Abstract
Endothelium forms a semi-permeable barrier that separates blood from the underlying tissue. Barrier function is largely determined by cell-cell and cell-matrix adhesions that define the limits of cell borders. Yet, such cell-cell and cell-matrix tethering is critically reliant upon the nature of adherence within the cell itself. Indeed, the actin cytoskeleton fulfills this essential function, to provide a strong, dynamic intracellular scaffold that organizes integral membrane proteins with the cell’s interior, and responds to environmental cues to orchestrate appropriate cell shape. The actin cytoskeleton is comprised of three distinct, but interrelated structures, including actin cross-linking of spectrin within the membrane skeleton, the cortical actin rim, and actomyosin-based stress fibers. This review addresses each of these actin-based structures, and discusses cellular signals that control the disposition of actin in different endothelial cell phenotypes.
Keywords: Membrane skeleton, Stress fibers, Rho GTPases, Microtubules, cAMP
Introduction
Actin microfilaments, microtubules and intermediate filaments are in constant and intimate communication with one other, and are principal determinants of cell shape (Chang and Goldman, 2004; Revenu et al., 2004; Dudek and Garcia, 2001). In quiescent endothelium, actin forms a cortical rim that interacts with both cell-cell and cell-matrix adhesion complexes, and tethers these structures to intracellular organelles. Actin tethering to adhesion complexes is absolutely essential to maintaining a functional endothelial cell barrier (Dudek and Garcia, 2001; Lee and Gotlieb, 2002; Lee and Gotlieb, 2003; Revenu et al., 2004). Indeed, agents that disrupt the cortical actin rim increase endothelial permeability(Shasby et al., 1982), whereas agents that stabilize the cortical actin rim prevent inflammatory agonists from disrupting the endothelial cell barrier (Phillips et al., 1989).
Inflammatory agonists, such as thrombin and histamine, reorganize the cortical actin rim as a necessary prerequisite to inter-endothelial cell gap formation. In many cases, inflammatory agonists increase cytosolic calcium, decrease cAMP and activate RhoA/Rho kinase, which collectively initiate actin reorganization from a cortical actin distribution into fibers that stretch throughout the cell body. These actin stress fibers increase centripetal tension and, along with the reorganization of adhesion complex architecture, mediate retraction of cell-cell borders into discernible gaps (Dudek and Garcia, 2001; Phillips et al., 1989; Shasby et al., 1982). Thus, actin reorganization from its cortical distribution into stress fibers is a principal component of the endothelial response to inflammation (Dudek and Garcia, 2001; Phillips et al., 1989), and preservation of the cortical actin cytoskeletal network is necessary for maintenance of endothelial barrier integrity (Fukuhara et al., 2005; Stelzner et al., 1989).
However, not all endothelial cells possess an equally restrictive barrier function. For many years endothelial cell biologists have recognized that endothelium can be characterized as continuous, discontinuous, or fenestrated, where continuous endothelium possesses a restrictive barrier function and fenestrated endothelium does not. However, in recent years it has become increasingly recognized that vast differences in barrier properties exist even within continuous endothelium. Capillary endothelial cells, such as those found within the pulmonary microcirculation, possess a much tighter barrier than do endothelial cells within conduit vessels, such as those found in pulmonary arteries and veins. Actin organization, and the signal transduction events that control actin disposition, are fundamentally different in pulmonary artery and microvascular endothelial cells, and contribute greatly to the difference in barrier properties among these cell types. This review therefore addresses the fundamental actin organization in endothelium, and addresses important molecular interactions that control actin distribution and, hence, the endothelial cell barrier.
Actin organization
Actin constitutes approximately 5–15% of the total protein in endothelial cells (Patterson and Lum, 2001). The actin cytoskeleton is a highly dynamic structure, and undergoes polymerization and depolymerization based upon cellular demand. In non-smooth muscle cells, individual globular β-actin and γ-actin subunits alternatively polymerize in a helical fashion to form a double stranded filamentous structure known as F-actin. In general the amount of globular(G)-actin and F-actin exist in an equal balance (Stossel et al., 1985). Actin polymerization is required for the formation of F-actin, which is a fundamental structural unit for actin-based cytoskeletal structures.
Actin polymerization occurs in two sequential processes, including nucleation and elongation (Stossel, 1993). Nucleation occurs when three actin monomers bind together in geometric configuration, and it provides a site for elongation, where ATP bound G-actin binds and grows to form F-actin. F-actin has a fast growing end known as the plus end, where actin monomers quickly bind and elongate the filament. The slow growing actin end is known as the minus end, where actin monomers are added at a relatively slower rate, making F-actin a polar structure (Pollard, 1984; Steinmetz et al., 1997; Wegner, 1976). F-actin depolymerizes primarily due to hydrolysis of bound ATP into ADP, resulting in loss of binding affinity between adjacent monomers (Lambrechts et al., 2004; Lee and Gotlieb, 2002; Lee and Gotlieb, 2003). Several actin-binding proteins have been identified which induce actin nucleation, elongation and cross-linking, processes that are responsible for modulating the organization and function of the actin cytoskeleton.
F-actin organizes into three distinct cytoskeletal structures, including the membrane skeleton, the cortical actin rim and stress fibers. Both the membrane skeleton and stress fibers are composed of relatively short F-actin filaments (Brenner and Korn, 1979; Brenner and Korn, 1980; Cramer et al., 1997; De Matteis and Morrow, 2000; Heimann et al., 1999), while the cortical actin rim is composed of long F-actin bundles (De Matteis and Morrow, 2000; Heimann et al., 1999). Each of these three distinct structures has a unique role in controlling endothelial cell shape.
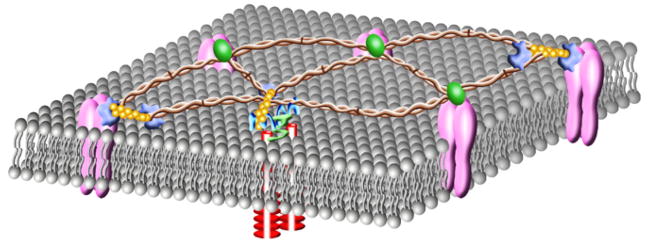
The membrane skeleton
The membrane skeleton determines membrane architecture, and enables for membrane distensibility (De Matteis and Morrow, 2000). This support structure is immediately adjacent to the plasma membrane, where it possesses a thickness of just a few nanometers. The molecular basis of the membrane skeleton was first identified in erythrocytes (De Matteis and Morrow, 2000), and subsequently resolved in non-erythroid cells (Bennett and Gilligan, 1993; Goodman, 1999; Goodman et al., 1981; Heltianu et al., 1986; Pratt et al., 1984; Wu et al., 2001). The non-erythroid membrane skeleton is primarily composed of spectrin and its binding proteins (Fig. 1), including F-actin, ankyrin, protein 4.1, adducin and α-catenin (De Matteis and Morrow, 2000; Pradhan et al., 2001; Ungewickell et al., 1979). Spectrin is a rod-shaped protein that exists as an α- and β-heterotetramer, and associates directly with short filamentous F-actin (Brenner and Korn, 1979; Brenner and Korn, 1980; De Matteis and Morrow, 2000; Heimann et al., 1999). Integral membrane proteins are linked to the spectrin-based membrane skeleton either directly, or through adaptor proteins such as protein 4.1 and ankyrin. Spectrin interaction with α-catenin provides a platform through which adhesion complexes connect sites of cell-cell tethering to the membrane skeleton and, ultimately, the cortical actin rim (see below). Thus, short actin filaments play a central role in controlling plasma membrane organization through their interaction with spectrin, and can provide a platform for interaction with the underlying cortical actin rim.
Fig. 1.
The spectrin-based membrane skeleton lies just beneath the plasma membrane, where it determines membrane architecture important for control of cell shape. Spectrin cross-links short F-actin forming a distensible network of scaffolding proteins that structurally support the plasma membrane. Spectrin ( ) is a rod-shaped anti-parallel heterotetramer comprised of α-spectrin and β-spectrin. β-spectrin binds to F-actin (
) is a rod-shaped anti-parallel heterotetramer comprised of α-spectrin and β-spectrin. β-spectrin binds to F-actin ( ), ankyrin (
), ankyrin ( ), protein 4.1 (
), protein 4.1 ( ), adducin and α-catenin (
), adducin and α-catenin ( ). Ankyrin, protein 4.1, adducin and α-catenin link transmembrane proteins to the spectrin membrane skeleton. Additionally, protein 4.1 mediates formation of a ternary complex with spectrin and actin.
). Ankyrin, protein 4.1, adducin and α-catenin link transmembrane proteins to the spectrin membrane skeleton. Additionally, protein 4.1 mediates formation of a ternary complex with spectrin and actin.
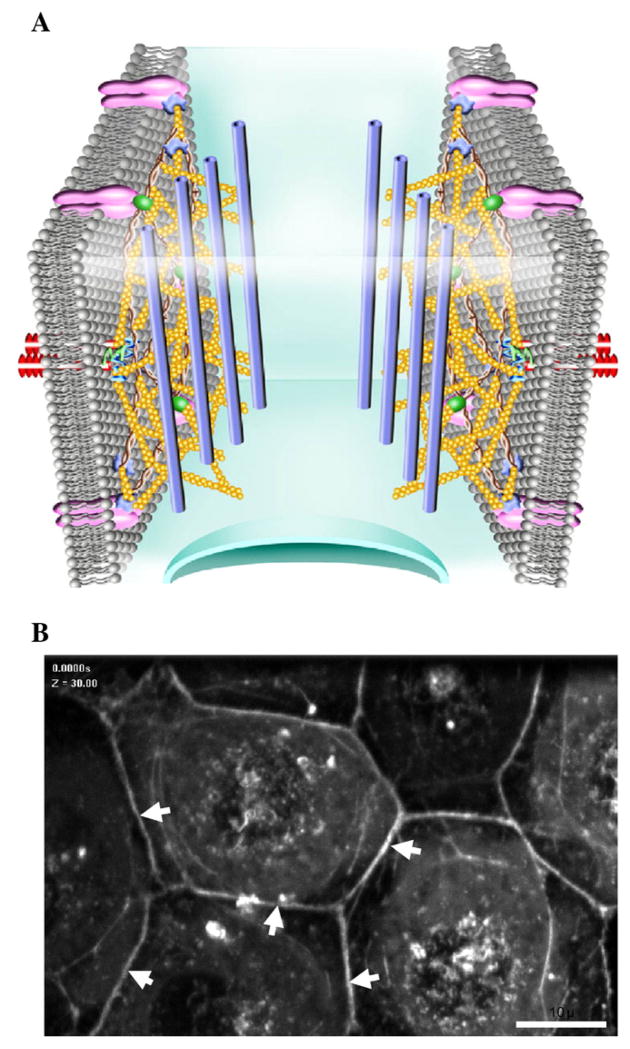
The cortical actin Rim
The cortical actin rim is organized and stabilized just beneath the spectrin-based membrane skeleton (Fig. 2). This dense rim of actin fibers is coordinated by actin-binding and cross-linking proteins, including spectrin, filamin, cortactin, Wiskott-Aldrich syndrome protein (WASP), vasodilator-stimulated phosphoprotein (VASP), gelsolin, cofilin, ezrin-radixin-moesin (ERM), and heat shock protein 27 (HSP27). The membrane skeleton and the cortical actin rim are distinct cytoskeletal structures, although they interact through the coordination of large multi-protein complexes.
Fig. 2.
The cortical actin rim lies just beneath the spectrin-based membrane skeleton where it determines cell shape. The cortical actin rim is associated with cell-cell and cell-matrix adhesion complexes that keep cells in juxtaposition to each other and to the matrix. The cortical actin rim provides strength for cell adhesion and generates outward centrifugal tension. A) Schematic of the cortical actin rim showing extensively cross-linked long F-actin ( ) networks. F-actin is shown interacting with micro-tubules (
) networks. F-actin is shown interacting with micro-tubules ( ). B) Organization of the cortical actin rim is shown in pulmonary microvascular endothelial cells that were infected with a retrovirus to stably express GFP-β-actin. White arrows illustrate the cortical actin rim organization in PMVECs.
). B) Organization of the cortical actin rim is shown in pulmonary microvascular endothelial cells that were infected with a retrovirus to stably express GFP-β-actin. White arrows illustrate the cortical actin rim organization in PMVECs.
Spectrin organizes and stabilizes the cortical actin rim by cross-linking F-actin to inter-endothelial junction proteins such as ZO-1 (Itoh et al., 1993; Itoh et al., 1991), α-catenin (Imamura et al., 1999; Muller et al., 2005), connexin-43 (Toyofuku et al., 1998; Wu et al., 2003), and VASP (Comerford et al., 2002). Cortactin and WASP activate the Arp2/3 complex responsible for nucleating new actin branches from preexisting F-actin, thereby organizing stable cortical actin cytoskeletal networks at the membrane, and promoting the assembly of endothelial cell-cell and cell-matrix adhesions (Millard et al., 2004; Weed and Parsons, 2001).
In addition, phosphorylated filamin cross-links F-actin to membrane glycoproteins and stabilizes the cortical actin rim at the plasma membrane, so that F-actin is available for the assembly of endothelial cell-cell and cell-matrix adhesions, but not available for the formation of stress fibers (Stossel et al., 2001). Filamin is constitutively phosphorylated by PKA. PKA phosphorylated filamin promotes organization and stabilization of the cortical actin rim and, consequently, stabilizes adhesion plaques. In contrast, oxidant stress, such as that induced by H2O2, inactivates PKA and dephosphorylates filamin (Hastie et al., 1997). Dephosphorylated filamin undergoes proteolytic degradation by calpain (Zhang et al., 1988), disrupting the cortical actin rim and increasing endothelial permeability(Hastie et al., 1997).
VASP activates actin polymerization, limits stress fiber formation, and promotes assembly and stabilization of cell-cell and cell-matrix adhesions (Krause et al., 2003). These functions of VASP rely upon the actions of actin binding proteins, such as cofilin (Reinhard et al., 1995; Salazar et al., 1999), vinculin (Reinhard et al., 1996), and gelsolin (Bearer et al., 2000; Salazar et al., 1999). Indeed, cofilin and gelsolin limit F-actin turnover (Lappalainen and Drubin, 1997) by capping the barbed ends of F-actin and inhibiting actin polymerization, hence limiting stress fiber formation (Gorovoy et al., 2005; Maekawa et al., 1999).
ERM proteins are distributed throughout the cortical actin rim and the adherens junctions, and are involved in directly or indirectly linking actin microfilaments to the cell membrane (Bretscher et al., 2002; Ivetic and Ridley, 2004; Niggli and Rossy, 2008). The amino-terminal domain of ERM proteins binds to membrane proteins on the cell surface, and connect them to the cortical actin rim by binding with F-actin through their carboxyl-terminal domains (Bretscher et al., 2002; Ivetic and Ridley, 2004; Niggli and Rossy, 2008). Thus, ERM proteins integrate cell surface proteins and receptors with the cortical actin rim, although their role in the regulation of endothelial barrier function is not well understood.
HSP27 is a member of the small heat shock protein family. When it is dephosphorylated, HSP27 inhibits actin polymerization (Miron et al., 1991). However, several edemagenic agents, such as thrombin, TNF-α, LPS and H2O2, induce HSP27 phosphorylation (Gilmont et al., 1996; Hirano et al., 2004; Huot et al., 1997; Mendelsohn et al., 1991). In its phosphorylated state, HSP27 relieves its inhibitory effect on actin polymerization (Benndorf et al., 1994) and stress fiber formation, causing endothelial barrier disruption. These findings suggest that in extreme cases, F-actin polymerization disrupts the endothelial barrier. However, extreme F-actin depolymerization also results in endothelial barrier disruption (Bogatcheva et al., 2003; Moy et al., 2004). Thus, a tightly controlled balance of polymerization and depolymerization is necessary to maintain cell shape and control the endothelial cell barrier function.
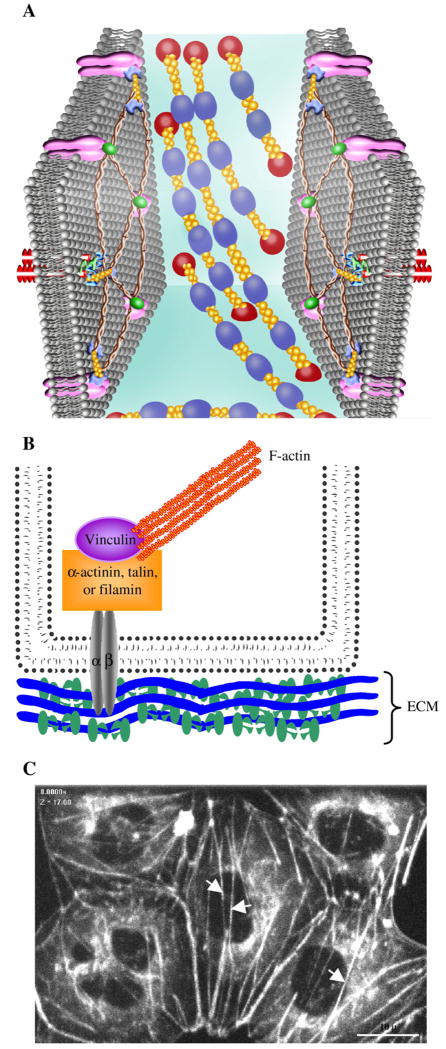
Stress Fibers
Stress fibers (Fig. 3) are actomyosin bundles that are necessary for inducing cell contraction (Hotulainen and Lappalainen, 2006); stress fibers dramatically influence the rate and size of the inter-endothelial cell gaps that form as cells retract from their borders (Dudek and Garcia, 2001; Patterson and Lum, 2001). Stress fibers consist of short F-actins with alternating polarity (Cramer et al., 1997), and are cross linked by α-actinin and other actin binding proteins (Hotulainen and Lappalainen, 2006). Myosin motor proteins track along cross-linked F-actin and form actomyosin contractile bundles necessary for generating centripetal tension (Dudek and Garcia, 2001), and for linking the cell to cell-matrix adhesions (Dudek and Garcia, 2001; Small et al., 1999). While the membrane skeleton and the cortical actin rim are situated close to the cell membrane, stress fibers extend throughout the cell cytoplasm. Indeed, stress fibers generate a centripetal (inward) tension that counteracts the centrifugal (outward) tension established by the cortical actin rim.
Fig. 3.
Stress fibers course through the cytosol, and also interact with focal adhesion complexes as they generate an inward centripetal tension. A) Schematic of stress fiber organization composed of long parallel bundles of actomyosin ( ) in the cytosol. B) Schematic of focal adhesion organization showing an integrin (α and β-heterodioamer)-mediated plaque. F-actin binds to vinculin, α-actinin, talin, and filamin and forms cytoplasmic focal adhesion plaques by binding to short cytoplasmic domain of transmembrane integrin receptor bound to extracellular matrix. C) Stress fibers are shown in pulmonary microvascular endothelial cells that were infected with a retrovirus to stably express GFP-β-actin. White arrows point the organization of stress fibers.
) in the cytosol. B) Schematic of focal adhesion organization showing an integrin (α and β-heterodioamer)-mediated plaque. F-actin binds to vinculin, α-actinin, talin, and filamin and forms cytoplasmic focal adhesion plaques by binding to short cytoplasmic domain of transmembrane integrin receptor bound to extracellular matrix. C) Stress fibers are shown in pulmonary microvascular endothelial cells that were infected with a retrovirus to stably express GFP-β-actin. White arrows point the organization of stress fibers.
Rho GTPases
Rho, Rac, and Cdc42 are Rho GTPases that belong to the superfamily of small GTP binding proteins. These proteins are responsible for transducing extracellular signals to control of the actin cytoskeleton (Tapon and Hall, 1997). Rho GTPases therefore regulate endothelial cell barrier function (Wojciak-Stothard and Ridley, 2002). While Rac and Cdc42 induce lamellipodia and filopodia formation (Nobes and Hall, 1995; Takai et al., 2001), Rho proteins (RhoA, RhoB, RhoC), and RhoA in particular, induces stress fiber and focal adhesion formation. RhoA activates its downstream kinase, rho kinase, which phosphorylates actin regulatory targets (Birukova et al., 2004a; Birukova et al., 2004d; Essler et al., 1998; Essler et al., 1999; Nobes and Hall, 1995; Verin et al., 2001; Wojciak-Stothard and Ridley, 2002). For example, activated Rho kinase inhibits myosin light chain (MLC) phosphatase by phosphorylating its myosin binding subunit (Birukova et al., 2004d; Essler et al., 1998; Essler et al., 1999; Verin et al., 2001). Inhibition of MLC phosphatase activity leads to a net increase in MLC phosphorylation induced by the calcium/CaM-dependent MLC kinase (Birukova et al., 2004d; Essler et al., 1998; Essler et al., 1999; Verin et al., 2001). Increased MLC phosphorylation increases actomyosin interaction, causing diffuse F-actin to bundle into stress fibers and increase endothelial permeability. Indeed, inhibition of RhoA activity by Clostridium botulinum toxin attenuates oxidant-induced MLC kinase activity and subsequent disruption in endothelial barrier function (Garcia et al., 1999). RhoA activation also induces actin polymerization and stress fiber formation by inhibiting the actin binding protein cofilin, independent of the Rho kinase-induced increase in MLC phosphorylation (Gorovoy et al., 2005; Maekawa et al., 1999). Dephosphorylated cofilin depolymerizes F-actin and limits stress fiber formation (Lappalainen and Drubin, 1997). RhoA effectors such as mDia (a mammalian diaphanous homolog) and Rho kinase activate LIM kinase, causing cofilin phosphorylation (Gorovoy et al., 2005; Maekawa et al., 1999). Phosphorylated cofilin is inactive, and RhoA-induced inactivation of cofilin promotes actin polymerization, stress fiber formation and endothelial barrier disruption. Additionally, localization of Round1, another family member of Rho, to adherens junctions induces F-actin depolymerization and causes cell rounding and loss of cell-cell adhesions in fibroblasts (Nobes et al., 1998).
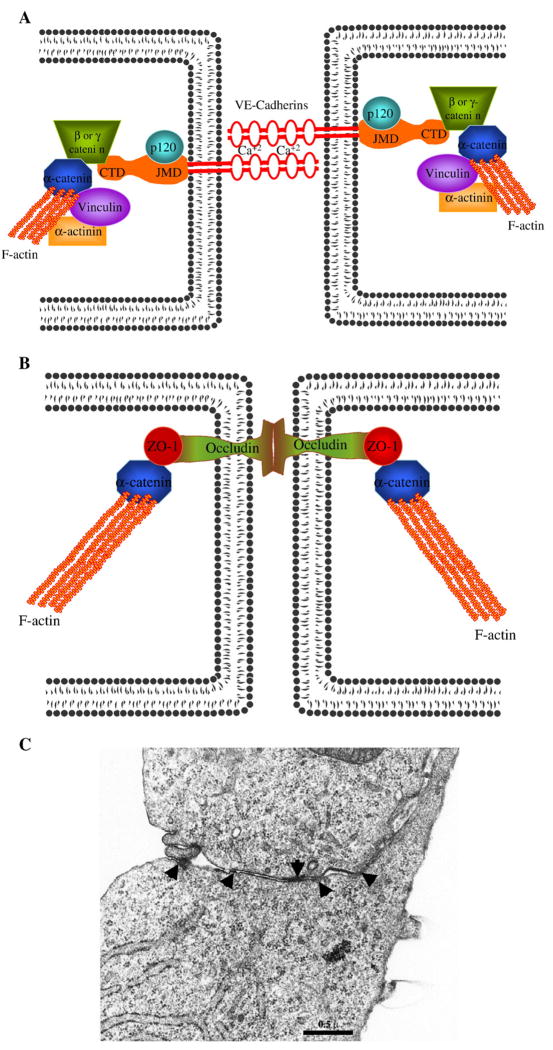
Cell-cell junctions
Direct assocation of the actin cytoskeleton with cell adhesion proteins is essential to barrier function (Fig. 4). Tight junctions and adherens junctions connect adjacent cells and regulate paracellular permeability (Bazzoni and Dejana, 2004). Adherens junctions are formed by calcium-dependent homophilic binding between extra-cellular amino-terminal domains of VE-cadherin molecules from adjacent cells (Bazzoni and Dejana, 2004; Dejana et al., 1999). VE-cadherin also possesses two cytosolic domains, known as a juxtra-membrane membrane domain and a carboxyl-terminal domain. The cytosolic domains of cadherin are connected to the actin cytoskeleton through intracellular anchoring proteins such as p120-catenin, α-catenin, β-catenin and γ-catenin (Bazzoni and Dejana, 2004). p120-catenin binds to the juxtramembrane domain, whereas β-catenin or γ-catenin bind to the carboxyl-terminal domain; in this latter case, β-catenin and γ-catenin binding is mutually exclusive. β-catenin and γ-catenin also interact with α-catenin (Dejana et al., 2001). α-catenin binds to F-actin directly or indirectly through actin binding proteins, including α-actinin (Knudsen et al., 1995), vinculin (Watabe-Uchida et al., 1998), and zona occludens protein-1 (ZO-1) (Imamura et al., 1999). Finally, α-catenin links the VE-cadherin-β-catenin complex or the VE-cadherin-γ-catenin complex to the actin cytoskeleton(Bazzoni and Dejana, 2004). Formation of leaky adherens junctions after ectopic expression of VE-cadherin lacking the extracellular domain (Venkiteswaran et al., 2002), disruption of VE-cadherin homotypic binding (Corada et al., 2001; Gotsch et al., 1997; Hordijk et al., 1999), or the chelation of extracellular calcium from homotypic junctions (Gao et al., 2000), all suggest that VE-cadherin plays an essential role in maintaining integrity of the endothelial barrier. Moreover, VE-cadherin null mice die in utero due to immature vascular development, indicating a central role for VE-cadherin in proper adherens junction assembly (Carmeliet et al., 1999; Vittet et al., 1997).
Fig. 4.
VE-cadherin-mediated adherens junctions and occludin-mediated tight junctions generate tethering forces that keep cells in juxtaposition to each other, and oppose centripetal contractile forces. A) Schematic of VE-cadherin-mediated adherens junctions. Adherens junctions are formed by the calcium-dependent homophilic binding between extracellular amino-terminal domains of VE-cadherin molecules from the adjacent cells. While the VE-cadherin juxtramembrane domain (JMD) binds p120, the carboxyl-terminal domain (CTD) binds mutually exclusively to either β-catenin or γ-catenin. β-catenin or γ-catenin binds to α-catenin, which then interacts with F-actin. α-catenin also binds to several actin binding proteins including vinculin and α-actinin, further strengthening the link between adherens junction complexes and F-actin. B) Schematic of the occludin-mediated tight junctions. Occludin-mediated tight junctions are formed by the homotypic binding between extracellular amino terminal domains of occludin molecules from the adjacent cells. The cytoplasmic carboxyl domain of occludin binds to ZO-1. ZO-1, by binding with α-catenin, connects the tight junction complexes to the F-actin. C) TEM analysis shows organization of electron dense adhesion complexes (black arrows). Adherens junctions and tight junctions form zipperlike electron dense structures between the adjacent cells and regulate paracellular permeability.
The actin cytoskeleton plays a crucial role in assembly and organization of adherens junctions. In non-endothelial cells, homo-typic binding of E-cadherin molecules from adjacent cells promote outside-in signal transduction, activating Cdc42 and causing cortactin and WASP activation (Erickson and Cerione, 2001; Millard et al., 2004). Cortactin and WASP, in turn, induce Arp2/3 activation, causing increased actin polymerization and promoting stabilization of adherens junctions (Erickson and Cerione, 2001; Millard et al., 2004). Additionally, cadherin homotypic binding between adjacent cells recruits other members of Rho GTPases, such as Rac, close to adherens junctions to further promote actin polymerization and junctional stability (Lampugnani et al., 2002; Noren et al., 2001).
Anchoring adherens junction proteins to F-actin is particularly important in regulation of endothelial barrier function (Navarro et al., 1995; Takeichi, 1990). The β–catenin binding site in the cytoplasmic domain of VE-cadherin is essential for maintaining a restrictive barrier, since transfection of VE-cadherin lacking amino acid sequences responsible for β–catenin binding causes loss of barrier function, while cell-cell contacts remain intact (Navarro et al., 1995). Also, localization of actin polymerizing proteins such as VASP (Comerford et al., 2002), α-actinin (Knudsen et al., 1995), vinculin (Imamura et al., 1999; Watabe-Uchida et al., 1998), and formin (Kobielak et al., 2004) close to adherens junctions enable linkage with α-catenin, and support the important role of actin polymerization in the formation and stabilization of adherens junctions.
The actin cytoskeleton is invariably involved in the assembly and stabilization of tight junctions. Endothelial tight junctions, first described by Staehelin (Staehelin, 1975) and by Yee and Revel (Yee and Revel, 1975), constitutes nearly 20% of the total endothelial junctional complexes (Wojciak-Stothard et al., 2001), and are involved in the regulation of macromolecular permeability (Milton and Knutson, 1990). Tight junctions are formed by the homotypic or heterotypic binding of the amino terminal domains of transmembrane adhesion molecules, such as claudin, occludin and junctional adhesion molecules from the adjacent endothelial cells. Claudin is the major transmembrane protein and forms homotypic or heterotypic binding (Furuse et al., 1999; Tsukita and Furuse, 2000). Occludin was the first tight junction transmembrane adhesion protein identified, and forms only homotypic binding with another occludin from an adjacent cell (Furuse et al., 1993). The intracellular linker protein ZO-1 links the carboxyl terminal of transmembrane adhesion proteins (Chen et al., 1997; Furuse et al., 1994) with α-catenin (Muller et al., 2005; Schmidt et al., 2004), spectrin (Mattagajasingh et al., 2000) and F-actin (Chen et al., 1997; Furuse et al., 1994). ZO-1 also binds to the actin polymerizing protein VASP (Comerford et al., 2002), indicating an essential role of actin polymerization in the assembly and stabilization of tight junctions.
Cell-matrix adhesion
Intimate interaction of the actin cytoskeleton with integrins and, consequently, the extracellular matrix, also contributes to endothelial barrier integrity (Curtis et al., 1995). Association of F-actin with the actin binding proteins vinculin, talin, α-actinin, zyxin, tensin, and filamin form cytoplasmic focal adhesion plaques, which in turn bind to the short cytoplasmic domain of the transmembrane integrin receptor that is tethered to the extracellular matrix (Burridge et al., 1988; Geiger et al., 2001). In non-activated confluent endothelial cells, focal adhesion plaques and associated cytoskeleton binding proteins are arranged around the cell periphery and appear as punctuate structures (Schaphorst et al., 1997; Curtis et al., 1995; Patterson and Lum, 2001). Upon endothelial cell activation by permeability inducing agonists such as thrombin or phorbol myristate acetate, focal adhesion plaques and the associated actin binding proteins from the cell periphery reorganize to sites where stress fibers anchor to the extracellular matrix and generate tension (Garcia et al., 1995; Schaphorst et al., 1997). Although the specific role of the F-actin-to-matrix attachment in the assembly of inter-endothelial junctions is still incompletely understood, the F-actin-matrix attachment clearly favors cell adhesion over cell proliferation (Form et al., 1986).
Microtubules and actin
Microtubules are rigid hollow tubes that originate from the microtubule organizing center (MTOC) near the nucleus, and radiate from the nucleus to the cell periphery (Wade and Hyman, 1997). Microtubules extend throughout the cytosol and utilize the motor proteins dynein and dynactin to interact with the spectrin-based membrane skeleton (Lambert et al., 1997; Malchiodi-Albedi et al., 1993; Wechsler and Teichberg, 1998; Drenckhahn and Merte, 1987). However, microtubules do not themselves extend to the plasma membrane (Conacci-Sorrell et al., 2002; Dejana, 2004; Dudek and Garcia, 2001; Lampugnani et al., 2002; Small and Kaverina, 2003).
Microtubules undergo frequent assembly and disassembly (Wade and Hyman,1997). Each microtubule is 25 nm in diameter, and its wall is made from approximately 13 parallel protofilaments formed by the self-assembly of alternating α- and β-tubulin heterodimers (Wade and Hyman, 1997). Endothelial cell shape and, consequently, barrier integrity is critically determined by organization of the actin cytoskeleton and microtubules (Dudek and Garcia, 2001; Lee and Gotlieb, 2003). As with the cortical actin network, microtubule disruption impairs endothelial cell barrier function (Birukova et al., 2004a; Verin et al., 2001), whereas microtubule stabilization strengthens barrier function (Suzuki et al., 2004). Interestingly, microtubule disruption reorganizes the cortical actin rim into stress fibers, and the combination of disrupted microtubules and increased actomyosin-based tension causes endothelial barrier disruption (Birukova et al., 2004a; Verin et al., 2001). These findings reveal an important role for microtubule-actin cross talk in regulation of endothelial barrier function.
Indeed, microtubule architecture directly influences organization of the actin cytoskeleton. Microtubule disruption frees Rho activators, such as Rho guanine exchange factors (RhoGEFs), from bound tubulin, causing RhoA activation and stress fiber formation (Krendel et al., 2002; van Horck et al., 2001), whereas microtubule polymerization sequesters LIM kinase 1 and limits its access to the actin cytoskeleton (Gorovoy et al., 2005; Maekawa et al., 1999). LIM kinase 1 reorganizes the cortical actin rim into stress fibers by phosphorylating and inhibiting activity of the actin depolymerizing factor cofilin (Gorovoy et al., 2005; Maekawa et al., 1999). Interestingly, administration of anticancer vinca alkaloids, which disrupt microtubules, result in sudden development of pulmonary edema (Cattan and Oberg, 1999). Thus, the interaction between microtubules and actin greatly influence the nature of cytoskeletal interactions that control endothelial cell barrier integrity.
Microtubule and actin coupling
For many years, microtubules and actin were thought to be separate entities, however recent findings indicate they are intricately inter-related (Fuchs and Yang, 1999; Goode et al., 2000; Klymkowsky, 1999). The physical interaction between microtubules and actin occurs directly, or indirectly through intermediate proteins and signaling molecules (Goode et al., 2000; Lee and Gotlieb, 2002; Lee and Gotlieb, 2003). Deepetch electron microscopy studies in neurons revealed direct interaction between microtubules and actin cytoskeleton (Goode et al., 2000). In these studies, microtubule-associated proteins were found to cross-link microtubules to the actin cytoskeleton. Indirect cross-linking of microtubules with actin is also mediated by intermediate linker or scaffolding proteins that bind to both microtubules and actin, such as coronin (Goode et al., 1999), centractin (Clark and Meyer, 1992), and IQGAP1 (Brunner, 2002; Fukata et al., 2002). Among these known interactions, the best understood is IQGAP1 (Brunner, 2002; Fukata et al., 2002; Tirnauer, 2004). Recent studies have established a role for IQGAP1 in integrating signals that control microtubule and actin organization, such as Ca2+, calmodulin, and Cdc42 (Briggs and Sacks, 2003; Mateer et al., 2003; Watanabe et al., 2004). IQGAP1 is named for its sequence homology to GTPase activating proteins, and for the presence of calmodulin binding motifs (IQ domain), although it does not possess GAP activity (Briggs and Sacks, 2003; Mateer et al., 2003; Tirnauer, 2004). While the amino terminus of IQGAP1 directly binds and cross links F-actin (Bashour et al., 1997), its carboxyl terminus binds to microtubule binding proteins such as CLIP-170 and EB1 (Brunner, 2002; Fukata et al., 2002; Watanabe et al., 2004). IQGAP1 cross-links the actin cytoskeleton to microtubules by binding to microtubule bound CLIP-170 and EB1 (Watanabe et al., 2004). CLIP-170 and EB1 are microtubule plus end tracking proteins (+tips) that localize to the plus end of growing microtubules and form polarized microtubule arrays (Gundersen, 2002; Schuyler and Pellman, 2001), which can be captured by IQGAP1 (Watanabe et al., 2004). Additionally, near the cell cortex, peripheral microtubules are stabilized by RhoA and its effector mDia (Palazzo et al., 2001). Thus, scaffolding proteins bind to both microtubules and actin, and integrate signals to achieve a coordinated cytoskeletal response to environmental cues.
In addition to the control of endothelial cell barrier function, the coordinated interaction between microtubules and actin is important for vesicular transport (Allan and Schroer,1999; Kuznetsov et al.,1992; Langford, 1995). Microtubule and actin motor proteins physically associate with each other to form hetero-motor complexes (Benashski et al., 1997; Huang et al., 1999) that indirectly cross-link microtubules and actin cytoskeleton, providing an explanation for how two separate motor systems influence transport of a single vesicle or organelle.
cAMP and the endothelial barrier
cAMP is an important second messenger that critically influences cytoskeletal disposition and, hence, endothelial cell barrier function. cAMP is synthesized by the activity of adenylyl cyclases (ACs), and is degraded by the activity of phospodiesterases (PDEs), which together establish the concentration of whole cell cAMP (Chetham et al., 1997; Stevens et al., 1995; Thompson et al., 2002). High membrane cAMP levels are established by the coordinated activities of membrane-associated ACs (AC1 to AC9) which generate cAMP near the plasma membrane (Ludwig and Seuwen, 2002; Sayner and Stevens, 2006; Sayner et al., 2006; Sayner et al., 2004). PDEs degrade cAMP before it enters into the bulk cytosol (Creighton et al., 2003; Insel, 2003; Stevens et al., 1999). In pulmonary microvascular endothelial cells, AC6 (Ludwig and Seuwen, 2002) and PDE4 (Thompson et al., 2002) are the principal adenylyl cyclase and phospodiesterases isoforms which establish a membrane-delimited cAMP pool that is barrier protective (Ludwig and Seuwen, 2002; Sayner and Stevens, 2006; Sayner et al., 2006; Sayner et al., 2004). Calcium inhibition of AC6 (calcium-inhibited) is sufficient to reduce membrane cAMP and increase permeability (Cioffi et al., 2002; Stevens et al., 1995), as expression of a calcium-stimulated isoform (e.g. AC8) maintains membrane cAMP levels and blocks calcium-induced permeability (Cioffi et al., 2002). These data collectively suggest that high plasma membrane cAMP levels are barrier protective.
Paradoxically, while increased membrane cAMP levels are barrier protective, recent studies have shown that cytosolic AC activity is barrier disruptive. ExoY is a bacterial toxin from Pseudomonas aeruginosa that acts as a soluble adenylyl cyclase (sAC). When ExoY is introduced into pulmonary microvascular endothelial cells, it combines with a mammalian co-factor and specifically increases cytosolic cAMP that disrupts the barrier (Sayner et al., 2004). Like ExoY, sACI/II is a soluble AC. This enzyme is a chimeric mammalian soluble AC that is generated by linking the C1a loop of ACI to the C2a loop of ACII using a 14 amino acid linker. When this enzyme is stimulated by forskolin it increases total cAMP by a modest 1.5 fold (Dessauer and Gilman, 1996; Sayner and Stevens, 2006; Sayner et al., 2006; Tang and Gilman,1995). The sACI/II-generated increase in cAMP originates in the cytosolic compartment, and in contrast to increased cAMP that is generated by endogenous membrane ACs, the increase in cytosolic cAMP generated by chimeric sACI/II disrupts the barrier function in a manner that is remarkably similar to that observed with bacterial toxins (Sayner and Stevens, 2006; Sayner et al., 2006). These findings highlight the importance of maintaining cAMP within discrete cellular compartments, and provide insight into why cAMP concentrations are 12-fold higher in membrane compartment than they are in the cytosolic compartment (Rich et al., 2001).
cAMP, actin and microtubules
It is unclear how cAMP interacts with actin microfilaments and microtubules to regulate endothelial barrier function. There are few studies addressing the mechanism by which increased cAMP strengthens the cortical actin cytoskeletal network that enhances the endothelial barrier. Stelzner and colleagues originally implicated cAMP in stabilizing the cortical actin rim (Stelzner et al., 1989). Recently, Fukuhara and coworkers reported that cAMP acts through an Epac-Rap1 signaling pathway to enhances the endothelial barrier function by facilitating cell-cell contact (Fukuhara et al., 2005). However, specific cAMP effectors that control microtubule organization remain largely unexplored. Our recent studies have begun to address this important issue. Interestingly, we observed that sAC/II activation disassembles microtubules and induces inter-endothelial gaps. However, in contrast to other agents that disassemble microtubules, F-actin did not reorgnize from its cortical rim into stress fibers (Stevens, unpublished). Our findings illustrate the preferential targeting of cAMP that is synthesized within the cytosol to microtubule structures, and demonstrate that cell border retraction can occur in the absence of concomitant stress fiber formation.
Endothelial cell retraction that occurs in the absence of stress fiber formation, while the cortical actin rim remains intact, is a surprising observation. As indicated, pharmacological agents that directly disrupt microtubules also result in stress fiber formation (Birukova et al., 2004d; Verin et al., 2001). Similarly, microtubule disruption by neurohumoral inflammatory mediators induces stress fiber formation (Birukova et al., 2004a; van Nieuw Amerongen et al., 1998; van Nieuw Amerongen et al., 2000). Endothelial cell retraction can be attenuated by either stabilizing microtubules (Birukova et al., 2004a) or inhibiting stress fiber formation (Phillips, 1994; Phillips et al., 1989; van Nieuw Amerongen et al., 1998; van Nieuw Amerongen et al., 2000), suggesting both processes are essential for cell retraction to proceed. Microtubule disruption causes myosin light chain (MLC) kinase and RhoA activation, ultimately leading to increased MLC20 phosphorylation that promotes actomyosin interaction and endothelial cell retraction (Birukova et al., 2005; Birukova et al., 2004c; Verin et al., 2001). cAMP opposes the actions of both myosin light chain kinase and RhoA; PKA directly phosphorylates both myosin light chain kinase and RhoA, and decreases MLC20 phosphorylation (Birukova et al., 2004b). Activation of sACI/II increases cytosolic cAMP that decreases MLC20 phosphorylation preventing reorganization of the cortical actin rim into stress fibers. Indeed, our recent findings provide new information regarding how compartmentalized signals may be exploited to reveal important regulatory pathways that uniquely uncouple the microtubule and actin tangle.
Endothelial cell heterogeneity and barrier function
All organs possess a vascular system composed of complex artery, capillary, and vein networks that supply blood constituents to meet the local requirements of underlying tissues (Butcher et al., 1980; Leach, 2002; Thurston et al., 2000). Endothelium forms the innermost cellular lining of blood vessels and lymphatics, and is involved in variety of physiological processes, including the regulation of vasomotor tone, leukocyte trafficking, homeostatic balance, angiogenesis, innate and adaptive immunity and permeability (Aird, 2003, 2008; Cines et al., 1998). While endothelial cell-cell and cell-matrix contacts establish the semipermeable barrier that regulates passage of proteins, fluid and leukocytes between the blood and interstitium (Patterson and Lum, 2001), the role that actin plays in regulating these processes, particularly within individual endothelial cell phenotypes, is not well understood.
Every organ is specialized to perform a unique function(s), and the endothelium within each organ is similarly specialized. For example, in brain, unrestricted access of blood constituents to cerebrospinal tissue is deleterious, and therefore adjacent endothelial cells form tight adhesions giving rise to a highly restrictive, continuous endothelium known as blood-brain barrier, in which only limited exchange between blood and brain is allowed (Gloor et al., 2001). By contrast, glomerular endothelium in the kidney is fenestrated to allow for increased fluid filtration, while restricting protein and large molecule filtration (Stan et al., 1999). It is therefore apparent that endothelial cell barrier function differs substantially among vascular bed within different organs (Aird, 2007a; Gebb and Stevens, 2004; Ofori-Acquah et al., 2008).
In addition, endothelial barrier function differs within an organ. Each of the segments within the pulmonary vascular tree- arteries, capillaries, and veins- are structurally and functionally distinct (Aird, 2007b; Gebb and Stevens, 2004). Muscularized arteries function as conduit vessels regulating the distribution of blood volume and flow to the lung. As these arteries descend into the lung parenchyma they divide into smaller, less muscularized vessels, and ultimately, into a fine capillary network composed of just a single endothelial cell layer immediately adjacent to the distal epithelial cell lining. This alveolar-capillary network optimizes gas exchange, enabling efficient delivery of O2 from the alveoli into the capillary and CO2 from the capillary into the alveoli. Oxygenated blood is collected by venules, which are poorly muscularized, and ultimately delivered to the left heart by larger conduit veins, which become increasingly muscularized.
Although the endothelium lining each of these segments is continuous, barrier function is quite diverse. About 20% of fluid filtration occurs in the arteries, about 40% in the capillaries, and the remaining 40% occurs in veins (Parker and Yoshikawa, 2002). While pulmonary capillary endothelial cells form a tighter barrier compared to arterial and venous endothelial cell barriers (Chetham et al., 1999; Kelly et al., 1998; Moore et al., 1998), the large capillary surface area is responsible for the higher amount of fluid filtration in this segment (Parker and Yoshikawa, 2002). Thus, when fluid and protein filtration is corrected for unit surface area, the fluid and protein filtration is significantly lower in lung capillaries than in arterial and venous segments. These in vivo observations parallel results obtained using cultured endothelium isolated from these discrete compartments (Chetham et al., 1999; Kelly et al., 1998; Moore et al., 1998). This apparent difference in endothelial barrier function for capillary and conduit endothelial cells underscores the presence of stable phenotypic differences that exist between endothelial cells isolated from capillary and conduit vessels.
Despite an increasing appreciation for heterogeneity among different endothelial cell populations, the underlying mechanism conferring such heterogeneity is not fully understood. Different mechanisms ranging from environment to endothelial cell origin have been proposed for explaining endothelial cell heterogeneity (Gebb and Stevens, 2004). For example, the mechano-chemical milieu differs substantially from arterial to capillary to venous segments. Endothelial cells sense these stimuli and can activate downstream signaling pathways that contribute site-specific endothelial cell behavior (Davies, 1995). However, the cause for endothelial functional heterogeneity cannot be fully ascribed to environmental cues. For example, histamine induces inter-endothelial gap formation in post-capillary venules of the systemic circulation, but immediately adjacent endothelial cells do not respond to histamine with gap formation (Majno and Palade, 1961). Similarly, thapsigargin, an edema-inducing plant alkaloid known to activate store-operated calcium entry (Chetham et al., 1999) by inhibiting the sarcoplasmic/endoplasmic reticulum calcium ATPase (Thastrup et al., 1990), induces inter-endothelial gap formation in intermediate to large arteries and veins, but not in capillaries, both in vivo and in vitro (Gebb and Stevens, 2004; Lowe et al., 2007; Kelly et al., 1998). Collectively, these data support the idea that environmental cues are not sufficient to cause endothelial functional heterogeneity.
Fluid and protein transport across the endothelial barrier occurs through paracellular and transcellular pathways. Transcellular transport of fluid and proteins occurs through vesicular carriers, whereas paracellular transport of fluid and proteins occurs through gaps between adjacent endothelial cells (Lum and Malik, 1994; Stevens et al., 2000). While the overall breadth and scope of transcellular transport remains an area of important study, the paracellular pathway is a major route of bulk fluid and large protein transport (Lum and Malik, 1994; Patterson and Lum, 2001; Stevens et al., 2000). Paracellular transport of fluid and proteins is initiated following loss of cell-cell and/or cell-matrix adhesions (Lum and Malik, 1994; Mehta and Malik, 2006; Stevens et al., 2000). Since the actin cytoskeleton stabilizes cell-cell and cell-matrix adhesion, stimuli that disrupt actin in the membrane skeleton, disrupt the cortical actin rim, or promote stress fiber formation also increase paracellular transport. Our recent studies indicate there is no difference in the amount of total actin that is present in cell lysates from microvascular and macrovascular endothelial cells (Ofori-Acquah et al., 2008). Moreover, the amount of circumferential actin that is directly involved in the formation and stabilization of cell-cell and cell-matrix adhesions is similar in microvascular endothelial cells when compared to macrovascular endothelial cells. However, a 5-fold greater cytochalasin D concentration is required to disrupt the pulmonary microvascular endothelial cell barrier than is needed to disrupt the pulmonary artery endothelial cell barrier. Thus, it appears that either signaling events which control actin organization, the increased expression or function of actin binding proteins that stabilize cell-cell and cell-matrix adhesion, or more effective coupling between the membrane skeleton/cortical actin rim and junctional plaques, generate a more restrictive barrier in microvascular endothelial cells (Ofori-Acquah et al., 2008).
In support of the latter idea, cell-cell adhesion molecules, such as N-cadherin and activated leukocyte cell adhesion molecule (ALCAM), are recruited to the adherens junction in pulmonary microvascular endothelial cells, where they interact with VE-cadherin to form a multi-protein complex that strengthens the barrier function (Ofori-Acquah et al., 2008). N-cadherin expression was 16.5-fold higher in pulmonary microvascular endothelial cells than it was in pulmonary artery endothelial cells, and N-cadherin protein was not detected in pulmonary artery endothelial cells. Similarly, ALCAM expression was higher in microvascular than in conduit cells. Further, ALCAM was concentrated at cell-cell contacts in pulmonary microvascular endothelial cells, but was not at cell-cell contacts in pulmonary artery endothelial cells. Thus, unique actin regulatory properties and protein complexes are found in microvascular endothelial cells, that collectively conribute to the greater barrier strength of these cells when compared with their macrovacular counterparts.
Summary
The actin cytoskeleton represents a collection of highly dynamic structures that contribute to the architecture of the membrane skeleton, the cortical actin rim, and actomyosin based stress fibers. Actin interacts with many secondary proteins, and its function is highly controlled by the nature of these interactions. In quiescent endothelium, the actin cytoskeleton cross-links spectrin in the membrane skeleton, which is then linked to the underlying cortical actin rim. These structures interact with transmembrane proteins, such as cell-cell and cell-matrix adhesion complexes, providing structural support for the endothelial barrier. In response to physico-chemical signals that induce cell border retraction, the cortical actin rim typically reorganizes into stress fibers, where it increases the centripetally directed tension that promotes gap formation. Recent identification of mechanisms which enable cell retraction without reorganization of the cortical actin rim into stress fibers bring into question which cell signaling networks are responsible for controlling actin disposition. Indeed, given the broad importance of cytoskeletal control over cell shape, it will be essential to further probe the intricacies of actin dynamics, to better understand its regulatory signals and proteins and, ultimately, how it exerts control over endothelial cell shape.
Acknowledgments
This work was supported by HL-60024 and HL-66299 (to T.S.), and by an American Heart Association Southeast Consortium Predoctoral Fellowship (to N.P.). The authors thank Frank Vogtner for his assistance in completing schematic representations in the figures.
References
- Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007a;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007b;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- Allan VJ, Schroer TA. Membrane motors. Curr Opin Cell Biol. 1999;11:476–482. doi: 10.1016/s0955-0674(99)80068-4. [DOI] [PubMed] [Google Scholar]
- Bashour AM, et al. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137:1555–1566. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Bearer EL, et al. VASP protects actin filaments from gelsolin: an in vitro study with implications for platelet actin reorganizations. Cell Motil Cytoskeleton. 2000;47:351–364. doi: 10.1002/1097-0169(200012)47:4<351::AID-CM8>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benashski SE, et al. Dimerization of the highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. doi: 10.1074/jbc.272.33.20929. [DOI] [PubMed] [Google Scholar]
- Benndorf R, et al. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004a;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2004b;287:L86–93. doi: 10.1152/ajplung.00441.2003. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004c;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004d;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, et al. Phorbol esters increase MLC phosphorylation and actin remodeling in bovine lung endothelium without increased contraction. Am J Physiol Lung Cell Mol Physiol. 2003;285:L415–426. doi: 10.1152/ajplung.00364.2001. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Korn ED. Spectrin-actin interaction. Phosphorylated and depho-sphorylated spectrin tetramer cross-link F-actin. J Biol Chem. 1979;254:8620–8627. [PubMed] [Google Scholar]
- Brenner SL, Korn ED. Spectrin/actin complex isolated from sheep erythrocytes accelerates actin polymerization by simple nucleation. Evidence for oligomeric actin in the erythrocyte cytoskeleton. J Biol Chem. 1980;255:1670–1676. [PubMed] [Google Scholar]
- Bretscher A, et al. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- Brunner D. How to grab a microtubule on the move. Dev Cell. 2002;3:2–4. doi: 10.1016/s1534-5807(02)00209-5. [DOI] [PubMed] [Google Scholar]
- Burridge K, et al. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Butcher EC, et al. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980;10:556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene inmice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Cattan CE, Oberg KC. Vinorelbine tartrate-induced pulmonary edema confirmed on rechallenge. Pharmacotherapy. 1999;19:992–994. doi: 10.1592/phco.19.11.992.31580. [DOI] [PubMed] [Google Scholar]
- Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–613. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetham PM, et al. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+entry. Am J Physiol. 1999;276:L41–50. doi: 10.1152/ajplung.1999.276.1.L41. [DOI] [PubMed] [Google Scholar]
- Chetham PM, et al. Ca(2+)-inhibitable adenylyl cyclase and pulmonary microvascular permeability. Am J Physiol. 1997;273:L22–30. doi: 10.1152/ajplung.1997.273.1.L22. [DOI] [PubMed] [Google Scholar]
- Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Cioffi DL, et al. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol. 2002;157:1267–1278. doi: 10.1083/jcb.200204022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SW, Meyer DI. Centractin is an actin homologue associated with the centrosome. Nature. 1992;359:246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- Comerford KM, et al. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002;16:583–585. doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, et al. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Cramer LP, et al. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J Cell Biol. 1997;136:1287–1305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton JR, et al. Coordinate regulation of membrane cAMP by Ca2+-inhibited adenylyl cyclase and phosphodiesterase activities. Am J Physiol Lung Cell Mol Physiol. 2003;284:L100–107. doi: 10.1152/ajplung.00083.2002. [DOI] [PubMed] [Google Scholar]
- Curtis TM, et al. Fibronectin attenuates increased endothelial monolayer permeability after RGD peptide, anti-alpha 5 beta 1, or TNF-alpha exposure. Am J Physiol. 1995;269:L248–260. doi: 10.1152/ajplung.1995.269.2.L248. [DOI] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113 (Pt 13):2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Dejana E, et al. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- Dejana E, et al. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Hae-most. 2001;86:308–315. [PubMed] [Google Scholar]
- Dessauer CW, Gilman AG. Purification and characterization of a soluble form of mammalian adenylyl cyclase. J Biol Chem. 1996;271:16967–16974. doi: 10.1074/jbc.271.28.16967. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Merte C. Restriction of the human kidney band 3-like anion exchanger to specialized subdomains of the basolateral plasma membrane of intercalated cells. Eur J Cell Biol. 1987;45:107–115. [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA. Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol. 2001;13:153–157. doi: 10.1016/s0955-0674(00)00192-7. [DOI] [PubMed] [Google Scholar]
- Essler M, et al. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Essler M, et al. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J Biol Chem. 1999;274:30361–30364. doi: 10.1074/jbc.274.43.30361. [DOI] [PubMed] [Google Scholar]
- Form DM, et al. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986;55:521–530. [PubMed] [Google Scholar]
- Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Fukata M, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, et al. Direct association of occludinwith ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, et al. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, et al. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1218–1225. doi: 10.1152/ajplung.2000.279.6.L1218. [DOI] [PubMed] [Google Scholar]
- Garcia JG, et al. Vascular endothelial cell activation and permeability responses to thrombin. Blood Coagul Fibrinolysis. 1995;6:609–626. doi: 10.1097/00001721-199510000-00001. [DOI] [PubMed] [Google Scholar]
- Garcia JG, et al. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src) Am J Physiol. 1999;276:L989–998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res. 2004;68:1–12. doi: 10.1016/j.mvr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Geiger B, et al. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gilmont RR, et al. TNF-alpha potentiates oxidant and reperfusion-induced endothelial cell injury. J Surg Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- Gloor SM, et al. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev. 2001;36:258–264. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- Goode BL, et al. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Goode BL, et al. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SR. Discovery of nonerythroid spectrin to the demonstration of its key role in synaptic transmission. Brain Res Bull. 1999;50:345–346. doi: 10.1016/s0361-9230(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Goodman SR, et al. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981;78:7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovoy M, et al. LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem. 2005;280:26533–26542. doi: 10.1074/jbc.M502921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsch U, et al. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110 (Pt 5):583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- Gundersen GG. Evolutionary conservation of microtubule-capture mechanisms. Nat Rev Mol Cell Biol. 2002;3:296–304. doi: 10.1038/nrm777. [DOI] [PubMed] [Google Scholar]
- Hastie LE, et al. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol. 1997;172:373–381. doi: 10.1002/(SICI)1097-4652(199709)172:3<373::AID-JCP11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Heimann K, et al. Specific isoforms of actin-binding proteins on distinct populations of Golgi-derived vesicles. J Biol Chem. 1999;274:10743–10750. doi: 10.1074/jbc.274.16.10743. [DOI] [PubMed] [Google Scholar]
- Heltianu C, et al. Endothelial cells express a spectrin-like cytoskeletal protein. Circ Res. 1986;58:605–610. doi: 10.1161/01.res.58.4.605. [DOI] [PubMed] [Google Scholar]
- Hirano S, et al. Endothelial barrier dysfunction caused by LPS correlates with phosphorylation of HSP27 in vivo. Cell Biol Toxicol. 2004;20:1–14. doi: 10.1023/b:cbto.0000021019.50889.aa. [DOI] [PubMed] [Google Scholar]
- Hordijk PL, et al. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112 (Pt 12):1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JD, et al. Direct interaction of microtubule- and actin-based transport motors. Nature. 1999;397:267–270. doi: 10.1038/16722. [DOI] [PubMed] [Google Scholar]
- Huot J, et al. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Imamura Y, et al. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA. Location, location, location. Trends Endocrinol Metab. 2003;14:100–102. doi: 10.1016/s1043-2760(03)00029-8. [DOI] [PubMed] [Google Scholar]
- Itoh M, et al. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, et al. A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol. 1991;115:1449–1462. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JJ, et al. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+and permeability. Am J Physiol. 1998;274:L810–819. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW. Weaving a tangled web: the interconnected cytoskeleton. Nat Cell Biol. 1999;1:E121–123. doi: 10.1038/12950. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, et al. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, et al. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, et al. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Krendel M, et al. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, et al. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Lambert S, et al. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts A, et al. The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol. 2004;36:1890–1909. doi: 10.1016/j.biocel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, et al. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford GM. Actin- and microtubule-dependent organelle motors: interrelationships between the two motility systems. Curr Opin Cell Biol. 1995;7:82–88. doi: 10.1016/0955-0674(95)80048-4. [DOI] [PubMed] [Google Scholar]
- Lappalainen P, Drubin DG. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. doi: 10.1038/40418. [DOI] [PubMed] [Google Scholar]
- Leach L. The phenotype of the human materno-fetal endothelial barrier: molecular occupancy of paracellular junctions dictate permeability and angiogenic plasticity. J Anat. 2002;200:599–606. doi: 10.1046/j.1469-7580.2002.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Gotlieb AI. Microtubule-actin interactions may regulate endothelial integrity and repair. Cardiovasc Pathol. 2002;11:135–140. doi: 10.1016/s1054-8807(01)00080-1. [DOI] [PubMed] [Google Scholar]
- Lee TY, Gotlieb AI. Microfilaments and microtubules maintain endothelial integrity. Microsc Res Tech. 2003;60:115–127. doi: 10.1002/jemt.10250. [DOI] [PubMed] [Google Scholar]
- Lowe K, et al. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res. 2007;143:70–77. doi: 10.1016/j.jss.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MG, Seuwen K. Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J Recept Signal Transduct Res. 2002;22:79–110. doi: 10.1081/rrs-120014589. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- Maekawa M, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Majno G, Palade GE. Studies on inflammation. 1 The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchiodi-Albedi F, et al. The 270 kDa splice variant of erythrocyte beta-spectrin (beta I sigma 2) segregates in vivo and in vitro to specific domains of cerebellar neurons. J Cell Sci. 1993;106 (Pt 1):67–78. doi: 10.1242/jcs.106.1.67. [DOI] [PubMed] [Google Scholar]
- Mateer SC, et al. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh SN, et al. Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J Biol Chem. 2000;275:30573–30585. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, et al. The 29-kDa proteins phosphorylated in thrombin-activated human platelets are forms of the estrogen receptor-related 27-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991;88:11212–11216. doi: 10.1073/pnas.88.24.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard TH, et al. Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem J. 2004;380:1–17. doi: 10.1042/BJ20040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton SG, Knutson VP. Comparison of the function of the tight junctions of endothelial cells and epithelial cells in regulating the movement of electrolytes and macromolecules across the cell monolayer. J Cell Physiol. 1990;144:498–504. doi: 10.1002/jcp.1041440318. [DOI] [PubMed] [Google Scholar]
- Miron T, et al. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TM, et al. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am JPhysiol. 1998;275:L203–222. doi: 10.1152/ajplung.1998.275.2.L203. [DOI] [PubMed] [Google Scholar]
- Moy AB, et al. Phorbol ester-mediated pulmonary artery endothelial barrier dysfunction through regulation of actin cytoskeletal mechanics. Am J Physiol Lung Cell Mol Physiol. 2004;287:L153–167. doi: 10.1152/ajplung.00292.2003. [DOI] [PubMed] [Google Scholar]
- Muller SL, et al. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- Navarro P, et al. Catenin-dependent and-independent functions of vascular endothelial cadherin. J Biol Chem. 1995;270:30965–30972. doi: 10.1074/jbc.270.52.30965. [DOI] [PubMed] [Google Scholar]
- Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008;40:344–349. doi: 10.1016/j.biocel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, et al. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, et al. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- Ofori-Acquah SF, et al. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res. 2008;75:391–402. doi: 10.1016/j.mvr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo AF, et al. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3:723–729. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- Parker JC, Yoshikawa S. Vascular segmental permeabilities at high peak inflation pressure in isolated rat lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1203–1209. doi: 10.1152/ajplung.00488.2001. [DOI] [PubMed] [Google Scholar]
- Patterson CE, Lum H. Update on pulmonary edema: the role and regulation of endothelial barrier function. Endothelium. 2001;8:75–105. doi: 10.3109/10623320109165319. [DOI] [PubMed] [Google Scholar]
- Phillips PG. Thrombin-induced alterations in endothelial cell cytoarchitectural and functional properties. Semin Thromb Hemost. 1994;20:417–425. doi: 10.1055/s-2007-1001930. [DOI] [PubMed] [Google Scholar]
- Phillips PG, et al. Phallacidin prevents thrombin-induced increases in endothelial permeability to albumin. Am J Physiol. 1989;257:C562–567. doi: 10.1152/ajpcell.1989.257.3.C562. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Polymerization of ADP-actin. J Cell Biol. 1984;99:769–777. doi: 10.1083/jcb.99.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan D, et al. alpha-Catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem. 2001;276:4175–4181. doi: 10.1074/jbc.M009259200. [DOI] [PubMed] [Google Scholar]
- Pratt BM, et al. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984;117:349–354. [PMC free article] [PubMed] [Google Scholar]
- Reinhard M, et al. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M, et al. VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. FEBS Lett. 1996;399:103–107. doi: 10.1016/s0014-5793(96)01295-1. [DOI] [PubMed] [Google Scholar]
- Revenu C, et al. The co-workers of actin filaments: from cell structures to signals. Nat Rev Mol Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- Rich TC, et al. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R, et al. Coordinate induction of the actin cytoskeletal regulatory proteins gelsolin, vasodilator-stimulated phosphoprotein, and profilin during capillary morphogenesis in vitro. Exp Cell Res. 1999;249:22–32. doi: 10.1006/excr.1999.4460. [DOI] [PubMed] [Google Scholar]
- Sayner S, Stevens T. Soluble adenylate cyclase reveals the significance of compartmentalized cAMP on endothelial cell barrier function. Biochem Soc Trans. 2006;34:492–494. doi: 10.1042/BST0340492. [DOI] [PubMed] [Google Scholar]
- Sayner SL, et al. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res. 2006;98:675–681. doi: 10.1161/01.RES.0000209516.84815.3e. [DOI] [PubMed] [Google Scholar]
- Sayner SL, et al. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- Schaphorst KL, et al. Thrombin-mediated focal adhesion plaque reorganization in endothelium: role of protein phosphorylation. Am J Respir Cell Mol Biol. 1997;17:443–455. doi: 10.1165/ajrcmb.17.4.2502. [DOI] [PubMed] [Google Scholar]
- Schmidt A, et al. Occludin binds to the SH3-hinge-GuK unit of zonula occludens protein 1: potential mechanism of tight junction regulation. Cell Mol Life Sci. 2004;61:1354–1365. doi: 10.1007/s00018-004-4010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler SC, Pellman D. Microtubule “plus-end-tracking proteins”: the end is just the beginning. Cell. 2001;105:421–424. doi: 10.1016/s0092-8674(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Shasby DM, et al. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982;51:657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Small JV, Kaverina I. Microtubules meet substrate adhesions to arrange cell polarity. Curr Opin Cell Biol. 2003;15:40–47. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Small JV, et al. Cytoskeleton cross-talk during cell motility. FEBS Lett. 1999;452:96–99. doi: 10.1016/s0014-5793(99)00530-x. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. A new occludens-like junction linking endothelial cells of small capillaries (probably venules) of rat jejunum. J Cell Sci. 1975;18:545–551. doi: 10.1242/jcs.18.3.545. [DOI] [PubMed] [Google Scholar]
- Stan RV, et al. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A. 1999;96:13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz MO, et al. Actin: from cell biology to atomic detail. J Struct Biol. 1997;119:295–320. doi: 10.1006/jsbi.1997.3873. [DOI] [PubMed] [Google Scholar]
- Stelzner TJ, et al. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol. 1989;139:157–166. doi: 10.1002/jcp.1041390122. [DOI] [PubMed] [Google Scholar]
- Stevens T, et al. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol. 1999;277:L119–126. doi: 10.1152/ajplung.1999.277.1.L119. [DOI] [PubMed] [Google Scholar]
- Stevens T, et al. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- Stevens T, et al. Ca(2+)-inhibitable adenylyl cyclase modulates pulmonary artery endothelial cell cAMP content and barrier function. Proc Natl Acad Sci U S A. 1995;92:2696–2700. doi: 10.1073/pnas.92.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Stossel TP, et al. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Stossel TP, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- Suzuki S, et al. Paclitaxel prevents loss of pulmonary endothelial barrier integrity during cold preservation. Transplantation. 2004;78:524–529. doi: 10.1097/01.tp.0000131951.72851.57. [DOI] [PubMed] [Google Scholar]
- Takai Y, et al. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. Construction of a soluble adenylyl cyclase activated by Gs alpha and forskolin. Science. 1995;268:1769–1772. doi: 10.1126/science.7792604. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Thastrup O, et al. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WJ, et al. Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4) Biochem Pharmacol. 2002;63:797–807. doi: 10.1016/s0006-2952(01)00914-5. [DOI] [PubMed] [Google Scholar]
- Thurston G, et al. Determinants of endothelial cell phenotype in venules. Microcirculation. 2000;7:67–80. [PubMed] [Google Scholar]
- Tirnauer JS. A new cytoskeletal connection for APC: linked to actin through IQGAP. Dev Cell. 2004;7:778–780. doi: 10.1016/j.devcel.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, et al. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann NY Acad Sci. 2000;915:129–135. doi: 10.1111/j.1749-6632.2000.tb05235.x. [DOI] [PubMed] [Google Scholar]
- Ungewickell E, et al. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979;280:811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- van Horck FP, et al. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J Biol Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, et al. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, et al. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- Venkiteswaran K, et al. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am J Physiol, Cell Physiol. 2002;283:C811–821. doi: 10.1152/ajpcell.00417.2001. [DOI] [PubMed] [Google Scholar]
- Verin AD, et al. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L565–574. doi: 10.1152/ajplung.2001.281.3.L565. [DOI] [PubMed] [Google Scholar]
- Vittet D, et al. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade RH, Hyman AA. Microtubule structure and dynamics. Curr Opin Cell Biol. 1997;9:12–17. doi: 10.1016/s0955-0674(97)80146-9. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, et al. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Wechsler A, Teichberg VI. Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J. 1998;17:3931–3939. doi: 10.1093/emboj/17.14.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976;108:139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- Wu JC, et al. Role of catenins in the development of gap junctions in rat cardiomyocytes. J Cell Biochem. 2003;88:823–835. doi: 10.1002/jcb.10390. [DOI] [PubMed] [Google Scholar]
- Wu S, et al. Essential control of an endothelial cell ISOC by the spectrin membrane skeleton. J Cell Biol. 2001;154:1225–1233. doi: 10.1083/jcb.200106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee AG, Revel JP. Endothelial cell junctions. J Cell Biol. 1975;66:200–204. doi: 10.1083/jcb.66.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. Phosphorylation of platelet actin binding protein protects against proteolysis by calcium dependent sulfhydryl protease. Biochem Biophys Res Commun. 1988;151:355–360. doi: 10.1016/0006-291x(88)90601-8. [DOI] [PubMed] [Google Scholar]