Abstract
Uveitis is an inflammatory condition that can lead to blindness. It is therefore, important to understand the pathophysiology on which to develop targeted therapy. Herein, we tested whether the oxidant-responsive transcription factor Nrf2 is involved in regulating the innate immune response and oxidative damage in the LPS uveitis model. With Dihydroethidium staining, intraperitoneally injected LPS increased reactive oxygen species in the retina and iris-ciliary body of Nrf2+/+ and Nrf2−/− mice. After LPS injection, ICAM-1, IL-6, TNF-α, COX-2, iNOS, and MCP-1 mRNA were increased more in the retina and iris-ciliary body of Nrf2−/− than Nrf2+/+ mice. NQO-1 and GCLM, two Nrf2 responsive anti-oxidant enzymes, had reduced expression in Nrf2+/+ retinas after LPS injection, but no change in expression in Nrf2−/− mice. The number of FITC-con A labeled leukocytes adherent to the retinal vascular endothelium increased after LPS treatment in both Nrf2+/+ and Nrf2−/− mice compared to control injections, with the more adherent leukocytes in Nrf2−/− than Nrf2+/+ mice. Pretreatment with the Nrf2 activator 1-(2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl)imidazole, increased antioxidant gene expression in the retina, reduced inflammatory mediator expression, and reduced leukocyte adherence to retinal vasculature after LPS treatment in Nrf2+/+ mice, but had no effect on Nrf2−/− mice. Treatment targeting the Nrf2 pathway may be new therapy for uveitis.
Keywords: Adhesion molecules, Cytokines, Lipopolysaccharide (LPS), Nuclear factor erythroid-2 related factor 2 (Nrf2), Rodent, Transcription factors, Transgenic/knockout mice, triterpenoids, uveitis
Introduction
Endotoxin-induced uveitis is an established model of acute ocular inflammation caused by LPS, a component of Gram-negative bacterial outer membranes[1, 2, 3]. The innate immune system is a critical first line defense system for sensing and eliminating inflammatory stimuli. Dysregulation of the innate immune response can result in tissue injury to ocular structures during uveitis. Host factors that regulate the innate immune system may protect against an abnormal inflammatory response. However, at present, few of these factors are known. This lack of understanding in ocular inflammatory diseases is an impediment to the development of novel anti-inflammatory targets.
Reactive oxygen species (ROS) play a key role in the pathophysiology of inflammation. For example, ROS through toll-like receptor 4, primes immune cells[4]. Importantly, the response to ROS in mounting an appropriate innate immune response is a determining factor for survival from sepsis[5]. Nrf2, a basic leucine zipper redox-sensitive transcription factor, regulates the inducible expression of antioxidant and cytoprotective genes by binding to the cis-acting enhancer sequence known as the antioxidant response element[6, 7, 8]. Normally, Nrf2 levels are low, but with an oxidative stimulus, nuclear accumulation of Nrf2 increases and activates transcription of its downstream targets, resulting in upregulation of cellular antioxidants. Disruption of Nrf2 decreases the constitutive expression and adaptive response to stress. Multiple studies in murine models indicate that the Nrf2-dependent transcriptional response influences the host response to oxidative and inflammatory stress, and that Nrf2 is a critical determinant of susceptibility to several inflammatory diseases including cigarette smoke-induce emphysema[8], hyperoxia-induced acute lung injury[9], allergen-induced asthma[10], bleomycin-induced lung fibrosis[11], experimental sepsis[5], and recently neuroinflammation[12]. In this study, we tested the hypothesis that the Nrf2 pathway protects against damage in experimental uveitis by attenuating oxidative damage and regulating the innate immune response. To address this hypothesis, we measured the inflammatory response in wild type and Nrf2 deficient mice after injection of LPS. We also tested whether enhancing Nrf2 signaling by the extremely potent synthetic triterpenoid activator, CDDO-Im (1-(2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl)imidazole)[13] protects against an exaggerated innate immune response in experimental uveitis.
Materials and Methods
Mice
Nrf2 deficient (Nrf2−/−) mice were generated as described[14] and backcrossed into the C57Bl6 background. Wild type (Nrf2+/+) and Nrf2−/− mice were fed an AIN-76A diet and water ad libitum, and were housed under controlled conditions (25°C, 12 hour light-dark cycles). All experimental protocols were performed in accordance with NIH guidelines and were approved by the Johns Hopkins University Animal Care and Use Committee.
Treatment
Mice received a single intraperitoneal injection of 60 μg LPS from Escherichia coli (Sigma-Aldrich, St. Louis, MO) in 0.15 ml PBS. Control mice received an equal volume of PBS. Some mice were pretreated either with 3 doses of CDDO-Im (Reata Pharmaceuticals, Irving, TX; 3μmol/kg bodyweight, dissolved in 10% DMSO, 10% cremophor-EL, PBS) or vehicle 24 hours apart.
Histochemical evaluation of Reactive Oxygen Species
Dihydroethidium (DHE; Invitrogen-Molecular Probes, Eugene, OR) staining for ROS was performed on cryosections. DHE, the chemically reduced form of ethidium bromide, shows a blue fluorescence (absorption/emission: 355/420nm) in the cell cytoplasm, but displays red fluorescence (absorption/emission: 518/605nm) after it is oxidized and intercalated into DNA. Cryosections (5μm) were incubated with DHE 5μM stabilized in DMSO at 37°C for 30 minutes, then rinsed in PBS and visualized using a Zeiss Axiovert 200M microscope (Zeiss Microimaging, Inc., Thornwood, NY). Fluorescent pixel intensity was determined for each retina or ciliary body using ImageJ software (version 1.34, NIH), and the mean pixel intensity for retina or ciliary body was calculated after subtracting background, as described previously[15]. Mean values were averaged for each group.
Real Time RT-qPCR
Total RNA was isolated using TRIZOL reagent (Invitrogen Corp, Calsbad, CA), and reverse transcription was performed using random hexamers and SuperScript II reverse transcriptase (SuperScript II; Invitrogen). First strand cDNA was assayed for inflammatory mediators using the LightCycler apparatus (Roche Diagnostics, Nutley, NJ) using our previously published protocol[16]. For inflammatory mediators, primers from QuantiTect Primer Assays (QIAGEN, Inc., Hilden, Germany) were obtained for ICAM-1, IL-6, TNF-α, MCP-1, COX-2, iNOS. Expression of cyclopilin A was used for normalization. For antioxidant enzymes, NQO-1, GCLM, and HO-1 were examined using Assay-on-Demand primers and probe sets from Applied Biosystems (Foster City, CA) with the ABI Taqman system (Applied Biosystems). Expression of β-actin was used for normalization.
Lectin Labeling of Retinal Vasculature and Adherent Leukocytes
Leukocytes adherent to retinal vasculature were evaluated by perfusion labeling with FITC-coupled to Con A; Vector, Burlingame, CA), as previously described[17]. Briefly, after deep anesthesia with ketamine (75–100 mg/kg) and xylazine (10 mg/kg), the chest cavity was opened and using a 27-gauge cannula, 2 ml of PBS was injected into the left ventricle to remove erythrocytes and nonadherent leukocytes, followed by injecting 2 ml FITC-con A lectin. Mice were euthanized and the eyes were enucleated. Whole retinas were dissected and flat mounted. The flat mounts were imaged with an epifluorescence microscope (Zeiss Axiovert 200M), and the total number of con A-stained adherent leukocytes per retina was counted by a masked observer.
Statistical Analysis
Results are expressed as the mean ± SD. The number of leukocytes adherent to retinal vasculature in each flat mount was counted, and the data were analyzed using the Mann-Whitney test. Differences were considered to be significant at P<0.05.
Results
Retinal and Ciliary Body ROS is increased in Nrf2 deficient mice given an LPS challenge
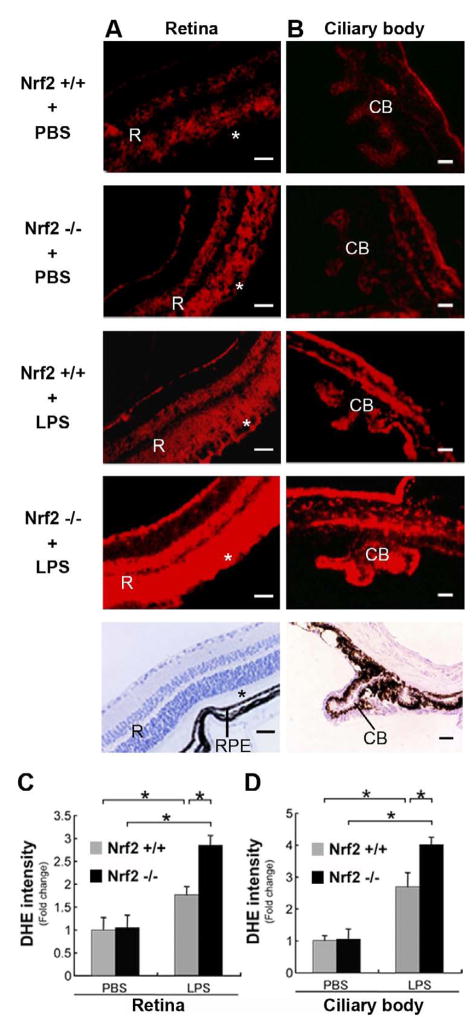
Using DHE histochemical staining for ROS, Fig 1A shows that the neurosensory retinas of Nrf2+/+ and Nrf2−/− mice have a low level of detectable ROS at baseline. After LPS stimulation, the degree of detectable ROS increased dramatically in all layers of the neurosensory retina. Interestingly, the retinal pigmented epithelium, which has a robust antioxidant capability, shows little increase in ROS. The increase in ROS was significantly greater in Nrf2−/− than Nrf2+/+ mice stimulated with LPS (p = 0.02 Fig 1C). A similar increase in ROS is seen in the ciliary body (Fig 1B, D).
Figure 1.
Evidence of ROS in the Neurosensory Retina and Ciliary Body. DHE histochemical staining of ROS. A. Neurosensory retina of wild-type (Nrf2+/+; n=4) and Nrf2 deficient (Nrf2−/−; n=4) mouse shows minimal red label in the retina (R), photoreceptor layer of the retina (*), and retinal pigmented epithelium (RPE) when unstimulated (PBS treated). Increased DHE labeling in the retina of Nrf2+/+ (n=4) and Nrf2−/− (n=4) mice after LPS stimulation. Note marked increase in Nrf2−/− mouse retina. B Ciliary body processes of Nrf2+/+ and Nrf2−/− mice showed increase in DHE labeling after LPS treatment compared with PBS treatment. Note marked increase in Nrf2−/− mouse after LPS stimulation. The lowest panels show the hematoxylin staining in Nrf2+/+ mice. Bar = 100μm. (C, D) The graphs show the ratio of DHE fluorescent intensity to PBS-treated Nrf2+/+ mice. *p<0.05.
Mixed expression of Nrf2 responsive antioxidant genes to mice given LPS an challenge
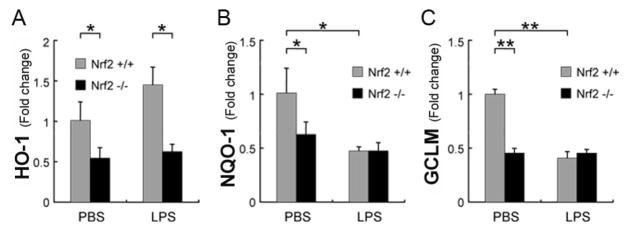
Nrf2 responsive antioxidant enzymes can either increase or decrease after an inflammatory insult, and this response is an important factor when mounting the innate immune response[5]. We evaluated the expression of NQO-1, GCLM, and HO-1 in the neurosensory retina 3 hours after LPS injection in Nrf2+/+ and Nrf2−/− mice because they are three genes that can be activated by Nrf2. The expression of NQO-1 and GCLM in Nrf2+/+ mice treated with LPS was significantly reduced compared to control injections (Fig 2). On the other hand, HO-1 was increased. In Nrf2−/− mice, there was no significant change in retinal expression of these three genes, whether given an LPS or control injections. Interestingly, HO-1 expression however, was significantly lower in Nrf2−/− compared to Nrf2+/+ LPS-treated mice (p<0.05).
Figure 2.
Decreased mRNA expression of antioxidant enzymes in the neurosensory retina 3 hours after LPS injection. RT-qPCR analysis of A) HO-1, B) NQO-1, and C) GCLM expression from retinas of wild-type (Nrf2+/+; n=5 each group) and Nrf2 deficient (Nrf2−/−; n=5 each group) mice that were given an intraperitoneal injection of 60 μg LPS. The graphs show the ratio of expression to PBS-treated Nrf2+/+ mice. Note the decreased expression of Nrf2 specific enzymes NQO-1 and GCLM in Nrf2+/+ mice. HO-1 expression is unchanged (p<0.09). On the other hand, basal expression of NQO-1, GCLM, and HO-1 was significantly greater in the retinas of Nrf2+/+ mice than Nrf2−/− mice. As expected, NQO-1 and GCLM expression were not affected by LPS injection in Nrf2−/− mice. In LPS-treated mice, the expression of HO-1 was significantly lower in Nrf2−/− compared to in Nrf2+/+. *p<0.05, **p<0.01.
Exaggerated Retinal and CB expression of inflammatory mediators in Nrf2−/− mice in response to LPS Challenge
Several cytokines and inflammatory mediators are involved in the pathogenesis of experimental uveitis[18]. At first, we examined the time course for mRNA induction of these inflammatory mediators in the neurosensory retina of Nrf2+/+ mice at 1, 3, and 6 hours after LPS injection. Maximum induction was seen 3 hours after LPS injection for ICAM-1, MCP-1, IL-6, COX-2 and TNF-α (p<0.05, data not shown). Similar results are seen for the upregulation of these factors in the iris-ciliary body of Nrf2+/+ mice (p<0.05).
We next compared the induction of these inflammatory mediators 3 hours after LPS injection in Nrf2+/+ and Nrf2−/− mice. Fig 3 shows significant increased expression in the retina and iris-ciliary body of all inflammatory markers in Nrf2−/− compared to Nrf2+/+ mice following LPS injection.
Figure 3.
Deletion of Nrf2 increases mRNA expression of inflammatory mediators in the neurosensory retina and iris-ciliary body by LPS. RT-qPCR analysis of inflammatory mediators was performed on retinas that were removed 3 hours after intraperitoneal injection of LPS (60 μg) or PBS control from wild-type (Nrf2+/+; n=5 each group) and Nrf2 deficient (Nrf2−/−; n=5 each group) mice. *p<0.05; **p<0.01; ***p<0.001.
Retinal vascular leukocyte adhesion is increased in Nrf2 deficient mice
LPS induces inflammatory mediators which break down the blood ocular barrier, resulting in leukocyte infiltration. A key early event of leukocyte invasion is adhesion of the leukocyte to the vascular endothelium which is mediated in part, by ICAM-1[19]. FITC-con A labeled leukocytes that were adherent to retinal vasculature were visualized by epifluorescence microscopy of retinal flatmounts (Fig 4A). Unstimulated Nrf2+/+ and Nrf2−/− mice had few adherent leukocytes to retinal vascular endothelium. In contrast, the number of labeled leukocytes significantly increased after LPS treatment in both Nrf2+/+ (p=0.001) and Nrf2−/− mice (p=0.002). The number of adherent leukocytes in LPS treated Nrf2−/− mice was 60% greater than LPS treated Nrf2+/+ mice (p=0.009; See Fig 4B), which is consistent with the increased retinal ICAM-1 expression in Nrf2−/− mice (Fig 3A).
Figure 4.
Deletion of Nrf2 potently increases leukocyte adherence to retinal vasculature is increased by LPS treatment. A. Retinal flatmounts of wild-type (Nrf2+/+; n=8 each group) and Nrf2 deficient (Nrf2−/−; n=8 each group) mice 24 hours after receiving either LPS (60 μg) or PBS control intraperitoneally. Arrowheads show leukocytes which are increased after LPS treatment compared to controls. Bar = 100 μm. B. Quantification of adherent leukocytes expressed as number of cells per retina. LPS increased the number of adherent leukocytes in Nrf2−/− (Black bars) more than Nrf2+/+ mice (Gray bars). **p<0.01; ***p<0.001.
Protection with the Nrf2 activator CDDO-Im
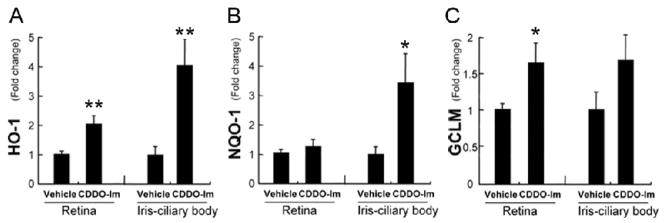
CDDO-Im is a synthetic triterpenoid that activates Nrf2[13]. To test whether CDDO-Im influences Nrf2 in the eye, Nrf2+/+ mice were treated with CDDO-Im for 3 days, and NQO-1, GCLM, and HO-1 expression was measured in the retina and iris-ciliary body. In the retina, GCLM and HO-1 were significantly upregulated while in the iris-ciliary body, NQO-1 and HO-1 were significantly increased by CDDO-Im treatment (Fig 5). As a control, Nrf2−/− mice stimulated with LPS were also given either CDDO-Im or vehicle. There was no difference in expression of HO-1, GLCM, and NQO-1 between CDDO-Im and vehicle treated Nrf2−/− mice (data not shown).
Figure 5.
CDDO-Im increases the expression of antioxidant enzymes. Nrf2+/+ mice were given CDDO-Im (3 μmol/kg bodyweight; n=5) or vehicle control (n=5) once daily for 3 days, and the expression of antioxidant enzymes (HO-1 (A), NQO-1 (B) and GCLM (C)) was evaluated by RT-qPCR in the retina and iris-ciliary body. *p<0.05, **p<0.01.
We next explored whether the expression of ICAM-1, CCL-2, IL-6, INOS, COX-2, and TNF-α was significantly reduced after pretreatment with CDDO-Im following LPS stimulation in the retina and iris-ciliary body (Fig 6). Except for iNOS expression in the retina, all of the inflammatory mediators were significantly downregulated by CDDO-Im treatment 3 hours after LPS injection. There was no difference in expression of ICAM-1, CCL-2, IL-6, INOS, COX-2, and TNF-α by CDDO-Im compared to vehicle control in Nrf2−/− mice (data not shown).
Figure 6.
CDDO-Im reduces the expression of inflammatory mediators in the neurosensory retina and the iris-ciliary body. Nrf2+/+ mice were given CDDO-Im (3 μmol/kg bodyweight; n=5) or vehicle control (n=5) once daily for 3 days, and the expression of inflammatory mediators was evaluated by RT-qPCR in the neurosensory retina and the iris-ciliary body. All of the genes except iNOS in the retina were significantly decreased by CDDO-Im. *p<0.05; **p<0.01.
Finally, we examined whether CDDO-Im treatment had a functional impact by measuring leukocyte adhesion to the retinal vasculature after LPS stimulation. Fig 7 shows that CDDO-Im treatment reduced by 45% (p = 0.038), the adhesion of leukocytes to retinal vasculature compared to vehicle control LPS stimulated mice. There was no difference in leukocyte adhesion to retinal vasculature of Nrf2−/− mice stimulated with LPS and treated with CDDO-Im compared to vehicle control.
Figure 7.
CDDO-Im decreases leukocyte adherence to retinal vasculature. A) Flatmounted retinas of Nrf2+/+ mice show FITC-Con A labeled leukocytes that were adherent to the retinal vasculature. Bar = 100μm. B) The graph depicts the percent of adherent leukocytes after pretreatment with CDDO-IM to vehicle control for both LPS stimulated Nrf2−/− and Nrf2+/+ mice (n=5 in each group). The number of adherent leukocytes from Nrf2+/+ mice pretreated with CDDO-Im is decreased compared to vehicle control eyes (*p<0.01). On the other hand, the number of adherent leukocytes in retinas of Nrf2−/− mice was not different between CDDO-Im and vehicle control. The number of adherent leukocytes from CDDO-Im to vehicle control treatment was significantly different between Nrf2+/+ and Nrf2−/− mice (**p<0.001).
Discussion
In this study, we demonstrate that Nrf2 is a critical host factor in modulating the innate immune response to LPS stimulation in a murine model of uveitis. LPS increased ROS, disrupted the coordinated Nrf2 mediated anti-oxidant gene expression response, induced a cluster of inflammatory mediators in the neurosensory retina, and finally, enhanced leukocyte adhesion to the retinal vascular endothelium. While we focused our studies on the retina, we found a similar response in the ciliary body. Deficiency of Nrf2 magnified the changes induced by LPS. Importantly, activation of the transcription factor Nrf2 by CDDO-Im increased antioxidant enzyme expression, reduced cytokine expression, and decreased leukocyte adhesion elicited by LPS. These results suggest that Nrf2 is associated with oxidative regulation of the innate immune response induced by LPS in the neurosensory retina. Importantly, activation of Nrf2 by CDDO-Im provided protection from inflammation caused by LPS. The triterpenoids, therefore, represent a novel class of therapeutics with potential for treating ocular inflammatory diseases.
An early critical event in endotoxin induced uveitis is the adhesion of leukocytes to the retinal vascular endothelium, which is mediated by ICAM-1[19]. LPS importantly, induces leukocytes to produce ROS[20, 21], and ROS in turn, primes the immune cell for an augmented innate immune response. Inhibition of ROS has been found to partially inhibit this response[21]. In our study, LPS clearly increased ROS production in the retina, and LPS increased the number of leukocytes that were adherent to the retinal vasculature. From our experimental design, we are not able to determine whether the increased ROS results from increased influx of leukocytes into the retina after LPS treatment. Nonetheless, we believe that part of influx of leukocytes is mediated by the upregulated expression of ICAM-1 in the retina by LPS. Nrf2 signaling appears to have a protective role that controls ocular ROS and subsequent inflammation because leukocyte adhesion was exaggerated by deficiency of Nrf2 and reduced by pretreatment of Nrf2+/+ mice with the Nrf2 activator CDDO-Im. The reduction of ICAM-1 expression after CDDO-Im could mechanistically participate in minimizing the influx of leukocytes into the retina, and therefore decrease leukocyte generated ROS and cytokine production. Interrupting leukocyte adhesion is a logical treatment target because terminating the inflammatory process at this early stage could reduce the cascade of inflammatory events caused by the influx of leukocytes that leads to local tissue injury.
The specific genes that respond from Nrf2 signaling are dependent in part, upon the cell type and stressor. Decreased expression of Nrf2 responsive genes to an insult is an inadequate response that could result in tissue injury. An extreme example is in sepsis, where an inappropriate Nrf2 signaling response magnifies the innate immune response and increases mortality[5]. In general, the basal expression of antioxidant enzymes are not appreciably regulated by Nrf2 signaling[6]. With oxidative stimulation, Nrf2 signaling can activate a large number of antioxidant related genes that could neutralize ROS accumulation. It is clear from our experiments that ROS accumulates at a higher rate in Nrf2−/− than Nrf2+/+ mice. We examined the expression of NQO-1, GCLM, and HO-1 because they are established Nrf2 responsive genes[8]. In the retina, we observed decreased expression of NQO-1 and GCLM, but increased expression of HO-1 by LPS. We note however, that the increased HO-1 expression was lower in Nrf2−/− than Nrf2+/+ mice treated with LPS. Since HO-1 can be activated by other transcription factors[22], it is likely that other activation pathways play a role in this protective response. Regardless, HO-1 plays a central role in regulating inflammation, as increased HO-1 is known to blunt the inflammatory response[12, 23]. The induction of HO-1 after LPS stimulation indicates an immediate protective response in these tissues that is also seen in microglia and monocytes[12, 24]. Our findings are consistent with the work of Ohta et al, who found that HO-1 induction protected against the ocular inflammation induced by LPS[25]. This protective response alone however, was insufficient to quench ROS in the retina because we observed increased ROS and a marked inflammatory response after LPS. The decreased expression of NQO-1 and GCLM suggests an inadequate response by Nrf2 mediated genes to LPS. NQO-1 exhibits anti-inflammatory activity by inhibiting LPS induction of TNF, IL-1β, and IL-6[5, 24], and could have played a role in regulating the induction of inflammatory genes in our study. However, the lack of induction by CDDO-Im in our experiments would suggest against a significant role in reducing the inflammatory response from LPS.
We were intrigued by both the decreased GCLM expression after LPS, but also its induction by CDDO-Im in Nrf2+/+, but not Nrf2−/− mice. GCLM is the modifier subunit of the glutamate cysteine ligase (GCL) complex that is also composed of the catalytic subunit GCLC[26]. The rate limiting step in glutathione synthesis[27], GCL is critical for maintaining cellular redox homeostasis. In endotoxemia, cellular glutathione levels drop not only from oxidative stress, but also from a fall in GCL activity[5, 28]. We suspect that the drop in GCLM after LPS contributes to diminished GCL activity in the retina. The induction of GCLM by CDDO-Im suggests that GCL could be involved in a protective response that we observed after this treatment. The Nrf2 mediated response however, likely involves many genes. Since this was an exploratory investigation, future studies should be directed at determining the global response by Nrf2 responsive genes to establish which genes in the retina reduce oxidative stress and blunt the inflammatory response.
An inappropriate innate immune response resulting in local tissue injury is a characteristic of uveitis. In the LPS model, ROS production and activation of inflammatory cytokines, and leukocyte infiltration must be appropriately neutralized in order to prevent tissue injury. Currently, the mainstay of therapy for uveitis involves either local or systemic corticosteroids, which has numerous local and systemic unwanted effects including cataract, glaucoma, diabetes, hypertension and osteoporosis. A mechanistic role for the Nrf2 pathway in the protection from an exaggerated innate immune response is suggested by the loss- and gain-of-function experiments using Nrf2 deficient mice and the pharmacologic Nrf2 activator CDDO-Im, respectively. Inadequate Nrf2 transcriptional activity during uveitis could result in incomplete control of the antioxidant and anti-inflammatory response. Correction of this response by CDDO-Im could represent a novel treatment strategy for uveitis as an alternative to corticosteroids.
Acknowledgments
This work is supported by Grants EY14005 (JTH), The Robert Bond Welch Professorship (JTH); Bausch and Laumb Fellowship Award (NN), Keio University Medical Science Fund (NN), and The Uehara Memorial Foundation (NN); NIH HL081205 (SB), NIH/NHLBI SCCOR grant P50HL084945 (SB), Clinical Innovator award from FAMRI (SB); NIH grant CA-78814 (MS), and a grant from Reata Pharmaceuticals (MS); and generous gifts from Ric and Sandy Forsythe, the Kwok family, the Merlau family, and Aleda Wright, and Research to Prevent Blindness to Wilmer Eye Institute.
Abbreviations
- CDDO-Im
1-(2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl)imidazole
- COX-2
cyclo-oxygenase-2
- DHE
Dihydroethidium
- GCLM
Glutamate-cysteine ligase, modifier subunit
- HO-1
heme oxygenase-1
- ICAM-1
intercellular adhesion molecule 1
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- MCP-1
monocyte chemotactic protein -1
- NQO-1
NADPH: quinine oxidoreductase-1
- Nrf-2
Nuclear factor erythroid-2 related factor 2
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacherjee P, Williams RN, Eakins KE. An evaluation of ocular inflammation following the injection of bacterial endotoxin into the rat foot pad. Invest Ophthalmol Vis Sci. 1983;24:196–202. [PubMed] [Google Scholar]
- 3.Hoekzema R, Verhagen C, van Haren M, Kijlstra A. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Invest Ophthalmol Vis Sci. 1992;33:532–539. [PubMed] [Google Scholar]
- 4.Mitra S, Abraham E. Participation of superoxide in neutrophil activation and cytokine production. Biochim Biophys Acta. 2006;1762:732–741. doi: 10.1016/j.bbadis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 7.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 8.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 10.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. Faseb J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 12.Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 13.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 15.Berent-Spillson A, Russell JW. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J Neurochem. 2007;101:342–354. doi: 10.1111/j.1471-4159.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamada Y, Ishibashi K, Ishibashi K, Bhutto IA, Tian J, Lutty GA, Handa JT. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840–848. doi: 10.1016/j.exer.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan RD, ’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 18.Nagai N, Oike Y, Noda K, Urano T, Kubota Y, Ozawa Y, Shinoda H, Koto T, Shinoda K, Inoue M, Tsubota K, Yamashiro K, Suda T, Ishida S. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:2925–2931. doi: 10.1167/iovs.04-1476. [DOI] [PubMed] [Google Scholar]
- 19.Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566. [PubMed] [Google Scholar]
- 20.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadhav A, Torlakovic E, Ndisang JF. Interaction among heme oxygenase, nuclear factor-kappaB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension. 2008;52:910–917. doi: 10.1161/HYPERTENSIONAHA.108.114801. [DOI] [PubMed] [Google Scholar]
- 23.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 24.Rushworth SA, MacEwan DJ, O’Connell MA. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008;181:6730–6737. doi: 10.4049/jimmunol.181.10.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta K, Kikuchi T, Arai S, Yoshida N, Sato A, Yoshimura N. Protective role of heme oxygenase-1 against endotoxin-induced uveitis in rats. Exp Eye Res. 2003;77:665–673. doi: 10.1016/j.exer.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2008 doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Payabvash S, Ghahremani MH, Goliaei A, Mandegary A, Shafaroodi H, Amanlou M, Dehpour AR. Nitric oxide modulates glutathione synthesis during endotoxemia. Free Radic Biol Med. 2006;41:1817–1828. doi: 10.1016/j.freeradbiomed.2006.09.010. [DOI] [PubMed] [Google Scholar]