates of the peak voxel based on the brain template provided by the Montreal Neurological Institute (MNI).
| L-OFC/VLPFC | R-OFC/VLPFC | |||||
|---|---|---|---|---|---|---|
| equivk | T | x,y,z | equivk | T | x,y,z | |
| Combined Group | 10 | 2.02 | -38 14 -4 | - | - | - |
| Adolescents | 10 | 3.56 | -28 60 -2 | 11 | 2.02 | 46 36 -4 |
| Adults | 16 | 2.61 | -48 18 0 | - | - | - |
| L-ACC | R-ACC | |||||
| Combined Group | 19 | 2.87 | -4 38 22 | 19 | 2.60 | 2 38 24 |
| Adolescents | 19 | 2.62 | -6 40 20 | 19 | 2.39 | 2 38 24 |
| Adults | - | - | - | - | - | - |
With all subjects combined, the number of risky choices negatively correlated with activation in the left OFC/VLPFC and bilateral ACC (Table 4). By group, this performance score was negatively correlated with activation in the left OFC/VLPFC in adults and adolescents and with bilateral ACC in adolescents only (Table 4). The specific locations of the activations that correlated with performance were very proximal to those showing significant group differences (as depicted in Fig. 4). Finally, these correlations did not differ significantly between adolescents and adults.
Figure 4.
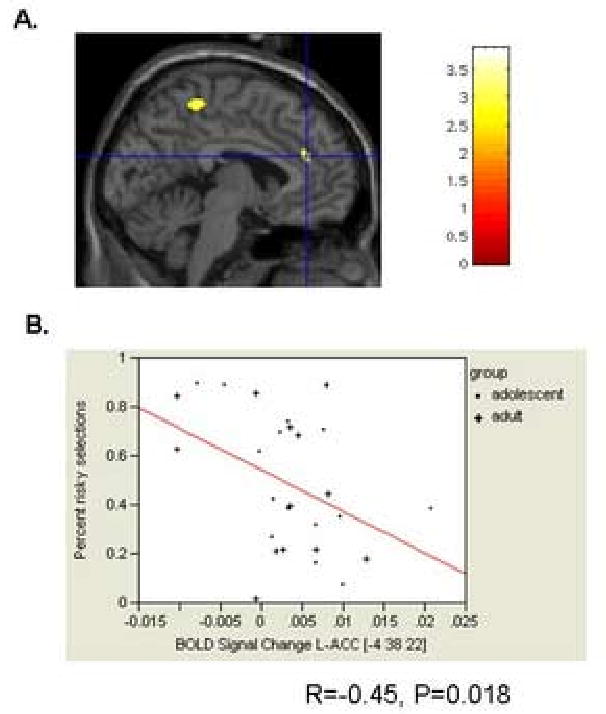
Scatter plot of the negative correlation between percent risk taking and BOLD percent signal change in the right anterior cingulate cortex in the whole group.
Dorsolateral prefrontal cortex (See Figure 3)
For the sake of specificity, a region of interest encompassing the left dorsolateral prefrontal cortex was examined. Significant activation was present in the combined group, and for each group separately, but no group differences could be detected (see Figure 3, x y z MNI coordinates in mm: -26 24 46).
Amygdala and ventral striatum
Both regions were significantly activated in the combined sample and each group separately by the selection of risky options vs. the selection of safe options. However, these activations did not differ in adolescents and adults. These results are provided for completeness, but will not be discussed below.
Discussion
This is the first paper to examine differences in brain activation between adolescents and adults while making decisions to obtain rewards of varying magnitude and probability. We used a monetary decision-making task that allowed the analysis of choice selection separately from anticipation of outcome or response to reward. Based on models of developmental discontinuities in reward-related brain structures (Ernst et al., 2006; Bjork et al., 2004; van Leijenhorst et al., 2006; Galvan et al., 2006), we predicted that adolescents would display lower activation than adults in a system encompassing OFC/VLPFC and ACC that contributes to behavioral control. The data generally supported this prediction: Risky choice selection was associated with significantly greater activation in the OFC/VLPFC and ACC in adults compared to adolescents. In addition, risk-taking performance scores in adolescents, adults and in the sample as a whole negatively correlated with activity in these areas. However, other regions of frontal cortex (dorsolateral prefrontal cortex) did not differ as a function of age, demonstrating the regional specificity of the effect.
Adults and adolescents performed similarly on the task: they made similar numbers of risky choices and won similar cumulative amounts of money. However, percent of risky choices at the moderate level of risk (option 30 of the 30/70 condition) was significantly negatively correlated with age, suggesting that a larger sample might have been able to detect stronger group differences. The relative weakness of behavioral differences in risk-taking was somewhat surprising, given adolescents' propensity for risk-taking (Arnett, 1992). However, we have demonstrated in a sample of similar size as that presented here that healthy adolescents (n=19, mean age 13.5 years) were making more risky decisions than healthy adults (n=11, mean age 29.7 years), F(1,28)=46.7, P=0.02, when the task was administered in the clinic, a less stressful environment than in the scanner (unpublished data, available upon request).
At present, five studies have examined brain activation in reward-related behavior in adolescents (May et al., 2004; Bjork et al., 2004; van Leijenhorst et al., 2006; Ernst et al., 2005; Galvan et al., 2006). Using a monetary card-guessing task in children and adolescents, May and colleagues (2004) found that the ventral striatum, medial and lateral OFC, and ACC were more active in rewarded trials than in neutral or punished trials, which is consistent with earlier findings using the same task in adults (Delgado, Nystrom, Fissell, Noll, Fiez, 2000). This suggested that adolescents use similar circuits to process reward as adults. The second study (Bjork et al., 2004) employed a monetary reaction-time task to compare adults and adolescents directly, and found that adolescents recruited less strongly the right ventral striatum and insula when anticipating possible gains. Neural activity in response to outcomes did not differ between groups. Bjork et al. (2004) concluded that adolescents might have weak motivational, but not consummatory, reward-related behavior, and that their propensity for risk-taking might compensate for low activity levels in these motivational structures.
Van Leijenhorst (2006) examined neural activation associated with the selection of the option with the most likely positive outcome. Compared to adults (18-26 years), children (9-12 years) showed greater activation of the dorsal ACC and no differences in medial orbitofrontal cortical activation when selecting between two less probable outcomes (33% vs. 66% or 44% vs. 55% chance of winning one point), compared to selecting between two more probable outcomes (11% vs. 88% or 22% vs. 77% chance of winning one point). The group difference analysis of selection did not include lateral OFC. The authors suggested that the greater activation of the ACC might reflect a higher degree of response conflict in children than in adults during decision-making under uncertainty. In addition, children recruited lateral OFC more strongly for negative relative to positive feedback compared to adults, suggesting enhanced sensitivity to losses. In contrast to this study, Galvan et al's work (2006) reported reduced activation of the lateral OFC in response to rewards in adolescents and children compared to adults. In addition to the very different paradigms used in these studies (i.e., decision-making based principally on probability vs. reaction time based on learned reward outcome), the discrepancy between results may reflect different developmental trajectories of responses to negative vs. positive feedbacks. Furthermore, similarly to the study by Ernst et al. (2005), Galvan et al., reported enhanced nucleus accumbens activation in response to rewards in adolescents compared to adults.
Finally, using the same sample as in the current study, our group (Ernst et al., 2005) previously showed differences in brain activation between adolescents and adults in response to outcomes (consummatory phase). The left nucleus accumbens, prominently associated with reward processing (Di Chiara, Bassareo, Fenu, De Luca, Spina, Cadoni, Acquas, Carboni, Valentini, Lecca, 2004), was recruited more strongly in adolescents, and the left amygdala, prominently associated with avoidance (LeDoux, 2000), was affected more strongly in adults. This suggested that adolescent risk-taking might be partly explained by adolescents' greater sensitivity for reward, and adults' heightened sensitivity to harm. Indeed, when asked in the current task to rate their feelings after receiving feedback, adolescents tended to feel happier than adults in response to gains and less upset than adults in response to not winning (data not shown). Taken together, these five studies indicate that adults and adolescents engage similar neural networks during reward-related performance, but to lower or higher degrees. The discrepancies among these studies need to be understood in light of differences in sample characteristics, including age ranges and sizes, paradigms (e.g., reaction time task as in Bjork et al. vs. decision-making task as in Ernst et al.), and methods of analysis.
The present study expands on our previous results and examines the activity in cortical regulatory structures during an earlier stage of decision-making: the formation of a preference between two choices, and the execution of this preference (Ernst and Paulus, 2005). When directly comparing adults to adolescents, we found that the lateral OFC/VLPFC (BA 47, including frontal operculum) and dorsal ACC (BA 32) were consistently engaged more strongly in adults. Furthermore, this activation in left OFC/VLPFC was negatively correlated with risky performance in the entire sample and in each group separately, and activation in the ACC was negatively correlated with risky performance in the combined group and adolescents bilaterally. This result is opposite to van Leijenhorst et al.'s (2006) finding of reduced ACC activation (BA 24) during selection of uncertain options in adults compared to children. This discrepancy could inform the role of age, puberty, and paradigm features in modulating circuits of cognitive control. Van Leijenhorst et al. (2006) used a younger group of children (11.3 years) than ours (13.2 years), and tested a simpler paradigm that did not manipulate the magnitude of outcomes to increase the salience of risk. Future studies will be able to disentangle the effect of these study parameters on age-related differences in neural responses to risky decision-making.
Neuropsychological, lesion, and functional neuroimaging studies in humans have identified the OFC/VLPFC (Arana et al., 2003; Elliott et al., 1999; Ernst et al., 2002; Paulus et al., 2001; Rogers et al., 1999; Walton et al., 2004; Rushworth, Buckley, Gough, Alexander, Kyriazis, McDonald, Passingham, 2005; Elliott, Rees, Dolan, 1999) and ACC (Bush et al., 2002; Ernst et al., 2004; Rogers et al., 2004; Walton et al., 2004; Williams et al., 2004) as key neural substrates of decision-making processes. Often modulated by both risk (Critchley et al., 2001; Cohen et al., 2005) and reward (Rogers et al., 2004), these structures are purported to support specific functions that are specialized by subregions (e.g., lateral vs. medial OFC, dorsal vs. ventral ACC).
Lateral OFC/VLPFC is generally involved in response inhibition and cognitive flexibility (Nagahama et al., 2001; Casey et al., 2001; Cools et al., 2002; O'Doherty et al., 2003), arguing for its role in controlling action in a conflictual context (O'Doherty et al., 2003; Schoenbaum, Roesch, 2005). The OFC/VLPFC continues to evolve through childhood and adolescence and has strong connections to other frontal and limbic areas, making it particularly suited to integrate affective and non-affective information (Happaney, Zelazo, Stuss, 2004). A lag in maturation of this structure, suggested by the present findings, might contribute to the commonly observed difficulty in adolescents of controlling decision-making as a function of potential consequences (Chambers et al., 2003).
Of note, the OFC/VLPFC activation in the present study almost always extended posteriorly and superiorly into the anterior insula. Generally, the insula is thought to be involved in the detection and interpretation of internal bodily states (Craig, 2002; Bar-On, Tranel, Denburg, Bechara, 2003; Paulus, Rogalsky, Simmons, Feinstein, Stein, 2003), particularly for unfair (Sanfey, Rilling, Aronson, Nystrom, Cohen, 2003) or aversive (Adolphs, 2002) stimuli. The neural representation of physical states can activate motivational circuits (e.g., ventral striatum) that determine engagement in or avoidance of risk-taking behavior (Craig, 2002). This is consistent with the observation that the insula is often activated concurrently with the OFC/VLPFC in decision-making tasks, and is similarly modulated by risk or reward (Ernst, Bolla, Mouratidis, Contoreggi, Matochik, Kurian, Cadet, Kimes, London, 2002; Critchley, Mathias, Dolan, 2001; Paulus, Feinstein, Leland, Simmons, 2005; O'Doherty et al., 2003). Our results suggest that anterior insula activation occurs in both adults and adolescents during decision-making, but that the activation is stronger in adults. This bolsters previous findings of age-related differences in the function of harm avoidance circuitry (Ernst et al., 2005).
The ACC has been associated with a number of regionally segregated functions in cognitive and affective control (Bush, Luu, Posner, 2000). The dorsal division (BA 24, 32) is commonly thought of as the “cognitive” ACC and is involved in monitoring behavior for response conflicts (Krawczyk, 2002; Kerns, Cohen, MacDonald, III, Cho, Stenger, Carter, 2004; e.g., Bush, Vogt, Holmes, Dale, Greve, Jenike, Rosen, 2002). The ventral division (BA 24, 25, 32, 33), on the other hand, is considered to be the “affective” ACC, and is predominantly involved in the representation of the emotional salience of stimuli (Phillips, Drevets, Rauch, Lane, 2003). Previous work has shown engagement of both regions in studies of reward-related processes (e.g., Cohen, Heller, Ranganath, 2005; Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter, Smith, 2004) and the selection of options (e.g., Ernst et al., 2004; Williams, Bush, Rauch, Cosgrove, Eskandar, 2004; Hadland, Rushworth, Gaffan, Passingham, 2003). The current results show activation in the dorsal ACC. This is consistent with a previous study using the same task (Ernst et al., 2004), and with single-unit recordings in humans demonstrating the dorsal ACC's role in linking reward information with appropriate actions (Williams et al., 2004). The dorsal ACC activation suggests that the wheel of fortune task taxes cognitive capacity, particularly the ability to resolve conflicts to take action, rather than the emotional processing of stimulus salience.
Of interest, a ventral region of the ACC was found to be more active in adolescents than in adults when making risky selections. We propose that the increased activation of this region in adolescents may reflect greater sensitivity to emotional stimuli, which may also contribute to risk-taking behavior (Ernst et al., 2005), as well as to the well-known peak of onset of mood disorders in this age group (Glied, Pine, 2002b).
Several caveats need to be mentioned. First, the age range of the adolescent sample covers early to late adolescence. It will be important to also include younger children in future studies to better characterize early maturation of the neural circuits underlying cognitive control. Second, each selection event is preceded by a feedback event with a fixed 1 sec interval (ITI), possibly causing feedback-related activation to influence selection-related activation. However, this effect is mitigated by the fact that feedback comprised a number of different conditions (win and lose associated with different wheels and selections) that were randomly distributed, and thus unlikely to give rise to a systematic bias. Third, while the various options are characterized as a function of risk, they also differ on expected value. For example, the 30 option has an expected value (EV) of 60 (probability of 30% X reward magnitude of $2), compared to the 70 option, which has an EV of 70 (probability of 70% X reward magnitude of $1). Similarly, the 90 option has an EV of 45, and the 10 option has an EV of 40. Two reasons mitigate the possible contribution of the EV to the findings. First, these differences are relatively small, and it is unlikely that they would generate significant differences in activation. Second, we would predict a positive relationship between activation and EV, since EV represents the net reward value of a potential outcome. This contradicts our finding that the lower EV option (risky selection) is associated with greater activation than the higher EV option (safe selection). Furthermore, in a recent study we demonstrated that activation of the ACC was driven by conflict rather than by the representation of reward value (Smith et al., under review).
In conclusion, the present results demonstrate that adults engage prefrontal regulatory structures to a greater extent than adolescents when contemplating options and making high-risk choices, and that this engagement negatively correlates with risky selections. This difference in brain activation, along with previous results suggesting differential engagement of circuits underlying approach behavior (favored in adolescents) and avoidance behavior (favored in adults) (Ernst et al., 2005), may explain the propensity for risk-taking and novelty-seeking in this age group. The neural developmental changes and associated plasticity during this transition period also suggests that adolescence may be a prime time for interventions aimed at reducing poor decision-making behavior.
In addition to informing the neural substrates of risk-taking, the results may help clarify processes involved in the etiology of mood and anxiety disorders, many of which first appear in adolescence (Chambers et al., 2003; Pine, Cohen, Gurley, Brook, Ma, 1998; Glied, Pine, 2002a), and may result from abnormalities in neural systems underlying decision-making. To determine specific deviations in such clinical populations, normal development must first be characterized. This study provides a first step toward that goal. Future research with tasks such as this will increase our understanding of both normative adolescence and its pathological correlates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Reckless Behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Benthin A, Slovic P, Severson H. A psychometric study of adolescent risk perception. J Adolesc. 1993;16:153–168. doi: 10.1006/jado.1993.1014. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Event-related fMRI and the hemodynamic response. Neuroimage. 1998;6:373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JP. The development of decision-making. J Adolesc Health. 2002;31:208–215. doi: 10.1016/s1054-139x(02)00503-7. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport J. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. The Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. J Gambl Stud. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Brain Res Cogn Brain Res. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the Neural Mechanisms of Probabilistic Reversal Learning Using Event-Related Functional Magnetic Resonance Imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. Journal of Neuroscience. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Crone EA, Bunge SA, Latenstein H, van der Molen MW. Characterization of Children's Decision Making: Sensitivity to Punishment Frequency, Not Task Complexity. Child Neuropsychol. 2005;11:245–263. doi: 10.1080/092970490911261. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47 1:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Eshel N, Zarahn E, Leibenluft E, Zametkin AJ, Towbin K, Charney DS, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision-making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci 2006. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Glied S, Pine DS. Consequences and correlates of adolescent depression. Arch Pediatr Adolesc Med. 2002;156:1009–1014. doi: 10.1001/archpedi.156.10.1009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, Harris WA, McManus T, Chyen D, Collins J. Youth risk behavior surveillance--United States, 2003. MMWR Surveill Summ. 2004;53:1–96. [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The anterior cingulate and reward-guided selection of actions. J Neurophysiol. 2003;89:1161–1164. doi: 10.1152/jn.00634.2002. [DOI] [PubMed] [Google Scholar]
- Happaney K, Zelazo PD, Stuss DT. Development of orbitofrontal function: current themes and future directions. Brain Cogn. 2004;55:1–10. doi: 10.1016/j.bandc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Dept. of Sociology; 1975. Thesis/Dissertation. [Google Scholar]
- Holmes A, Friston KJ. Generalisability, random effects, and population inference. Neuroimage. 1998;7 [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents' performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Dev Psychol. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of Widely Distributed Brain Function Subserves Cognitive Development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H. Dissociable Mechanisms of Attentional Control within the Human Prefrontal Cortex. Cerebral Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, Passingham RE. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neuroscience. 2005;25:11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DL, Hersen M, Van Hasselt VB. Reliability of the Structured Clinical Interview for DSM-III-R: an evaluative review. Compr Psychiatry. 1994;35:316–327. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain Cogn. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl RE, Keating D, Kupfer D, Masten A, Pine DS. In: Psychopathology in adolescence: integrating affective neuroscience with the study of context. Cicchetti D, editor. New York: Wiley; 2005. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers Inc.; 1988. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. In Press. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Zarahn E. Testing for neural responses during temporal components of trials with BOLD fMRI. Neuroimage. 2000;11:783–796. doi: 10.1006/nimg.2000.0560. [DOI] [PubMed] [Google Scholar]


