Abstract
Experimentally-induced diabetes can modify the behavioral and neurochemical effects of drugs acting on dopamine systems, possibly through insulin-related regulation of dopamine transporter activity. In this study, several behavioral procedures were used to examine possible changes in sensitivity to amphetamine and other drugs in rats rendered diabetic by a single injection of streptozotocin. Conditioned place preference developed to food (Froot Loops®) in both control and diabetic rats, demonstrating that conditioned place preference with tactile stimuli can occur in streptozotocin-treated rats. Baseline locomotion was lower in streptozotocin-treated as compared to control rats, although amphetamine significantly increased locomotion in all rats. Conditioned place preference developed to amphetamine regardless of whether rats had received streptozotocin or saline. A second study compared the potency of drugs to decrease lever pressing maintained by food, before and after streptozotocin treatment. Gamma-hydroxybutyrate and amphetamine were less potent after streptozotocin while the potency of raclopride, quinpirole, ketamine, haloperidol and cocaine was not significantly changed by streptozotocin. While markedly affecting locomotion, body weight and blood glucose, streptozotocin only modestly affected sensitivity to the behavioral effects of amphetamine and other drugs; these results fail to confirm previous reports of decreased behavioral actions of stimulants in diabetic rats.
Keywords: streptozotocin, amphetamine, conditioned place preference, locomotion, dopamine transporter
1. Introduction
Abnormal dopamine neurotransmission is implicated in several psychiatric disorders including substance abuse. Dopamine systems are regulated by several hormonal factors, and a growing body of evidence suggests that insulin-signaling pathways play an especially prominent role in regulating dopamine neurotransmission. In addition to well-characterized effects in the periphery (e.g., regulation of glucose), insulin can also have effects in the brain. It can cross the blood-brain barrier and act on receptors (i.e., insulin and IGF-1) that are densely concentrated in brain regions enriched with dopamine neuron cell bodies, receptors and transporters (Figlewicz et al., 2003; Schulingkamp et al., 2000; Ciliax et al., 1995).
The anatomical proximity and overlap of insulin and dopamine systems also has functional significance. For example, dopamine transporter mRNA was significantly elevated in the substantia nigra in hyperinsulinemic (Zucker fa/fa) rats and in rats chronically treated with insulin i.c.v. (Figlewicz et al., 1994; 1998). Conversely, rats with low circulating insulin had reduced dopamine transporter activity and mRNA (Owens et al., 2005; Patterson et al., 1998). Dopamine uptake was decreased in synaptosomes from rats made hypoinsulinemic by food deprivation and was restored by administration of insulin (Patterson et al., 1998). These effects appear to be the result of insulin acting on receptors (insulin, IGF-1) since inhibition of insulin signaling caused dopamine transporters to be translocated away from the plasma membrane, thereby reducing dopamine transport (Carvelli et al., 2002; Garcia et al., 2005). In addition to effects on dopamine uptake, insulin can also modulate other components of the dopamine system; for example, hypoinsulinemic rats show decreased synthesis (Saller, 1984) and turnover (Kwok and Juorio, 1986; Lim et al., 1994) of dopamine and they are hyporesponsive to the behavioral effects of direct-acting dopamine drugs (Sevak et al., 2007a).
Changes in insulin status can modify sensitivity to the behavioral effects of drugs acting on dopamine systems (e.g., Sevak et al., 2005). For example, alloxan-induced diabetes attenuated amphetamine-induced stereotypy and locomotor-stimulation; insulin restored sensitivity to these drug effects (Marshall, 1978). The behavioral effects of drugs acting directly at dopamine receptors also are changed by streptozotocin (Rowland et al., 1985) or by restricted access to food (Sevak et al., 2007a), and food restriction is known to enhance self-administration as well as the motor-activating effects of several drugs of abuse, including cocaine and amphetamine (Campbell and Fibiger, 1971; Carroll et al., 1981; Carroll and Stotz, 1983). Amphetamine and cocaine decrease the threshold for lateral hypothalamic self-stimulation and this effect is further augmented by food restriction (Carr, 2002). Streptozotocin also increased sensitivity to methamphetamine-induced conditioned place preference, and food restriction enhanced amphetamine-induced conditioned place preference (Kamei and Ohsawa, 1996; Stuber et al., 2002).
The current study examined the generality of changes in sensitivity to the behavioral effects (conditioned place preference and schedule-controlled responding) of amphetamine and related drugs in rats made diabetic by streptozotocin. An initial study using food determined that conditioned place preference could be established with tactile stimuli in streptozotocin-treated rats. Control and streptozotocin-treated rats also were compared for their sensitivity to the locomotor-stimulating and conditioned place preference effects of amphetamine and to the rate-decreasing effects of amphetamine and several other compounds. Given the high comorbidity of substance abuse and eating disorders (e.g., Wolfe and Maisto, 2000) and the fact that glucose and insulin levels can vary dramatically (e.g., dieting, diabetes), it is important to establish the functional relationships among nutritional status, insulin signaling, glucose, and the behavioral effects of drugs.
2. Materials and Methods
2.1 Animals
One hundred and eleven male rats (Harlan Sprague-Dawley Inc., Indianapolis, IN), weighing 250–350 g, were individually housed in an environmentally-controlled room (24 ± 1 °C, 50 ± 10% relative humidity) under a 12-h light/dark cycle with water available continuously. Seven rats were used for studies on schedule-controlled responding and the remaining rats were used for the locomotion and conditioned place preference studies. Food (rat sterilizable diet, Harlan Teklad) was continuously available in the home cage with the exception of the 7 rats that were used in the study of schedule-controlled responding (8–10 g of food per day in the home cage immediately after experimental sessions).
Fifty-nine rats (52 in the locomotion and conditioned place preference study and 7 in the study on schedule-controlled responding) were rendered diabetic by an i.p. injection of 50 mg/kg streptozotocin. Behavioral experiments began one week after administration of streptozotocin or saline (control group); blood glucose concentrations were measured one week after the administration of streptozotocin or saline in order to confirm hyperglycemia (i.e., diabetes) in streptozotocin-treated rats. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2 Apparatus
Locomotion and conditioned place preference were studied using custom-made (Instrumentation Services, University of Texas Health Science Center, San Antonio, TX) Lexan® polycarbonate (General Electric Structured Products, Mt. Vernon, IN, USA) chambers (26 × 61 × 23 cm high) located within sound-attenuating cubicles (MED Associates Inc., St. Albans, VT). The metal floors of the chambers were removable and varied in texture across conditions. For training sessions the entire floor was the same texture: 6 mm diameter holes (9 mm center-to-center) or a grid of 6 × 6 mm wire mesh supported by 5 mm diameter metal rods spaced 16 mm center-to-center. For test sessions half (26 × 30.5 cm) of the floor had the hole texture and half the grid texture. Horizontal activity (locomotion) and location in the chamber (e.g., conditioned place preference) were measured with 4 pairs of infrared photo beams (Multi-Varimex, Columbus Instruments, Columbus, OH) positioned 4 cm above the floor of the chamber. The photo beams were separated by 15 cm with two of the photo beams located 8 cm from the ends of the chamber.
Schedule-controlled responding studies were conducted in operant chambers located within sound-attenuating, ventilated chambers (Model #ENV-018M and ENV-008CT; MED Associates Inc., St. Albans, VT) and equipped with two levers, associated stimulus lights and a food hopper that delivered food pellets (45 mg, PJAI-0045, Noyes Precision Pellets; Research Diets Inc., New Brunswick, NJ) to a 5 × 5 cm opening located equidistant between the two levers. Data were recorded and experimental events controlled by a computer, interface and software (MED Associates Inc., St. Albans, VT).
2.3 Procedure
2.3.1 Locomotion and conditioned place preference
Locomotion and conditioned place preference were studied according to methods described previously for rats (Borman and Cunningham, 1998; Owens et al., 2005; Sevak et al., 2007b). Briefly, conditioning sessions were preceded by a single 30-min habituation session; rats received an i.p. injection of saline prior to being placed in a chamber equipped with a floor covered with a sheet of paper, thereby preventing contact with the conditioning floor textures. For the next 8 daily 30-min sessions, rats (n=10–12/group) received either amphetamine or vehicle and were placed in a chamber equipped with a floor comprising only one texture (hole or grid, alternating across days). For the study with food, on alternate days each of the floor textures was paired with the presence or absence of 5 g of Froot Loops® (Kellogg’s, Battle Creek, MI) placed in the center of the chamber (n=12/group). In separate groups (n=10/group) of saline-treated (control) and streptozotocin-treated rats, amphetamine was studied at doses of 0.178, 0.56, and 5.6 mg/kg. Two additional groups of 10 rats each (one saline-treated, one streptozotocin-treated) received saline only in all eight conditioning trials to see whether there was any preference for floor texture or side of the chamber. The day after the last (eighth) conditioning trial all rats received saline i.p. and were placed in the center of a chamber equipped with a floor comprising both textures; the terminal (test) session was 30-min in duration.
2.3.2 Schedule-controlled responding
Seven rats were initially trained in the presence of a light (2.8 watts, 20.1 lumens) to press either of two levers for a food pellet (45 mg; Research Diets; New Brunswick, NJ) under a schedule of continuous reinforcement and in 60-min sessions. After responding occurred reliably (i.e., at least 50 pellets delivered in a session), rats were trained to press the left lever for food with the response requirement increasing progressively across days once performance was stable. The final response requirement was a fixed-ratio (FR) 10. Daily sessions comprised multiple cycles with each cycle including a 10-min timeout, when the chamber was dark and lever presses had no programmed consequence, followed by a 5-min response period, when the light was illuminated and a maximum of 10 food pellets could be delivered under the FR10 schedule. Once responding stabilized under the FR10 schedule (i.e., 10 consecutive session in which the average daily rate of responding did not vary by more than ±20% for each session), sensitivity to the rate-altering effects of drugs was assessed in sessions where increasing doses of drug were administered during the first minute of timeout periods with the cumulative dose increasing by 0.25 or 0.5 log units per cycle. In general, test sessions were conducted every third day if responding on intervening days was stable and not different from established control rates. The drugs studied included the following: the dopamine receptor antagonists raclopride and haloperidol; the indirect-acting dopamine agonists cocaine and amphetamine; the direct-acting dopamine receptor agonist quinpirole; the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine; and gamma-hydroxybutyric acid (GHB). All 7 rats then received a single i.p. injection of 50 mg/kg of streptozotocin and beginning 11 days later (when the baseline rate of responding was no longer different from that prior to streptozotocin treatment) sensitivity to the same drugs that were studied before streptozotocin treatment, was assessed under conditions identical to those described above.
2.4 Drugs
The following drugs were used: d-amphetamine hydrochloride and cocaine hydrochloride (The Research Technology Branch, National Institute of Drug Abuse, Rockville, MD), streptozotocin, raclopride tartrate, haloperidol, quinpirole dihydrochloride, gamma-hydroxybutyric acid (Sigma-Aldrich, St. Louis, MO), and ketamine hydrochloride (Fort Dodge Laboratories, Fort Dodge, IA). With the exception of ketamine, which was purchased as a commercially-available solution, compounds were dissolved in sterile 0.9% saline; compounds were administered i.p, except raclopride, which was administered s.c.
2.5 Data analyses
2.5.1 Blood glucose concentration and body weight
Blood glucose concentration and body weights of rats used to study locomotion and conditioned place preference were analyzed with an analysis of variance (ANOVA). A paired t-test was performed to analyze blood glucose concentrations before and 7 days after the administration of streptozotocin in rats that were used in the scheduled-controlled responding study. Body weight data for these rats were analyzed with a one-factor ANOVA with days (1, 8, and 46) as the within-subjects factor.
2.5.2 Locomotion and conditioned place preference
Unpaired t-tests were used to analyze locomotion in habituation sessions between streptozotocin-treated and saline-treated rats. Locomotor activity data were analyzed by a four-factor ANOVA with group (streptozotocin- or saline-treated) and dose as between-subjects factors and conditioning trial (1 to 4) and treatment (drug and vehicle) as within-subject factors. The effects of amphetamine on locomotion in the first conditioning trial were analyzed by a two-factor ANOVA with group and dose as between-subjects factors. Data for locomotor activity of rats that received only saline across eight conditioning trials were analyzed by a two-factor (group and trial) ANOVA. Preference (for the Froot Loops®-associated floor [i.e., difference in the time spent on each floor texture]) was analyzed by one-sample t-tests to determine whether the mean difference was significantly different from zero. The effects of amphetamine on floor preference were analyzed by a two-factor ANOVA with group and dose as between-subjects factors.
2.5.3 Schedule-controlled responding
The rate of responding of 7 rats in 14 saline/sham sessions before and after the administration of streptozotocin was analyzed by a one-factor ANOVA with days as the within-subjects factor. The effects of drugs on rate of responding are plotted as a percentage of the control rate (control rate for an individual rat was the mean rate for the three saline sessions immediately preceding a test). Saline/sham sessions on the day immediately following a test session were excluded from the calculation of control values. Dose-response data determined before and after streptozotocin administration were analyzed by a two-factor ANOVA with dose and treatment as within-subjects factors. For all ANOVA tests, Tukey-Kramer post hoc tests were conducted when appropriate, and a two-tailed P<0.05 was considered significant.
3. Results
3.1 Blood glucose concentration and body weight
A single injection of 50 mg/kg of streptozotocin markedly increased blood glucose concentrations (>300 mg/dl) and decreased body weight. Statistical analyses of blood glucose concentration of rats used to study locomotion and conditioned place preference (n=104) showed a significant main effects of group (P<0.001). A subsequent post-hoc test showed that one week after streptozotocin blood glucose concentration was significantly greater than in rats that received saline. Body weight was significantly different between treatment groups, with body weight increasing in rats that received saline and decreasing in those receiving streptozotocin (P<0.001).
In rats used for the study on scheduled-controlled responding (n=7), blood glucose concentration was markedly increased (P<0.001) one week after streptozotocin (542.4 ± 16 mg/dl) compared with blood glucose measured immediately before streptozotocin (123.1± 11.9 mg/dl). The average weight of these seven rats was 353.0 ± 2.9 g immediately before streptozotocin; body weight decreased to an average of 317.4 ± 2.1 g 7 days after streptozotocin and decreased further to 239.1 ± 7.4 g 46 days after streptozotocin (i.e., on the last day of the study).
3.2 Conditioned place preference with food
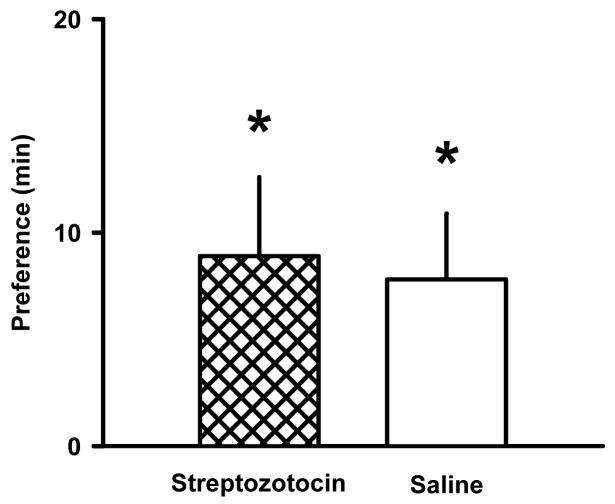
Place preference for the floor texture that was paired with food (Froot Loops®) developed in rats that received streptozotocin and in rats that received saline (Fig. 1). The mean (± S.E.M.) preference for the Froot Loops®-paired floor in streptozotocin- and saline-treated rats was 8.9 ± 3.7 and 7.8 ± 3.2 min, respectively (n=12/group; P<0.05).
Fig. 1.
Conditioned place preference with Froot Loops® (5 g) in streptozotocin-treated and saline-treated rats. Bars represents the average difference (min) in the time spent on the floor-texture associated with Froot Loops® minus the time spent on the floor-texture not associated with Froot Loops® (mean ± S.E.M. of 12 rats per condition). *P<0.05 significantly different from zero.
3.3 Basal locomotion
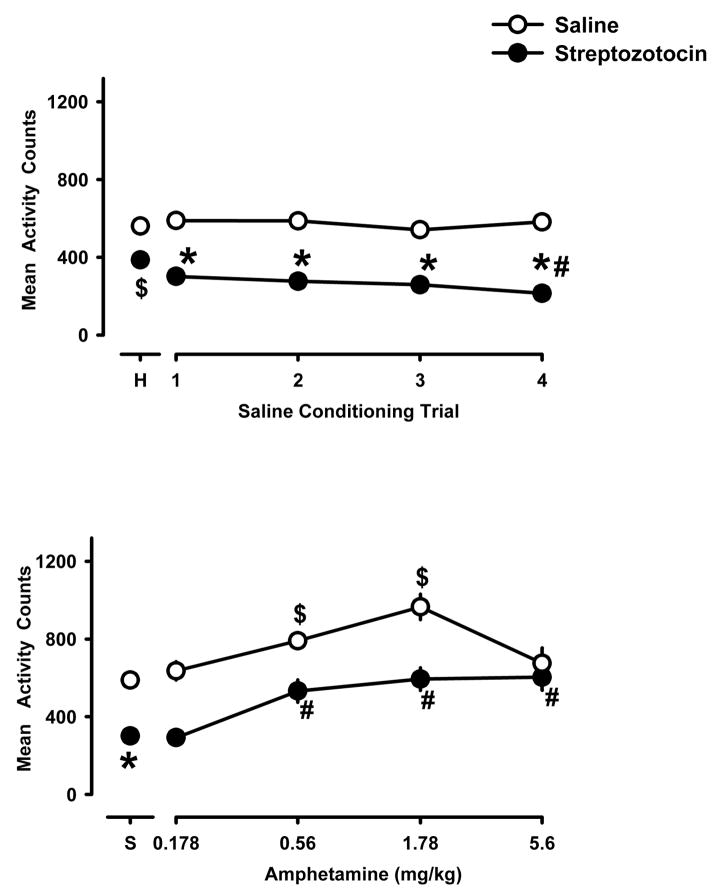
Fig. 2 shows results obtained in the habituation session (“H”, upper panel) and in every-other-day saline conditioning sessions (upper panel) in separate groups of rats (n=10/group) that received either streptozotocin or saline. Streptozotocin-treated rats showed significantly less locomotion compared with control (saline-treated) rats in the habituation session (Fig. 2, upper panel, P<0.01) and in each of the four saline conditioning trials (P<0.05). Locomotor activity of rats that received only saline in eight conditioning trials, was significantly less in saline-treated rats than in streptozotocin-treated rats (P<0.001; data not shown). Locomotion decreased significantly over the four saline conditioning trials in streptozotocin-treated rats.
Fig. 2.
Locomotor activity (counts per 30-min session) in saline-treated (open symbols) and in streptozotocin-treated (closed symbols) rats during an initial habituation session (“H”), during each of the four every-other-day saline conditioning trials (upper panel), and during the first conditioning trail with amphetamine (lower panel). Each data point in the upper panel is the mean ± S.E.M. for 40 rats (same rats as shown in the lower panel) and with the exception of data above “S” (which shows the baseline activity in the first saline conditioning trial for all 40 rats) each data point in the lower panel is the mean ± S.E.M. for 10 rats. Data for 1.78 mg/kg amphetamine in both groups are replotted from a published study (Owens et al., 2005) that was conducted at the same time as this study. $P<0.05 compared with the habituation session (upper panel) or with the first saline-conditioning trial (lower panel) in control rats; *P<0.05 compared with saline-treated rats in the corresponding conditioning trial; #P<0.05 compared with streptozotocin-treated rats in saline conditioning trial 1.
3.4 Effects of acute administration of amphetamine on locomotion
Amphetamine increased locomotion in the first drug conditioning trial in control rats and in streptozotocin-treated rats (lower panel, Fig. 2). In control rats, locomotion increased in a dose-related manner with maximum increases occurring with a dose of 1.78 mg/kg of amphetamine (P<0.01); a larger dose did not result in greater locomotion. Although baseline (non-drug) locomotion was less in streptozotocin-treated rats as compared with saline-treated control rats, (P<0.001), amphetamine also significantly increased locomotion in streptozotocin-treated rats in a manner that resulted in a similar percentage increase in the two groups of rats. In streptozotocin-treated rats locomotion was increased significantly over non-drug values at doses of 0.56, 1.78 and 5.6 mg/kg of amphetamine (P<0.01).
3.5 Effects of repeated administration of amphetamine on locomotion
With two exceptions, the locomotor-stimulating effects of amphetamine did not change significantly over the four every-other-day conditioning trials in saline-treated (upper panel, Fig. 3) or streptozotocin-treated (lower panel, Fig. 3) rats. The locomotor-stimulating effect of the largest dose of amphetamine (5.6 mg/kg) decreased significantly across the four trials in saline-treated rats (squares, upper panel) and the locomotor-stimulating effect of 1.78 mg/kg of amphetamine increased significantly between the first and fourth trials in streptozotocin-treated rats (squares, lower panel). The effects of other doses of amphetamine did not change significantly over trials. In control rats amphetamine significantly increased locomotion in each of the conditioning trials at doses of 0.56 and 1.78, and not 0.178 and 5.6 mg/kg of amphetamine. In streptozotocin-treated rats, not all doses of amphetamine increased locomotion in all conditioning trials. For example, 0.178 mg/kg amphetamine did not significantly increase locomotion in any trial, 0.56 mg/kg increased locomotion in the first and third trials, 5.6 mg/kg increased locomotion in the first and third trials, and 1.78 mg/kg amphetamine increased locomotion significantly in all trials.
Fig. 3.
Locomotor activity during the first (circles) and last (fourth, squares) conditioning trials with amphetamine in saline-treated (upper) and in streptozotocin-treated (lower) rats (mean ± S.E.M. of 10 rats per condition). *P<0.05 compared with corresponding data for the first conditioning trial with the same dose. Data for 1.78 mg/kg in both groups are replotted from Owens et al. (2005). See Fig. 2 for other details.
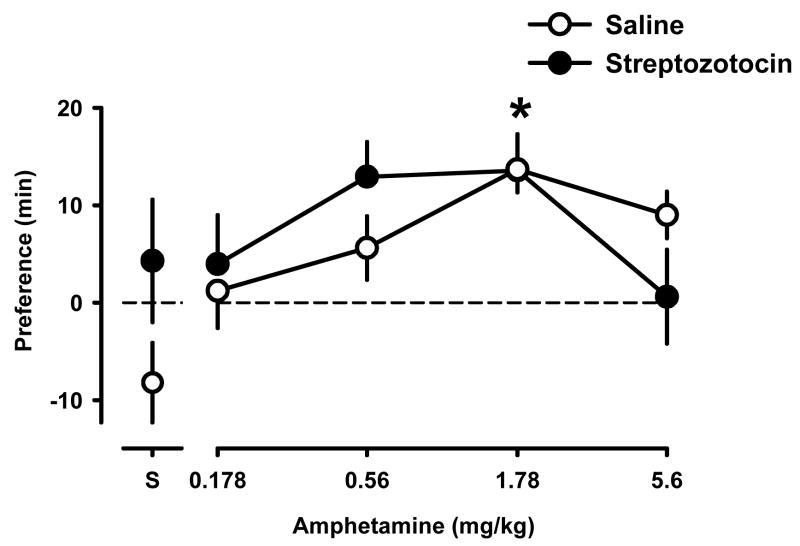
3.6 Conditioned place preference with amphetamine
Saline-treated and streptozotocin-treated rats that received only saline for all eight conditioning trials, showed no significant preference for either floor texture (data points above “S”); however, conditioned place preference developed with amphetamine in saline-treated and in streptozotocin-treated rats (Fig. 4). A dose of 1.78 mg/kg amphetamine produced a significant place preference in saline-treated (control) as well as streptozotocin-treated rats (P<0.01). There was a slight and non-significant shift leftward in the ascending and descending limbs of the amphetamine dose-effect curve in streptozotocin-treated, as compared to saline-treated rats.
Fig. 4.
Conditioned place preference studies with saline (“S”) and different doses of amphetamine in saline-treated (open) and streptozotocin-treated (closed) rats. Each data point represents the average difference (min) in time spent on the drug-paired floor minus the time spent on the saline-paired floor (mean ± S.E.M. of 10 rats per condition). Data above “S” represent the time spent on the grid floor minus time spent on the hole floor for rats that received saline in all eight conditioning trials. *P<0.05 significantly different from rats that received saline only.
3.7 Schedule-controlled responding
The average rate of lever pressing in seven rats responding under an FR 10 schedule of food presentation (mean of 14 sessions before streptozotocin) was 0.72 ± 0.02 responses per second. Streptozotocin significantly decreased responding with rats responding at an average rate of 0.29 ± 0.13 and 0.43 ± 0.06 responses per second 6 and 7 days, respectively, after receiving streptozotocin (P<0.001). The mean rate of responding recovered by day 10 and, thereafter, was not significantly different from the control rate determined prior to streptozotocin (data not shown).
Prior to streptozotocin, all compounds decreased responding in a dose-related manner (open symbols, Fig. 5). All compounds also decreased responding in a dose-related manner after streptozotocin (closed symbols, Fig. 5). Rats were significantly less sensitive to the rate-decreasing effects of amphetamine and GHB after streptozotocin treatment; sensitivity to the rate-decreasing effects of other drugs was not changed significantly after streptozotocin treatment.
Fig. 5.
Rate-decreasing effects of raclopride, haloperidol, ketamine (left panel), quinpirole, amphetamine, cocaine and GHB (right panel) before (open symbols) and after (closed symbols) streptozotocin treatment on lever pressing maintained under the fixed-ratio schedule of food presentation. Data above “S” represent the rate of responding after vehicle (saline) injections, given at the beginning of the first cycle of a cumulative dose-effect curve. Rate is plotted as a percentage of the average rate in the three control (saline) sessions immediately preceding the test. Each symbol represents mean ± S.E.M. for 7 rats. *P<0.05 compared with corresponding data obtained before streptozotocin treatment.
4. Discussion
This study compared the behavioral effects of amphetamine and related drugs in normal rats and in rats made diabetic by an acute injection of streptozotocin and showed that streptozotocin treatment does not significantly affect all (e.g., conditioned place preference) behavioral effects of amphetamine. Conditioned place preference developed with food and with amphetamine both in streptozotocin-treated rats and in control rats. Similarly, the locomotor-stimulating and rate-altering effects of amphetamine and other drugs were largely unaffected by streptozotocin, although there was a small (statistically significant) decrease in sensitivity to the rate-decreasing effects of amphetamine and GHB after streptozotocin treatment.
Dopamine systems play an important role in controlling movement and locomotion (Ranje and Ungerstedt, 1977; Uhl, 2003) and those systems are markedly changed by hypoinsulinemia; for example, synthesis, turnover, and uptake of dopamine are all decreased in hypoinsulinemic animals (Lim et al., 1994; Saller, 1984; Owens et al., 2005). Thus, reduced dopamine neurotransmission likely accounts for the significantly lower spontaneous locomotion of streptozotocin-treated rats. Deletion of dopamine transporters markedly attenuated amphetamine-stimulated locomotion (Giros et al., 1996), suggesting that dopamine was critical for this behavioral effect of amphetamine. However, despite reduced spontaneous locomotion in streptozotocin-treated rats and the fact that streptozotocin significantly decreases dopamine clearance, presumably by decreasing dopamine transporter activity (Owens et al., 2005), streptozotocin-treated rats showed a marked response (locomotion) to amphetamine that was very similar to results obtained in control rats. That dopamine transporter activity is restored after several injections of amphetamine (Owens et al., 2005) suggests that a mechanism other than or in addition to altered dopamine transporter activity contributes to the acute behavioral effects of amphetamine. For example, other components of dopamine neurotransmission (receptor number or sensitivity) are changed after streptozotocin treatment (Sevak et al., 2007a), perhaps compensating for changes in dopamine transporter activity. Norepinephrine and serotonin also might play a role in the behavioral effects of stimulants since amphetamine has affinity for several types of monoamine transporters (Han and Gu, 2006; Rothman and Baumann, 2003; Uhl et al., 2002). The norepinephrine transporter, for example, is structurally related to dopamine and is thought to mediate some of the effects of amphetamine (Kuczenski and Segal, 2001; Salomon et al., 2006). While dopamine transporter expression is decreased by streptozotocin, norepinephrine transporter expression is increased by streptozotocin (Figlewicz et al., 1996). Thus, particularly in streptozotocin-treated rats, the norepinephrine transporter could be an important site of action for amphetamine and related drugs.
Results from the current study fail to confirm earlier reports showing decreased sensitivity to amphetamine in diabetic rats (Marshall, 1978; Rowland et al., 1985). Unlike some earlier studies that examined changes in sensitivity to a single dose of drug, the present study examined the effects of a range of doses of amphetamine and failed to detect significant changes. Temporal parameters also were different among studies: whereas data from the first 30-min after the drug injection were used in the current study, other studies used data from much longer periods. There also could be a gender-related difference insofar as positive results in a prior study (Marshall, 1978) were obtained in female rats whereas the current study used males.
Under some conditions sensitivity to stimulant drugs increases (i.e., sensitization) over repeated treatments (Segal and Mandell, 1974; Shuster et al., 1977). In this study, with the exception of the largest dose (5.6 mg/kg), sensitivity of control rats to the locomotor-stimulating effects of amphetamine did not change over the four every-other-day injections comprising the place preference conditioning. A decreased response over repeated administrations of this dose could result from the emergence of competing behavior (e.g., stereotypy) that interfered with locomotion. After the same four every-other-day injections, sensitivity of streptozotocin-treated rats to the locomotor-stimulating effects of amphetamine was not changed for three doses (0.178, 0.56 and 5.6 mg/kg) and was increased for one dose (1.78 mg/kg). This injection regimen (four every-other-day injections of 1.78 mg/kg) for amphetamine normalizes streptozotocin-induced decreases in dopamine transporter activity (Owens et al., 2005). However, the first and last (fourth) locomotor activity dose-effect curves for amphetamine were remarkably similar, suggesting that sensitization did not develop under these dosing conditions, despite its development under other conditions (Robinson and Becker, 1986; Shuster et al., 1977; Segal and Mandell, 1974). The fact that the same injection schedule that normalizes dopamine clearance (transporter activity) failed to alter sensitivity to amphetamine further supports the view that sites other than the dopamine transporter might be important for some behavioral actions of amphetamine.
A variety of conditioned place preference procedures have been developed and preference has been reported for drugs (e.g., amphetamine, cocaine, morphine) and for non-drug stimuli (e.g., sucrose) in normal rats (Gaiardi et al., 1987; Bell et al., 1997; Figlewicz et al., 2001; Stuber et al., 2002). The conditioned place preference procedure used in this study was adapted from a procedure that was developed for mice (Chester and Cunningham, 1998), although it also has been used with rats (Borman and Cunningham, 1998; Owens et al., 2005). Rather than training with distinctive visual stimuli in separate compartments of a multi-compartment chamber and testing with free access to all compartments, this study varied only floor texture while keeping chamber size the same between training and testing. One possible advantage of such a procedure is that by keeping the same chamber (size) for training and testing, subjects are not exposed to a highly novel environment for testing. Using this procedure, conditioned place preference developed with food (Froot Loops®), thereby demonstrating the sensitivity of this apparatus and these tactile stimuli to a non-pharmacological conditioning stimulus. Conditioned place preference is thought to be related to and predictive of the positive reinforcing effects of drugs since many drugs that serve as positive reinforcers under other conditions (e.g., maintain i.v. self-administration responding) can be used to establish conditioned place preference (Bardo and Bevins, 2000). That streptozotocin does not significantly alter the development of conditioned place preference to amphetamine while decreasing amphetamine self administration (Galici et al., 2003), suggests that these two procedures might not be sensitive to the same effects, at least for some drugs. There are other examples, some thought to be related to route of administration, of little or no conditioned place preference with drugs that are self administered and abused (Hemby et al., 1992; Mayer and Parker, 1993; Nomikos and Spyraki, 1988).
Operant procedures provide a simple and highly quantitative method for evaluating sensitivity to drug effects and for evaluating the neurobiological mechanisms underlying drug action in vivo. One advantage of scheduled-controlled responding is that, at sufficiently large doses, most if not all drugs affect responding; one disadvantage is the possible loss of pharmacological selectivity at the comparatively large doses that sometimes are needed to affect responding. In the current study, sensitivity to rate-decreasing effects was not changed significantly by streptozotocin with the exceptions of GHB and amphetamine; however, even for those two compounds the change in sensitivity was very modest. Because the primary mechanism of action of GHB is different from the primary mechanism of action of amphetamine, it seems unlikely that a common mechanism underlies the altered potency of these drugs in streptozotocin-treated rats. Streptozotocin-treated rats used for the schedule-controlled responding study were food-restricted, which might have contributed to the lack of significant change observed with these drugs. Indeed, sensitivity to behavioral effects of other drugs is dramatically changed by streptozotocin and by food restriction (Carr et al., 2002; Sevak et al., 2005, 2007a).
In summary, this study compared amphetamine and several related drugs in control and hypoinsulinemic (streptozotocin) rats in order to test the generality of diabetes-induced changes that have been reported by others and also to determine whether neurochemical changes parallel changes in behavioral effects. Importantly, conditioned place preference with tactile stimuli developed in control and in streptozotocin-treated rats, demonstrating the sensitivity of this procedure to pharmacologic as well as non-pharmacologic stimuli and showing that food remains an effective reinforcer in streptozotocin-treated rats. Streptozotocin had very little effect on the sensitivity of rats to the behavioral effects of amphetamine and other drugs, despite well-documented neurochemical changes that occur after streptozotocin treatment and after amphetamine treatment, and despite well-documented behavioral changes that occur with other drugs after streptozotocin treatment or food restriction. Collectively, these results strongly suggest that other neurochemical mechanisms contribute to some of the behavioral effects of amphetamine in rats.
Acknowledgments
Supported by USPHS grants DA14684 (AG) and DA17918 (Senior Scientist Award to CPF). The authors also thank Daniel Mojica, Ginger Truitt and Tracey Cawthorn for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Borman NM, Cunningham CL. Ethanol-induced conditioned place aversion in rats: effect of interstimulus interval. Pharmacol Biochem Behav. 1998;59:427–432. doi: 10.1016/s0091-3057(97)00455-3. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Fibiger HC. Potentiation of amphetamine-induced arousal by starvation. Nature. 1971;233:424–425. doi: 10.1038/233424a0. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Carroll ME, Stotz DC. Oral d-amphetamine and ketamine self-administration by rhesus monkeys: effects of food deprivation. J Pharmacol Exp Ther. 1983;227:28–34. [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LMF, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Modulation of corticosterone does not affect the acquisition or expression of ethanol-induced conditioned place preference in DBA/2J mice. Pharmacol Biochem Behav. 1998;59:67–75. doi: 10.1016/s0091-3057(97)00320-1. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Brot MD, McCall AL, Szot P. Diabetes causes differential changes in CNS noradrenergic and dopaminergic neurons in the rat: a molecular study. Brain Res. 1996;736:54–60. doi: 10.1016/0006-8993(96)00727-5. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73:229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Res Bull. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res. 1994;464:331–334. doi: 10.1016/0006-8993(94)91698-5. [DOI] [PubMed] [Google Scholar]
- Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Babbini M. Increased sensitivity to the stimulus properties of morphine in food deprived rats. Pharmacol Biochem Behav. 1987;26:719–723. doi: 10.1016/0091-3057(87)90603-4. [DOI] [PubMed] [Google Scholar]
- Galici R, Galli A, Jones DJ, Sanchez TA, Saunders C, Frazer A, Gould GG, Lin RZ, France CP. Selective decreases in amphetamine self-administration and regulation of dopamine transporter function in streptozotocin-treated rats. Neuroendocrinology. 2003;77:132–140. doi: 10.1159/000068650. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Jones GH, Justice BJ, Jr, Neill DB. Conditioned locomotor activity but not conditioned place preference following intra-accumbens infusions of cocaine. Psychopharmacology. 1992;106:330–336. doi: 10.1007/BF02245413. [DOI] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M. Effects of diabetes on methamphetamine-induced place preference in mice. Eur J Pharmacol. 1996;318:251–256. doi: 10.1016/s0014-2999(96)00804-7. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- Kwok RP, Juorio AV. Concentration of striatal tyramine and dopamine metabolism in STZ-treated rats and effect of insulin administration. Neuroendocrinology. 1986;43:590–596. doi: 10.1159/000124586. [DOI] [PubMed] [Google Scholar]
- Lim DK, Lee KM, Ho IK. Changes in the central dopaminergic systems in the streptozotocin-induced diabetic rats. Arch Pharm Res. 1994;17:398–404. doi: 10.1007/BF02979114. [DOI] [PubMed] [Google Scholar]
- Marshall JF. Further analysis of the resistance of the STZ-treated rat to d-amphetamine. Pharmacol Biochem Behav. 1978;8:281–286. doi: 10.1016/0091-3057(78)90317-9. [DOI] [PubMed] [Google Scholar]
- Mayer LA, Parker LA. Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology. 1993;112:189–194. doi: 10.1007/BF02244909. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology. 1988;94:119–125. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- Owens WA, Sevak RJ, Galici R, Chang X, Javors MA, Galli A, France CP, Daws LC. Deficits in dopamine clearance and locomotion in hypoinsulinemic rats unmask novel modulation of dopamine transporters by amphetamine. J Neurochem. 2005;94:1402–1410. doi: 10.1111/j.1471-4159.2005.03289.x. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68:11–20. doi: 10.1159/000054345. [DOI] [PubMed] [Google Scholar]
- Ranje C, Ungerstedt U. High correlations between number of dopamine cells, dopamine levels and motor performance. Brain Res. 1977;134:83–93. doi: 10.1016/0006-8993(77)90927-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rowland N, Joyce JN, Bellush LL. Stereotyped behavior and diabetes mellitus in rats: reduced behavioral effects of amphetamine and apomorphine and reduced in vivo brain binding of [3H]spiroperidol. Behav Neurosci. 1985;99:831–841. doi: 10.1037//0735-7044.99.5.831. [DOI] [PubMed] [Google Scholar]
- Saller CF. Dopaminergic activity is reduced in STZ-treated rats. Neurosci Lett. 1984;49:301–306. doi: 10.1016/0304-3940(84)90306-9. [DOI] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci USA. 2006;103:7476–7481. doi: 10.1073/pnas.0600839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, France CP. Streptozotocin-induced diabetes differentially modifies haloperidol- and gamma-hydroxybutyric acid (GHB)-induced catalepsy. Eur J Pharmacol. 2005;517:64–67. doi: 10.1016/j.ejphar.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Galli A, France CP. Insulin replacement restores the behavioral effects of quinpirole and raclopride in streptozotocin-treated rats. J Pharmacol Exp Ther. 2007a;320:1216–1223. doi: 10.1124/jpet.106.115600. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Owens WA, Koek W, Galli A, Daws L, France CP. Evidence for D2 receptor mediation of amphetamine-induced normalization of locomotion and dopamine transporter function in hypoinsulinemic rats. J Neurochem. 2007b;101:151–159. doi: 10.1111/j.1471-4159.2006.04358.x. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology. 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and Parkinsonism. Mov Disord. 2003;18:S71–S80. doi: 10.1002/mds.10578. [DOI] [PubMed] [Google Scholar]
- Wolfe WL, Maisto SA. The relationship between eating disorders and substance abuse: moving beyond co-prevalence research. Clin Psychol Rev. 2000;20:617–631. doi: 10.1016/s0272-7358(99)00009-4. [DOI] [PubMed] [Google Scholar]