Abstract
The syntheses of an important class of hitherto unreported 1,3,5-pyrazoles, inspired by an unanticipated eliminatory ring opening are described. The reported pyrazole compounds were constructed through the Huisgen cyclization of 2-methylene-1,3,3-trimethylindoline and an in situ generated nitrile imine. The newly formed spiro-pyrazoline intermediate presumably then undergoes a ring opening/elimination process to afford a pyrazole, as evidenced by single X-ray crystal data. The current report constitutes the first formal observation of this kind of ring opening involving a spiro-pyrazoline intermediate.
Keywords: Cycloaddition, Ring Fragmentation, Regioselectivity, Heterocycles, Spirocycles
Introduction
Heterocycles are popularly known for displaying a wide range of biological properties.1 The recent success of pyrazole based COX-II inhibitors and their application in medicinal chemistry have amplified the importance of pyrazoles to even a greater extent.2 Several pharmaceutical drugs including celecoxib2 and rimonabant3 utilize the pyrazole as their core molecular entity,4,5 and a regioselective synthetic method for the synthesis of trisubstituted pyrazoles is still in demand.3,5-7 Pyrazoles and pyrazolines are often synthesized by the 1,3-dipolar cycloaddition reaction of nitrilimines with alkynes,8 alkyne surrogates,6,9 or alkenes,5,7,8 and other methods.4 Special attention is warranted towards the synthetic design and development of pyrazoles because of their high demand in academic and pharmaceutical sectors. Herein we report the synthesis of 1,3,5-trisubstituted pyrazoles through a 1,3-dipolar cycloaddition/ring opening process.
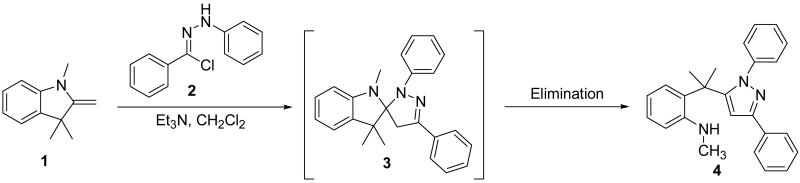
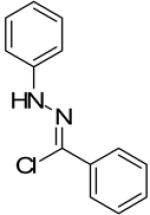
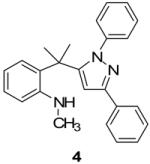
In continuance of our interest in the 1,3-dipolar cycloaddition chemistry, we describe our endeavor towards the synthesis of 1,3,5-trisubstituted pyrazoles on the basis of the 1,3-dipolar cycloaddition protocol,8,10 which was then followed by an unforeseen ring opening. During our investigation on the synthesis of spiro-pyrazolines, we discovered that when 2-methylene-1,3,3-trimethylindoline (1) was reacted with the in situ generated nitrile imine from hydrazonyl chloride11 (2), pyrazole (4) was the only product that was isolated rather than the expected spiro-pyrazoline (3) (Scheme 1). The formation of a trisubstituted pyrazole is in quite contrast to the expected spiro-pyrazoline product. On the basis of this result, we decided to investigate the generality of this 1,3-dipolar cycloaddition /ring opening reaction towards the synthesis of 1,3,5-trisubstituted pyrazoles.
Scheme 1.
Pyrazole synthesis from the spiro-pyrazoline intermediate 3.
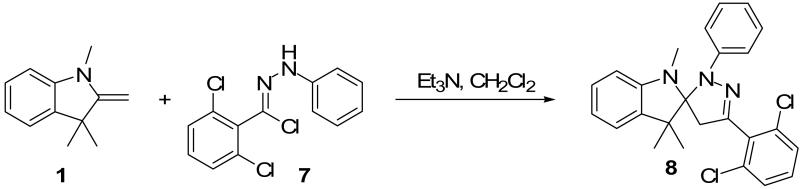
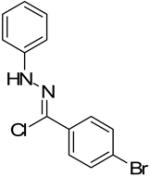
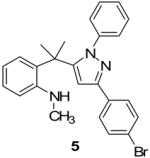
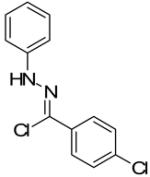
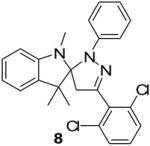
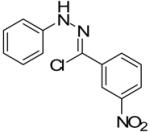
Two additional hydrazonyl chlorides containing an electron withdrawing group in the para position of the aromatic ring were exposed to triethylamine to afford the analogous nitrile imines in situ. These nitrile imines then followed the aforementioned 1,3-dipolar cycloadditon/ring opening reaction pathway to afford the corresponding pyrazoles. (Table 1) Interestingly, when 2,6-dichlorophenyl hydrazonyl chloride (7) was used, only the spiro-pyrazoline (8) was isolated. (Scheme 2) Two additional hydrazonyl chlorides possessing electron withdrawing groups in either the ortho- or meta- positions as well as electron donating groups at the ortho- and para-positions reacted with 1 to give the expected spiro-pyrazoline as the only product. (Table 2) It appears that either a hydrogen or an electron withdrawing group in the para- position of the aromatic ring of the spiro-pyrazoline intermediate is required for the rupturing of the spirocyclic ring to give rise to the pyrazole product.
Table 1.
1,3,5-trisubstituted pyrazoles isolated from the 1,3-dipolar cycloaddition reaction.
| Entry | Alkene | α-Chloro hydrazone | Product | Yield |
|---|---|---|---|---|
| 1 | 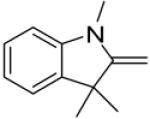 |
 |
 |
88% |
| 2 | 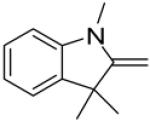 |
 |
 |
84% |
| 3 | 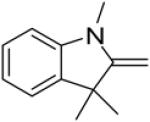 |
 |
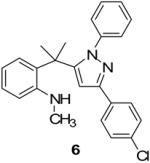 |
75% |
Scheme 2.
Synthesis of spiro-pyrazoline (8) without ring cleavage.
Table 2.
Spiro-pyrazolines isolated from the 1,3-dipolar cycloaddition reaction.
| Entry | Alkene | α-Chloro hydrazone | Product | Yield |
|---|---|---|---|---|
| 4 |  |
 |
 |
89% |
| 5 | 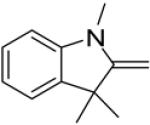 |
 |
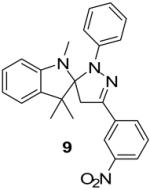 |
78% |
| 6 | 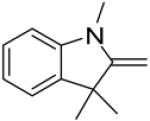 |
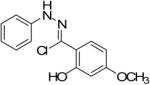 |
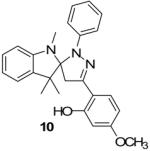 |
76% |
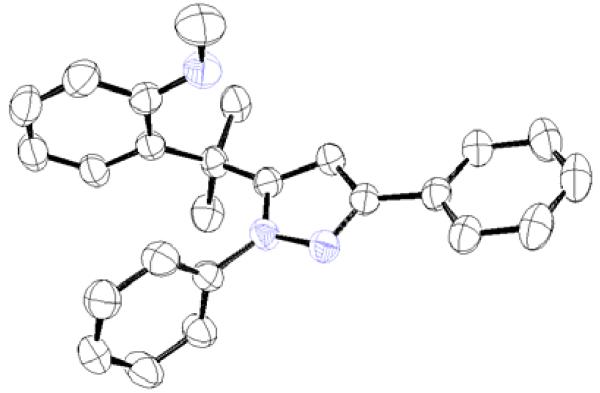
The existence of pyrazole (4) as a crystalline solid enabled us to perform the X-ray studies to reveal its stereo-structural features. Compound 4 was unambiguously confirmed by the X-ray structural analysis as a trisubstituted pyrazole rather than the expected spiro-pyrazoline. (Figure 1) The structures of the remaining pyrazoles were elucidated based upon their NMR spectroscopic data and their comparison to pyrazole (4).
Figure 1.
Thermal ellipsoid plot of the structure of pyrazole (4).12
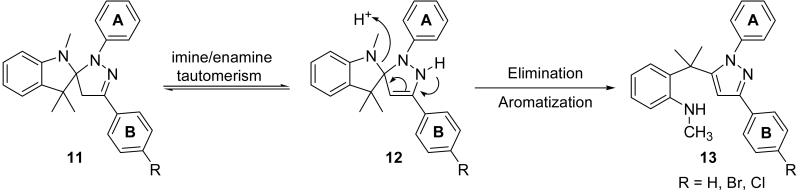
A proposed mechanism for formation of the pyrazole via spiro-pyrazoline ring opening is shown in Scheme 3. After the formation of the cycloadduct (11), an imine to enamine tautomerization occurs to form the enamine tautomer (12). (Scheme 3) Instead of compound 12 reverting to 11, the enamine tautomer undergoes an eliminatory ring cleavage of the indoline ring at the spirocyclic carbon affording pyrazole (13). The X-ray crystal data from 4 show the benzene ring B is nearly coplanar to the pyrazole. The presence of a coplanar relationship between phenyl ring B in the spiro-pyrazoline intermediates 11 and 12 would assist in pi-orbital contact between the phenyl ring B and the spiro-pyrazoline imine and enamine pi systems. On the other hand, an 84 degree twisting of the phenyl ring A occurs relative to the pyrazole (4) in order to avoid any potential steric interaction with the substitutents on the adjacent quaternary carbon. If the aforementioned interactions are present in the spiro-pyrazoline intermediate, the driving force behind the ring opening could be a combination of the relief of strain at the spirocyclic carbon along with concomitant formation of the aromatic pyrazole ring.
Scheme 3.
Proposed mechanism for the spiro-pyrazoline ring fragmentation to form the corresponding pyrazole.
Conclusions
In summary, we report a unique route for the synthesis of novel 1,3,5-trisubstituted pyrazoles13 through a novel 1,3-dipolar cycloaddition/ring opening of a spiro-pyrazoline intermediate.14 The construction of the aromatic pyrazole ring could be the driving force behind the eliminative ring opening process. Along with NMR data, X-ray crystallographic analysis also confirms the structure of the distinctive pyrazole compounds. Future investigations of the ring opening mechanism and the proposed imine/enamine tautomerization of spiro-pyrazolines are in progress.
Acknowledgements
We thank the National Institutes of Health MBRS-SCORE and RCMI programs (3S06 GM 008047-34S1 and G12RR13459 (NMR and Analytical CORE facilities)) and the National Science Foundation NSF-RISE program (HRD-0734645). EJV gratefully acknowledges the support of the National Science Foundation grant MRI 0618148 and the W. M. Keck Foundation for crystallographic resources.
References
- 1.Shen D, Shu M, Chapman KT. Org. Lett. 2000;2:2789. doi: 10.1021/ol006197h. [DOI] [PubMed] [Google Scholar]
- 2.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. J. Med. Chem. 1997;40:1347. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 3.Deng X, Mani NS. Org. Lett. 2008;10:1307. doi: 10.1021/ol800200j. [DOI] [PubMed] [Google Scholar]
- 4.(a) Katritzky AR, Wang M, Zhang S, Voronkov MV. J. Org. Chem. 2001;66:6787. doi: 10.1021/jo0101407. [DOI] [PubMed] [Google Scholar]; (b) Elguero J. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 3. Pergamon Press; Oxford: 1996. p. 1. [Google Scholar]
- 5.Deng X, Mani NS. Org. Lett. 2006;8:3505. doi: 10.1021/ol061226v. [DOI] [PubMed] [Google Scholar]
- 6.Donohue AC, Pallich S, McCarthy TD. J. Chem. Soc., Perkin Trans. 2001;1:2817. [Google Scholar]
- 7.Chiericato M, Croce PD, Carganico G, Maiorana S. J. Heterocyclic Chem. 1979;16:383. [Google Scholar]
- 8.(a) Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:565. [Google Scholar]; (b) Huisgen R. Angew. Chem. Int.Ed. Engl. 1963;2:633. [Google Scholar]
- 9.Oh L. Tetrahedron Lett. 2006;47:7943. [Google Scholar]
- 10.General Cycloaddition Procedure: A solution of the 2-Methylene-1,3,3-trimethylindoline (3 mmol) and the hydrazonyl chloride (3 mmol) in 10 mL of either dry chloroform or dichloromethane was treated with triethylamine (0.46 mL, 3.3 mmol). The reaction mixture was stirred at rt until the disappearance of the starting materials, as evidenced by TLC. After the reaction was complete, the solvent was evaporated under reduced pressure. For pyrazoles 4, 5, and 6, the crude products were purified by triturating with hexanes. For Spiro-pyrazolines 8, 9, and 10, the crude products were purified by flash column chromatography over silica gel by respectively using a 4:1, 9:1, and 7:3 hexanes-ethyl acetate eluant system.
- 11.Sibi PM, Stanley ML, Jasperse PC. J. Am. Chem. Soc. 2005;127:8276. doi: 10.1021/ja051650b. [DOI] [PubMed] [Google Scholar]
- 12.Structural information for pyrazole (4) has been deposited with the CCDC as 701051, available free of charge from www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CambridgeCrystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033).
- 13.Representative analytical data for a pyrazole: 2-(2-(1,3-diphenyl-1H-pyrazol-5-yl)propan-2-yl)-N-methylaniline (4): After concentrating the reaction mixture, the residual product was purified by triturating with hexanes, this compound was obtained as light yellow crystalline solid (0.97 g, 88%), mp 180-181°C; IR (KBr; selected peaks) ν 3407, 3132, 2967, 2816, 1600, 1579 cm−1. 1H NMR (CDCl3): δ 1.68 (s, 6H), 2.71 (s, 3H), 4.15 (br s, 1H), 6.40 (t, J = 7.5 Hz, 1H), 6.52 (d, J = 7.5 Hz, 1H), 6.67 (d, J = 8 Hz, 1H), 6.79–6.82 (m, 3H), 7.07 (q, J = 7.3 Hz, 3H), 7.19 (t, J = 7.5 Hz, 1H), 7.33-7.46 (m, 3H), 7.91 (d, J = 7Hz, 2H); 13C NMR: δ 28.9, 31.1, 38.0, 101.03, 110.8, 116.8, 125.9, 126.0(3C), 128.12(3C), 128.16(2C), 128.19, 128.7, 128.9(2C), 129.4, 133.3, 140.3, 147.2, 150.9, 153.0; HRMS (EI): m/z calcd for C25H26N3 (MH+): 368.2121; found: 368.2118.
- 14.Representative analytical data for a spiro-pyrazoline: 5′-(2,6-dichlorophenyl)-1,3,3-trimethyl-2′-phenyl-2′,4′-dihydrospiro[indoline-2,3′-pyrazole] (8): After column chromatography (hexanes-ethyl acetate, 4:1), this compound was obtained as a dark brown semi-oily liquid (1.1 g, 89%), IR (KBr; selected peaks): ν 3113, 2967, 1622, 1506 cm−1. 1H NMR: δ 1H NMR (CDCl3): δ 1.33 (s, 3H), 1.47 (s, 3H), 2.68 (s, 3H), 3.42 (d, J = 19.5 Hz, 1H), 3.78 (d, J = 19.5 Hz, 1H), 6.19 (d, J = 7.6 Hz, 1H), 6.76 (d, J = 8.9 Hz, 3H), 6.92 (t, J = 7.5 Hz, 2H), 7.08 (d, J = 7.4 Hz, 2H), 7.22-7.30 (m, 2H) 7.40 (d, J = 8.7 Hz, 2H); 13C NMR: δ 21.3, 29.0, 29.3, 31.1, 41.4, 48.3, 100.6, 104.6 118.5, 118.6, 119.2, 121.4, 121.6, 127.8, 127.9, 128.30, 128.34, 128.5, 130.6, 132.4, 135.9, 136.1, 142.6, 145.0, 149.7. HRMS (EI): m/z calcd for C25H24Cl2N3 (MH+): 436.1347; found: 436.1344.